ice1-1 is a widely accepted mutant allele known to abolish cold-inducible expression of DREB1A, which encodes a key transcription factor involved in plant cold stress adaptation, but DREB1A repression in ice1-1 is a case of transgene-induced silencing and not genetic regulation by ICE1.
Abstract
DREB1/CBFs are key transcription factors involved in plant cold stress adaptation. The expression of DREB1/CBFs triggers a cold-responsive transcriptional cascade, after which many stress tolerance genes are expressed. Thus, elucidating the mechanisms of cold stress–inducible DREB1/CBF expression is important to understand the molecular mechanisms of plant cold stress responses and tolerance. We analyzed the roles of a transcription factor, INDUCER OF CBF EXPRESSION1 (ICE1), that is well known as an important transcriptional activator in the cold-inducible expression of DREB1A/CBF3 in Arabidopsis (Arabidopsis thaliana). ice1-1 is a widely accepted mutant allele known to abolish cold-inducible DREB1A expression, and this evidence has strongly supported ICE1-DREB1A regulation for many years. However, in ice1-1 outcross descendants, we unexpectedly discovered that ice1-1 DREB1A repression was genetically independent of the ice1-1 allele ICE1(R236H). Moreover, neither ICE1 overexpression nor double loss-of-function mutation of ICE1 and its homolog SCRM2 altered DREB1A expression. Instead, a transgene locus harboring a reporter gene in the ice1-1 genome was responsible for altering DREB1A expression. The DREB1A promoter was hypermethylated due to the transgene. We showed that DREB1A repression in ice1-1 results from transgene-induced silencing and not genetic regulation by ICE1. The ICE1(R236H) mutation has also been reported as scrm-D, which confers constitutive stomatal differentiation. The scrm-D phenotype and the expression of a stomatal differentiation marker gene were confirmed to be linked to the ICE1(R236H) mutation. We propose that the current ICE1-DREB1 regulatory model should be revalidated without the previous assumptions.
INTRODUCTION
Cold stress is an environmental condition that affects plant growth, development, and productivity. Under cold stress conditions, the expression of numerous genes that function in the stress response and in tolerance is induced in various plant species. The products of these genes function to enhance freezing stress tolerance and to regulate gene expression under cold stress conditions (Thomashow, 1999; Yamaguchi-Shinozaki and Shinozaki, 2006). The dehydration-responsive element (DRE)/C-repeat with the common core motif A/GCCGAC has been identified as a cis-acting promoter element that regulates gene expression in response to both cold and dehydration stresses in plants (Baker et al., 1994; Yamaguchi-Shinozaki and Shinozaki, 1994). Three transcription factors, DREB1A/CBF3, DREB1B/CBF1, and DREB1C/CBF2, bind to the DRE, activating the expression of many downstream cold-inducible genes. Overexpression of DREB1/CBFs improves stress tolerance to freezing, drought, and high salinity in transgenic Arabidopsis (Arabidopsis thaliana; Jaglo-Ottosen et al., 1998; Liu et al., 1998). More than 100 target genes of DREB1s have been identified by transcriptome analyses (Maruyama et al., 2009; Park et al., 2015). Many of the products of these target genes have been reported to function in the acquisition of stress tolerance and in the further regulation of stress responses. Moreover, double and triple genome-edited DREB1 mutants presented a severe reduction in freezing tolerance (Jia et al., 2016; Zhao et al., 2016). Thus, these three DREB1 transcription factors reportedly act as master switches in cold-inducible gene expression (Yamaguchi-Shinozaki and Shinozaki, 1994).
Since all three DREB1 genes are rapidly and significantly induced by cold stress, their induction is considered to be the first switch in the cold-responsive expression of numerous genes (Yamaguchi-Shinozaki and Shinozaki, 1994). Therefore, elucidating the mechanisms of DREB1 induction in response to cold stress is important. Some transcription factors have been identified to regulate the cold-inducible expression of DREB1s. CALMODULIN BINDING TRANSCRIPTION ACTIVATOR3/Arabidopsis thaliana SIGNAL-RESPONSIVE GENE1 (CAMTA3/AtSR1), along with CAMTA1 and CAMTA2, has been indicated to activate the expression of CBF1/DREB1B and CBF2/DREB1C (Doherty et al., 2009). The expression of many cold-inducible genes, including DREB1s and their downstream genes, has also been revealed to be regulated by the circadian clock. CCA1 and its close homolog LHY, which are key components of the circadian oscillators and morning-expressed MYB transcription factors, have been shown to bind to the promoter regions of DREB1s, and cold-inducible expression of DREB1s has been reported to be significantly reduced in cca1 lhy double mutant plants (Dong et al., 2011; Kidokoro et al. 2017), suggesting that the key circadian components, such as CCA1 and LHY, also function as important transcriptional activators in the cold-responsive expression of DREB1s. We recently revealed that plants recognize cold stress as two different signals, rapid and gradual temperature decreases, and that each of the three DREB1 genes is differently induced in response to these two stress signals. CAMTA3 and CAMTA5 respond to a rapid temperature decrease and induce the expression of DREB1B and DREB1C. By contrast, CCA1 and LHY strongly induce the expression of DREB1A and DREB1C in response to rapid and gradual temperature decreases (Kidokoro et al. 2017). The presence of the two different signaling pathways leading to the expression of DREB1s in response to cold stress has made it difficult to elucidate the regulatory mechanisms of their expression.
The MYC-like basic helix-loop-helix transcription factor INDUCER OF CBF EXPRESSION1/SCREAM (ICE1/SCRM) is also a well-known regulator of DREB1/CBF expression. An ice1-1 mutant was first isolated in a screen for mutations that impair the cold-induced transcription of a firefly luciferase (LUC) reporter gene driven by the CBF3/DREB1A promoter (Chinnusamy et al., 2003). The cold-inducible expression of endogenous CBF3/DREB1A clearly decreased in the ice1-1 mutant, but that of DREB1B and DREB1C did not. The ice1-1 mutant showed a significant decrease in plant chilling and freezing tolerance. Moreover, overexpression of the ICE1 gene in the wild-type Arabidopsis plants enhanced the expression of the CBF/DREB1 regulon in response to cold stress and improved the freezing stress tolerance of the transgenic plants. It was concluded that the mutation of one amino acid residue, Arg, at amino acid 236 to His (R236H) in the ICE1 protein caused this decreased expression of CBF3/DREB1A (Chinnusamy et al., 2003).
Kanaoka et al. (2008) isolated a scrm-D mutant whose stomatal development was abnormal. In this mutant, nearly all cells in the epidermis developed into guard cells. The authors revealed that this phenotype was caused by the same missense mutation (R236H) in the same ICE1 protein by using map-based cloning. In the scrm-D mutant, increased expression of stomatal differentiation marker genes such as FAMA and EPF1 was tightly linked to the ICE1(R236H) mutation (Pillitteri et al. 2011). The ICE1(R236H) mutation dominantly and semidominantly affects DREB1A expression and stomatal development, respectively, but how this mutation results in two different phenotypes is unclear. Additionally, a T-DNA insertion double mutant of ICE1 and its homologous gene, SCRM2/ICE2, did not exhibit stomatal differentiation in the epidermis, which was opposite to the effect observed in the scrm-D mutant (Kanaoka et al., 2008). This double mutation caused a slight decrease in the expression of all three CBF/DREB1 genes (Kim et al., 2015), while the ice1-1 mutant showed a strong decrease in only CBF3/DREB1A expression (Chinnusamy et al., 2003).
Because the DREB1A promoter contains several typical MYC-type transcription factor binding sequences (CANNTG), it is possible that ICE1 targets these sequences and regulates cold-inducible DREB1A expression (Chinnusamy et al., 2003; Kim et al., 2015). In addition, various reports have shown that the activity of the ICE1 protein is modulated by various posttranslational modifications, such as phosphorylation, SUMOylation, and ubiquitination, to regulate cold stress tolerance (Dong et al., 2006; Miura et al., 2007, 2011; Ding et al., 2015). Thus, many factors have been reported to regulate the cold-responsive expression of DREB1 genes. Among these factors, the cold-inducible expression of DREB1A is activated by both ICE1 and circadian components, while that of DREB1B and DREB1C is activated by CAMTAs and circadian components. Therefore, the mechanism underlying the regulation of the cold-inducible expression of DREB1A may differ from that of the other two DREB1s, but this supposition has not yet been clarified.
In this study, we focused on the regulatory mechanism of the cold-inducible expression of DREB1A/CBF3 and tried to analyze the role of ICE1 in the regulation of this expression. However, we unexpectedly discovered that DREB1A repression in ice1-1 is genetically independent of the known ICE1(R236H) mutation. Using genomic analysis, we deduced that a T-DNA allele from the ice1-1 genome is associated with DREB1A repression. Our subsequent analyses demonstrated that DREB1A repression in ice1-1 is achieved by DNA methylation-mediated gene silencing triggered by T-DNA, not genetic regulation.
