Abstract
Since the initial release of miRPathDB, tremendous progress has been made in the field of microRNA (miRNA) research. New miRNA reference databases have emerged, a vast amount of new miRNA candidates has been discovered and the number of experimentally validated target genes has increased considerably. Hence, the demand for a major upgrade of miRPathDB, including extended analysis functionality and intuitive visualizations of query results has emerged. Here, we present the novel release 2.0 of the miRNA Pathway Dictionary Database (miRPathDB) that is freely accessible at https://mpd.bioinf.uni-sb.de/. miRPathDB 2.0 comes with a ten-fold increase of pre-processed data. In total, the updated database provides putative associations between 27 452 (candidate) miRNAs, 28 352 targets and 16 833 pathways for Homo sapiens, as well as interactions of 1978 miRNAs, 24 898 targets and 6511 functional categories for Mus musculus. Additionally, we analyzed publications citing miRPathDB to identify common use-cases and further extensions. Based on this evaluation, we added new functionality for interactive visualizations and down-stream analyses of bulk queries. In summary, the updated version of miRPathDB, with its new custom-tailored features, is one of the most comprehensive and advanced resources for miRNAs and their target pathways.
INTRODUCTION
Understanding the mechanisms of gene regulation is one of the major challenges in molecular biology and bioinformatics. In order to get the big picture, diverse sub-fields emerged to study the underlying principles of transcriptional, post-transcriptional, translational and post-translational levels of gene regulation. Short, conserved and non-coding RNA families, so-called microRNAs (miRNAs), were shown to orchestrate major pathways in a post-transcriptional manner by targeting 3′ untranslated regions (UTRs) of mRNAs in mammals and plants (1,2). While early studies focused on the validation of human microRNAs and those found in important model organisms such as mouse and rat, the focus has been broadly expanded to characterize miRNAs in a larger set of metazoan species (3). To this end, several reference databases such as miRBase, miRCarta and miRGeneDB and different nomenclatures were established (4–6). Since the number of miRNAs discovered is steadily rising (7), a remarkable amount of studies already validated microRNA target genes and their function in a multitude of cell-types, tissues and disease phenotypes (8,9). These global research efforts have led to an accumulation of novel data. To scale up with these developments and to gain deeper insights into miRNA functionality, robust statistical methods and curated databases are in great demand, especially to integrate all the important findings from miRNA discovery, target validation and target gene function (10,11).
One of the key questions of functional miRNA analysis is which pathways or cellular functions are regulated by a given miRNA (miRNA-centric view), or conversely, which miRNAs regulate a given gene set or pathway (pathway-centric view) (12,13). To solve these problems, several tools and databases have been proposed so far. From a miRNA-centric view, the miRTar database, which links individual miRNAs to metabolic pathways (14) and miRSystem, providing pre-computed enrichments of target genes in pathways (15), should be noted. Moreover, pure enrichment-based tools like miEAA, the bioconductor package miRNApath, or BUFET that are based on many-to-many relationships can process lists of miRNA identifiers to compute pathway associations (16–18). More specialized miRNA-centric tools include miRNet (19), which is a networks-based approach and miTALOS v2 (20) that annotates miRNA functions in a tissue-specific manner. PolymiRTS (21) is a pathway-centric database that maps SNPs in target sites to gene categories and phenotypes, i.e. disease traits.
Only a minor fraction of the tools and databases support both miRNA- and pathway-centric applications. These include the online database miRNApath (22), the R package CORNA (23), DIANA-miRPath v3.0 (24) incorporating GO and KEGG enrichments derived from predicted and validated miRNA-target interactions, and finally miRPathDB v1 (25), which in turn is based on our very first dictionary on miRNAs and target pathways (26).
After the initial release of miRPathDB, miRNA research has made notable progress. Novel miRNAs have been discovered, the number of experimentally validated target genes has increased tremendously. Most importantly, new reference databases emerged that either catalog validated miRNAs with high confidence (6), or that contain thousands of novel miRNA candidates (5). Additionally, we evaluated publications, citing our database, to identify common application scenarios, new visualizations and useful downstream applications (27–29). An overview of these publications can be found in Supplementary Table S1.
The new version of miRPathDB, provides access to target genes and regulated pathways not only for miRNAs from miRBase (Version 22.1), but also from miRCarta (Version 1.1). This increases the provided information by more than a factor of ten compared to the original version. Second, our database now also provides similarity information for all miRNAs based on their sequence, genomic position, target genes and target pathways. This information not only allows to query miRNAs with similar properties and to cluster miRNAs based on their similarity, but also to assess the regulatory potential of new candidate miRNAs. On top of the new data compilation, miRPathDB provides several interactive tools for user-specific analyses. From a miRNA perspective, we developed an appealing miRNA-to-pathway heatmap visualization that intuitively shows which pathways are regulated by a given set of miRNAs. To serve the pathway-centric use-case as well, we have formulated and implemented an Integer Linear Program (ILP) to automatically extract a set of miRNAs whose targetome covers a user-provided pathway or set of genes. Taken together, the new version of miRPathDB is a comprehensive resource to study the function of miRNAs in human and mouse.
