In addition to its role in sister chromatid cohesion, the cohesin complex subunit ZmSMC3 participates in meiotic centromere pairing in maize to ensure accurate chromosome segregation.
Abstract
Meiosis consists of two highly conserved nuclear divisions, which allow eukaryotes to maintain their chromosome number through sexual reproduction. The successful completion of meiosis depends on homologous chromosome pairing. Centromere interactions during early meiotic prophase I facilitate homologous chromosome pairing, but the underlying mechanism is unclear. Here, we performed chromatin immunoprecipitation-mass spectrometry analysis of maize (Zea mays) anthers during early meiotic prophase I using anti-centromeric histone H3 (CENH3) antibodies and determined that the cohesin subunit Structural Maintenance of Chromosome3 (SMC3) interacts with CENH3 during this period. SMC3 is enriched at centromeres and along chromosome arms in threads from leptotene to pachytene and might promote interactions between homologous centromeres. We observed dysfunctional SMC3 assembly in meiotic-specific maize mutants with defective centromere pairing. In SMC3 RNAi meiocytes, centromere pairing defects were observed during early meiotic prophase I, SMC3 was weakly associated with centromeres, and SMC3 did not localize to the chromosome arms. In wild-type mitosis, SMC3 is associated with chromatin and is enriched at centromeres from prophase to anaphase. CRISPR-Cas9-induced Zmsmc3 mutants showed premature loss of sister chromatid cohesion and mis-segregation of chromosomes in mitotic spreads. Our findings suggest that in addition to sister chromatid cohesion, ZmSMC3 participates in meiotic centromere pairing.
INTRODUCTION
Meiosis is a highly conserved eukaryotic cell division process by which diploid cells undergo a single round of DNA replication and two rounds of chromosome separation, yielding haploid gametes. This process allows the proper species-specific chromosome number to be maintained across generations. To ensure the accurate distribution of the replicated genome, homologous chromosomes must recognize each other, identify their partners, and align lengthwise during the first meiotic division. This process is known as homologous pairing (for review, see Stewart and Dawson 2008; Da Ines et al., 2014; Da Ines and White, 2015; Zhang et al., 2014). However, how chromosomes identify their correct partners, and how an accurate physical linkage between partners is established at the beginning of meiosis, remain unresolved.
At the onset of meiosis, homologous chromosomes are spatially separated from each other in the nucleus. Thus, these chromosomes must be brought into close proximity via interactions of particular chromosomal regions such as telomeres, centromeres, or specialized pairing centers (Scherthan, 2007; Ronceret and Pawlowski, 2010; Tsai and McKee, 2011; Zhang et al., 2013). In most eukaryotes, telomeres attach to the inner nuclear envelope and cluster at a particular region of the nuclear periphery to form a structure termed the “telomere bouquet” during early meiotic prophase I (Roberts et al., 2013). Telomere bouquet formation is thought to facilitate the initiation of homologous chromosome pairing by bringing chromosome ends into close proximity (Bass, 2003; Ding et al., 2004, 2007; Scherthan, 2007). However, telomere interactions alone may not be sufficient to bring long chromosomes together (Penfold et al., 2012) and cannot be responsible for the specificity of the chromosome recognition process.
Centromere interactions during early meiotic prophase I have been described in a number of species, including fission yeast (Schizosaccharomyces pombe; Scherthan et al., 1994; Ding et al., 2004), budding yeast (Saccharomyces cerevisiae; Kemp et al., 2004; Tsubouchi and Roeder, 2005), Drosophila melanogaster (Takeo et al., 2011; Unhavaithaya and Orr-Weaver, 2013), onion (Allium cepa; Church and Moens, 1976), wheat (Triticum aestivum; Martínez-Pérez et al., 1999, 2001), Arabidopsis (Arabidopsis thaliana; Da Ines et al., 2012; Ronceret et al., 2009), Brachypodium distachyon (Wen et al., 2012), barley (Hordeum vulgare; Phillips et al., 2012), and maize (Zea mays; Zhang et al., 2013). Centromere interaction processes during early meiotic prophase I include centromere clustering (association of centromeres into groups), centromere coupling (association of nonhomologous centromeres), and centromere pairing (association of homologous centromeres; reviewed in Da Ines and White, 2015). These early meiotic centromere interactions occur before telomere bouquet formation and chromosome arm pairing. Thus, this common feature during early meiosis suggests that centromere interactions play a key role in facilitating the initiation of homologous chromosome pairing (reviewed in Zhang et al., 2014; Da Ines and White, 2015). However, the molecular mechanisms that influence centromere interactions are largely unknown.
The cohesin complex has been reported to facilitate chromosome pairing during meiosis in addition to sister-chromatids cohesion (Nasmyth, 2011). This ring-shaped structure plays an important role in determining chromosome morphology (Mainiero and Pawlowski, 2014). The cohesin complex consists of a core cohesin ring and several associated proteins. The core cohesin ring includes Structural Maintenance of Chromosomes (SMC) proteins SMC1 and SMC3 and one kleisin alpha family protein SCC1 (MCD1 or RAD21) and a stromalin domain protein SCC3 (SA1 and SA2; reviewed in Nasmyth and Haering, 2005, 2009). Mitotic and meiotic cohesin complexes are different. In cohesin complexes from budding yeast and fission yeast that function during meiosis, SCC1 (or RAD21) is replaced by REC8 (Watanabe and Nurse 1999; Schleiffer et al., 2003; Watanabe, 2004). In vertebrates, RAD21 has an additional meiosis-specific ortholog called “RAD21L” (Ishiguro et al., 2011). In the cohesin complexes of mammalian systems that function during meiosis, SMC1 is replaced by SMC1β and SCC3 by STAG3 (Revenkova et al., 2004; Biswas et al., 2013; Hopkins et al., 2014). In Arabidopsis, SYN1 (DIF1) is an ortholog of REC8, and SYN2, SYN3, and SYN4 are three paralogs of SCC1 (Lam et al., 2005).
Differences in the dynamics and localization of different cohesin subunits have been identified in a number of organisms. In human oocytes, cohesin subunits REC8, STAG3, SMC1β, and SMC3 are loaded onto chromatin before the lateral element protein SCP3, and colocalize with SCP3 from leptotene to diplotene during meiotic prophase, suggesting that the cohesin subunits may serve as a basis for synaptonemal complex assembly (Garcia-Cruz et al., 2010). In Arabidopsis, REC8 colocalizes with chromatin conformation during meiosis I (Prusicki et al., 2019), SMC3 and SYN1 localize to axial elements during pachytene stage (Lam et al., 2005), SMC3 is detected in the centromere region during mitosis (Schubert et al., 2016), suggesting that they are essential for chromosome cohesion and meiotic chromosome pairing.
The major role of the cohesin complex is holding sister chromatids together before mitosis (Schubert et al., 2009). The cohesin complex also has a wide range of functions in meiotic homologous chromosome pairing, double-strand break repair, meiotic synapsis, and centromere mono-orientation during meiotic prophase I (Nasmyth 2011). Studies in plants, animals, and fungi suggest that meiotic cohesin subunits SYN1, REC8, STAG3, and RAD21L are required for correct meiotic chromosome pairing and might serve as “barcodes” for the proper spatial alignment of homologous chromosomes (Cai et al., 2003; Chelysheva et al., 2005; Xu et al., 2005; Golubovskaya et al., 2006; Ishiguro et al., 2011; Hopkins et al., 2014; Ding et al., 2016). However, little is known about the relationships between centromere interactions and the cohesin complex during early meiosis.