RESULTS
An R236H Mutation within ICE1 Is Independent of DREB1A Repression
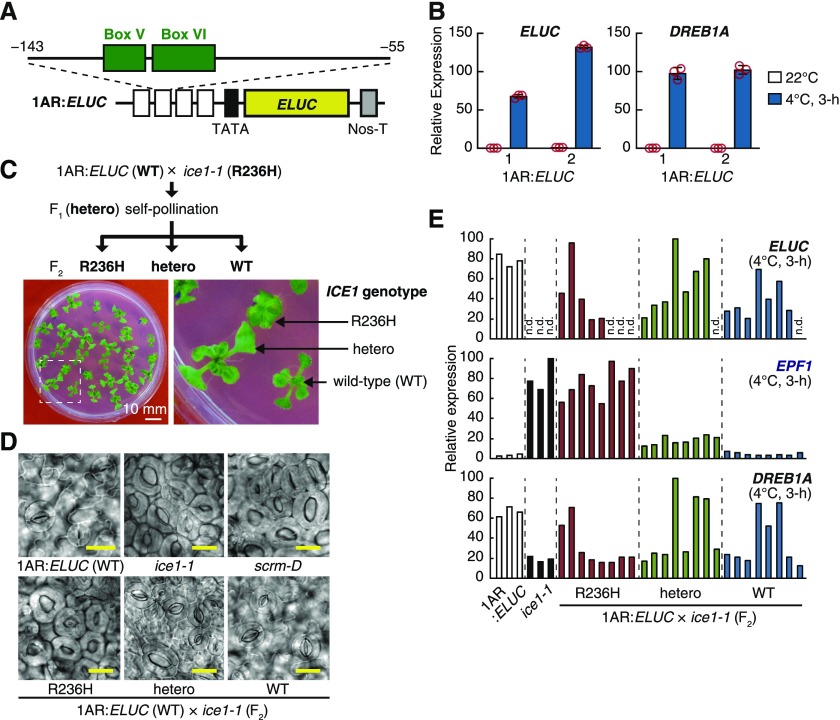
To identify the cis-acting elements involved in the cold-inducible expression of DREB1A, we generated transgenic Arabidopsis plants that express an emerald luciferase (ELUC) reporter gene driven by four tandem repeats of the DREB1A promoter fragment (−143 to −55 bp from the transcription start site), including two conserved sequences (boxes V and VI) among the promoters of three DREB1s, and its minimal promoter (−57 to +118 bp) and named it 1AR:ELUC (Figure 1A). We detected obvious cold-inducible expression of the ELUC gene in the generated 1AR:ELUC plants in the Col-0 background, indicating that the 1AR fragment governs the cold-inducible expression of DREB1A (Figure 1B). ICE1 has been assumed to be a candidate transcription factor that targets E-box sequences (CANNTG) within the DREB1A promoter and regulates the cold-inducible expression of DREB1A (Chinnusamy et al., 2003). 1AR:ELUC contains one E-box sequence (CACCTG); therefore, to investigate whether ICE1 can regulate cold-responsive transcription via the fragment, we crossed an 1AR:ELUC plant with an ice1-1 mutant plant. The F2 population segregated into three subgroups that exhibited different rosette phenotypes, the wild type, intermediate (heterozygous), and ice1-1 (homozygous), at an ∼1:2:1 ratio (Figure 1C). We observed the epidermal development of these three subgroups and found that they showed normal development, increased stomata, and a stomata-only phenotype similar to that of ice1-1 or scrm-D, respectively (Figure 1D). These results were consistent with the reported effect of the ICE1(R236H) mutation, which is known to strongly upregulate ICE1 target genes, including EPF1 (Kanaoka et al., 2008; Pillitteri et al. 2011).
Figure 1.
Dissociation of DREB1A Repression from the ice1-1 Allele.
(A) Schematic diagram of the reporter constructs. The ELUC reporter gene is driven by four tandem repeats of an 89-bp fragment around boxes V and VI and a minimal promoter sequence of the DREB1A promoter. Nos-T indicates the nopaline synthase terminator.
(B) Expression of the ELUC reporter gene in response to cold stress. The transcript level of each gene in the transgenic Arabidopsis seedlings was measured by RT-qPCR. Two representative lines are shown. The bars refer to means ± sds; experiments were performed in triplicate. Line 2 was used for crossing with the ice1-1 mutant plants.
(C) Process flow of the F2 population generated by an 1AR:ELUC × ice1-1 cross. Two-week-old seedlings of the F2 population grown on agar medium are shown. Bar = 10 mm.
(D) Abaxial rosette leaf epidermis of 1AR:ELUC, ice1-1, scrm-D, and the F2 population generated by an 1AR:ELUC × ice1-1 cross. Bars = 20 μm.
(E) Related gene expression in F2 individuals evaluated by RT-qPCR. ICE1 alleles were determined by the apparent rosette and stomatal phenotypes. n.d., not detected; WT, wild type.
We then analyzed the expression of 1AR:ELUC under cold stress conditions at 4°C for 3 h in the three F2 subgroups (Figure 1E). Because the coding sequence of ELUC that we introduced is not homologous to that of firefly LUC, which was originally included in the ice1-1 mutant as a reporter gene, ELUC expression could be specifically analyzed by quantitative RT-PCR (RT-qPCR). We expected that ELUC expression was repressed depending on the presence of the ICE1(R236H) mutation. However, the expression of ELUC did not seem to be associated with the observed rosette phenotypes. Many F2 plants homozygous for ICE1(wild type) presented unexpectedly low levels of ELUC expression—levels that were similar to those in the ice1-1 mutant. Moreover, some F2 plants with ICE1(R236H) presented high levels of ELUC expression—levels that were as high as those in the wild type (1AR:ELUC) plants (Figure 1E). To confirm the expression of endogenous ICE1/SCRM target genes in the three classes of F2 plants, we measured the expression of DREB1A and EPF1 in those plants. The expression of DREB1A was unexpectedly low in many F2 plants with homozygous ICE1(wild type) in response to cold stress but was highly induced in some F2 plants with ICE1(R236H; Figure 1E). These expression patterns of DREB1A in the F2 plants were similar to those of the ELUC reporter genes driven by 1AR. By contrast, the expression of EPF1, one of the target genes of ICE1 in stomatal development, was increased in the heterozygous plants and greatly increased in the homozygous R236H plants (Figure 1E). These results implied that DREB1A repression in the ice1-1 mutant is genetically independent of the ICE1(R236H) mutation, while the elevated expression of EPF1 is tightly associated with this mutation as well as with stomatal development.
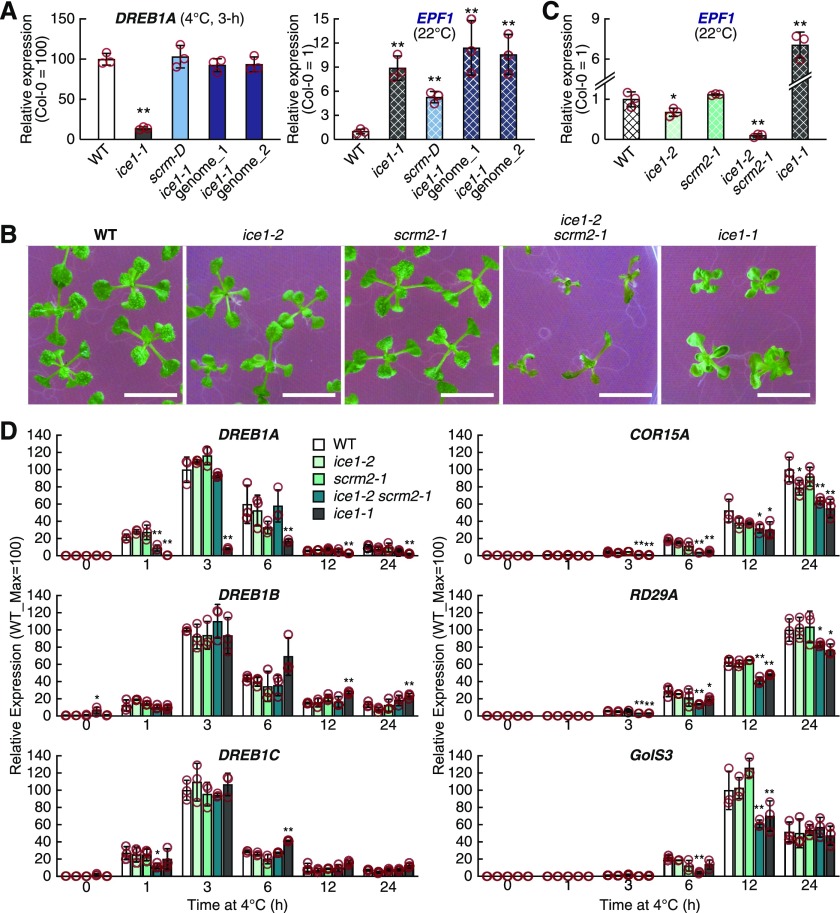
We further analyzed the cold-inducible expression of DREB1A in the scrm-D mutant plants and two lines of transgenic plants (Col-0) harboring the ICE1 genomic fragment prepared from the ice1-1 mutant genomic DNA (Figure 2A). Compared with that in the wild type (Col-0) plants, EPF1 expression in the scrm-D mutant and transgenic plants was significantly induced, whereas the expression levels of DREB1A did not significantly change in these plants. Therefore, we concluded that the DREB1A repression in the ice1-1 mutant is not due to the ICE1(R236H) mutation; rather, the repression is attributed to unknown independent genetic variation.
Figure 2.
Cold-Induced Expression of DREB1s and Their Downstream Genes in Mutant Plants of ICE1 and Its Homolog.
(A) Expression of EPF1 and DREB1A in ice1-1, scrm-D, and two lines of transgenic plants (Col-0) harboring the ICE1(R236H) genomic DNA fragment prepared from the genomic DNA extracted from the ice1-1 mutant.
(B) Plant growth of the mutant plants of ICE1 and its homolog SCRM2/ICE2. Two-week-old seedlings grown on agar medium are shown. Bar = 10 mm.
(C) and (D) Gene expression in single and double mutant plants of ICE1 and SCRM2/ICE2. Gene expression levels of EPF1 under unstressed conditions (C) and those of three DREB1s and their downstream genes under cold stress conditions (D) were detected.
The transcript level of each gene was measured by RT-qPCR. The bars refer to means ± sds; experiments were performed in triplicate. The asterisks indicate significant differences (**P < 0.01 or *P < 0.05 according to Student’s t test; Supplemental Data Set) in the expression of each gene in the transgenic plants compared with the wild-type (WT; Col-0) plants.