MATERIALS AND METHODS
Our database integrates information of miRNAs, miRNA–target interactions (MTIs), and signaling pathways from several third-party resources. In the following sections, we describe the respective data sources and all processing steps performed to create the underlying data collection. Additionally, we describe the methodology of new downstream analysis features.
miRNA resources
The database stores information on all human and mouse miRNAs from miRBase (Version 22.1) and from miRCarta (Version 1.1), including miRNA candidates. Validated MTIs were acquired from miRTarBase (Version 7) (30) and pre-processed to create two subsets for each miRNA: all MTIs independent of their type of experimental evidence and only those with a strong level of evidence. On top of this, we predicted target genes for each miRNA sequence using TargetScan (Version 7.1) (31) and MiRanda (Version 3.3a) (32). Based on the prediction output, we also created two further list of MTIs: the intersection and the union of all predictions, which is a common strategy to account for putative sources of bias from target prediction tools and to balance sensitivity versus specificity (25,33). As 3′ UTR input target set for the two algorithms, we used the curated annotations from targetscan.org for both human and mouse runs. Each program was executed using its default set of parameters.
Pathway databases and enrichment analysis
In order to determine whether a specific miRNA is associated with a particular biological process or signaling pathway, we used the enrichment analysis functionality of the GeneTrail2 C++ library (34). To this end, we analyzed functional categories from the Gene Ontology (35), as well as signaling pathways from KEGG (36), Reactome (37) and WikiPathways (38). For each pair of miRNA and functional category, we applied a hypergeometric test to check if the pathway contains significantly more target genes than expected by chance. Resulting p-values were FDR-adjusted (39) and a significance level of 0.05 was selected.
miRNA similarities
We also calculated similarities between all miRNAs and miRNA candidates based on their seed sequence, mature sequence, target genes and target pathways. For the string comparison, we calculated the Hamming distance between the sequences of all miRNA pairs, once using the full mature sequences and once the 7-nt substrings starting at position 2 from the 5′ end of the mature sequence. Given the hamming distance Hd between two sequences of length l, we defined the pairwise sequence similarity as 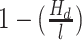 . The similarity of two sets containing either target genes or pathways was calculated using the Jaccard coefficient. Moreover, we compared miRNAs according to the positions of their genomic loci by computing the minimal distance between miRNAs annotated to the same chromosome.
. The similarity of two sets containing either target genes or pathways was calculated using the Jaccard coefficient. Moreover, we compared miRNAs according to the positions of their genomic loci by computing the minimal distance between miRNAs annotated to the same chromosome.
Customized pathway heatmaps
The custom heatmap depicts which pathways are regulated by a user defined set of miRNAs. To create a heatmap, we first select all pathways that are significantly enriched for the targets of at least one of the specified miRNAs. The obtained p-values are used to construct a matrix that contains the −log10-transformed and discretized P-values for the set of miRNAs and all enriched pathways. Finally, similar miRNAs and pathways are clustered together by applying an hierarchical approach (Ward’s method with Euclidian distance) to both rows and columns of the matrix. The clustered matrix is subsequently displayed as an interactive heatmap, implemented using the Highcharts JavaScript library.
Maximum targetome coverage analysis
A noteworthy issue in functional miRNA research is to find a small number of miRNAs that are sufficient to regulate a given gene set, e.g. a particular signaling cascade or pathway. To solve this problem, we first search for the ‘best’ miRNA (k = 1) that regulates the maximal number of genes of the given target set. Next, we increase the considered number of miRNAs step-by-step (k ≔ k + 1) until all target genes are covered or a predefined kmax is reached. For each k, we report an optimal set of miRNAs and the regulated target genes.
The problem to find the optimal set of miRNAs for one particular k is closely related to the maximum coverage problem, which can be solved using Integer Linear Programming (ILP). A formal definition of this problem can be found in the online documentation and Supplement S2. The ILP was implemented in C++ using the CPLEX optimization framework. Finally, results of an analysis are visualized by an interactive plot using the Highcharts JavaScript library.