We previously observed centromere pairing during early meiotic prophase I in maize and demonstrated that this process is crucial for the initiation of homologous chromosome pairing (Zhang et al., 2013). In this study, to explore which proteins participate in this process, we performed chromatin immunoprecipitation (ChIP)-mass spectrometry (MS) using anti-centromeric histone H3 (CENH3) antibodies and determined that SMC3 interacts with functional centromeres during early meiotic prophase I, a time when centromere pairing occurs in maize. The loading of ZmSMC3 was affected in meiosis-specific mutants with abnormal centromere interactions. In smc3 mutants, precocious separation of sister chromatids occurred during mitotic pro-metaphase, and defective centromere pairing and altered chromosome morphology were observed during early meiotic prophase I. Thus, we uncovered a novel role for SMC3 in meiotic centromere pairing in addition to its known role in sister chromatid cohesion in maize.
RESULTS
SMC3 Interacts with CENH3 during Early Meiotic Prophase I
To identify proteins involved in centromere pairing during early meiotic prophase I in maize, we collected ∼5 g of fresh anthers in early meiotic prophase I and subjected them to native ChIP-MS (chromatin immunoprecipitation mass spectrometry). The same amount of anthers after metaphase I was used as a control. We subjected immunoprecipitates obtained with anti-CENH3 antibodies or IgG to liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS) analysis. Proteins detected by LC-MS/MS analysis after digestion with trypsin peptidase are listed in Supplemental Data Sets 1 and 2. ChIP-MS against CENH3 identified 414 interactors in early meiotic prophase I anthers, including the conserved kinetochore assembly protein Centromere protein C and CENH3 itself (Supplemental Data Set 1). ChIP-immunoblot analysis also indicated that centromere proteins were immunoprecipitated with anti-CENH3 antibody.
Based on the coverage of proteins, we identified a protein that interacted with CENH3 during early meiotic prophase I that was not detected in the control samples (Supplemental Data Set 2). The gene ID is ZEAMMB73_Zm00001d039189. BLAST analysis (https://blast.ncbi.nlm.nih.gov) revealed the identity of the protein as SMC3 (LOC103630807). Because SMC3 and SMC1 are the two most extensively studied subunits of the core cohesin complex, we cloned the SMC3 gene in maize and investigated its functions during early meiosis.
SMC3 Cloning in Maize
We cloned the maize SMC3 gene based on the sequence of LOC103630807. The maize genome contains one copy of SMC3. The ZmSMC3 genomic sequence is ∼18 kb long, and the ZmSMC3 cDNA is 3,615 bp long. ZmSMC3 is located on the end of the long arm of chromosome 6 and contains 28 exons and 27 introns. ZmSMC3 encodes a 1,204-amino acid protein with a calculated molecular weight of 138 kD. Based on its genomic sequence, ZmSMC3 contains all the characteristic features of SMC proteins: an ATP binding cassette domain at the N terminus (amino acids 3 to 166) and a P-loop NTPase at the C terminus (amino acids 1,070 to 1,184) as well as two extended coiled-coil domains separated by a hinge (amino acids 521 to 634) located in the middle of the protein.
Using the leaves, shoots, and anthers of maize inbred line B73, we analyzed the expression pattern of ZmSMC3 by RT-PCR. ZmSMC3 was expressed in all three tissues, with higher expression levels in tissues with vigorous cell division, i.e. shoots and anthers. Therefore, ZmSMC3 expression is not meiosis-specific (see Supplemental Figure 1A).
As ChIP-MS cannot discriminate between direct versus indirect interactions, we performed a yeast two-hybrid assay to verify the interaction between SMC3 and CENH3. Three overlapping ZmSMC3 truncations including ZmSMC3-N (1 to 1,950 bp), ZmSMC3-M (901 to 2,700 bp), and ZmSMC3-C (1,951 to 3,615 bp) were used to detect interactions with CENH3. All three fragments failed to interact with CENH3, indicating that SMC3 does not directly interact with CENH3.
ZmSMC3 Dynamics during the Mitotic Cell Cycle
To investigate the subcellular distribution and localization of SMC3 in maize, we generated an antibody against ZmSMC3. We synthesized the polypeptide C-SKEEALDFIERDQTYN based on the C-terminus region (amino acids 1,187 to 1,202) of ZmSMC3 and used it to raise a monoclonal antibody in mouse. This antibody was specific for ZmSMC3. Immunoblot analysis using this antibody detected a single 138-kD protein band in nucleoprotein extracts isolated from B73 shoots (see Supplemental Figure 1B).
We performed immunolocalization experiments using root tips that had been fixed in 4% formaldehyde. Immunostaining revealed strong background SMC3 signals in these somatic cells. However, when we performed immunostaining experiments with anthers, which contained numerous somatic cells, dynamic changes in ZmSMC3 signals were clearly observed in these somatic cells. Immunostaining of somatic cells from anthers initially revealed SMC3 signals as diffuse staining patterns in the nucleus at interphase (Figure 1A), when chromosomes are replicated and sister chromatids are held together by the cohesin complex. During this stage, strong labeling of ZmSMC3 was observed in the centromere region, as revealed by labeling of CENH3 (Figure 1A). When the cells entered into prophase, fluorescent signals of SMC3 were associated with chromatin and were detected in chromosome arms and centromere regions (Figure 1B). This localization pattern was maintained until metaphase, when ZmSMC3 signals became weaker (Figure 1C). According to these results, the cohesin subunit SMC3 likely plays a role in mitotic cell division.
Figure 1.
SMC3 Localizes to Chromosomes and Is Enriched at the Centromere Regions in Somatic Maize Cells.
(A) Interphase.
(B) Prophase.
(C) Pro-metaphase.
CENH3 is shown in red; SMC3 is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. The merged images are shown on the left. Scale bar = 10 μm.
ZmSMC3 Is Continuously Enriched at the Centromere Region during Early Meiosis
To further investigate the localization of SMC3 during early meiotic prophase I in maize, we performed double immunostaining analysis of pollen mother cells using anti-SMC3 and anti-CENH3 antibodies. SMC3 was first detected as weakly diffuse signals in the nucleus and as strong signals at the centromere regions at the pre-meiotic interphase stage (Figure 2A). When meiocytes entered into meiotic prophase I, SMC3 was present in the centromeric regions of all chromosomes; and during leptotene, when centromere pairing occurred, SMC3 was also detected along the chromosome arms (Figure 2B). During the zygotene stage, when homologous chromosomes form synapses (Figure 3), SMC3 localized to homologous chromosomes that had not yet synapsed (Figures 3B and 3C), suggesting that SMC3 was located between sister chromatids. During this stage, strong labeling of SMC3 was detected in the centromeric regions of all chromosomes and localized to chromatin threads as discontinuous dots, which formed a threadlike pattern along the chromosome arm (Figure 2C). At pachytene, fully synapsed homologs were labeled with SMC3, and enriched SMC3 signals were detected at all centromeres (Figure 2D). Remarkably, SMC3 localized onto chromatin threads and showed enrichment at the centromere regions of all chromosomes from the leptotene to pachytene stages, a period of centromere pairing, with fluorescent signals from SMC3 at the centromere regions becoming stronger (Figures 2B to 2D). During metaphase I and anaphase I, SMC3 signals were still observed in the centromeric regions, but not along the chromosome arms, and the fluorescence intensity of these signals at the centromeric regions was weak compared with that during meiotic prophase I (Figures 2E and 2F). In summary, SMC3 signals were dynamic during meiosis and were continuously enriched in the centromere region during early meiotic prophase I, suggesting that SMC3 participates in centromere interactions during meiotic chromosome pairing.
