Abstract
Background
New treatments for stress-related disorders including depression, anxiety, and substance use disorder are greatly needed. Kappa opioid receptors are expressed in the central nervous system, including areas implicated in analgesia and affective state. Although kappa opioid receptor agonists share the antinociceptive effects of mu opioid receptor agonists, they also tend to produce negative affective states. In contrast, selective kappa opioid receptor antagonists have antidepressant- and anxiolytic-like effects, stimulating interest in their therapeutic potential. The prototypical kappa opioid receptor antagonists (e.g., norBNI, JDTic) have an exceptionally long duration of action that complicates their use in humans, particularly in tests to establish safety. This study was designed to test dose- and time-course effects of novel kappa opioid receptor antagonists with the goal of identifying short-acting lead compounds for future medication development.
Methods
We screened 2 novel, highly selective kappa opioid receptor antagonists (CYM-52220 and CYM-52288) with oral efficacy in the warm water tail flick assay in rats to determine initial dose and time course effects. For comparison, we tested existing kappa opioid receptor antagonists JDTic and LY-2456302 (also known as CERC-501 or JNJ-67953964).
Results
In the tail flick assay, the rank order of duration of action for the antagonists was LY-2456302 < CYM-52288 < CYM-52220 << JDTic. Furthermore, LY-2456302 blocked the depressive (anhedonia-producing) effects of the kappa opioid receptor agonist U50,488 in the intracranial self-stimulation paradigm, albeit at a higher dose than that needed for analgesic blockade in the tail flick assay.
Conclusions
These results suggest that structurally diverse kappa opioid receptor antagonists can have short-acting effects and that LY-2456302 reduces anhedonia as measured in the intracranial self-stimulation test.
Keywords: JDTic, LY-2456302, U50, 488, analgesia, ICSS
Significance Statement.
There is a need for novel antidepressant medications. In both humans and laboratory animals, kappa opioid receptor (KOR) activation produces depressive and anxiety-like effects. These findings have stimulated efforts to develop novel, selective, and short-acting KOR antagonists. Here we tested 2 novel KOR antagonists developed at The Scripps Research Institute, CYM-52220 and CYM-52288, for dose and time course effects in the warm water tail flick assay (TFA) in rats. For comparison, we tested the prototypical and long-lasting KOR antagonist, JDTic, and the short-acting KOR antagonist LY-2456302 (also known as CERC-501 or JNJ-67953964). Although the CYM compounds have oral bioavailability and a shorter duration of action than JDTic, they are not as short-acting in the TFA as LY-2456302. As such, we used intracranial self-stimulation to show that LY-2456302 dose-dependently reduces the depressive (anhedonia-producing) effects of the KOR agonist U50,488, demonstrating that the protracted time course of prototypical KOR antagonists is not necessary for antidepressant-like effects.
Introduction
Kappa opioid receptors (KORs) are expressed throughout the body, including within brain areas implicated in analgesia, motivation, and emotion (Mansour et al., 1994, 1995; Svingos et al., 1999). Although selective KOR agonists share the antinociceptive effects of mu opioid receptor (MOR)-selective opioids (Han and Xie, 1982), they tend to produce depressive-, aversive,- and stress-like effects in humans (Pfeiffer et al., 1986; MacLean et al., 2013) and laboratory animals (Todtenkopf et al., 2004; Bruchas et al., 2010; Ebner et al., 2010; Knoll and Carlezon, 2010). These KOR-mediated negative affective states are thought to contribute to drug-seeking and relapse in animal models used to study addictive behaviors (Negus, 2004; Schindler et al., 2012; Chartoff and Carlezon, 2014) and may have similar effects in humans. In contrast, selective KOR antagonists or genetic deletion of KORs have antidepressant- and anxiolytic-like effects (Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003; Van’t Veer et al., 2013; Rorick-Kehn et al., 2014), and they block stress-induced reinstatement of drug-seeking behavior in rats (Beardsley et al., 2005; Carey et al., 2007; Walker and Koob, 2008; Nygard et al., 2016; Le et al., 2018). Inasmuch as drug withdrawal is stressful (Chartoff and Carlezon, 2014), a broad ability to reduce the impact of stress may explain how KOR antagonists can have efficacy in models that would appear to represent a wide variety of disease states, ranging from mood disorders to anxiety to addiction.
It is important to emphasize that the profile presented by KOR antagonists—combined antidepressant and anxiolytic effects—is a unique and distinguishing feature that could have a transformative impact on treatment strategies for stress-related disorders. For comparison, standard antidepressant drugs (e.g., selective serotonin reuptake inhibitors) often produce anxiogenic effects in preclinical screens, especially on acute administration (Knoll et al., 2007). Although these anxiogenic effects undergo tolerance with repeated administration and may even be replaced by anxiolytic effects, they are also seen in humans and can create tolerability/adherence problems in patients with co-morbid depression and anxiety. This unique profile suggests that if KOR antagonists were developed to treat depressive disorders, they would be better tolerated, particularly at the beginning of a treatment regimen. Moreover, if they do not produce initial anxiogenic side-effects, they may be effective more quickly than selective serotonin reuptake inhibitors. As such, the ability to rapidly treat both depressive- and anxiety-related signs and symptoms would represent a major advance (Carlezon and Krystal, 2016).
While data from preclinical studies provide a strong rationale for clinical studies, there are well-characterized issues with the prototypical KOR antagonists (norBNI, JDTic) that complicate their use in humans. Foremost, these agents have a slow onset of KOR specificity (Munro et al., 2012) and an exceptionally long duration of action (Potter et al., 2011; Carroll and Carlezon, 2013). Perhaps owing to the exciting potential of KOR antagonists as therapeutic agents, National Institute on Drug Abuse (NIDA) sponsored a phase I clinical trial of JDTic despite its long-lasting effects (http://clinicaltrials.gov/ct2/show/NCT01431586). Unfortunately, this study was terminated because JDTic administration was associated with a single episode of nonsustained ventricular tachycardia in 2 of 6 subjects receiving the active dose (Chavkin and Martinez, 2015), likely reflecting off-target effects at ion channels in the heart. Another KOR antagonist, LY-2456302 (also known previously as CERC-501 and currently as JNJ-67953964), was one of a series of aminobenzyloxyarylamide KOR antagonists produced by Eli Lily (Mitch et al., 2011). It was the first compound that tested and passed the newly implemented National Institute of Mental Health (NIMH) FAST-FAIL initiative (Krystal et al., 2018), which supports early-phase drug development methodologies designed to lower the risk of failure in large clinical trials. It is currently being tested in a phase II proof of concept clinical trial for treating patients with treatment-resistant depression (NCT03559192). LY-2456302 displays in vitro subnanomolar KOR antagonism with a selectivity of approximately 21-fold over the MOR and 135-fold over the delta opioid receptor (DOR) and efficacy in animal models of substance use disorder (SUD) and depression (see Guerrero et al., 2019). Finally, a proprietary formulation of buprenorphine (a combination of buprenorphine with the MOR antagonist samidorphan; Wentland et al., 2009), owned by Alkermes (ALKS 5461), has antidepressant efficacy in clinical studies (Ehrich et al., 2015; Fava et al., 2016), an effect that is attributed to KOR antagonism. While the work with ALKS 5461 has provided a crucial proof-of-principle that KOR antagonists may have efficacy as antidepressants in people, development and widespread use of this particular agent may be complicated by potential abuse-liability of the parent compound (buprenorphine) and the challenges of formulating complex mixtures that contain multiple drugs. As such, the development of selective KOR antagonists, which appear to have no abuse liability of their own, is a high priority (Guerrero et al., 2019).