To analyze the effect of ICE1 loss of function on DREB1 expression, we obtained T-DNA insertion alleles of ICE1 (ice1-2) and its homolog SCRM2 (scrm2-1) and generated a double loss-of-function mutant of ICE1 and SCRM2 (ice1-2 scrm2-1; Kanaoka et al., 2008). These single and double mutant plants were grown on germination medium (GM) agar plates with ice1-1 and the wild-type plants. Among these plants, ice1-1 showed growth inhibition, and ice1-2 scrm2-1 exhibited more severe growth inhibition, as previously reported by Kanaoka et al. (2008; Figure 2B). Using the plants grown on the agar plates, we examined the expression of EPF1 and found that its expression was reduced in ice1-2 and more reduced in ice1-2 scrm2-1 (Figure 2C). The expression levels of the three DREB1 genes and their downstream genes (COR15A, RD29A, and GolS3) in these plants were subsequently analyzed at 4°C for 24 h (Figure 2D). The expression of DREB1A in ice1-2, scrm2-1, and ice1-2 scrm2-1 was not significantly changed, except for a decrease in expression in ice1-2 scrm2-1 when treated at 4°C for 1 h. By contrast, DREB1A expression remained at extremely low levels in the ice1-1 mutant during the 24 h, even under cold stress conditions. The expression patterns of DREB1B and DREB1C in all these mutants were similar to those in the wild type during the 24 h under cold stress conditions. These results indicated that among the three DREB1 genes, only DREB1A showed decreased expression in ice1-1 compared with that in the wild-type plants. By contrast, the expression levels of the DREB1-downstream genes COR15A, RD29A, and GolS3 were slightly but significantly decreased in the ice1-1 and double mutant plants.
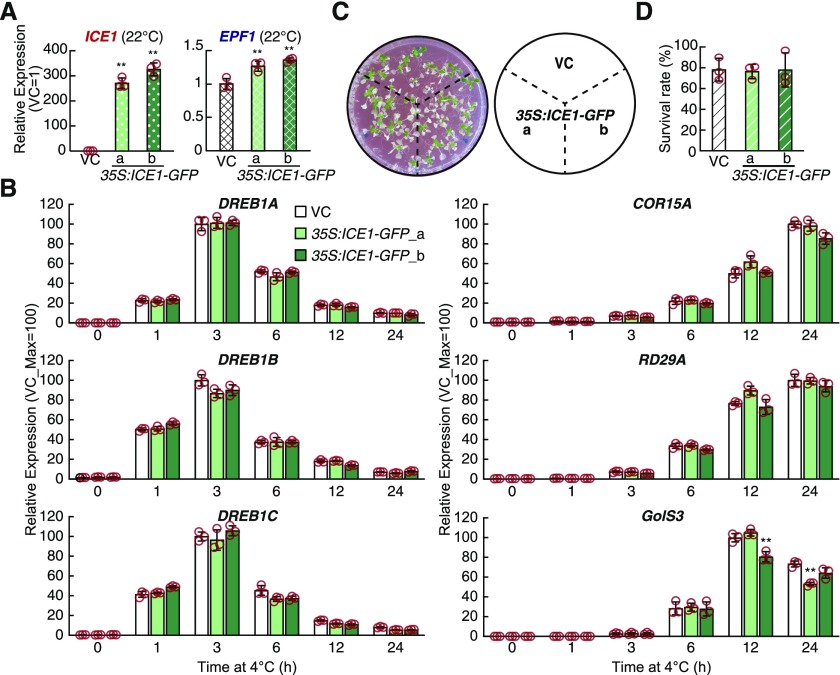
In addition, we tested the effects of overexpression of ICE1 in transgenic Arabidopsis plants. Using the cauliflower mosaic virus (CaMV) 35S promoter, we generated two ICE1 overexpression lines that presented high levels of ICE1 expression (Figure 3A). Compared with the vector control plants, neither overexpression line presented significant increases in the expression of the three DREB1 genes or their downstream genes, while the expression of EPF1 was slightly but significantly increased under the control conditions (Figures 3A and 3B). We examined the freezing tolerance of the overexpression lines and found that these plants also did not show significant differences in stress tolerance (Figures 3C and 3D). These results suggest that the overexpression of ICE1 using the 35S promoter does not confer significant effects on the expression of DREB1 or its downstream genes, nor does it confer freezing stress tolerance in Arabidopsis plants.
Figure 3.
Cold-Induced Expression of DREB1s and Their Downstream Genes in ICE1-Overexpressing Plants.
(A) and (B) Gene expression in ICE1-GFP–overexpressing plants. Gene expression levels of ICE1 and EPF1 under unstressed conditions (A) and those of the three DREB1s and their downstream genes under cold stress conditions (B) were detected. The transcript level of each gene was measured by RT-qPCR. The bars refer to means ± sds; experiments were performed in triplicate.
(C) and (D) Freezing tolerance of ICE1-GFP overexpression plants. Nonacclimated seedlings were treated at −9°C for 0.5 h. Representative images (C) and survival rates (D) of plants after recovery are shown. The bars refer to means ± sds; experiments were performed in triplicate.
The asterisks indicate significant differences (**P < 0.01 according to Student’s t test; Supplemental Data Set) in the expression of each gene in the transgenic plants compared with that of the vector control (VC) plants, in which only GFP was expressed under the CaMV 35S promoter.
A T-DNA Insertion on Chromosome 1 of ice1-1 Is Associated with DREB1A Repression
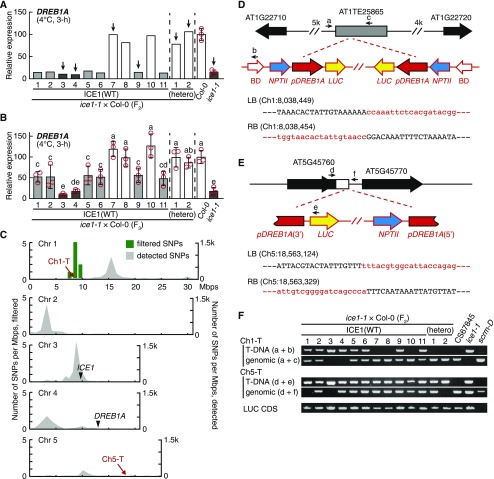
To understand the genetic characteristics of the novel locus for DREB1A repression, we propagated a new F2 population from an ice1-1 × Col-0 cross. Eight of the 11 F2 plants that exhibited the ICE1(wild type) rosette phenotype showed repressed DREB1A expression as the ice1-1 plant did, and we subsequently found two heterozygous ICE1(R236/ wild type) plants having Col-0–like DREB1A expression (Figure 4A). These results are consistent with our observations in the 1AR:ELUC × ice1-1 progeny (Figure 1), again providing strong evidence that DREB1A repression is not due to the ICE1(R236H) allele. DREB1A expression was analyzed further in the self-pollinated F3 generation by pooling six to eight plants into a sample. The progeny of the F2 plants with Col-0–like DREB1A expression uniformly presented similar fully activated DREB1A expression, indicating that they were null segregants (Figure 4B). By contrast, the progeny of the F2 plants with repressed DREB1A presented variable DREB1A expression. Two of the progeny showed similar repression to that of ice1-1, while the other six showed only modest repression of DREB1A, indicating that the pooled plants were heterogeneous (Figure 4B). Thus far, the observed segregation ratio was 2:6:3 in the F2 ICE1(wild type) plants, suggesting that the DREB1A repression of ice1-1 is regulated by a single genetic locus in a dominant-negative manner (χ2, P > 0.95). This is the same characteristic that the original ice1-1 report mentioned (Chinusamy et al., 2003). We named this novel locus New ICE1 (NICE1).
Figure 4.
Identification of the NICE1 Locus.
(A) Cold-induced expression of DREB1A in the F2 progeny of an ice1-1 × Col-0 cross. The lines indicated with arrows were used for genome resequencing.
(B) Cold-induced expression of DREB1A in the bulked F3 progeny (six to eight seedlings) of the F2 individuals in (A). The white, light gray, and dark gray bars indicate null segregants and heterogeneous and homogenous mutants of NICE1, respectively. The transcript level of each gene was measured by RT-qPCR. The bars refer to means ± sds; experiments were performed in triplicate. The different letters above the bars designate significant differences (two-tailed t test with Bonferroni–Holm correction, P < 0.05; Supplemental Data Set).
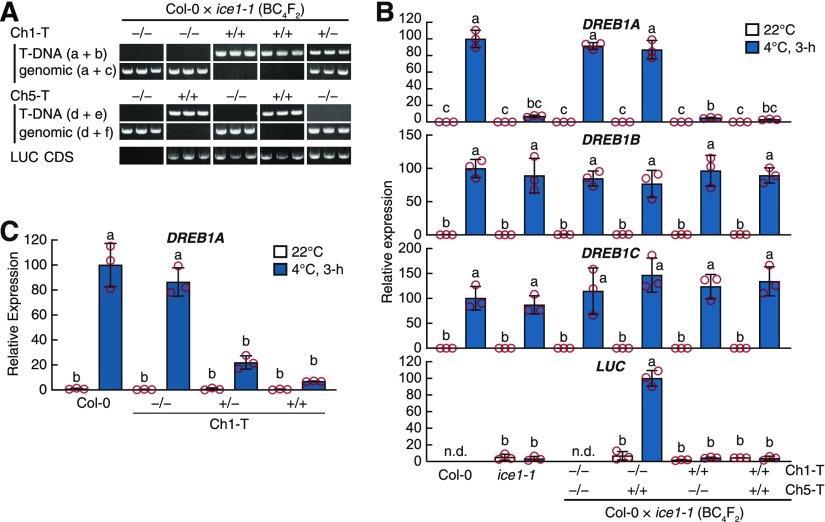
(C) Chromosomal distribution of SNPs and genetic loci related to DREB1A repression in ice1-1. The gray fills and green bars refer to the numbers of SNPs detected in total and of SNPs with perfect association with DREB1A repression in a bin (Mbp), respectively. The approximate loci of the ICE1 and DREB1A genes and of the two discovered T-DNAs (Ch1-T, Ch5-T) are given. Chr, chromosome.