Figure 2.
Dynamics of SMC3 in Maize during Meiosis I.
(A) Pre-meiotic interphase.
(B) Leptotene.
(C) Zygotene.
(D) Pachytene.
(E) Metaphase I.
(F) Anaphase I.
CENH3 is shown in red; SMC3 is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. The merged images are shown on the left. Scale bar = 10 μm.
Figure 3.
SMC3 Localizes along Sister Chromatids during Meiosis.
(A) to (D) Zygotene stage.
(A) Flat projections of three-dimensional images of whole nuclei.
(B) to (D) Single optical section from nuclei in (A).
Arrows indicate unsynapsed homologous chromosomes. SMC3 is shown in red; ZYP1 is shown in green. Blue, chromosomes counterstained with DAPI. Scale bar = 10 μm.
SMC3 Participates in Centromere Pairing and the Meiosis-specific Cohesin Subunit REC8 Is Required for SMC3 Assembly
To explore the role of SMC3 in early meiotic prophase I, we examined its distribution in the meiosis-specific Absence of First Division1 (AFD1) maize mutant afd1-1. This null mutant carries a deletion in the afd1 gene, encoding a homolog of the meiosis-specific α-kleisin REC8. This mutant exhibits defective chromosome structure and lacks the meiotic-specific sister-chromatid cohesin subunit REC8 (Golubovskaya et al., 2006). We previously detected ∼18 to 20 centromere signals in this mutant from leptotene to pachytene, indicating a failure of centromere pairing during early prophase I (Zhang et al., 2013). Immunostaining of the afd1-1 mutant revealed no SMC3 on meiotic chromatin from leptotene to pachytene (Figure 4). These results indicate that SMC3 is not located in chromatin during early meiotic prophase I when centromere pairing is disrupted in the absence of REC8, suggesting that SMC3 assembly onto meiotic chromosomes is dependent on the meiosis-specific cohesin subunit REC8.
Figure 4.
In afd1-1, Which Exhibits the Absence of Centromere Pairing during Early Prophase I, SMC3 Is Not Detectable from Leptotene to Pachytene.
CENH3 is shown in red, SMC3 is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. Scale bar = 10 μm.
SMC3 Assembly is Dysfunctional in a Meiotic-specific Mutant with Defective Chromosome Pairing and Recombination
To further investigate the role of SMC3 in maize during early meiotic prophase I, we investigated the localization of SMC3 in the maize poor homologous synapsis1 (phs1) mutant. The phs1 gene encodes a protein required for homologous chromosome pairing and for preventing synapsis between nonhomologous chromosomes (Pawlowski et al., 2004; Ronceret et al., 2009). The phs1 mutant almost completely lacks chromosomal foci of the recombination protein RAD51 and exhibits defects in early recombination; synapsis primarily takes place between nonhomologous chromosomes in this mutant (Pawlowski et al., 2004; Ronceret et al., 2009). In phs1, we observed 16.8 ± 1.7 centromere signals during leptotene (n = 34), 17.1 ± 1.8 signals during zygotene (n = 40), and 17 ± 1.7 signals during pachytene (n = 33; Figures 5C and 5D). These results indicate that phs1 exhibits incomplete centromere pairing in early prophase I compared with the wild type (Figures 5A and 5B). Immunostaining showed that SMC3 signals at the centromeric regions became very weak from leptotene to pachytene compared with the wild type (Figures 5C and 5D). Moreover, SMC3 signals disappeared from the chromosome arms during this period. These results suggest that the defects in SMC3 assembly in phs1 during early meiotic prophase I are related to improper homologous chromosome recombination. In immunostaining experiments with the maize afd1-1 mutant, SMC3 signals disappeared or became very weak when centromere pairing was disrupted or incomplete. Together, these findings suggest that SMC3 is involved in the centromere pairing process in maize during early meiotic prophase I.
Figure 5.
In phs1, Which Exhibits Incomplete Centromere Pairing during Early Prophase I, SMC3 Signals in Centromere Region Are Weak, and SMC3 Signals along the Chromosome Arms Are Absent from Leptotene to Pachytene.
(A) Leptotene in wild-type meiocytes.
(B) Pachytene in wild-type meiocytes.
(C) Leptotene in phs1 meiocytes
(D) Pachytene in phs1 meiocytes.
CENH3 is shown in red; SMC3 is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. The merged images are shown on the left. Scale bar = 10 μm.
Maize smc3 Mutants Show Premature Sister Chromatid Separation and Mis-segregation of Mitotic Chromosomes
To further explore the role of SMC3 in maize, we obtained SMC3 mutants that were generated using the maize Mutator (Mu) transposon system. A Mu insertion (mu1015674) occurred 105 bp upstream of the transcription starting site in UFMu-01801. Homozygous Mu insertion mutants showed defects in both embryos and endosperm and early arrest during seed development.
We performed targeted modification of ZmSMC3 using a high-efficiency clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-based genome editing system designed for maize (Feng et al., 2018). In this system, the Cas9 gene is controlled by the maize DNA Meiotic Recombinase1 (DMC1) promoter. Because the DMC1 promoter is expressed during the callus stage of plant regeneration (Feng et al., 2018), mutations in ZmSMC3 could lead to callus lethality. We obtained only two transgenic events and 20 seedlings.
For transgenic event #1, we obtained only one transgenic seedling, which was very small and ultimately died (see Supplemental Figure 2B). We analyzed the transgenic seedling by PCR-restriction enzyme analysis followed by cloning and sequencing of the PCR product. Sequencing revealed that the seedling was a mosaic mutant with three mutated alleles, with a mutation ratio of 100% (see Supplemental Figures 2A and 2C). For transgenic event #2, we obtained 19 transgenic seedlings, which grew slowly (see Supplemental Figure 2B). The mutation efficiency of this event was 100% according to PCR-restriction enzyme analysis. We analyzed all 19 transgenic seedlings by sequencing, finding that they were mosaic mutants with insertion and deletion mutations in the target site. The mutation ratios of these mutants from transgenic event #2 ranged from 25% to 50% (see Supplemental Figures 2A and 2C).
To investigate the effects of the smc3 mutation on mitosis, we harvested the root tips of SMC3 CRISPR/Cas9-generated transgenic seedlings and used them to prepare mitotic spreads. In the smc3 mutants, three classes of mitotic spreads were observed at prometaphase. The first class was normal (Figure 6A), the second contained completely separated sister chromatids at high frequency (Figure 6B), and the third displayed a loss of arm cohesion (Figure 6C). At anaphase, all smc3 mutants exhibited high ratios of mis-segregation (Figures 6E and 6F). Micronuclei were detected at telophase and interphase in the smc3 mutants (Supplemental Figure 3), apparently representing the products of mis-segregation. When we performed immunostaining of the smc3 mutants using anti-tubulin antibody, atypical spindle morphology was observed at metaphase and anaphase (Figures 7B and 7D) compared with the wild type (Figures 7A and 7C), perhaps due to incorrect attachment of kinetochores to the spindles. The precocious separation of sister chromatids at pro-metaphase and the mis-segregation of chromosomes at anaphase in the smc3 mutants indicate that SMC3 is important for sister chromatid cohesion and chromosome segregation during mitosis.