The purpose of this study was to compare in vivo pharmacological profiles of 2 existing KOR antagonists of different chemical classes (JDTic and LY-2456302) with 2 novel KOR antagonists synthesized at The Scripps Research Institute (CYM-52220 and CYM-52288). These novel compounds were chosen because they are approximately 1500-fold more selective for KOR receptors than other opioid receptors and devoid of off-target effects at key ion channels. We conducted dose- and time-course effects of the 4 KOR antagonists in the warm water tail flick assay (TFA) in rats. Considering the favorable results from those studies, we also tested the effects of LY-2456302 in the operant paradigm intracranial self-stimulation (ICSS) to examine in vivo efficacy in a behavioral test sensitive to changes in reward function and motivated behavior.
Materials and Methods
Animals
Adult, male Sprague Dawley rats (Charles River Laboratories) weighing 350 to 375 g on arrival to the facility were used. Rats were group housed (4 rats/cage) unless they were being used in ICSS studies, in which case they were singly housed. Rats were acclimatized for 1 week in a 12-hour-ligh/-dark cycle (lights on at 7:00 am) vivarium with free access to food and water, and experiments were conducted during the light phase. Procedures were approved by the McLean Hospital Animal Care and Use Committee and consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
KOR antagonists were administered i.p. or via oral gavage (p.o.) at a volume of 1 mL/kg. Rats were habituated to oral gavage insertion for 5 days prior to testing. JDTic was synthesized at Research Triangle Institute (Research Triangle Park, NC; gift of F. I. Carroll) and dissolved in 0.9% saline. Previous work in our laboratory has shown that i.p. injection of JDTic at a dose of 10 mg/kg produces significant anxiolytic effects in the elevated plus maze in rats (see Knoll et al., 2007). Here we used a dose of 20 mg/kg (based on the salt form of the drug) to compensate for oral administration. LY-2456302 was synthesized at The Scripps Research Institute (TSRI, La Jolla, CA) and dissolved in 3% lactic acid in water; doses were based on previous reports (Rorick-Kehn et al., 2014). The novel KOR antagonists CYM-52220 and CYM-52288 were synthesized at TSRI and dissolved in either 10% dimethyl sulfoxide (DMSO) + 10% Tween-80 in water (CYM-52220) or in water (CYM-52288); doses were based on in vitro affinities and potencies at the KOR (Guerrero et al., 2019). The KOR agonist (±)-trans-U-50,488 methanesulfonate salt (U50,488, Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water and administered i.p. at a volume of 1 mL/kg; doses were based on (Barrett et al., 2002).
Tail Flick Assay
A water bath (Fisher Scientific) was set to a temperature of 52oC. Rats were held gently but firmly around the shoulders with a towel, and the bottom 1 inch of the rat’s tail was dipped in the warm water. The latency for the rat to flick its tail out of the water was recorded with a stopwatch. To prevent tissue damage, a cut-off time of 15 seconds was used. After testing, rats were returned to their home cages. A baseline TFA measurement was performed 24 hours before the drug test. For comparison of temporal and route of administration parameters for JDTic, the drug (vehicle or 20 mg/kg JDTic) was administered either i.p. or p.o. 3 or 71 hours prior to an i.p. injection of U50,488 (15 mg/kg). The TFA was conducted 1 hour after U50,488 administration. Note that initial experiments with JDTic (Figure 1) used a within-subjects design in which the same rats were tested with U50,488 at each time point. Subsequent experiments with LY-2456302, CYM-52220, and CYM-52288 used a higher dose of U50,488 (30 mg/kg) and hence a between-subjects design to avoid U50,488-induced tolerance. This increase in U50,488 dose was triggered by our finding that, based on a variety of factors (batch of rats, batch of U50,488, experimenter, time of year, etc.), 15 mg/kg U50,488 produced highly variable analgesia in the TFA. For dose response experiments of LY-2456302, CYM-52220, and CYM-52288, the KOR antagonist was administered p.o. 1 hour prior to an i.p. injection of U50,488. The TFA was conducted 1 hour after U50,488 administration. For time course experiments, the AD80 dose (determined from dose response) of each KOR antagonist was used, which is defined as the dose of antagonist that produces an effect in 80% of the population. We chose to use the AD80 to ensure that each antagonist would have an effect in sufficient rats to observe reliable temporal effects. LY-2456302, CYM-52220, and CYM-52288 were administered either 1, 2, 4, 8, or 24 hours prior to TFA testing. Regardless of the antagonist pretreatment time, U50,488 was administered 1 hour prior to TFA testing.
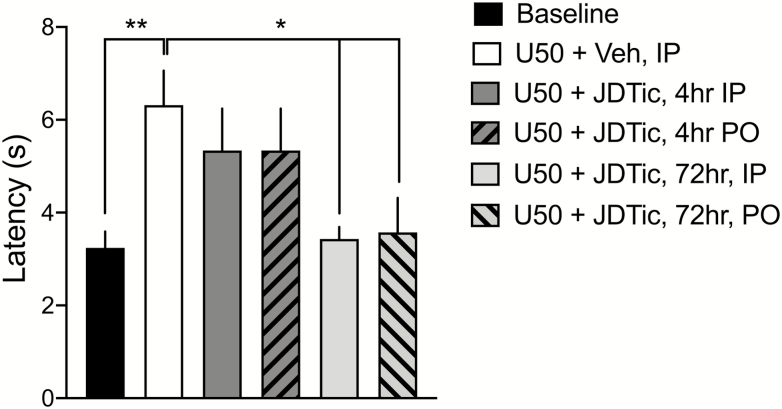
Figure 1.
Effects of systemic (i.p.) vs oral (p.o.) administration of JDTic on U50,488-induced analgesia in the warm water tail flick assay (TFA). Baseline TFA latencies were obtained at least 2 hours prior to any drug treatment. JDTic was administered before the TFA was performed at the times indicated in the legend. U50,488 (15 mg/kg, i.p.) was injected 1 hour before the TFA was performed in each group. n = 12/group for baseline and U50,488 alone, n = 6/group for JDTic groups. **P < .01 compared withU50,488 alone (U50, white bar).
Intracranial Self-Stimulation
ICSS is an operant paradigm in which rodents self-administer rewarding electrical stimulation through electrodes implanted in the medial forebrain bundle (Carlezon and Chartoff, 2007). Rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.; Abbott Laboratories, North Chicago, IL) supplemented with subcutaneous atropine (0.25 mg/kg) to minimize bronchial secretions and implanted with stainless-steel monopolar electrodes (0.25-mm diameter; Plastics One, Roanoke, VA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to midline, 7.8 mm below dura) as described in (Ebner et al., 2010). After 1 week of recovery from surgery, rats began ICSS training using a continuous reinforcement schedule at 141 Hz, where each lever press earned a 500-ms train of square wave cathodal pulses (100 μs per pulse).