(D) and (E) Schematic view of the Ch1-T (D) and Ch5-T (E) loci. The gray bar and open arrows indicate the TE and genes, respectively. The primer sets and positions for confirming the T-DNA insertion are shown with small black arrows (a to f). BD, T-DNA border; LB, T-DNA left border; RB, T-DNA right border.
(F) Genotypes of the two transgenes in the F2 individuals from the ice1-1 × Col-0 cross in (A). LUC CDS refers to amplification of the transgenic luciferase coding DNA sequence.
To characterize the NICE1 locus within the Arabidopsis genome, we resequenced the individual genomes of ice1-1, scrm-D, six of the F2 plants described above, two homozygous NICE1 mutants (NICE1+/+), one heterozygous NICE1 mutant (NICE1+/−), and three null segregants (NICE1−/−). More than 4000 biallelic single-nucleotide polymorphisms (SNPs) were detected from each resequenced genome, and they were filtered based on the known NICE1 genotypes. The filtration yielded eight SNPs of cytosine-to-thymine conversion. These SNPs were gathered within a range of 8.0 to 10.5 Mbp on chromosome 1 (Figure 4C), which was a different chromosome than those containing the loci of DREB1A (chromosome 4) and ICE1 (chromosome 3). Among the eight SNPs, four were found in each coding region of four genes, and the other four were located within intergenic regions (Supplemental Figure 1A). The SNPs from the coding regions were further evaluated for their association with DREB1A repression by the use of the other nonsequenced F2 segregants. However, only incomplete association was observed in all of these cases, indicating that the detected SNPs are near NICE1 but are not causal alleles for the DREB1A repression of ice1-1 (Supplemental Figure 1B).
We therefore attempted to investigate the other possibility: a T-DNA insertion. The original report of ice1-1 indicated that the ice1-1 genome harbors a single T-DNA locus for reporter gene expression, but the locus has not been elucidated (Chinnusamy et al., 2003). To identify the T-DNA locus, we prepared another set of genome sequencing data from ice1-1 in the paired-end form. We searched for the T-DNA locus by screening abnormal singleton mapping features in the Arabidopsis reference genome, and the candidates were verified by Sanger sequencing analysis. We discovered two T-DNA loci from the ice1-1 sequencing data on chromosome 1 and chromosome 5 and named them Ch1-T and Ch5-T, respectively (Figures 4C to 4E). Ch1-T was positioned in the middle of a transposable element (TE) on chromosome 1 (AT1TE25865) and overlapped with the range where the eight candidate SNPs accumulated (Figure 4D). Ch5-T was positioned in the 3′ untranslated region of a protein-coding gene (AT5G45760) on chromosome 5 and was accompanied by a 204-bp genomic deletion (Figure 4E). Our Sanger sequencing analysis revealed that Ch1-T contains at least two copies of the reporter gene (DREB1A promoter-driven LUC), each at the left and right borders of the T-DNA in an inverted repeat form. Ch5-T was found to contain one copy of the reporter gene, and the DREB1A promoter was divided into two fragments and flanked the T-DNA (Figures 4D and 4E). Both T-DNA sequences remain incomplete, since the extended sequences from the T-DNA borders could not be extended any further. To investigate the association between these two T-DNA loci and DREB1A repression, the genotypes of Ch1-T and Ch5-T were analyzed in the 11 ICE1(wild type) F2 plants described above (Figure 3A). In contrast to the candidate SNPs, the Ch1-T genotype showed a complete association with DREB1A repression (Figure 4F). Notably, the germline distributed as the progenitor of ice1-1 (CS67845) did not contain either of the two ice1-1 T-DNAs, although its genome still harbored the ectopic LUC gene as part of the reporter gene (Figure 4F).
For further validation, we analyzed the expression of DREB1A in BC4F2 segregating plants derived from the backcross of ice1-1 to Col-0. The association between Ch1-T and DREB1A repression was also maintained in the BC4F2 population, although the expression of two neighboring homologs, DREB1B and DREB1C, was not affected by Ch1-T (Figures 5A and 5B). The association occurred in a dominant-negative manner (Figure 5C), as our F2 population suggested (Figures 4A and 4B) and the original ice1-1 report indicated (Chinnusamy et al., 2003). The Ch5-T genotypes were not associated with DREB1A repression (Figure 5B). Moreover, the BC4F2 plants harboring only Ch5-T presented solid LUC induction in response to cold treatment, indicating that Ch5-T contains the active reporter gene (Figure 5B). Overall, we identified a T-DNA on chromosome 1 (Ch1-T) as the NICE1 locus responsible for the dominant-negative DREB1A regulation of ice1-1.
Figure 5.
DREB1A Repression Is Dominantly Associated with a T-DNA Insertion in Chromosome 1.
(A) T-DNA insertions in BC4F2 segregants of ice1-1 × Col-0. Three segregants in four genotypes are shown. The primer sets and positions are described in Figures 4D and 4E. CDS, coding sequence.
(B) Cold-inducible gene expression of DREB1s and luciferase in the BC4F2 segregants. The bars refer to the means ± sds; experiments were performed in triplicate.
(C) Cold-inducible expression of DREB1A in the Ch1-T segregants. The transcript level was measured by RT-qPCR. Bars refer to the means ± sds from triplicate experiments. The different letters above the bars indicate significant differences (two-tailed t test with Bonferroni–Holm correction, P < 0.01; Supplemental Data Set).
Induced DNA Methylation of the DREB1A Promoter Represses Its Activity
The next question was how the single T-DNA allele regulates both native and transgenic DREB1A promoters on remote chromosomes. We first suspected the regional influence of the T-DNA insertion on the behavior of nearby genes, which probably influences subsequent DREB1A promoter activity. However, two neighboring genes on either side (AT1G22710 and AT1G22720) are relatively distant (>4 kb) from the NICE1 locus (Figure 4D), and the NICE1 genotype seemed to have little effect on the expression of these genes whether the plants were treated with cold stress or not (Supplemental Figure 2). In addition, the expression of AT1G22710 was downregulated in ice1-1, while the same downregulation was also observed in the scrm-D mutant, showing that AT1G22710 regulation is independent of DREB1A repression (Supplemental Figure 2). We attempted to reconstruct the NICE1 transgene in transgenic plants to analyze the mechanism of DREB1A repression. Cold-induced DREB1A expression was not altered in transgenic Arabidopsis plants to which a TE (AT1TE25865) or a TE with DREB1Apro:LUC was introduced (Supplemental Figure 3). On the other hand, we could not obtain any transgenic plants when we introduced a TE (AT1TE25865) with an inverted repeat of DREB1Apro:LUC similar to the NICE1 locus (Supplemental Figure 3).
DNA methylation was another hypothesis, inspired by previous reports describing the silencing of both a transgene and the associated endogenous gene by ectopically induced DNA methylation (Sidorenko and Peterson, 2001; Gong et al., 2002; Wang et al., 2011). 5-Methylcytosine (5mC) is a prominent form of methylated DNA in eukaryotes and is a stable but reversible epigenetic mark responsible for various biological processes and silencing of repetitive genomic features, including TEs (Zhang et al., 2018). Most DNA methylation in mammals occurs at CG sites, whereas DNA methylation in plants occurs at every cytosine base via multiple specified pathways for each sequence context, CG, CHG, and CHH (where H is A, C, or T). Plants also have evolved an RNA-directed DNA methylation (RdDM) pathway, which is capable of triggering de novo 5mC accumulation in particular genomic regions from a remote methylated origin with DNA sequence similarity across the genome (Matzke et al., 2015; Zhang et al., 2018). Accordingly, we hypothesized that the NICE1 locus, including the transgenic DREB1A promoter, underwent rapid 5mC-mediated silencing and triggered RdDM to silence the native DREB1A promoter.