Figure 6.
Mitotic Spreads Prepared from smc3 Mutants Generated By CRISPR/Cas9 Genome Editing.
(A) to (C) Pro-metaphase.
(D) to (F) Anaphase.
(A) and (D) show normal mitotic spreads.
(B) Complete loss of cohesion.
(C) Loss of arm cohesion.
(E) and (F) Mis-segregation.
Scale bar = 10 μm.
Figure 7.
Spindle Morphology in the smc3 Mutants.
(A) and (B) Metaphase.
(C) and (D) Anaphase.
(A) and (C) show normal spindle morphology.
(B) and (D) Atypical spindle morphology.
Tubulin is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. Scale bar = 10 μm.
Among the smc3 CRISPR/Cas9-generated transgenic mutants, transgenic seedlings with high mutagenesis efficiency developed abnormally and eventually died. This result confirms former observations for different organisms that knockout of core cohesin subunits leads to lethality (Lam et al., 2005). All of these transgenic plants were mosaic mutants, and we did not obtain any homozygous CRISPR/Cas9-generated transgenic mutants.
Knock-down of SMC3 Causes Defective Centromere Pairing during Early Meiotic Prophase I and Abnormal Chromosome Morphology
To further investigate the functions of ZmSMC3 in meiosis, we generated RNA interference (RNAi) transgenic lines of ZmSMC3. We cloned a 418-bp fragment of the maize ZmSMC3 coding sequence into the pUCCRNAi vector and obtained six SMC3 RNAi transgenic events and 149 seedlings. Of these SMC3 RNAi transgenic lines, three grew more slowly and were shorter than the wild type (Figure 8A), and one line (transgenic event #3) exhibited severe sterility (Figures 8B to 8D). RT-PCR revealed that the RNAi lines had substantially reduced SMC3 transcript levels.
Figure 8.
Phenotypes of Wild-Type and SMC3 RNAi Transgenic Plants.
(A) Phenotypes of three SMC3 RNAi transgenic lines and the wild type. #1-2, #2-10-1, and #3-4 are plants selected from SMC3 RNAi transgenic events #1, #2, and #3, respectively.
(B) Phenotypes of SMC3 RNAi transgenic event #3 and the wild type.
(C) Tassel of plant #3-6-1 from SMC3 RNAi transgenic event #3.
(D) Tassel of plant #3-8-1 from SMC3 RNAi transgenic event #3.
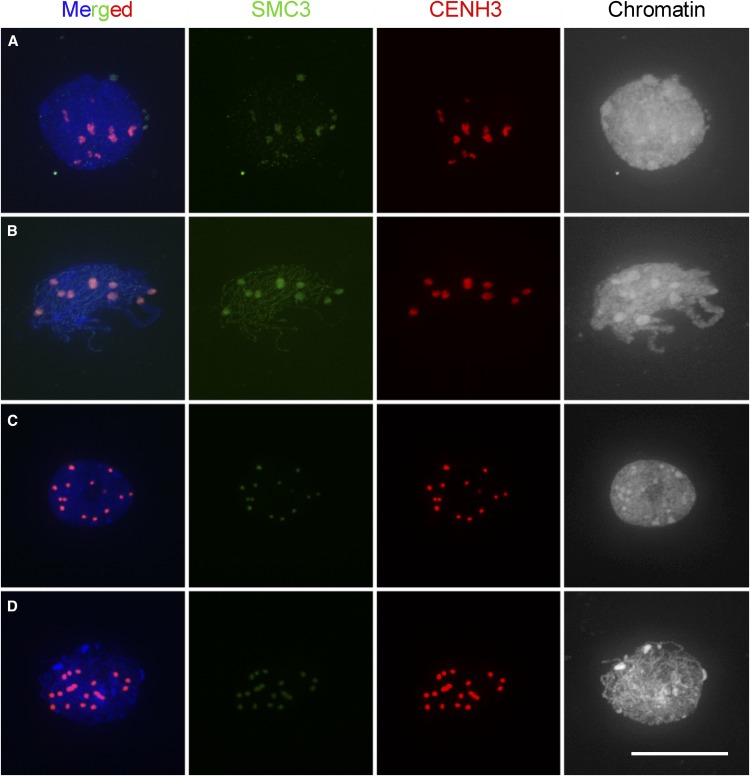
Cytological examination of meiocytes in SMC3 RNAi transgenic event #3 revealed that, in contrast with the wild type, the centromeres were not paired completely in early meiotic prophase I (Figure 9). We observed 16.1 ± 1.1 centromere signals at the leptotene stage (n = 34; Figure 9A), 16.7 ± 1.2 signals at the zygotene stage (n = 35; Figures 9B and 9C), and 15.7 ± 2.8 signals at the pachytene stage (n = 35; Figure 9D). In the SMC3 RNAi transgenic mutants, the fluorescence intensity of SMC3 signals at the centromere regions became very weak from leptotene to pachytene compared with the wild type, and SMC3 staining was no longer observed on chromosome arms (Figures 9B to 9D). Chromosome morphology was particularly altered at pachytene (Figure 9D). ZYP1 signals were also absent in the SMC3 RNAi lines, indicating that SC assembly was affected in these mutants. The centromere pairing defects in the SMC3 RNAi transgenic mutants indicate that ZmSMC3 plays an important role in meiotic centromere pairing in maize. Thus, our work uncovered a novel role of SMC3 in participating in meiotic centromere pairing in plants for the first time.
Figure 9.
Centromere Behavior and SMC3 Localization in SMC3 RNAi Transgenic Event #3 Meiocytes during Meiosis I.
(A) Leptotene.
(B) Zygotene.
(C) Late Zygotene.
(D) Pachytene.
(E) Metaphase I.
CENH3 is shown in red; SMC3 is shown in green. Images are flat projections of three-dimensional images of whole nuclei. Blue, chromosomes counterstained with DAPI. The merged images are shown on the left. Scale bar = 10 μm.
DISCUSSION
We previously demonstrated that homologous centromere pairing persists during early meiotic prophase I and is essential for initiating homologous chromosome pairing in maize (Zhang et al., 2013). Centromere interactions during early meiotic prophase are considered to be a general feature of eukaryotes, as suggested by studies in diverse species (Martínez-Pérez et al., 1999; Tsubouchi and Roeder, 2005; Ronceret et al., 2009; Takeo et al., 2011; Da Ines et al., 2012; Phillips et al., 2012; Wen et al., 2012), although the molecular mechanism underlying this phenomenon is poorly understood. Our findings show that in maize, the major core cohesin complex subunit SMC3 facilitates centromere pairing during early meiotic prophase I.