After a minimally effective stimulation current was found for each rat, it was kept constant throughout training and testing. Rats were then trained using the rate-frequency method, in which a series of 15 descending stimulation frequencies (141–28 Hz, in 0.05 log10 Hz increments) was presented to the rat. For detailed descriptions of these procedures, including threshold analysis measures, see (Carlezon and Chartoff, 2007; Ebner et al., 2010). Rats were trained for an average of 3 to 4 weeks until mean ICSS thresholds remained stable (±10% for 4 consecutive days). In addition to achieving continued, stable stimulation thresholds, rats needed to press through at least the top 6 highest frequencies, with ≥20 presses for each of these frequencies (i.e., ≥20 presses/50 s). We have shown that these parameters reflect reliable electrode placement in the medial forebrain bundle at the level of the lateral hypothalamus (Ebner et al., 2010; Russell et al., 2014).
To determine the effects of the KOR antagonist LY-2456302 on U50,488-induced increases in ICSS thresholds, rats first performed 3 rate-frequency curves (45 minutes) to establish pre-drug baseline thresholds. Immediately following this, 1 dose of LY-2456302 (0, 1, 10, or 30 mg/kg, p.o.) was administered, and rats were returned to the ICSS chambers for 2 additional rate-frequency curves (30 minutes). Immediately following this, U50,488 (10 mg/kg, i.p.) was administered and 4 additional rate frequency curves (60 minutes) were obtained. Data are presented as percent change from the average of the latter 2 of the 3 pre-drug baseline thresholds. The doses of LY-2456302 were determined initially from the TFA experiment, in which 1.0 mg/kg LY-2456302 produced the strongest reduction in U50,488-induced analgesia. However, this dose failed to reduce the effects of U50,488 in ICSS, so we increased the range of doses tested in order to observe a behavioral effect. The dose of U50,488 (10 mg/kg, i.p.) was chosen because it reliably produces a robust increase in ICSS thresholds but does not completely block operant responding, as a higher dose such as 30 mg/kg would do (Russell et al., 2014). Drug treatments were administered in random order and separated by 2 or 3 nondrug days during which baseline ICSS thresholds were maintained.
Data Analysis
TFA data are presented as either latency (seconds) to withdraw tail (Figure 1) or the change in latency (test minus pre-drug baseline; Figures 2 and 3). To analyze the effects of treatment or time in the TFA studies, 1-way ANOVAs were used followed by Dunnett’s multiple comparison post-hoc tests in the event of significant effects. Nonlinear regression of dose response data were used to calculate the dose of antagonist that produced 80% antagonism of U50,488-induced analgesia (AD80). For ICSS data in which drug effects were compared across time (90 minutes), mixed effects ANOVAs were performed with repeated measures on time and treatment, followed by Tukey’s multiple comparison tests in the event of significant interactions. For the ICSS study, some data points were missing because not all rats were tested with each combination of drug treatments (2 rats stopped responding during baseline sessions towards the end of the experiment and were eliminated from further testing). For ICSS data in which percent baseline thresholds are represented as an average over time (Figure 4e–f), 1-way ANOVAs were used followed by Dunnett’s multiple comparison posthoc tests in the event of significant effects.
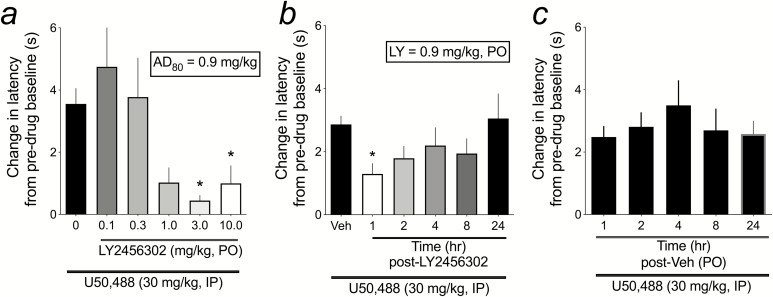
Figure 2.
Dose response and time course effects of LY-2456302 on U50,488-induced analgesia in the warm water tail flick assay (TFA). (a) LY-2456302 was administered orally (p.o.) 1 hour prior to injection of U50,488 (30 mg/kg, i.p.), and TFA latencies were determined 1 hour after U50,488 administration. n = 12 (0.0 mg/kg), 6 (0.1 mg/kg), 7 (0.3 mg/kg), 6 (1.0 mg/kg), 6 (3.0 mg/kg), and 8 (10.0 mg/kg). *P < .05 compared withLY-2456302 (0.0 mg/kg). (b) Based on the dose response, nonlinear regression was used to determine the AD80 dose, which was then used to test the ability of LY-2456302 to attenuate U50,488-induced analgesia when administered 1 hour (n = 18), 2 hours (n = 16), 4 hours (n = 12), 8 hours (n = 7), and 24 hours (n = 12) prior to the TFA test. LY-2456302 (0.9 mg/kg, p.o.) significantly reduced U50,488-induced increases in TFA latency when administered 1 hour before the TFA test. *P < .05 compared withVeh. (c) For each LY-2456302 pretreatment time point, a separate vehicle (3% lactic acid in water) group was tested; total n = 38. There was no effect of Veh pretreatment on TFA latency at any time. As such, Veh groups for each time point were collapsed for the analysis shown in (b).
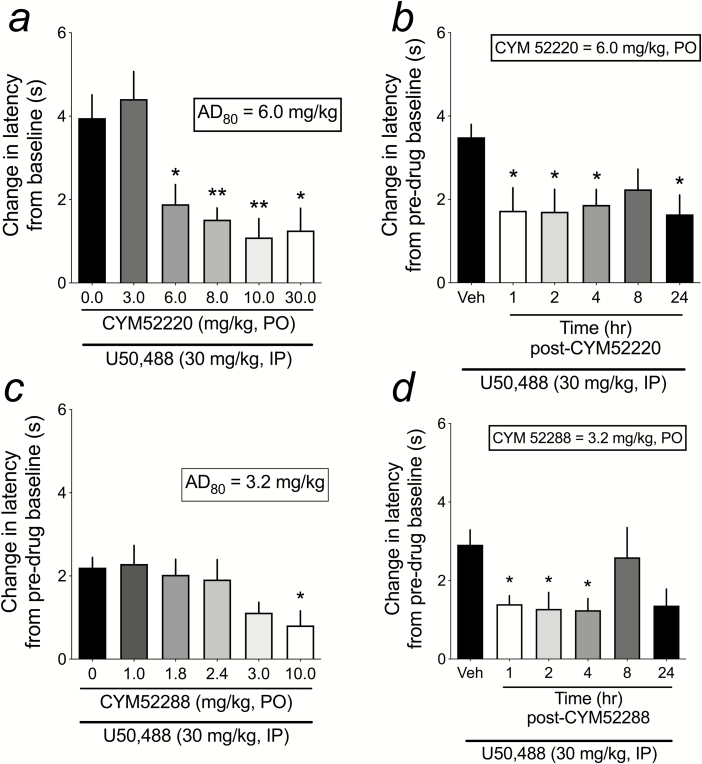
Figure 3.