OVERVIEW OF miRPathDB 2.0
miRPathDB stores information on (candidate) miRNAs, their target genes and their target pathways. To access this information, our database offers users two distinct representations: a miRNA-centric and a pathway-centric view. An overview table and a detailed description of each miRNA or pathway are available. The representations can either be accessed through the overview tables or a query in the quick-search bar. A general description of these representations has already been presented in the original manuscript (26). Hence, we describe the extensive changes of the miRNA-centric view, the interactive analysis tools, and the new export functionality in the following sections.
NEW MIRNA-CENTRIC VIEW
Here, we explain the different levels of information miRPathDB offers for each miRNA or miRNA candidate.
General information
On the top of each miRNA page, we provide general information about the respective miRNA: the precursor mapping, the sequence of the mature miRNA, seed and corresponding parent stem loops, and all annotated genomic loci. Additionally, specific links to external reference database (miRBase and miRCarta) entries for all miRNAs and for corresponding precursors and family assignments are deposited. On top of this, each miRNA entry is linked to other third-party databases, like the TissueAtlas (40) or miRTargetLink (41), not only to improve the usability, but also to complement the features of miRPathDB with other essential tools for miRNA analysis.
miRNA-target interactions (MTIs)
Below the general information section, the website renders a responsive, sortable, and fully searchable table containing all target genes of an examined miRNA. For each gene, we also highlight in which of the four evidence sets it is contained. Table rows can be filtered using the text boxes below each column. Users can export both filtered and unfiltered tables in different file formats (CSV, Excel and PDF).
Targeted pathways
One major focus of our database is to provide information on associations between miRNAs and their putative target pathways. Likewise to the table for target genes, the pathways are shown in another fully responsive table. It contains, for the different evidence sets, all pathways that are significantly enriched with targets of the examined miRNAs. For each pathway, the number of contained target genes, the number of target genes that are expected by chance, and a FDR-adjusted P-value are listed. Since users might be interested in a specific subset of results, the table can be filtered with respect to all fields. For example, users can select significant pathways for a certain MTI evidence level, or only pathways that contain a specific gene of interest. Each pathway cell is linked to the corresponding external database entry, which often displays additional information like a description of the pathway or the underlying gene network.
miRNA similarities
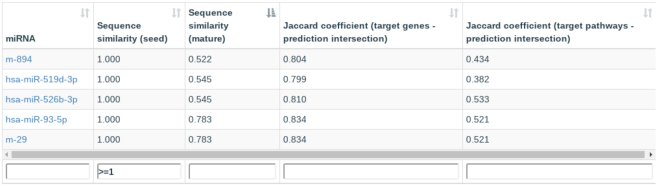
At the bottom of each miRNA page, a novel table containing similarity information of the selected miRNA with respect to all other miRNAs from the same organism, including miRNA candidates, is displayed (Figure 1). The table lists the seed and full sequence similarities, the chromosomal distance, in case miRNAs are annotated on the same chromosome and eight Jaccard coefficients, measuring the similarity of target genes and target pathways for the different evidence sets of MTIs. Analogously to information about target genes and pathways, this table can be filtered, searched, sorted, resized, and exported for further usage.
Figure 1.
Example of the new pairwise miRNA similarity table. The figure shows the pre-computed similarities for hsa-miR-106a-5p sorted by sequence similarity (mature) in increasing order. Furthermore, the table is filtered to show only miRNAs and miRNA candidates having 100% seed similarity. The Jaccard index provides additional information about the functional similarity of each miRNA and miR-106a-5p for predicted targets and target pathways.
NEW INTERACTIVE DATABASE FUNCTIONALITY
In addition to a new data compilation, miRPathDB features several new interactive tools for advanced user-specific database queries and analyses.
Custom pathway heatmaps
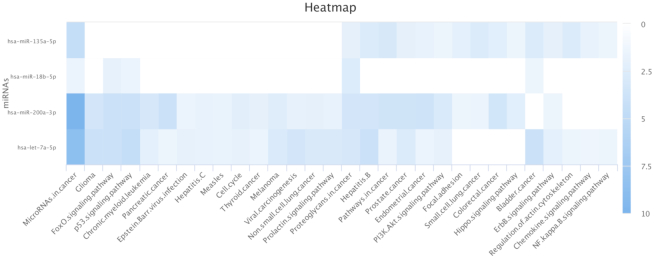
A common question in miRNA research is, whether targets of deregulated miRNAs are similarly enriched in certain biological processes or are associated with distinct molecular functions (27,28). In order to help users to tackle this question, we developed an interactive heatmap visualization. To create this plot, a user needs to specify a list of miRNAs as well as the evidence level for the MTIs. miRPathDB automatically selects all functional categories that are significantly enriched for the targets of at least one of the specified miRNAs. Results are represented as a heatmap, where each row depicts enrichment results for the respective functional categories. The color of individual entries corresponds to the p-value of the associated enrichment result. Darker colors indicate more significant enrichments of miRNA target genes in the corresponding biological processes. On top of this, users may specify the resolution of the resulting heatmap and download the image in different file formats (PNG, JPEG, PDF and SVG). An example heatmap is shown in Figure 2. Our customized heatmap feature provides a rapid overview of molecular functions and signaling pathways that are potentially regulated by a specific miRNA set. This analysis might even be helpful to assess possible downstream effects of deregulated miRNAs in high-throughput studies.