In Addition to Sister Chromatid Cohesion, Cohesin Contributes to Meiotic Centromere Pairing
Cohesin is an evolutionarily conserved multiprotein complex that plays a pivotal role in chromosome dynamics. The cohesin complex is essential for sister chromatid cohesion and for establishing higher-order chromosome architecture, and it also plays specific roles in various meiosis-associated chromosomal events. During meiosis, cohesin contributes to chromosome axis formation (Fukuda et al., 2014; Hopkins et al., 2014; Ward et al., 2016; Biswas et al., 2018), chromosome pairing (Golubovskaya et al., 2006; Ishiguro et al., 2011; Hopkins et al., 2014), meiotic recombination (Prieto et al., 2001; Yan and McKee, 2013), homolog association (Golubovskaya et al., 2006), and centromere mono-orientation (Watanabe and Nurse, 1999; Chelysheva et al., 2005; Tachibana-Konwalski et al., 2013).
Cohesin has been shown to localize to the centromere region in several species. Cohesin is localized to the core centromere region in fission yeast (Sakuno et al., 2009), in mouse (Kim et al., 2015), and in Arabidopsis (Schubert et al., 2016). In D. melanogaster oocytes, cohesins establish centromeric cohesion before the onset of meiosis and are enriched at the centromere region; this centromeric cohesin can promote centromere clustering (Yan and McKee, 2013; Hatkevich et al., 2019). The dynamics and localization of SMC3 have been reported in a number of organisms. Previous studies on the function of SMC3 in plants and nonplant organisms did not focus on meiotic centromere pairing. In this study, we analyzed the effects of SMC3, a major core cohesin subunit, on mitosis and meiosis in maize. ChIP-MS revealed that SMC3 binds to CENH3-chromatin during centromere pairing. Immunostaining showed that during early meiotic prophase I, SMC3 was enriched in the centromeric region from leptotene to pachytene, a period of meiotic centromere pairing. These findings suggest that SMC3 takes part in the progression of meiotic centromere pairing. The smc3 mutants exhibited premature loss of sister chromatid cohesion, mis-segregation of chromosomes, and abnormal spindle morphology during mitosis and showed incomplete centromere pairing and altered chromosome structure during early meiotic prophase I. These loss-of-function phenotypes indicate that SMC3 is involved in sister chromatid cohesion, as well as in meiotic centromere pairing.
Possible Mechanisms Underlying the Regulation of Chromosome Pairing by Meiotic Cohesin Subunits
During meiotic prophase I, homologous chromosomes must recognize each other and align along their lengths. During this period, these chromosomes undergo a series of movements and rearrangements to overcome topological challenges. The cohesin complex is essential for these meiotic chromosomal rearrangements (Rankin, 2015).
A recent report on Drosophila describes a model in which the enrichment of SMC1 at the centromere combined with chromosome movements induce centromere clustering at the onset of meiosis and that this SMC1-dependent centromere clustering stabilizes the initial homologous chromosomal pairing during early meiosis (Hatkevich et al., 2019). The mitotic cohesin complex contains four subunits: SMC1, SMC3, SCC1, and SCC3. However, the properties of cohesin complexes and their roles in meiosis might differ depending on the organism.
In our work, SMC3 was identified by ChIP-MS analysis. We performed ChIP-MS more than 15 times and SMC3 could be detected with high coverage of peptides every time. Another cohesin subunit SMC1 was also detected by ChIP-MS several times. We also cloned SMC1 of maize and generated antibodies. In maize, SMC3 is enriched at the centromere regions during meiotic prophase I. However, immunolocalization results revealed that SMC1 signals were distributed along chromosome arms without obvious enrichment in centromere regions (see Supplemental Figure 4A). The possibility for the different observations between SMC1 and SMC3 signals could be due to antibodies behaving differently, or accessibility of the epitope. However, AFD1, the meiosis-specific kleisin of maize, showed a similar signal pattern to SMC1 during early meiotic prophase I (Golubovskaya et al., 2006). These findings indicate that different cohesin subunits may play different roles during meiosis in maize, and that centromeric SMC3 may contribute to meiotic interactions of homologous centromeres in maize. This process may be independent of SMC3′s role as a cohesin subunit, because other cohesin subunits were not enriched in centromere regions. Furthermore, SMC3 colocalizes with the synaptonemal complex during meiotic prophase I in different plant and nonplant organisms, suggesting a function of SMC3 in synaptonemal complex assembly.
In SMC3 RNAi transgenic meiocytes, centromere pairing was perturbed, and SMC3 enrichment at the centromere was reduced, but SMC3 was absent along the chromosome arm. The localization of SMC3 in SMC3 RNAi transgenic meiocytes was the same as that in the maize phs1 mutant, which exhibits early recombination defects and synapsis primarily between nonhomologous chromosomes. SMC3 localized along the chromosome arm can form an axial element with other cohesin complexes. Axial elements play an important role in providing a barrier to recombination between sister chromatids. Cohesin is important for recombination and repair of DNA double-strand breaks. In SMC3 RNAi transgenic meiocytes, synaptonemal complex assembly is also defective, suggesting that loading of SMC3 is essential for synaptonemal complex assembly. In Drosophila, two meiosis-specific cohesin complexes are required for synaptonemal complex assembly, and one complex is essential for promoting interactions between homologs (Gyuricza et al., 2016). Meiotic chromosome pairing, recombination, and synapsis are core events during meiotic progression and are highly interrelated. In maize, SMC3 is loaded onto the centromere regions during pre-meiotic interphase, which occurs before the onset of meiosis. Perhaps centromeric SMC3 initiates meiotic centromere pairing, and the SMC3 along the chromosome arms stabilizes meiotic chromosome synapsis and recombination.
The SMC Family and Centromere Pairing
We previously demonstrated that SMC6 takes part in early meiotic prophase I, colocalizes with the synaptonemal complex, and is also required for early meiotic centromere pairing (Zhang et al., 2013). In Arabidopsis, Brassica (Brassica rapa L.), and rye (Secale cereale), SMC5/6 show synaptonemal complex localization and might facilitate the formation and/or stabilization of synapsis (Hesse et al., 2019; Zelkowski et al., 2019). SMC5/6 are also reported to be involved in meiotic double-strand breaks, meiotic recombination, centromere cohesion, and heterochromatin maintenance (Hesse et al., 2019). In maize, SMC3 and SMC6 participate in the progression of meiotic centromere pairing, suggesting that higher-order interactions between meiotic chromosome structures function not only in maintaining chromatin structure, but also in meiotic chromosome dynamics including chromosome pairing. A recent study in maize on condensin subunits SMC2 and SMC4 shows centromeric enrichment during mitosis (Wang et al., 2019), suggesting a centromere-related function of SMC2 and SMC4. Cohesin, condensin, and the SMC5/6 complex are members of the SMC protein family, which is evolutionarily conserved among different organisms and act in basic biological processes such as sister chromatid cohesion, chromosome condensation, transcription, replication, DNA repair, and recombination (Jeppsson et al., 2014). In the future, more work is needed to fully elucidate the role(s) of the SMC protein family during meiotic progression.
METHODS
Plant Materials
Maize (Zea mays) inbred lines KYS and B73 were used as wild type for cytological analysis. Genotyping of the afd1-1 mutants was conducted with rec8-specific primers: forward 5′-GGTGGCTCTGGATCGGCGTTTATAC-3′ and reverse: 5′-GCCCCCTTTTGGACCTGTGTTCA-3′. After MboII digestion of the resulting PCR product, a 360-bp band was produced in the wild type, whereas a 280-bp band was produced in the homozygous mutants.