Dose response and time course effects of CYM-52220 and CYM-52288 on U50,488-induced analgesia in the warm water tail flick assay (TFA). (a) CYM-52220 [n = 12 (0.0 mg/kg), 12 (3.0 mg/kg), 13 (6.0 mg/kg), 16 (8.0 mg/kg), 9 (10 mg/kg), and 6 (30.0 mg/kg)] was administered p.o. 1-hour prior to injection of U50,488 (30 mg/kg, IP), and TFA latencies were determined 1-hour after U50,488 administration. (b) Based on the CYM-52220 dose response, nonlinear regression was used to determine the AD80 dose, which was then used to test the ability of CYM-52220 to attenuate U50,488-induced analgesia when administered 1 hour (n = 14), 2 hours (n = 14), 4 hours (n = 14), 8 hours (n = 14), and 24 hours (n = 8) prior to the TFA test. CYM-52220 vehicle control groups were included for each time point, but similar to data shown in Figure 2C there was no significant difference in tail flick latencies at any vehicle time point, so the values were combined (total n = 60). CYM-52220 (6.0 mg/kg, p.o.) significantly reduced U50,488-induced increases in TFA latency when administered 1, 2, 4 (but not 8), or 24 hours before the TFA test. (c) CYM-52288 [n = 16 (0.0 mg/kg), 17 (1.0 mg/kg), 16 (1.8 mg/kg), 16 (2.4 mg/kg), 19 (3.0 mg/kg), and 16 (10.0 mg/kg)] was administered p.o. 1 hour prior to injection of U50,488 (30 mg/kg, IP), and TFA latencies were determined 1 hour after U50,488 administration. *P < .05, **P < .01 compared with the 0.0-mg/kg dose. (d) Based on the CYM-52288 dose response, nonlinear regression was used to determine the AD80 dose, which was then used to test the ability of CYM-52288 to attenuate U50,488-induced analgesia when administered 1 hour (n = 13), 2 hours (n = 11), 4 hours (n = 9), 8 hours (n = 12), and 24 hours (n = 10) prior to the TFA test. CYM-52288 vehicle control groups were included for each time point, but similar to data shown in Figure 2C, there was no significant difference in tail flick latencies at any vehicle time point, so the values were combined (total n = 20). CYM-52288 (3.2 mg/kg, p.o.) significantly reduced U50,488-induced increases in TFA latency when administered 1, 2, and 4 (but not 8) hours before the TFA test. There was a strong trend (P = .06) for CYM-52288 to reduce U50,488-induced increases in TFA latency when administered 24 hours before testing. *P < .05, **P < .01 compared with vehicle.
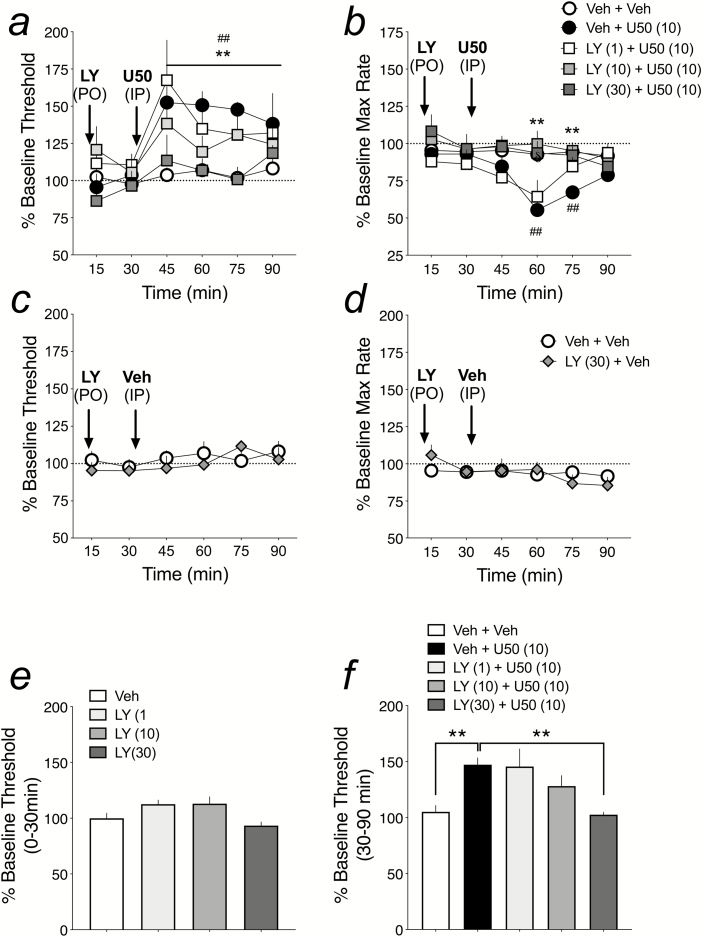
Figure 4.
LY-2456302 attenuates U50,488-induced increases in ICSS stimulation thresholds and decreases in maximum rates of responding. LY-2456302 was administered p.o. 30 minutes before injection of U50,488 (10 mg/kg, i.p.). Shown are effects on (a) ICSS stimulation thresholds (Theta 0) and (b) maximum rates of operant responding for: Veh + Veh (n = 9), Veh + U50 (n = 8), and LY-2456302 [1 mg/kg (n = 5), 10 mg/kg (n = 5), and 30 mg/kg (n = 5)] + U50. ICSS thresholds and rates of responding were measured for a total of 90 minutes and data are presented as percent pre-drug baseline. ##P < .01 comparing [Veh + U50] to [Veh + Veh]; **P < .01 comparing [LY (30) + U50] to [Veh + U50] (a), and [LY (10) and LY (30) + U50] to [Veh + U50] (b). LY-2456302 (30 mg/kg, n = 5) on its own had no effect on ICSS thresholds (c) or maximum rates of responding (d) over the 90-minute session. (e) No dose of LY-2456302 significantly altered percent baseline ICSS thresholds in the first 30 minutes post-LY injection, but before Veh was injected at 30 minutes: LY-2456302 [Veh (n = 9), 1.0 mg/kg (n = 5), 10.0 mg/kg (n = 5), 30 mg/kg (n = 8)]. (f) Average of changes in percent baseline ICSS thresholds for each treatment from 30 to 90 minutes post-LY-2456302 treatment, including LY-2456302 (1.0 mg/kg, n = 5). **P < .05 compared with Veh + U50.
Results
Comparing Effects of i.p. and p.o. Administration of Long-Lasting KOR Antagonist JDTic in TFA
Oral bioavailability is an important characteristic of compounds being tested for possible clinical use. To establish whether p.o. administration of JDTic has similar effects as i.p. administration on tail withdrawal latency in the TFA, baseline withdrawal latencies were taken at least 2 hours prior to any drug treatment, and then rats were treated with JDTic (20.0 mg/kg, i.p. or p.o.); either 3 or 71 hours prior to the KOR agonist U50,488 (15 mg/kg, i.p.). Tail flick latencies were measured 1 hour after U50,488 treatment, giving total pretreatment times before testing of 4 and 72 hours. Effects on TFA latencies depended on treatment (F5,42 = 4.21, P < .01; Figure 1). U50,488 had an analgesic effect as shown by a significant increase in tail withdrawal latency compared with baseline latency (P < .01) that was blocked by both i.p. and p.o. JDTic administered 72 hours (P < .05), but not 4 hours, prior to testing. This is consistent with reports that the specificity and potency of KOR antagonists such as JDTic increase with time (Carroll and Carlezon, 2013). These results demonstrate that systemic and oral administration of the same dose of a KOR antagonist can have identical effects blocking the analgesic effects of U50,488 in the TFA.