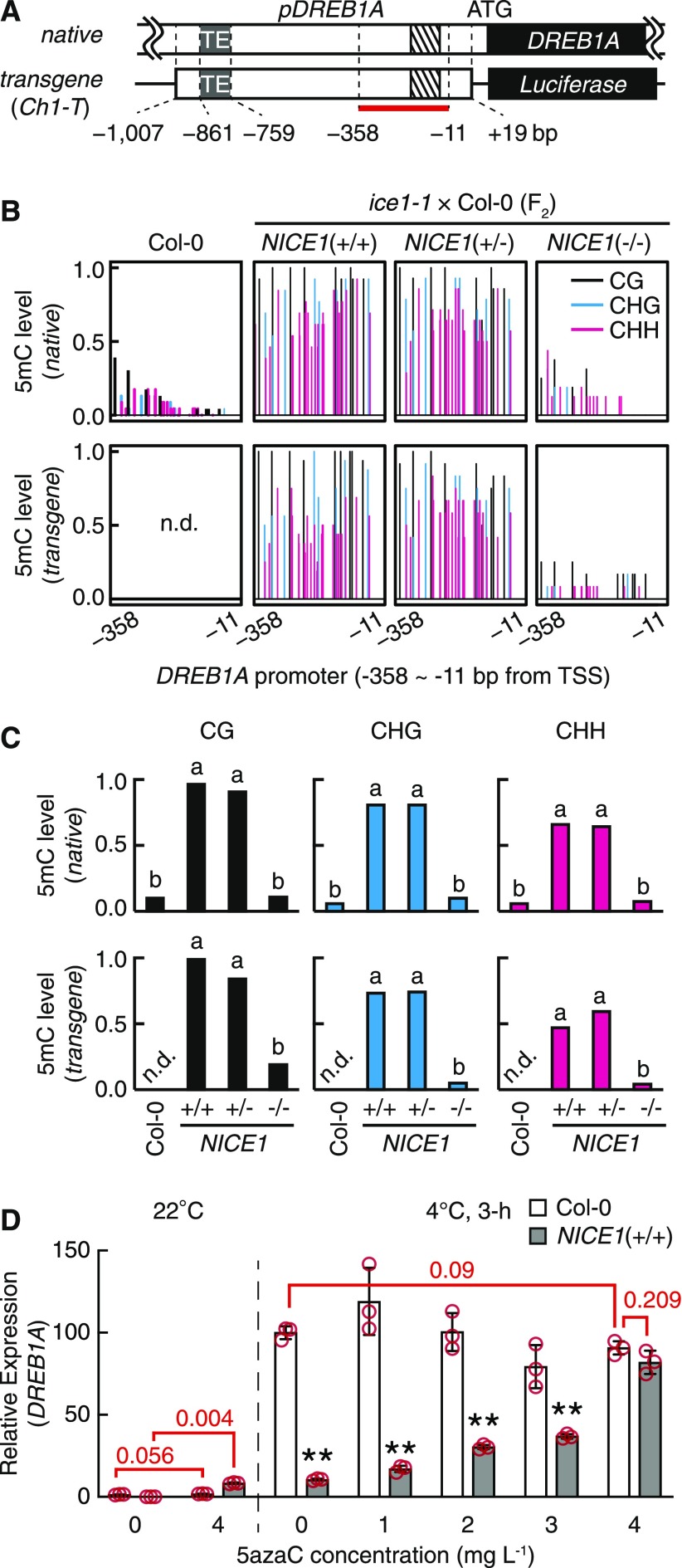
We first evaluated the 5mC levels of the DREB1A promoter attributed to NICE1 allelic variants. The DREB1A promoter in the NICE1 transgene contains the 1-kb DREB1A promoter region (from −1007 to +19 nucleotides), harboring both a TE fragment (AT4TE60970; from −861 to −759) and the 1AR fragment (from −143 to −55), which can regulate the cold-inducible expression of DREB1A (Figure 6A). Local bisulfite sequencing was conducted to analyze the 3′ region of the promoter including 1AR (from −350 to −11), which also benefited the assessment of the 5mC levels of the native and transgenic DREB1A promoters separately (Figure 6A). The analyzed DREB1A promoter region of the Col-0 plant was barely methylated, indicating that the DREB1A promoter is not a major target of Arabidopsis DNA methylation (Figures 6B and 6C). By contrast, NICE1+/+ and NICE1+/− plants displayed obvious 5mC accumulation in the same promoter region of both the transgenic and native DREB1A loci. This 5mC accumulation was observed in all three cytosine contexts, and the induced levels were comparable between NICE1+/+ and NICE1+/− (Figures 6B and 6C). This hypermethylation level recovered to Col-0–like levels in the NICE−/− plants (Figures 6B and 6C). We subsequently evaluated 5mC levels in the promoter regions of DREB1B and DREB1C. Although these two homologs neighbor DREB1A and share multiple homologous parts in their promoter region (Shinwari et al., 1998), the two promoter regions were barely methylated in all NICE1 genotypes (Supplemental Figure 4), as their activity was not altered by NICE1 genotypes (Figure 5B). Our results indicated that the DREB1A promoter becomes hypermethylated coincidently with the T-DNA allele of the NICE1 locus.
Figure 6.
Hypermethylation of the Repressed DREB1A Promoter.
(A) Schematic view of native and transgenic DREB1A promoters in ice1-1. The open and closed bars indicate the intergenic and coding regions, respectively. The gray shaded and striped boxes indicate the TE fragment (AT4TE60970) and the 1AR region within the DREB1A promoter, respectively. The horizontal bar of the transgene represents the anonymous backbone sequences of the T-DNA. The bisulfite sequencing target region is indicated by a red bar. Distances (bp) from the DREB1A transcription start site are given.
(B) and (C) Cytosine methylation levels in the DREB1A promoter of the wild type (Col-0) and the F2 segregants generated by an ice1-1 × Col-0 cross. (B) Bars indicate the relative positions (x axis) and the methylation levels (y axis) of each cytosine in the promoter. (C) Cumulative 5mC levels by cytosine context evaluated from local bisulfite sequencing. The different letters above the bars indicate significant differences (two-tailed Fisher’s exact test; P < 0.001; Supplemental Data Set). TSS, transcription start site.
(D) Cold-responsive DREB1A transcript levels of plants grown under various concentrations of 5azaC. The transcript level was measured by RT-qPCR. The bars refer to the means ± sds of triplicate experiments. The asterisks indicate significant differences (P < 0.01 according to Student’s t test; Supplemental Data Set) from the Col-0 data under the same conditions. Some P-values are shown in red; these values were determined by Fisher’s exact test (C) and Welch’s pairwise t test (D).
Next, we investigated the influence of hypermethylation on DREB1A promoter activity. 5-aza-2′-Deoxycytidine (5azaC) is a 5mC inhibitor that reduces global 5mC levels and releases 5mC-sensitive transcription in plant cells (Wang et al., 2011; Ikeda et al., 2017). Col-0 and NICE1+/+ seedlings were grown in various concentrations of 5azaC, after which cold-induced DREB1A expression in the seedlings was measured (Figure 6D). The repressed DREB1A expression in NICE1+/+ was significantly recovered by the addition of 5azaC in a dose-dependent manner, and the highest concentration of 5azaC (4 mg L−1) specifically recovered DREB1A expression to a level comparable to that of Col-0. Similar DREB1A recovery by 5azaC was observed under noncold conditions, indicating that the promoter hypermethylation in NICE1+/+ plants also repressed the basal expression of DREB1A (Figure 6D). The parental ice1-1 plants had a similar hypermethylated DREB1A promoter, and the repressed DREB1A expression was significantly recovered by 5azaC treatment (Supplemental Figure 5). By contrast, the DREB1A expression in Col-0 was not affected by 5azaC applications, regardless of cold treatment (Figure 6D). Accordingly, our results indicate that DREB1A promoter activity is 5mC sensitive and that promoter hypermethylation in ice1-1 actually represses the expression of DREB1A under both cold and noncold conditions. The observed hypermethylation levels that were similar between NICE1+/+ and NICE1+/− supported the dominant-negative effect of the NICE1 T-DNA allele on DREB1A expression.
RdDM Participates in DREB1A Promoter Hypermethylation by NICE1
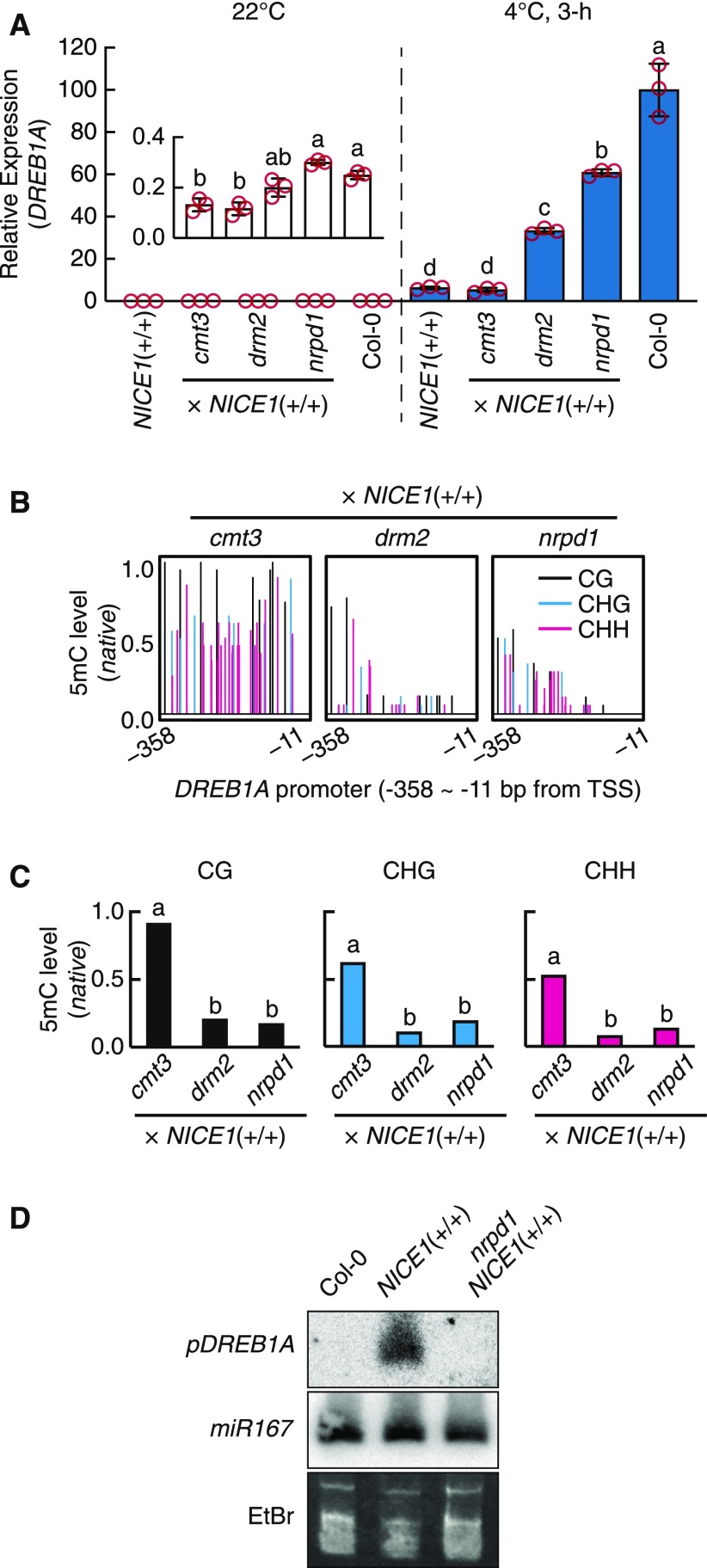
We next investigated whether the RdDM machinery participates in DREB1A repression. Previous studies have reported that the dysfunction of core RdDM components such as DOMAINS REARRANGED METHYLTRANSFERASE2 (DRM2) or NUCLEAR RNA POLYMERASE D1 (NRPD1), which is the largest subunit of RNA polymerase IV, can be used to relieve RdDM-mediated hypermethylation and associated promoter repression (Yamamuro et al., 2014; Tang et al., 2016). We introduced the drm2 or nrpd1 mutation into NICE1+/+ by crossing and then measured the DREB1A activity. The repressed DREB1A expression was significantly recovered in both the drm2 NICE1+/+ and nrpd1 NICE1+/+ double mutants under both cold and noncold conditions (Figure 7A). The drm2 and nrpd1 single mutants had little effect on cold-inducible DREB1A expression (Supplemental Figure 6). In addition, the effect of an RdDM-independent DNA methyltransferase CHROMOMETHYLASE3 (CMT3; Cao et al., 2003) was analyzed in the same way, although the double mutant cmt3 NICE1+/+ did not display any significant DREB1A recovery (Figure 7A; Supplemental Figure 6). We subsequently assessed the 5mC levels of the DREB1A promoter in the double mutant plants. Consistent with the recovered activity of the promoter, compared with the NICE1+/+ single mutant, the drm2 NICE1+/+ and nrpd1 NICE1+/+ plants presented largely reduced 5mC levels in all cytosine contexts, whereas the cmt3 NICE1+/+ plants presented only subtle changes in 5mC levels (Figures 6B, 6C, 7B, and C7C).