Genotyping of the phs1 mutants was conducted with primers Mu-TIR: 5′-CTCTTCGTCYATAATGGCAA-3′, PHS-LP: 5′-ACATTGCTTGCTTCCATCGT-3′, and PHS-RP: 5′-AAAGATCGACTCCACACCAAAT-3′. Plants containing insertions that are either homozygous or heterozygous yield a ∼650-bp product when using Mu-TIR/PHS-LP. An ∼300-bp product is amplified when using PHS-LP/PHS-RP in the wild type.
All of the maize plants were grown in the farm field or under greenhouse conditions (28°C, 16-h light/8-h dark) to obtain anthers containing pollen mother cells during meiosis.
ChIP-MS
ChIP was performed as previously described by Nagaki et al. (2003) with minor modifications. Approximately 5 g of fresh anthers in early meiotic prophase I and metaphase I from maize inbred line B73 was ground to a fine powder in liquid nitrogen and resuspended in 30 mL of nuclei isolation buffer 1 (10 mM of potassium phosphate at pH 7.0, 0.1 M of NaCl, 0.1% [v/v] β-mercaptoethanol, and 12% [v/v] hexylene glycol). The suspension was incubated at 4°C for 10 min and filtered through Miracloth (Millipore). The nuclei were pelleted by centrifugation at 1,250 g for 10 min at 4°C. The nuclei were washed three times with 5 mL of nuclei isolation buffer 2 (10 mM of potassium phosphate at pH 7.0, 0.1 M of NaCl, 10 mM of MgCl2, 0.1% [v/v] β-mercaptoethanol, 12% [v/v] hexylene glycol, and 0.5% [v/v] Triton X-100) and resuspended in 1 mL of micrococcal nuclease digestion buffer (10% [w/v] Suc, 50 mM of Tris-HCl at pH 7.5, 4 mM of MgCl2, 1 mM of CaCl2, and 0.1 mM of phenylmethanesulfonyl fluoride), followed by digestion with 10 units of micrococcal nuclease (cat. no. N3755; Sigma-Aldrich). The nucleosome samples were incubated with preimmune rabbit serum (1:100 dilution), followed by Protein A Dynabeads (cat. no. 10001D; Life Technologies) for 4 h. The supernatant was incubated with anti-CENH3 antibody (1:200 dilution) overnight and Protein A Dynabeads for 2 h. The Protein A Dynabeads that bound to the samples were sequentially washed with 1 mL of wash buffer (50 mM of Tris-HCl at pH 7.5, 0.1 mM of phenylmethanesulfonyl fluoride, 10 mM of EDTA, and protease inhibitor) containing 50, 100, and 150 mM of NaCl. The samples were centrifuged (18,000g for 1 min) to remove any supernatant that remained after the last washing. The beads were resuspended in ChIP-MS elution buffer (50 mM of Tris-HCl at pH 7.5, 10 mM of EDTA, and 1% [w/v] SDS), followed by SDS loading buffer containing 100 mM of DTT and incubated at 100°C for 10 min. The samples were centrifuged and separated by SDS/PAGE. The gel was stained with Coomassie blue for downstream MS analysis. The ChIP-MS experiment was repeated three times, and the results of one experiment are listed in Supplemental Data Sets 1 and 2.
Cloning of ZmSMC3 cDNA
RNA was isolated from the shoots of maize inbred line B73 using TRIzol (Invitrogen). Forward primer 5′-ATGGTAGCGTGACCTGTCT-3′ and reverse primer 5′-CCTGTCTGCGTTAGCATCT-3′ were used to amplify the predicted coding region of the ZmSMC3 transcript. The amplified fragment was cloned and sequenced. The ZmSMC3 coding sequence was deposited in the GenBank.
Yeast Two Hybrid Assay
The coding sequence of ZmSMC3 was divided into three fragments: ZmSMC3-N (1 to 1,950 bp), ZmSMC3-M (901 to 2,700 bp), and ZmSMC3-C (1,951 to 3,615 bp). These three truncated coding sequences were inserted into the pGADT7 vector, and the full-length coding sequence of ZmCENH3 was inserted into the pGBKT7 vector. A yeast two-hybrid assay was conducted with the Matchmaker Gold Yeast Two-Hybrid system (catalog no. 630489; Clontech) using yeast strain Y2HGOLD. Detailed procedures are described in the Yeast Handbook (Clontech).
Antibody Production and Immunoblot Analysis
To generate the anti-ZmSMC3 antibody, we synthesized polypeptide (C-SKEEALDFIERDQTYN) based on the C-terminal region (amino acids 1,187 to 1,202) of ZmSMC3 and used it to raise a monoclonal antibody in mouse. To generate the anti-ZmSMC1 antibody, we synthesized polypeptide (ADGRSGDFRVRGG-C) based on the N-terminal region (amino acids 4 to 16) of ZmSMC1 and used it to raise a polyclonal antibody in mouse. For immunoblot analysis, 30-mg protein samples were separated in 8% SDS-PAGE gels and transferred onto a polyvinylidene fluoride membrane (Millipore). The anti-ZMSMC3 antibody was used at a dilution of 1:5,000. An anti-mouse-IgG antibody produced in Goat and conjugated to horseradish peroxidase (1:20,000 dilution; cat. no. BE0102; EASYBIO) was used to detect the recombinant protein. Protein bands were visualized using the enhanced chemiluminescent substrate.
Immunolocalization
Immunolocalization experiments on meiocytes were performed as previously described by Zhang et al. (2013) using the following antibodies: mouse anti-ZmSMC3 diluted 1:200, mouse anti-ZmSMC1 diluted 1:200, rabbit anti-CENH3 diluted 1:200, guinea pig ZmZYP1 diluted 1:500, and mouse anti-tubulin diluted 1:100. Images were acquired under the Observer Z1 (Zeiss) and BX61 (Olympus) microscopes. Flat projection of multiple optical sections were created using the softwares ZEN 2011 (Zeiss) and MetaMorph (Molecular Devices) and processed with the software Photoshop CS 5.0 (Adobe).
SMC3 CRISPR/Cas9 Transgenic Seedlings
The single guide RNA (sgRNA; gaacctgcgaagtgaagat) was designed for a site located in the second exon of ZmSMC3. The sgRNA was driven by the maize U3 promoter, and Cas9 was driven by the maize DMC1 promoter. The sgRNA-Cas9 construct was transformed into immature maize embryos by Agrobacterium tumefaciens-mediated transformation as described by Feng et al. (2016, 2018).
SMC3 RNAi lines
To produce the RNAi construct, we used a 418-bp cDNA fragment of ZmSMC3, which was amplified with the following primers: RNAiF, 5′-CCGCTCGAGAACTCTTCGTCGGACCCTT-3′ (adding an XhoI site) and RNAiR, 5′-GGAAGATCTCCCATCCAAGTAGTATTCATCC-3′ (adding a BglII site). The fragment was cloned into the pUCCRNAi vector. The RNAi construct was transformed into maize as described by Vain et al. (1993).
Accession Numbers
The ZmSMC3 coding sequence was deposited in the GenBank (accession number: MN841022).
Supplemental Data
Supplemental Figure 1. Expression analysis of ZmSMC3.
Supplemental Figure 2. Analysis of smc3 mutants generated by CRISPR/Cas9.
Supplemental Figure 3. Micronuclei in somatic cells of the smc3 mutants.
Supplemental Figure 4. Distribution of SMC1 and SMC3 in maize at pachytene stage.