Dose- and Time Course-Effects of Short-Acting KOR Antagonist LY-2456302 in TFA
LY-2456302 is a selective and potent KOR antagonist with good oral availability (Rorick-Kehn et al., 2014). It has been shown to have selective antagonist activity at KORs vs MORs and DORs (Table 1). We established a dose effect function of LY-2456302 in the TFA to determine the AD80, which we then used in a TFA time course study. The purpose of this experiment was to serve as a benchmark for dose- and time-course effects of the novel KOR antagonists CYM-52220 and CYM-52288. For the dose effect determination, LY-2456302 (0.0, 0.1, 0.3, 1.0, 3.0, or 10.0 mg/kg, p.o.) was administered 1 hour prior to U50,488 (30 mg/kg, i.p.) and TFA latencies determined 1 hour after that. LY-2456302 produced a dose-dependent blockade of U50,488-induced increases in tail withdrawal latency (F5,39 = 5.03, P < .01; Figure 2a), with 3.0 and 10.0 mg/kg doses of LY-2456302 significantly reducing the analgesic effects of U50,488 (P < .05). Using nonlinear regression and setting F to 20 to identify the AD80 (dose of antagonist that produces an effect in 80% of the population), we found the AD80 of LY-2456302 to be 0.9 mg/kg. Using this dose, we then conducted a time course TFA study in which LY-2456302 (0.9 mg/kg, p.o.) was administered to rats 1, 2, 4, 8, or 24 hours prior to the TFA test. For each antagonist time point, a vehicle (3% lactic acid in water) group was also included. There was no significant effect of time on the change in latency from vehicle+U50,488 compared with baseline (F4,38 = 0.56, ns; Figure 2b). As such, vehicle+U50,488 latencies were collapsed for all time points for the LY-2456302 time course study (Figure 2c). LY-2456302 significantly reduced U50,488-induced analgesia only at the 1-hour pretreatment time (F5,97 = 2.75, P < .05), confirming that it is a short-acting KOR antagonist in this assay. To see raw latency (seconds) data for pretreatment baseline TF and posttreatment TF for both the LY-2456302 dose-response and time course studies, see Supplementary Figure 1.
Table 1.
Pharmacological Properties of KOR Antagonists
| KOR antagonist |
Selectivity for KOR vs MOR/DOR | Functional antagonism at KOR, Kb (nM) | t1/2 (h) | [Brain]/[plasma] |
|---|---|---|---|---|
| JDTic | 340/4900a | 0.098 (0.069)b | 28.4c | 6.9e |
| LY-2456302 | 30/190a | 0.813 (0.285)b | 3.8d | 4.3f |
| CYM-52220 | 1896g | (0.77)g* | 1.9h | >4h |
| CYM-52288 | >2222g | (4.5)g* | 1.6h | >4h |
Abbreviations: ***
a Receptor binding affinities (Ki, nM) of antagonists using human embryonic kidney (HEK) or Chinese hamster ovary (CHO) cells expressing human KOR, MOR, or DOR (Rorick-Kehn et al., 2014).
b Inhibition of agonist-stimulated [35S]GTPgS binding in membranes from HEK or CHO cells expressing human KOR, MOR, or DOR (Rorick-Kehn et al., 2014).
c Mean plasma elimination half-life of 5 mg/kg IP in rats (Owens et al., 2016).
d Mean plasma elimination half-life of 10 mg/kg p.o. in rats (Rorick-Kehn et al., 2014).
e Brain/plasma exposure (ng/g to ng/mL) of 5.0 mg/kg IP in rats (Owens et al., 2016).
f Brain/plasma exposure (ng/g to ng/mL) of 1.0 mg/kg p.o. in rats (Rorick-Kehn et al., 2014).
g From (Guerrero et al., 2019). g* This assay uses Tango OPRK1-bla Human Bone Osteosarcoma Epithelial Cells (U2OS) cells, which express KOR linked to a GAL4-VP16 transcription factor via a tobacco etch virus (TEV) protease site. Stimulation of the KOR by U-50488 causes migration of the β-arrestin fusion protein to the GPCR and through proteolysis liberates GAL4-VP16 from the receptor (Guerrero et al., 2010).
h Roberts and Rosen, unpublished.
Dose- and Time Course-Effects of Novel KOR Antagonists CYM-52220 and CYM-52288 in TFA
As with LY-2456302, we established dose effect functions for both CYM-52220 and CYM-52288 in the TFA to determine the AD80s, which we then used in TFA time course studies. The purpose of these experiments was to determine whether these novel KOR antagonists have oral bioavailability in a behavioral assay and whether they are short-acting KOR antagonists conducive to medication development. For the dose effect determination of CYM-52220, 0.0, 3.0, 6.0, 8.0, 10.0, or 30 mg/kg of drug was administered (p.o.) 1 hour prior to U50,488 (30 mg/kg, i.p.) and TFA latencies determined 1 hour after that. CYM-52220 dose-dependently blocked U50,488-induced increases in tail withdrawal latency (F5,62 = 8.23, P < .001; Figure 3a), with every dose except 3.0 mg/kg significantly reducing the analgesic effects of U50,488 (P < .05). Using nonlinear regression, the AD80 of CYM-52220 in the TFA is 6.0 mg/kg. We then conducted a time course TFA study in which CYM-52220 (6.0 mg/kg, p.o.) was administered to rats 1, 2, 4, 8, or 24 hours prior to the TFA test. For each time point, a vehicle (10% DMSO + 10% Tween-80 in water) group was also included. As was the case with LY-2456302, there was no significant effect of time point on the change in latency from vehicle+U50,488 compared with baseline (F4,52 = 0.58, ns), so the latencies were collapsed for all time points for the CYM-52220 time course study. CYM-52220 significantly reduced U50,488-induced analgesia at every time point (F5,118 = 3.94, P < .01; Figure 3b) except 8 hours post-antagonist. These data do not support the hypothesis that CYM-52220 is a short-acting KOR antagonist in vivo, although time points longer than 24 hours were not tested.
For the dose effect determination of CYM-52288, 0.0, 1.0, 1.8, 2.4, 3.0, or 10.0 mg/kg of drug was administered (p.o.) 1 hour prior to U50,488 (30 mg/kg, i.p.), and TFA latencies determined 1 hour after that. CYM-52288 dose-dependently reduced U50,488-induced increases in tail withdrawal latency (F5,94 = 2.86, P < .05; Figure 3c), with only the 10.0-mg/kg dose significantly blocking the analgesic effects of U50,488 (P < .05). Using nonlinear regression, the AD80 of CYM-52288 in the TFA is 3.2 mg/kg. We then conducted a time course TFA study in which CYM-52288 (3.2 mg/kg, p.o.) was administered to rats 1, 2, 4, 8, or 24 hours prior to the TFA test. For each time point, a vehicle (water) group was also included. As was the case with LY-2456302 and CYM-52220, there was no significant effect of time point on the change in latency from vehicle+U50,488 compared with baseline (F3,16 = 2.02, ns), so the latencies were collapsed for all time points for the CYM-52288 time course study (Figure 3d). CYM-52288 reduced U50,488-induced analgesia over time (F5,69 = 3.13, P < .05; Figure 3d), with significant blockade occurring at 1, 2, and 4 hours, and a trend (P = .07) for a blockade at 24 hours post-CYM52288 administration. These data suggest that CYM-52288 has a nominally shorter time course of action as a KOR antagonist compared with CYM-52220, with neither CYM compound approaching the clear short-acting effects of LY-2456302. To see raw latency (seconds) data for pretreatment baseline TF and posttreatment TF for both CYM 52220 and CYM 52288 dose-response and time course studies, see Supplementary Figure 2.