Figure 7.
Participation of RdDM Machinery in DREB1A Repression.
(A) Cold-inducible DREB1A transcription recovery via dysfunction of RdDM components. The transcript level was measured by RT-qPCR. The bars refer to the means ± sds of triplicate experiments. The different letters above the bars indicate significant differences under the same conditions (two-tailed t test with Bonferroni–Holm correction, P < 0.01; Supplemental Data Set).
(B) and (C) Cytosine methylation levels in the DREB1A promoters of NICE1+/+ plants with different DNA methylation component dysfunctions. (B) Bars indicate the relative positions (x axis) and methylation levels (y axis) of each cytosine within the promoter. (C) Cumulative 5mC levels by cytosine context evaluated from local bisulfite sequencing. The different letters above the bars refer to significant differences (two-tailed Fisher’s exact test, P < 0.001; Supplemental Data Set). Some P-values are shown in red. TSS, transcription start site.
(D) sRNA gel blot analysis of NICE1+/+ and nrpd1 NICE1+/+ plants. Non-sRNA bands stained with ethidium bromide (EtBr) and mature miR167 detection were used as controls.
Because RdDM targets are guided by small RNAs (sRNAs) from the methylated origin (Matzke et al., 2015; Zhang et al., 2018), we anticipated that the NICE1+/+ plants would accumulate unnatural sRNAs originating from the DREB1A promoter. sRNA gel blots were conducted with Col-0, NICE1+/+, and nrpd1 NICE1+/+ plants (Figure 7D). Using a probe of the 1AR promoter region, we detected a clear sRNA accumulation signal from NICE1+/+, but the signal was not detected from nrpd1 NICE1+/+ or Col-0. This finding indicated that NICE1+/+ causes the plants to generate sRNAs of the DREB1A promoter in a polymerase IV–dependent manner, which is in concordance with current knowledge of the RdDM pathway (Matzke et al., 2015; Zhang et al., 2018). Overall, our results support the participation of the RdDM machinery in DREB1A repression in ice1-1 (Supplemental Figure 7).
DISCUSSION
The expression of DREB1A/CBF3 is the key step of the cold-responsive transcriptional cascade, after which a large number of cold-inducible genes are expressed. In this study, to elucidate the role of ICE1 in the cold-inducible expression of DREB1A, we analyzed the relationship between DREB1A repression and the ice1-1 mutation (R236H). Unexpectedly, we found that DREB1A repression was not due to the ice1-1 mutation (R236H). ice1-1 (Chinnusamy et al., 2003) and scrm-D (Kanaoka et al., 2008) have the same missense mutation (R236H) of the basic helix-loop-helix–type transcription factor ICE1/SCRM, and both mutant plants typically present obvious defects in leaf and stomatal development. However, their genetic behaviors seemingly contrast: the ICE1(R236H) mutation in ice1-1 showed a dominant-negative effect on DREB1A expression, while the same mutation in scrm-D showed a semidominant positive effect on the expression of the downstream genes involved in stomatal development. This inconsistency has been explained by a model in which ICE1 could function as a convergence point integrating cold and other signal response pathways (Ding et al., 2015; Barrero-Gil and Salinas, 2017), although no convincing evidence to support the model has been provided. Recently, it was reported that the missense mutation R236H of SCRM/ICE1 in scrm-D increases its stability because its ability to interact with MITOGEN-ACTIVATED PROTEIN KINASE3 (MPK3) and MPK6 that negatively regulate ICE1/SCRM protein stability is abolished (Putarjunan et al., 2019). These results are consistent with the semidominant positive effect exhibited by scrm-D, but are not consistent with the dominant-negative effect on DREB1A expression exhibited by ice1-1. Our study revealed that DREB1A transcription was differentially affected in these two scrm-D and ice1-1 mutant plants; cold-induced DREB1A expression was repressed in ice1-1, whereas this expression was not repressed in scrm-D (Figure 2A). Moreover, we have provided a clear answer to this conflict by indicating that two mutant phenotypes of ice1-1 are able to be separated in the backcrossing progeny (Figures 1C to 1E). We demonstrated that a transgene position in chromosome 1 (NICE1) drives the repression of DREB1A expression (Figures 4 and 5) via RdDM machinery (Figures 6 and 7). Thus, our results indicated that the ice1-1 mutation is irrelevant to DREB1A transcriptional regulation.
The roles of ICE1 in the cold stress regulatory pathway are not negligible. After the discovery of the ice1-1 mutant (Chinnusamy et al., 2003), many reports by multiple research groups have agreed with the regulatory pathway (Lee et al., 2005; Miura et al., 2007; Kim et al., 2015) or have supported the positive roles of ICE1 in plant cold stress tolerance by the use of transgenic plants overexpressing ICE1 or ICE1 homologs (Miura et al., 2011; Xu et al., 2014; Huang et al., 2015). However, the dysfunction of ICE1 and its homolog ICE2/SCRM2 in the ice1-2 scrm2-2/+ double mutant resulted in only marginal effects on DREB1 expression (Kim et al., 2015), and most of the other reports did not analyze DREB1 expression. Furthermore, we observed little effect on DREB1 expression in the single and double T-DNA mutant plants (ice1-2, scrm2-1, and ice1-2 scrm2-1; Figure 2D), in contrast to the obvious defects in EPF1 regulation and subsequent stomatal development in the double mutant plants (Figure 2C; Kanaoka et al., 2008). The expression levels of three DREB1 downstream genes were slightly but significantly decreased in the double mutant and ice1-1 mutant plants. However, these expression patterns were inconsistent with the expression patterns of the DREB1 genes, indicating that the decreased expression of the downstream genes could be independent of the regulation of DREB1 expression. Their decreased expression might be due to the severe growth inhibition caused by the abnormal stomatal development of ice1-1 and ice1-2 scrm2-1 or due to other signaling pathways independent of DREB1/CBF. In fact, ICE1 was recently reported to function in various signaling pathways including abscisic acid signaling (Wei et al., 2018; Hu et al., 2019; MacGregor et al., 2019). The effects of these other pathways might affect the expression of the DREB1 downstream genes regardless of DREB1 expression. In addition, we could not detect any increased expression of the DREB1 genes or DREB1 downstream genes, and we did not observe any improvements in the freezing stress tolerance of transgenic Arabidopsis plants overexpressing ICE1 when we used the CaMV 35S promoter to overexpress ICE1 (Figure 3). Considering that the ICE1(R236H) mutation is not related to the repression of DREB1A expression and that neither ICE1 overexpression nor ice1-2 scrm2-1 altered DREB1A expression, we propose that the present ICE1-DREB1 regulatory model should be carefully revalidated without the previous assumption. By contrast, the scrm-D stomatal phenotype and expression of the stomatal differentiation marker gene EPF1 were confirmed to be linked to the ICE1(R236H) mutation.
The T-DNA and RdDM-mediated regulation indicated that the DREB1A repression of ice1-1 is another case of transgene-induced silencing. This phenomenon is widely observed during plant genetic engineering and occasionally suppresses the transgene itself and other cotransfected transgenes (Matzke et al., 1994; Daxinger et al., 2008; Mlotshwa et al., 2010). Some transgenes were reported to cause homology-dependent endogenous gene silencing in transgenic plants (Sidorenko and Peterson, 2001; Wang et al., 2011), similar to how NICE1 caused silencing of DREB1A (Figures 6 and 7). However, compared with most transgene-induced silencing that gradually occurs in generations, NICE1-induced silencing has strong characteristics in terms of its distinctively instant effects on DREB1A expression (Figures 4 and 5). The structure of the NICE1 transgene locus is intriguing and contains an inverted repeat of reporter genes (Figure 4D), which offers hints to interpret the instant DREB1A repression. The maize (Zea mays) MuDR transposon suppressor Muk locus generates an inverted repeat transcript homologous to MuDR, which is processed into sRNAs and initiates the heritable suppression of MuDR (Slotkin et al., 2005). In Arabidopsis, previous reports on targeted de novo DNA methylation demonstrated that expressing inverted repeats of the target promoter sequence is sufficient to provoke hypermethylation of the promoter and silencing of the following gene in a single generation (Kanno et al., 2004; Kinoshita et al., 2007). Therefore, it is suggested that the inverted repeat structure of NICE1 potentially accelerates transgene-induced silencing via rapid sRNA generation through the allocated pathway.