Supplemental Data Set 1. LC-MS/MS analysis of CENH3 co-immunoprecipitates in early meiotic prophase I anthers.
Supplemental Data Set 2. LC-MS/MS analysis of CENH3 co-immunoprecipitates in anthers after metaphase I.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank James Birchler and Grace Sun (University of Missouri-Columbia) for comments and critical reading of the article. We thank the Maize Genetics Cooperation Stock Center for the afd1 mutant and Wojciech Pawlowski (Cornell University) for the phs1 mutant. This work was supported by the National Natural Science Foundation of China (grants 31630049 and 31600994).
AUTHOR CONTRIBUTIONS
F.H. and J.Z. designed research; J.Z., C.F., and Yang Liu performed research; J.Z., C.F., H.S., and Yalin Liu analyzed data; J.Z. and F.H. wrote the article.
Footnotes
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Bass H.W.(2003). Telomere dynamics unique to meiotic prophase: Formation and significance of the bouquet. Cell. Mol. Life Sci. 60: 2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas U., Stevense M., Jessberger R.(2018). SMC1α substitutes for many meiotic functions of SMC1β but cannot protect telomeres from damage. Curr. Biol. 28: 249–261.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas U., Wetzker C., Lange J., Christodoulou E.G., Seifert M., Beyer A., Jessberger R.(2013). Meiotic cohesin SMC1β provides prophase I centromeric cohesion and is required for multiple synapsis-associated functions. PLoS Genet. 9: e1003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Dong F., Edelmann R.E., Makaroff C.A.(2003). The Arabidopsis SYN1 cohesin protein is required for sister chromatid arm cohesion and homologous chromosome pairing. J. Cell Sci. 116: 2999–3007. [DOI] [PubMed] [Google Scholar]
- Chelysheva L., et al. (2005). AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J. Cell Sci. 118: 4621–4632. [DOI] [PubMed] [Google Scholar]
- Church K., Moens P.B.(1976). Centromere behavior during interphase and meiotic prophase in Allium fistulosum from 3-D, E.M. reconstruction. Chromosoma 56: 249–263. [Google Scholar]
- Da Ines O., Abe K., Goubely C., Gallego M.E., White C.I.(2012). Differing requirements for RAD51 and DMC1 in meiotic pairing of centromeres and chromosome arms in Arabidopsis thaliana. PLoS Genet. 8: e1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ines O., Gallego M.E., White C.I.(2014). Recombination-independent mechanisms and pairing of homologous chromosomes during meiosis in plants. Mol. Plant 7: 492–501. [DOI] [PubMed] [Google Scholar]
- Da Ines O., White C.I.(2015). Centromere associations in meiotic chromosome pairing. Annu. Rev. Genet. 49: 8.1-8.20. [DOI] [PubMed] [Google Scholar]
- Ding D.Q., Matsuda A., Okamasa K., Nagahama Y., Haraguchi T., Hiraoka Y.(2016). Meiotic cohesin-based chromosome structure is essential for homologous chromosome pairing in Schizosaccharomyces pombe. Chromosoma 125: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D.Q., Yamamoto A., Haraguchi T., Hiraoka Y.(2004). Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev. Cell 6: 329–341. [DOI] [PubMed] [Google Scholar]
- Ding X., Xu R., Yu J., Xu T., Zhuang Y., Han M.(2007). SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12: 863–872. [DOI] [PubMed] [Google Scholar]
- Feng C., Su H., Bai H., Wang R., Liu Y., Guo X., Liu C., Zhang J., Yuan J., Birchler J.A., Han F.(2018). High-efficiency genome editing using a dmc1 promoter-controlled CRISPR/Cas9 system in maize. Plant Biotechnol. J. 16: 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Yuan J., Wang R., Liu Y., Birchler J.A., Han F.(2016). Efficient targeted genome modification in maize using CRISPR/Cas9 system. J. Genet. Genomics 43: 37–43. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Fukuda N., Agostinho A., Hernández-Hernández A., Kouznetsova A., Höög C.(2014). STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 33: 1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cruz R., Brieño M.A., Roig I., Grossmann M., Velilla E., Pujol A., Cabero L., Pessarrodona A., Barbero J.L., Garcia Caldés M.(2010). Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum. Reprod. 25: 2316–2327. [DOI] [PubMed] [Google Scholar]
- Golubovskaya I.N., Hamant O., Timofejeva L., Wang C.J., Braun D., Meeley R., Cande W.Z.(2006). Alleles of afd1 dissect REC8 functions during meiotic prophase I. J. Cell Sci. 119: 3306–3315. [DOI] [PubMed] [Google Scholar]
- Gyuricza M.R., Manheimer K.B., Apte V., Krishnan B., Joyce E.F., McKee B.D., McKim K.S.(2016). Dynamic and stable cohesins regulate synaptonemal complex assembly and chromosome segregation. Curr. Biol. 26: 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatkevich T., Boudreau V., Rubin T., Maddox P.S., Huynh J.R., Sekelsky J.(2019). Centromeric SMC1 promotes centromere clustering and stabilizes meiotic homolog pairing. PLoS Genet. 15: e1008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S., Zelkowski M., Mikhailova E.I., Keijzer C.J., Houben A., Schubert V.(2019). Ultrastructure and dynamics of synaptonemal complex components during meiotic pairing and synapsis of standard (A) and accessory (B) rye chromosomes. Front. Plant Sci. 10: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J., Hwang G., Jacob J., Sapp N., Bedigian R., Oka K., Overbeek P., Murray S., Jordan P.W.(2014). Meiosis-specific cohesin component, Stag3, is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet. 10: e1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K., Kim J., Fujiyama-Nakamura S., Kato S., Watanabe Y.(2011). A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep. 12: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson K., Kanno T., Shirahige K., Sjögren C.(2014). The maintenance of chromosome structure: positioning and functioning of SMC complexes. Nat. Rev. Mol. Cell Biol. 15: 601–614. [DOI] [PubMed] [Google Scholar]
- Kemp B., Boumil R.M., Stewart M.N., Dawson D.S.(2004). A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18: 1946–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., et al. (2015). Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 517: 466–471. [DOI] [PubMed] [Google Scholar]
- Lam W.S., Yang X., Makaroff C.A.(2005). Characterization of Arabidopsis thaliana SMC1 and SMC3: evidence that AtSMC3 may function beyond chromosome cohesion. J. Cell Sci. 118: 3037–3048. [DOI] [PubMed] [Google Scholar]
- Mainiero S., Pawlowski W.P.(2014). Meiotic chromosome structure and function in plants. Cytogenet. Genome Res. 143: 6–17. [DOI] [PubMed] [Google Scholar]
- Martínez-Pérez E., Shaw P., Moore G.(2001). The Ph1 locus is needed to ensure specific somatic and meiotic centromere association. Nature 411: 204–207. [DOI] [PubMed] [Google Scholar]
- Martínez-Pérez E., Shaw P., Reader S., Aragón-Alcaide L., Miller T., Moore G.(1999). Homologous chromosome pairing in wheat. J. Cell Sci. 112: 1761–1769. [DOI] [PubMed] [Google Scholar]