Effect of LY-2456302 on Brain Stimulation Reward Measured With ICSS
Of the 4 KOR antagonists tested in the TFA, LY-2456302 had the shortest duration of action, with its KOR-mediated antagonism of U50,488-induced analgesia lasting for less than 2 hours (Figure 2c). As such, we chose to test LY-2456302 in the ICSS paradigm, which is sensitive to increases and decreases in reward function and motivated behavior. Using the rate-frequency method of ICSS (Carlezon and Chartoff, 2007), an increase in percent baseline stimulation thresholds reflects a decrease in reward sensitivity and motivated behavior (i.e., anhedonia). We and others have shown that KOR activation increases percent baseline stimulation thresholds (Todtenkopf et al., 2004; Ebner et al., 2010), whereas the long-lasting KOR antagonists norBNI and JDTic block this KOR activation effect (Potter et al., 2011; Russell et al., 2016). Likewise, LY-2456302 was able to block U50,488-mediated increases in ICSS thresholds. Here, the effects of LY-2456302 pretreatment on U50,488-induced increases in ICSS thresholds depended on an interaction between treatment and time (F20,126 = 2.21, P < .05; Figure 4a). For graphical representation of the impact of LY-2456302 (30 mg/kg) on the U50,488-induced rightward shift in the rate-frequency function of ICSS, see Supplementary Figure 3. LY-2456302 itself had no effects on percent baseline ICSS thresholds over time (F5,54 = 0.88, ns) or when averaged over the first 30 minutes after administration (Figure 4c, e). Interestingly, only the 30-mg/kg dose of LY-2456302 significantly reduced U50,488-induced increases in stimulation thresholds (Figure 4a, f), even though doses of 3.0 and 10.0 mg/kg blocked U50,488-induced analgesic effects in the TFA (Figure 2a), suggesting that higher doses of antagonist are needed to block the motivational vs analgesic effects of this KOR agonist. In addition to decreasing motivated behavior and reward sensitivity as measured with ICSS, KOR activation is also known to reduce motor activity (Ebner et al., 2010; Russell et al., 2014). With ICSS behavior, we can observe this effect by examining drug effects on percent baseline maximum rates of responding. Figure 4b shows that U50,488 (10 mg/kg) significantly reduced percent baseline max rate at the 60- and 75-minute time points, while LY-2456302 (10 and 30 mg/kg, but not 1 mg/kg) significantly blocked the rate-suppressing effects of U50,488 [treatment x time interaction (F20,135 = 3.54, P < .001)]. As with effects on percent baseline stimulation thresholds, LY-2456302 (30 mg/kg) on its own had no effect on percent baseline max rates [no treatment x time interaction (F5,55 = 1.38, ns) and no main effect of treatment (F1,11 = 0.0004, ns)] (Figure 4d).
Discussion
This study shows that both CYM-52220 and CYM-52288 are structurally unique, potent, and selective KOR antagonists with approximately 2000-fold selectivity for KOR over MOR or DOR as assessed by in vitro binding and functional assays. In addition, these compounds have good oral bioavailability and encouraging pharmacokinetic properties. Prototypical KOR antagonists such as JDTic and norBNI have been shown to have pharmacokinetic and pharmacodynamic effects for weeks, with norBNI blocking the effects the KOR agonist Salvinorin A for at least 80 days (Endoh et al., 1992; Melief et al., 2011; Potter et al., 2011; Munro et al., 2012). Although numerous attempts have been made to understand the mechanisms underlying these long-lasting effects, they are still not completely understood. A viable and well-supported finding is that the long duration of action of these selective KOR antagonists is positively correlated with c-Jun N-Terminal Kinase-1 activation (Melief et al., 2011). In an effort to identify novel selective KOR antagonists with favorable properties—including a medication-like duration of action—a high-throughput screening campaign of the Molecular Libraries−Small Molecule Repository was carried out at TSRI. On the basis of an initial promising hit, a medicinal chemistry campaign generated numerous compounds including CYM-52220 and CYM-52288. When tested in this study in the TFA for their ability to block U50,488-induced analgesia in male rats, both CYM-52220 and CYM-52288 showed KOR antagonist effects for at least 24 hours after p.o. administration, albeit with a nonsignificant reduction in tail flick latencies at 8 hours for both drugs. Although time points longer than 24 hours were not tested, both CYM-52220 and CYM-52288 produced disruptions of KOR-induced analgesia longer than LY-2456302 (also known as CERC-501 or JNJ-67953964), which has been previously reported as a short-acting KOR antagonist. As such, under our study conditions, neither of these novel compounds demonstrated properties of short-acting KOR antagonists, which would be desirable for early-phase clinical (safety) trials. Given the need for short-acting KOR antagonists to advance translational studies testing their utility as anti-anxiety and antidepressive-like compounds, we tested the ability of LY-2456302 to reduce KOR agonist-induced anhedonia in the ICSS test. We found that LY-2456302 reduced KOR agonist-induced elevations in ICSS thresholds, indicating an antidepressant (anti-anhedonia) effect, although at a dose that was 10-fold higher than needed to block KOR agonist-induced analgesia.
Dosing and Oral Bioavailability of JDTic, LY-2456302, CYM-52220, and CYM-52288 in Warm Water TFA
JDTic was the first potent KOR antagonist not derived from an opioid class of compounds (Carroll et al., 2004). For comparison, another prototypical KOR antagonist, norBNI, is derived from the general opioid receptor antagonist naltrexone. In the original description of the pharmacological properties of JDTic, both subcutaneous and oral (p.o.) administration blocked the antinociceptive effects of enadoline, a selective KOR agonist, enadoline. The AD50s of the 2 routes of administration were 4.1 and 27.3 mg/kg, respectively (Carroll et al., 2004), indicating that JDTic has moderate oral bioavailability. In the current study, we confirmed the oral bioavailability of JDTic by showing that i.p. and p.o. administration blocked U50,488-induced analgesia in the TFA within roughly the same dose range (20 mg/kg) as was effective with enadoline.