The instant recovery of DREB1A after losing the NICE1 transgene implied that the DREB1A promoter became a target of active 5mC removal (Figures 4 and 6). The first report of Arabidopsis active DNA demethylase REPRESSOR OF SILENCING1 (ROS1) described that ros1 dysfunction brought instant repression of both transgenic and native RD29A promoters (Gong et al., 2002). This analogous feature suggested that ROS1 functions against transgene-induced silencing. Considering its enzyme activity, ROS1 would be a factor that supports the rapid demethylation and recovery of the DREB1A promoter activity in the outcrossing progeny that lack NICE1. With these unique features, future studies on NICE1 in terms of gene silencing and RdDM would provide a deeper understanding of transgene-induced silencing and its regulation.
Our results indicated that ICE1 is not involved in the regulation of cold-inducible expression of DREB1A/CBF3. The question arises as to what kind of transcription factors regulate DREB1A/CBF3 expression. The central oscillators of the circadian clock, CCA1 and LHY, play an important role in inducing the expression of DREB1A under cold conditions (Dong et al., 2011; Kidokoro et al., 2017). Considering that the expression of DREB1A exhibited a circadian rhythm under cold conditions, the major regulatory factors involved in cold-inducible DREB1A expression may be clock-related factors such as CCA1 and LHY. However, even in cca1 lhy double mutants, the cold-inducible expression of DREB1A persisted considerably and continued to exhibit a circadian rhythm, which indicates that other clock-related factors may be involved in DREB1A expression. Furthermore, CCA1 and LHY are known to function as repressors of the expression of several core clock genes such as TOC1, PRR5, PRR7, and PRR9 at 22°C (Nagel et al., 2015; Kamioka et al., 2016; Shalit-Kaneh et al., 2018). The mechanisms by which circadian clock–related factors including CCA1 and LHY activate the cold-specific expression of DREB1A have not yet been clarified. We speculate that unknown factors can alter the function of circadian clock–related factors in response to cold stress and that these unknown factors probably form complexes with the circadian clock–related factors under cold stress conditions. We expect that these factors will be clarified in the near future.
METHODS
Plant Materials
The Arabidopsis (Arabidopsis thaliana) seeds of ice1-1 (CS67843; Chinnusamy et al., 2003), the known parental line of ice1-1 (CS67845; Chinnusamy et al., 2003), ice1-2 (SALK_003155; Kanaoka et al., 2008), scrm2-1 (CS836083; Kanaoka et al., 2008), drm2 (SALK_150863; Yamamuro et al., 2014), cmt3 (SALK_148381; Cao et al., 2003), and nrpd1 (SALK_128428; Yamamuro et al., 2014) were obtained from the Arabidopsis Biological Resources Center. The scrm-D (Kanaoka et al., 2008) seeds were kindly provided by Keiko U. Torii (University of Washington). For the transgenic Arabidopsis plants, the 5030-bp genomic region (including the 2284-bp promoter region) of ice1-1 was amplified and cloned into the KpnI and NotI sites of a pGreen0029 vector (Hellens et al., 2000). The subsequent processes of transfection and antibiotic selection were in accordance with previous processes (Kidokoro et al., 2017). The plants were grown on GM agar plates at 22 ± 1°C under a 16-h-light/8-h-dark cycle and a photon flux density of 50 ± 10 μmol m−2 s−1 of white light. The oligomers applied are presented in the Supplemental Table.
Cold Treatment and Plant RNA Extraction
For the cold stress treatment, 12-d-old whole seedlings on agar plates were gradually chilled in the 4°C cold chamber for 3 h (Kidokoro et al., 2017). The treatments were started at 2 h after dawn. Four to eight plants, depending on the plant size, were pooled to obtain a single sample for RNA preparation. The plant total RNA extraction was conducted with RNAiso plus (Takara Bio) and supplemental DNase treatment. The extracted RNA was subjected to RT-qPCR and RNA gel blot assays.
RT-qPCR
cDNA was synthesized from the plant total RNA by a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-qPCR was performed using the QuantStudio 3 Real-Time PCR system and software version 1.2 (Applied Biosystems). Power SYBR Green Master Mix (Applied Biosystems) was used for amplification. Arabidopsis IPP2 was used as the quantitative control for the template. For each biological replicate in all RT-qPCR experiments, three independent RNA samples were analyzed. The oligomers applied are presented in Supplemental Table.
Whole-Genome Resequencing
A total of 1 μg of Arabidopsis genomic DNA for each sample was subjected to Illumina sequencing library construction according to the TruSeq DNA sample preparation guide (Illumina). Single-read sequencing was performed on a NextSeq 500 system, resulting in an average of 34.5 million reads (75 nucleotides) from each library. The manually cleaned reads were mapped to The Arabidopsis Information Resource (TAIR10) using bwa version 0.7.5a (Li and Durbin, 2009). Genetic variants of the samples were called using BCFtools version 1.3.1 (Li, 2011). Only biallelic SNPs supported by more than six reads were retained for the analysis. Supplemental paired-end sequencing was performed on a HiSeq 2500 system, which output 23.4 million reads (2 × 101 nucleotides) of the ice1-1 genome. The cleaned reads were mapped to TAIR10 using bwa, and abnormal singleton mapping features were retrieved to estimate where T-DNA was positioned. To determine the base pair-resolution borders of the T-DNA locus, targeted assembly by TASR version 1.6.2 (Warren and Holt, 2011) was performed on the flanking genomic region where singleton mapping features were observed.
Local Bisulfite Sequencing
A total of 0.5 μg of Arabidopsis genomic DNA was subjected to bisulfite treatment and subsequent Sanger sequencing analysis. The bisulfite treatment and DNA purification steps were achieved with an EpiTect Bisulfite Kit (Qiagen). Each region of interest was amplified from the bisulfite-treated DNA, and the amplicon was sequenced individually upon subcloning into a pGEM-T vector system (Promega). More than 12 independent clones were sequenced for each sample data. The oligomers applied are presented in Supplemental Table.
5azaC Treatment
Sterilized Arabidopsis seeds were sown on GM agar plates that were supplemented with 5azaC (A2232, Tokyo Chemical Industry). The same volume of dimethyl sulfoxide solvent was supplemented for the mock condition. The seedlings were grown for 12 d under the same growth conditions described above and then subjected to the cold treatment described above to evaluate the treatment effects.
sRNA Gel Blots
The details of the procedure followed that in a previous report by Schwab et al. (2006). In brief, 30 μg of total RNA per lane was run on a 17% (w/v) acrylamide gel that was supplemented with 7 M urea, after which the RNA was transferred to a Nytran SPC membrane (GE Healthcare). The blots were hybridized using 32P-end–labeled oligonucleotide probes of the DREB1A promoter sequence, which included boxes V and VI (Supplemental Table). Hybridization was performed in PerfectHyb Plus Hybridization Buffer (Sigma-Aldrich) at 38°C overnight.
Freezing Tolerance Test
The freezing treatment was performed as previously described by Kidokoro et al. (2015), with minor modifications. Ten-day-old whole seedlings grown on agar plates were chilled in a −2°C cold chamber for 2 h. After the generation of ice nuclei, the temperature was lowered by −1°C h−1 until −9°C was reached. The plates were then transferred to 4°C and thawed overnight. After recovery at 22°C for a week, the seedlings that generated new leaves were counted as having survived.
Accession Numbers
The raw sequence data from the whole genome resequencing analysis were deposited in National Center for Biotechnology Information Short Read Archive under a specific accession number (PRJNA489736). Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: ICE1 (AT3G26744), SCRM2 (AT1G12860), DREB1A (AT4G25480), DREB1B (AT4G25490), DREB1C (AT4G25470), EPF1 (AT2G20875), COR15A (AT2G42540), RD29A (AT5G52310), GolS3 (AT1G09350), NRPD1 (AT1G63020), DRM2 (AT5G14620), CMT3 (AT1G69770), and IPP2 (AT3G02780).
Supplemental Data
Supplemental Figure 1. The candidate SNPs were rejected by additional derived cleaved amplified polymorphic sequences (dCAPS) (Supports Figure 4.).
Supplemental Figure 2. NICE1 alleles have little effect on the expression of flanking genes (Supports Figure 4.).
Supplemental Figure 3. Cold-induced DREB1A expression in transgenic plants transformed with TE or TE and DREB1Apro:LUC (Supports Figure 4.).
Supplemental Figure 4. Cytosine methylation levels of the DREB1B and DREB1C promoters by NICE1 genotype (Supports Figure 6.).
Supplemental Figure 5. Effects of 5azaC treatment on DREB1A expression in ice1-1 (Supports Figure 6.).
Supplemental Figure 6. Dysfunctional effects of DNA methylation components on DREB1A expression (Supports Figure 7.).
Supplemental Figure 7. A working model of 5mC-mediated DREB1A repression and recovery via the Arabidopsis ice1-1 mutation (Supports Figures 4-7.).
Supplemental Table. Oligomers used in this study.
Supplemental Data Set. P values of statistical analyses in this study.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Yuriko Tanaka (University of Tokyo), Tomomi Shinagawa, Ayami Furuta (Chubu University), and Saho Mizukado and Fuyuko Shimoda (RIKEN) for providing excellent technical assistance and Etsuko Toma (University of Tokyo) for providing skillful editorial assistance. We also thank Tetsuji Kakutani (University of Tokyo) for the fruitful discussions and valuable suggestions concerning DNA methylation to prepare the article and Keiko U. Torii (University of Washington) for kindly providing scrm-D mutant seeds. This work was supported by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research for Young Scientists [B]17K15413 to S.K., for Scientific Research [A]; 18H03996 to K.Y.-S., and for Scientific Research on Innovative Areas 15H05960 to K.Y.-S.), and by RIKEN (Special Postdoctoral Researcher program and the Incentive Research Project to J.-S.K.).