- Nagaki K., Talbert P.B., Zhong C.X., Dawe R.K., Henikoff S., Jiang J.(2003). Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163: 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K.(2011). Cohesin: A catenase with separate entry and exit gates? Nat. Cell Biol. 13: 1170–1177. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Haering C.H.(2005). The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 74: 595–648. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Haering C.H.(2009). Cohesin: Its roles and mechanisms. Annu. Rev. Genet. 43: 525–558. [DOI] [PubMed] [Google Scholar]
- Pawlowski W.P., Golubovskaya I.N., Timofejeva L., Meeley R.B., Sheridan W.F., Cande W.Z.(2004). Coordination of meiotic recombination, pairing, and synapsis by PHS1. Science 303: 89–92. [DOI] [PubMed] [Google Scholar]
- Penfold C.A., Brown P.E., Lawrence N.D., Goldman A.S.(2012). Modeling meiotic chromosomes indicates a size dependent contribution of telomere clustering and chromosome rigidity to homologue juxtaposition. PLOS Comput. Biol. 8: e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D., Nibau C., Wnetrzak J., Jenkins G.(2012). High resolution analysis of meiotic chromosome structure and behaviour in barley (Hordeum vulgare L.). PLoS One 7: e39539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Suja J.A., Pezzi N., Kremer L., Martínez-A C., Rufas J.S., Barbero J.L.(2001). Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat. Cell Biol. 3: 761–766. [DOI] [PubMed] [Google Scholar]
- Prusicki M.A., Keizer E.M., van Rosmalen R.P., Komaki S., Seifert F., Müller K., Wijnker E., Fleck C., Schnittger A.(2019). Live cell imaging of meiosis in Arabidopsis thaliana. eLife 8: e42834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S.(2015). Complex elaboration: Making sense of meiotic cohesin dynamics. FEBS J. 282: 2426–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E., Eijpe M., Heyting C., Hodges C.A., Hunt P.A., Liebe B., Scherthan H., Jessberger R.(2004). Cohesin SMC1 β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6: 555–562. [DOI] [PubMed] [Google Scholar]
- Roberts N.Y., Osman K., Franklin F.C., Pradillo M., Varas J., Santos J.L., Armstrong S.(2013). Telomeres in plant meiosis: Their structure, dynamics and function. Annu. Plant Rev. 46: 191–228. [Google Scholar]
- Ronceret A., Doutriaux M.P., Golubovskaya I.N., Pawlowski W.P.(2009). PHS1 regulates meiotic recombination and homologous chromosome pairing by controlling the transport of RAD50 to the nucleus. Proc. Natl. Acad. Sci. USA 106: 20121–20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronceret A., Pawlowski W.P.(2010). Chromosome dynamics in meiotic prophase I in plants. Cytogenet. Genome Res. 129: 173–183. [DOI] [PubMed] [Google Scholar]
- Sakuno T., Tada K., Watanabe Y.(2009). Kinetochore geometry defined by cohesion within the centromere. Nature 458: 852–858. [DOI] [PubMed] [Google Scholar]
- Scherthan H.(2007). Telomere attachment and clustering during meiosis. Cell. Mol. Life Sci. 64: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H., Bähler J., Kohli J.(1994). Dynamics of chromosome organization and pairing during meiotic prophase in fission yeast. J. Cell Biol. 127: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffer A., Kaitna S., Maurer-Stroh S., Glotzer M., Nasmyth K., Eisenhaber F.(2003). Kleisins: A superfamily of bacterial and eukaryotic SMC protein partners. Mol. Cell 11: 571–575. [DOI] [PubMed] [Google Scholar]
- Schubert V., Weissleder A., Ali H., Fuchs J., Lermontova I., Meister A., Schubert I.(2009). Cohesin gene defects may impair sister chromatid alignment and genome stability in Arabidopsis thaliana. Chromosoma 118: 591–605. [DOI] [PubMed] [Google Scholar]
- Schubert V., Zelkowski M., Klemme S., Houben A.(2016). Similar sister chromatid arrangement in mono- and holocentric plant chromosomes. Cytogenet. Genome Res. 149: 218–225. [DOI] [PubMed] [Google Scholar]
- Stewart M.N., Dawson D.S.(2008). Changing partners: Moving from non-homologous to homologous centromere pairing in meiosis. Trends Genet. 24: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana-Konwalski K., Godwin J., Borsos M., Rattani A., Adams D.J., Nasmyth K.(2013). Spindle assembly checkpoint of oocytes depends on a kinetochore structure determined by cohesin in meiosis I. Curr. Biol. 23: 2534–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Lake C.M., Morais-de-Sá E., Sunkel C.E., Hawley R.S.(2011). Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Curr. Biol. 21: 1845–1851. [DOI] [PubMed] [Google Scholar]
- Tsai J.H., McKee B.D.(2011). Homologous pairing and the role of pairing centers in meiosis. J. Cell Sci. 124: 1955–1963. [DOI] [PubMed] [Google Scholar]
- Tsubouchi T., Roeder G.S.(2005). A synaptonemal complex protein promotes homology-independent centromere coupling. Science 308: 870–873. [DOI] [PubMed] [Google Scholar]
- Unhavaithaya Y., Orr-Weaver T.L.(2013). Centromere proteins CENP-C and CAL1 functionally interact in meiosis for centromere clustering, pairing, and chromosome segregation. Proc. Natl. Acad. Sci. USA 110: 19878–19883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vain P., McMullen M.D., Finer J.J.(1993). Osmotic treatment enhances particle bombardment-mediated transient and stable transformation of maize. Plant Cell Rep. 12: 84–88. [DOI] [PubMed] [Google Scholar]
- Wang H., Liu Y., Yuan J., Zhang J., Han F.(2019). The condensin subunits SMC2 and SMC4 interact for correct condensation and segregation of mitotic maize chromosomes. Plant J.. [DOI] [PubMed] [Google Scholar]
- Ward A., Hopkins J., Mckay M., Murray S., Jordan P.W.(2016). Genetic interactions between the meiosis-specific cohesin components STAG, REC8, and RAD21L. G3 (Bethesda) 6: 1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y.(2004). Modifying sister chromatid cohesion for meiosis. J. Cell Sci. 117: 4017–4023. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Nurse P.(1999). Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400: 461–464. [DOI] [PubMed] [Google Scholar]
- Wen R., Moore G., Shaw P.J.(2012). Centromeres cluster de novo at the beginning of meiosis in Brachypodium distachyon. PLoS One 7: e44681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Beasley M.D., Warren W.D., van der Horst G.T., McKay M.J.(2005). Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 8: 949–961. [DOI] [PubMed] [Google Scholar]
- Yan R., McKee B.D.(2013). The cohesion protein SOLO associates with SMC1 and is required for synapsis, recombination, homolog bias and cohesion and pairing of centromeres in Drosophila meiosis. PLoS Genet. 9: e1003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowski M., Zelkowska K., Conrad U., Hesse S., Lermontova I., Marzec M., Meister A., Houben A., Schubert V.(2019). Arabidopsis NES4 proteins act in somatic nuclei and meiosis to ensure plant viability and fertility. Front. Plant Sci. 10: 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Pawlowski W.P., Han F.(2013). Centromere pairing in early meiotic prophase requires active centromeres and precedes installation of the synaptonemal complex in maize. Plant Cell 25: 3900–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang B., Su H., Birchler J.A., Han F.(2014). Molecular mechanisms of homologous chromosome pairing and segregation in plants. J. Genet. Genomics 41: 117–123. [DOI] [PubMed] [Google Scholar]