Previous reports indicate that LY-2456302 has good oral bioavailability. It selectively and potently occupies central KORs in vivo (ED50 = 0.33 mg/kg), without evidence of MOR or DOR occupancy, at doses up to 30 mg/kg (Rorick-Kehn et al., 2014). In clinical studies, LY-2456302 was absorbed rapidly following oral administration and was eliminated within 48 hours of administration when administered at low, KOR-selective doses (Lowe et al., 2014). Furthermore, oral administration of LY-2456302 reversed the analgesic effects of U69,593 (a KOR agonist closely related to U50,488) with an ED50 of 0.4 mg/kg, whereas no KOR antagonist effects were observed after 1 week of pretreatment (Urbano et al., 2014), providing further support that LY-2456302 is a short-acting KOR antagonist. Our p.o. dose and time course study of LY-2456302 in the TFA confirms both the oral bioavailability and the short-acting effects of the drug. We found that LY-2456302 blocked U50,488-induced analgesia in the TFA with an AD80 of 0.9 mg/kg (p.o.). Furthermore, administration of this dose of LY-2456302 significantly blocked U50,488-induced increases in TFA latency when administered 1 hour, but not 2 to 24 hours, prior to testing. Interestingly, TFA latencies post-LY-2456302 were qualitatively (but not significantly) lower for up to 8 hours post-administration, with a complete return to Veh+U50,488 latencies at 24 hours post-LY-2456302 (see Figure 2B).
CYM-52220 and CYM-52288 were iteratively synthesized from the pyridine CYM-50202 by focusing on the piperidine linker (Guerrero et al., 2019). One promising compound from this series, CYM-51317, has good oral bioavailability with an F of 20% (Guerrero et al., 2019). In our studies, both CYM-52220 and CYM-52288 had oral bioavailability. Our TFA studies with the KOR agonist U50,488 (30 mg/kg, i.p.) demonstrate that the AD80 of p.o. CYM-52220 is 6.0 mg/kg and the AD80 of p.o. CYM-52288 is 3.2 mg/kg (see Figure 3A, C). Given our experimental design of testing 5 doses of each KOR antagonist (LY-2456302, CYM-52220, and CYM-52288), p.o. administration of doses of CYM-52220 ranging from 6.0 to 30.0 mg/kg all significantly decreased U50,488-induced analgesia in the TFA, whereas only 10.0 mg/kg CYM-52288 significantly reduced TFA latencies.
Time Course of Antinociceptive Effects of the Drugs
A major goal of these studies was to determine if the novel CYM compounds are short-acting KOR antagonists in an in vivo assay. A prior study in mice showed that CYM-51317 blocked U69,593-induced analgesia in the TFA with 1-hour, but not 24-hour or 7-day, pretreatment (Xie et al., 2017). These data, along with other in vitro pharmacokinetic data for this chemical series (Guerrero et al., 2019), supported our hypothesis that CYM-52220 and CYM-52288 would also be short-acting KOR antagonists. We designed the TFA experiments to test the efficacy of the AD80 doses of CYM-52220 and CYM-52288 at 1, 2, 4, 8, and 24 hours after administration and compare the time course to that of LY-2456302. We chose to stop testing at 24 hours on the basis that any compound that still had KOR antagonist effects beyond this could not be considered short-acting. Consistent with earlier findings for LY-2456302, we found that p.o. administration of 0.9 mg/kg significantly reduced U50,488-induced analgesia in the TFA at only the 1-hour pretreatment time point. Unlike CYM-51317 (Xie et al., 2017), both CYM-52220 and CYM-52288 reduced KOR agonist-induced analgesia in the TFA for 24 hours, with significant effects for both compounds at 1, 2, and 4 hours and also at 24 hours for CYM-52220 (see Figure 3). Interestingly, both compounds failed to significantly reduce U50,488 effects when administered 8 hours prior to testing, although there was a clear trend for tail flick latencies to be decreased relative to U50,488 alone. The 8-hour tests were effectively powered, with 12 to 14 rats used for each CYM compound. It is unclear why the effects of both CYM compounds appeared to be waning at 8 hours but were reinstated at 24 hours. Although untested here, 1 possibility invokes the known bi-phasic extracellular signal-regulated kinase (ERK) 1/2 activation profile for KORs (and other G protein-coupled receptors (GPCRs)) (Bruchas and Chavkin, 2010). On KOR activation, there is an arrestin-independent early phase and an arrestin-dependent late phase. Although the timing of these events in cell culture systems is within 2 to 3 hours, it is possible that, in vivo, complex interactions between the pharmacodynamics of the drugs and stress-induced plasticity in CNS KOR systems (Donahue et al., 2015) contribute to the consistently observed suppression of KOR antagonism at 8 hours and rebound at 24 hours.
LY-2456302 Reduces KOR Agonist-Induced Anhedonia in ICSS
Numerous preclinical studies using a variety of stress-related paradigms have shown that pretreatment with a KOR antagonist reduces stress-induced outputs including depressive-like, anxiety-like, and addictive-like behaviors (Bruchas et al., 2010; Knoll and Carlezon, 2010; Chartoff et al., 2012; Carroll and Carlezon, 2013). Most of these previous studies have been conducted with prototypical, long-lasting KOR antagonists such as JDTic and norBNI. Increasingly, emerging data indicate that shorter-acting small molecule or small peptide compounds with KOR antagonist properties also block stress-induced negative affective states (Browne and Lucki, 2019; Guerrero et al., 2019). We chose to test the ability of the shortest-acting KOR antagonist from our study, LY-2456302, in the ICSS test to determine if it would mitigate the reward-decreasing (anhedonia-producing) effects of U50,488 (an antidepressant effect) and whether it would have any reward-potentiating effects on its own. Identifying any reward-potentiating effects is particularly important, considering that drugs with abuse liability in humans share this feature (Chartoff and Carlezon, 2007). Although LY-2456302 (10 mg/kg; p.o.) demonstrated efficacy in the mouse forced swim test, an animal model predictive of antidepressant-like efficacy (Rorick-Kehn et al., 2014), there are no reports in the literature to indicate that this compound—or any other short-acting KOR antagonist—has ever been tested in the ICSS paradigm. We found that doses of LY-2456302 (3.0, 10.0 mg/kg; p.o.) that reduced U50,488-induced analgesia in the TFA had no effect on U50,488 (10 mg/kg; i.p.)-induced increases in ICSS stimulation thresholds. Only LY-2456302 at a dose of 30 mg/kg (p.o.) blocked U50,488-induced anhedonia, importantly, however, without having any effects on ICSS threshold on its own. Since KOR activation has been shown to have rate-suppressing effects in ICSS, which is indicative of reduced motor capacity (Ebner et al., 2010; Russell et al., 2014), we show that U50,488 (10 mg/kg) significantly reduced maximum rates of lever responding compared with pre-drug baseline. Unlike the anhedonic-like effect of U50,488, which lasted at least 60 minutes post-U50, the rate-decreasing effect of U50,488 emerged 30 minutes post-U50,488 injection and was significantly reduced compared with vehicle controls after only 30 minutes. Although U50,488 (10 mg/kg) has clear motor-suppressing effects, the temporal distinction from anhedonic effects suggests that the increase in percent baseline thresholds is not caused by a decrease in rates of responding. Furthermore, we found that LY-2456304 at both 10 and 30 mg/kg blocked the rate-suppressing effects of U50,488, whereas only the higher dose (30 mg/kg) of LY-2456302 blocked U50-induced anhedonia. LY-2456302 has excellent functional selectivity over MOR-mediated effects, with no evidence of appreciable MOR occupancy or associated pharmacodynamic effects at doses up to 30 mg/kg (Rorick-Kehn et al., 2014). Taken together, these findings suggest that the anti-depressant-like effect of LY-2456302 in ICSS is mediated through blockade of KORs, and, perhaps more importantly, that selective blockade of KORs does not produce the types of reward-facilitating effects in the ICSS that are shared by agents with abuse-liability in humans.