AUTHOR CONTRIBUTIONS
S.K., J.-S.K., and K.Y.-S. designed the study. S.K., J.-S.K., and T.I. performed the experiments and analyzed the data. T.S. contributed to the genome resequencing. S.K., J.-S.K., K.S., and K.Y.-S. wrote the article. All of the authors discussed the results and commented on the article.
Footnotes
Articles can be viewed without a subscription.
References
- Baker S.S., Wilhelm K.S., Thomashow M.F. (1994). The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol. Biol. 24: 701–713. [DOI] [PubMed] [Google Scholar]
- Barrero-Gil J., Salinas J. (2017). CBFs at the crossroads of plant hormone signaling in cold stress response. Mol. Plant 10: 542–544. [DOI] [PubMed] [Google Scholar]
- Cao X., Aufsatz W., Zilberman D., Mette M.F., Huang M.S., Matzke M., Jacobsen S.E. (2003). Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr. Biol. 13: 2212–2217. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Ohta M., Kanrar S., Lee B.H., Hong X., Agarwal M., Zhu J.K. (2003). ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L., Hunter B., Sheikh M., Jauvion V., Gasciolli V., Vaucheret H., Matzke M., Furner I. (2008). Unexpected silencing effects from T-DNA tags in Arabidopsis. Trends Plant Sci. 13: 4–6. [DOI] [PubMed] [Google Scholar]
- Ding Y., Li H., Zhang X., Xie Q., Gong Z., Yang S. (2015). OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 32: 278–289. [DOI] [PubMed] [Google Scholar]
- Doherty C.J., Van Buskirk H.A., Myers S.J., Thomashow M.F. (2009). Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 21: 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.H., Agarwal M., Zhang Y., Xie Q., Zhu J.K. (2006). The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 103: 8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M.A., Farré E.M., Thomashow M.F. (2011). Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 7241–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z., Morales-Ruiz T., Ariza R.R., Roldán-Arjona T., David L., Zhu J.K. (2002). ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111: 803–814. [DOI] [PubMed] [Google Scholar]
- Hellens R.P., Edwards E.A., Leyland N.R., Bean S., Mullineaux P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42: 819–832. [DOI] [PubMed] [Google Scholar]
- Hu Y., Han X., Yang M., Zhang M., Pan J., Yu D. (2019). The transcription factor INDUCER OF CBF EXPRESSION1 interacts with ABSCISIC ACID INSENSITIVE5 and DELLA proteins to fine-tune abscisic acid signaling during seed germination in Arabidopsis. Plant Cell 31: 1520–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Li K., Jin C., Zhang S. (2015). ICE1 of Pyrus ussuriensis functions in cold tolerance by enhancing PuDREBa transcriptional levels through interacting with PuHHP1. Sci. Rep. 5: 17620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Pélissier T., Bourguet P., Becker C., Pouch-Pélissier M.N., Pogorelcnik R., Weingartner M., Weigel D., Deragon J.M., Mathieu O. (2017). Arabidopsis proteins with a transposon-related domain act in gene silencing. Nat. Commun. 8: 15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. (1998). Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280: 104–106. [DOI] [PubMed] [Google Scholar]
- Jia Y., Ding Y., Shi Y., Zhang X., Gong Z., Yang S. (2016). The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 212: 345–353. [DOI] [PubMed] [Google Scholar]
- Kamioka M., Takao S., Suzuki T., Taki K., Higashiyama T., Kinoshita T., Nakamichi N. (2016). Direct repression of evening genes by CIRCADIAN CLOCK-ASSOCIATED1 in the Arabidopsis circadian clock. Plant Cell 28: 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka M.M., Pillitteri L.J., Fujii H., Yoshida Y., Bogenschutz N.L., Takabayashi J., Zhu J.K., Torii K.U. (2008). SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to arabidopsis stomatal differentiation. Plant Cell 20: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Mette M.F., Kreil D.P., Aufsatz W., Matzke M., Matzke A.J.M. (2004). Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 14: 801–805. [DOI] [PubMed] [Google Scholar]
- Kidokoro S., Watanabe K., Ohori T., Moriwaki T., Maruyama K., Mizoi J., Myint Phyu Sin Htwe N., Fujita Y., Sekita S., Shinozaki K., Yamaguchi-Shinozaki K. (2015). Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 81: 505–518. [DOI] [PubMed] [Google Scholar]
- Kidokoro S., Yoneda K., Takasaki H., Takahashi F., Shinozaki K., Yamaguchi-Shinozaki K. (2017). Different cold-signalling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell 29: 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Lee M., Lee J.H., Lee H.J., Park C.M. (2015). The unified ICE-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol. Biol. 89: 187–201. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Saze H., Kinoshita T., Miura A., Soppe W.J.J., Koornneef M., Kakutani T. (2007). Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. Plant J. 49: 38–45. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Henderson D.A., Zhu J.K. (2005). The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17: 3155–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2011). Improving SNP discovery by base alignment quality. Bioinformatics 27: 1157–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor D.R., Zhang N., Iwasaki M., Chen M., Dave A., Lopez-Molina L., Penfield S. (2019). ICE1 and ZOU determine the depth of primary seed dormancy in Arabidopsis independently of their role in endosperm development. Plant J. 98: 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., et al. (2009). Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol. 150: 1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A.J., Neuhuber F., Park Y.D., Ambros P.F., Matzke M.A. (1994). Homology-dependent gene silencing in transgenic plants: Epistatic silencing loci contain multiple copies of methylated transgenes. Mol. Gen. Genet. 244: 219–229. [DOI] [PubMed] [Google Scholar]
- Matzke M.A., Kanno T., Matzke A.J. (2015). RNA-directed DNA methylation: The evolution of a complex epigenetic pathway in flowering plants. Annu. Rev. Plant Biol. 66: 243–267. [DOI] [PubMed] [Google Scholar]
- Mlotshwa S., Pruss G.J., Gao Z., Mgutshini N.L., Li J., Chen X., Bowman L.H., Vance V. (2010). Transcriptional silencing induced by Arabidopsis T-DNA mutants is associated with 35S promoter siRNAs and requires genes involved in siRNA-mediated chromatin silencing. Plant J. 64: 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.J., Hasegawa P.M. (2007). SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19: 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Ohta M., Nakazawa M., Ono M., Hasegawa P.M. (2011). ICE1 Ser403 is necessary for protein stabilization and regulation of cold signaling and tolerance. Plant J. 67: 269–279. [DOI] [PubMed] [Google Scholar]
- Nagel D.H., Doherty C.J., Pruneda-Paz J.L., Schmitz R.J., Ecker J.R., Kay S.A. (2015). Genome-wide identification of CCA1 targets uncovers an expanded clock network in Arabidopsis. Proc. Natl. Acad. Sci. USA 112: E4802–E4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee C.M., Doherty C.J., Gilmour S.J., Kim Y., Thomashow M.F. (2015). Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 82: 193–207. [DOI] [PubMed] [Google Scholar]
- Pillitteri L.J., Peterson K.M., Horst R.J., Torii K.U. (2011). Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell 23: 3260–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putarjunan A., Ruble J., Srivastava A., Zhao C., Rychel A.L., Hofstetter A.K., Tang X., Zhu J.K., Tama F., Zheng N., Torii K.U. (2019). Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat. Plants 5: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit-Kaneh A., Kumimoto R.W., Filkov V., Harmer S.L. (2018). Multiple feedback loops of the Arabidopsis circadian clock provide rhythmic robustness across environmental conditions. Proc. Natl. Acad. Sci. USA 115: 7147–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwari Z.K., Nakashima K., Miura S., Kasuga M., Seki M., Yamaguchi-Shinozaki K., Shinozaki K. (1998). An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low-temperature-responsive gene expression. Biochem. Biophys. Res. Commun. 250: 161–170. [DOI] [PubMed] [Google Scholar]
- Sidorenko L.V., Peterson T. (2001). Transgene-induced silencing identifies sequences involved in the establishment of paramutation of the maize p1 gene. Plant Cell 13: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin R.K., Freeling M., Lisch D. (2005). Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat. Genet. 37: 641–644. [DOI] [PubMed] [Google Scholar]
- Tang K., Lang Z., Zhang H., Zhu J.K. (2016). The DNA demethylase ROS1 targets genomic regions with distinct chromatin modifications. Nat. Plants 2: 16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 571–599. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Xiong L., Li W., Zhu J.K., Zhu J. (2011). The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 23: 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R.L., Holt R.A. (2011). Targeted assembly of short sequence reads. PLoS One 6: e19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D., Liu M., Chen H., Zheng Y., Liu Y., Wang X., Yang S., Zhou M., Lin J. (2018). INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet. 14: e1007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Jiao Y., Li R., Zhang N., Xiao D., Ding X., Wang Z. (2014). Chinese wild-growing Vitis amurensis ICE1 and ICE2 encode MYC-type bHLH transcription activators that regulate cold tolerance in Arabidopsis. PLoS One 9: e102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57: 781–803. [DOI] [PubMed] [Google Scholar]
- Yamamuro C., Miki D., Zheng Z., Ma J., Wang J., Yang Z., Dong J., Zhu J.K. (2014). Overproduction of stomatal lineage cells in Arabidopsis mutants defective in active DNA demethylation. Nat. Commun. 5: 4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Lang Z., Zhu J.K. (2018). Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 19: 489–506. [DOI] [PubMed] [Google Scholar]
- Zhao C., Zhang Z., Xie S., Si T., Li Y., Zhu J.K. (2016). Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 171: 2744–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]