Our results showing that LY-2456302 can block KOR-mediated anhedonia measured with ICSS also supports development of this compound as a medication targeting negative affective states. A recent study in which LY-2456302 (10 mg/kg, p.o.) was repeatedly administered to cocaine-dependent persons or normal volunteers in a stress-minimized inpatient setting showed no evidence of changes in affective state or cocaine craving (Reed et al., 2018). Although at first these findings seem to contradict preclinical studies, the study was done in the absence of overt stressors, which appear to be required for KOR activation trigger negative affective states and drug craving. Additionally, the fact that there was no evidence that the KOR antagonist triggered drug craving is consistent with our finding that it had no reward-related effects of its own in the ICSS test. As such, this early clinical study served to demonstrate that this orally administered dose of LY-2456302 was well tolerated in humans, paving the way for future clinical development of this and other KOR antagonists.
Conclusions
In summary, the in vivo time courses of both CYM-52220 and CYM-52288 in the warm water TFA do not provide strong evidence that these particular KOR antagonist compounds possess shorter time courses of action relative to the previously described LY-2456302. To the extent that a short time course is a desirable feature for initial clinical trials, until drug safety is established, they may not represent a major improvement over the prototypical KOR antagonists as candidates for medication development. However, they are part of a series of compounds synthesized from a promising hit obtained in the high-throughput screening campaign of the Molecular Libraries−Small Molecule Repository that was recently published (Guerrero et al., 2019). From that series, the novel, selective, and short-acting KOR antagonist BTRX-335140 (CYM-53093) was identified and demonstrates a more favorable duration of action. It is now in a phase I clinical trial in healthy volunteers designed to evaluate safety and tolerability and the dose-exposure relationship of the compound (BlackThorn, 2018). Comparisons among the growing numbers of KOR antagonists available to the field may facilitate the development of optimized candidates for therapeutic development.
Supplementary Material
Acknowledgments
This work was supported by an administrative supplement to National Institute of Mental Health at the National Institutes of Health grant MH063266 (to W.A.C.).
Statement of Interest
Sarah Page, Maria Mavrikaki, Tania Lintz, Dan Puttick, F. Ivy Carroll, and Elena Chartoff: no conflicts of interest. Edward Roberts is an advisor to Blackthorn therapeutics and may receive compensation in stock and/or monetary payment. Hugh Rosen is an advisor to Blackthorn therapeutics and may receive compensation in stock and/or monetary payment. William Carlezon, Jr has served as a consultant for Psy Therapeutics within the past 2 years. F. Ivy Carroll provided JDTic for this study.
References
- Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ (2002) Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology (Berl) 160:170–181. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI (2005) Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 183:118–126. [DOI] [PubMed] [Google Scholar]
- BlackThorn (2018) Blackthorn therapeutics initiates phase 1 study of BTRX-335140, an Investigational Kappa Opioid Receptor (KOR) antagonist. In: BlackThorn Therapeutics; San Francisco, CA. https://www.blackthornrx.com/btrx-335140-kappa-opioid-receptor-antagonist/ [Google Scholar]
- Browne CA, Lucki I (2019) Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacol Ther 201:51–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP (2007) Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol 569:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr, Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Krystal AD (2016) Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety 33:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Carlezon WA Jr (2013) Development of κ opioid receptor antagonists. J Med Chem 56:2178–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS (2004) Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol 501:111–119. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA (2012) Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology 62:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Carlezon WA Jr (2014) Drug withdrawal conceptualized as a stressor. Behav Pharmacol 25:473–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Martinez D (2015) Kappa antagonist JDTic in phase 1 clinical trial. Neuropsychopharmacology 40:2057–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA Jr (2015) Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol 26:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH (2010) Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 210:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M (2015) Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology 40:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H (1992) Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther 316:30–42. [PubMed] [Google Scholar]
- Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, Leigh-Pemberton R, DiPetrillo L, Silverman B, Ehrich E (2016) Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am J Psychiatry 173:499–508. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Urbano M, Kim EK, Gamo AM, Riley S, Abgaryan L, Leaf N, Van Orden LJ, Brown SJ, Xie JY, Porreca F, Cameron MD, Rosen H, Roberts E (2019) Design and synthesis of a novel and selective kappa opioid receptor (KOR) antagonist (BTRX-335140). J Med Chem 62:1761–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Xie CW (1982) Dynorphin: potent analgesic effect in spinal cord of the rat. Life Sci 31:1781–1784. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA Jr (2007) Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323:838–845. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Mathew SJ, Sanacora G, Keefe R, Song A, Calabrese J, Goddard A, Goodman W, Lisanby SH, Smoski M, Weiner R, Iosifescu D, Nurnberger J Jr, Szabo S, Murrough J, Shekhar A, Potter W (2018) The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat Rev Drug Discov 18:82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Coen K, Tamadon S, Shaham Y (2018) Role of κ-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology 43:838–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, Rorick-Kehn L, Weller M, Stoltz RR, Royalty J, Tauscher-Wisniewski S (2014) Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol 54:968–978. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR (2013) Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl) 226:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS, Carlezon WA Jr (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305:323–330. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ (1994) Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci 5:124–144. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29. [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Béguin C, Carlezon WA Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C (2011) Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol 80:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch CH, Quimby SJ, Diaz N, Pedregal C, de la Torre MG, Jimenez A, Shi Q, Canada EJ, Kahl SD, Statnick MA, McKinzie DL, Benesh DR, Rash KS, Barth VN (2011) Discovery of aminobenzyloxyarylamides as κ opioid receptor selective antagonists: application to preclinical development of a κ opioid receptor antagonist receptor occupancy tracer. J Med Chem 54:8000–8012. [DOI] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van’t Veer A, Béguin C, Carroll FI, Zhao Z, Carlezon WA Jr, Cohen BM (2012) Long-acting κ opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. (2004) Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 176:204–213. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS (2002) Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22:10883–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard SK, Hourguettes NJ, Sobczak GG, Carlezon WA, Bruchas MR (2016) Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. J Neurosci 36:9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21:7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA Jr, Cohen BM, Chartoff EH (2011) Repeated exposure to the κ-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry 70:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Fry RS, Kimani R, Kreek MJ (2018) Repeated administration of opra kappa (LY2456302), a novel, short-acting, selective KOP-r antagonist, in persons with and without cocaine dependence. Neuropsychopharmacology 43:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, et al. (2014) LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 77:131–144. [DOI] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH (2014) Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Puttick DJ, Sawyer AM, Potter DN, Mague S, Carlezon WA Jr, Chartoff EH (2016) Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J Neurosci 36:5748–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C (2012) Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci 32:17582–17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM (1999) Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci 19:1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470. [DOI] [PubMed] [Google Scholar]
- Urbano M, Guerrero M, Rosen H, Roberts E (2014) Antagonists of the kappa opioid receptor. Bioorg Med Chem Lett 24:2021–2032. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY, Schütz G, Chartoff EH, Rudolph U, Cohen BM, Carlezon WA Jr (2013) Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology 38:1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, Ganorkar R, VanAlstine MA, Guo C, Cohen DJ, Bidlack JM (2009) Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett 19:2289–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F (2017) Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia 37:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.