Figure 2.
Example of the custom heatmap visualization. The figure depicts the enrichment results of hsa-miR-18b-5p, hsa-miR-135a-5p, hsa-let-7a-5p and hsa-miR-200a-3p for the categories of the KEGG database and strongly experimentally validated MTIs. Rows represent the enrichment results for the targets of the four miRNAs. Columns represent all KEGG pathways that are significant for the different miRNAs. For demonstration purposes, the heatmap was filtered to only show pathways with at least two associated miRNAs. The color of individual fields represent the −log10-transformed P-value of the respective enrichment results. Darker colors indicate more significant associations between miRNA and target pathway.
Maximum targetome coverage analysis
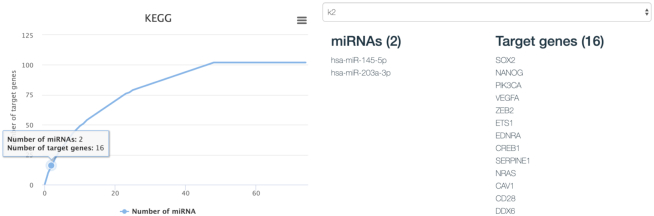
While the previous feature allows downstream analysis from a miRNA-centric view, by mapping a given miRNA set to enriched target pathways, miRPathDB also provides functionality for the reverse direction, i.e. given a set of target genes G, find a minimal set of miRNAs that target all genes in G. To this end, we provide a tool that iteratively computes k miRNAs (for all k ∈ {1, 2, ..., kmax}) with a maximal number of targets in G (see Materials and Methods). To start the maximum coverage analysis, a user must upload a list of genes, select the desired level of evidence that should be used to lookup the MTIs and set the largest k = kmax where the algorithm should stop. The results of such an analysis are displayed in an interactive line-graph that plots k against the number of covered target genes (Figure 3, left). For each k, a node is inserted in the graph that can be selected. Upon selection of a node, the website displays an optimal set of miRNAs of the corresponding size k along with the list of overlapping target genes (Figure 3, right).
Figure 3.
Example of the interactive visualization for a user-specific maximum-coverage analysis. The curve on the left indicates how many of the specified target genes can be targeted by an increasing number of miRNAs. Here the x-axis shows the increasing number of miRNAs and the Y-axis the number of covered target genes. Users are able to click on every point of the curve to inspect the corresponding miRNAs and targeted genes. An example for k = 2 is depicted on the right-hand side.
DATA EXPORT
Most of the views in miRPathDB offer dedicated export functionality. All tables in the miRNA-centric and the pathway-centric view can be filtered and downloaded in different formats (CSV, Excel and PDF). Additionally, we host downloads for all processing steps of the enrichment analyses. Users are able to acquire the unprocessed enrichment results, i.e. a table containing detailed information for each functional category. Furthermore, a table containing all pairs of miRNA and pathways and their −log10-transformed p-values is available. miRPathDB also supplies all functional categories in Gene Matrix Transposed (GMT) format (cf. Online documentation).
CONCLUSION
Recent advancements in miRNA research yielded huge numbers of novel miRNAs, miRNA candidates, and experimentally validated MTIs. This circumstance motivated a novel release of miRPathDB. Besides miRNAs and their targets, our database also provides information about associations between pathways and miRNAs. Beyond the ten-fold increase of data, our database now offers powerful tools for the visualization and downstream analysis of database queries. In particular, users are able to search similar miRNAs, create interactive clustered heatmaps and to determine a minimal set of candidate regulators that are sufficient to target a specified gene list. In summary, miRPathDB 2.0 is the most comprehensive publicly available resource to assess the relationship between microRNAs, their targets and cellular functions for human and mouse.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Internal funds of Saarland University.
Conflict of interest statement. None declared.
REFERENCES
- 1. Jonas S., Izaurralde E.. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015; 16:421–433. [DOI] [PubMed] [Google Scholar]
- 2. Bartel D.P. Metazoan microRNAs. Cell. 2018; 173:20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fehlmann T., Backes C., Pirritano M., Laufer T., Galata V., Kern F., Kahraman M., Gasparoni G., Ludwig N., Lenhof H.P. et al.. The sncRNA Zoo: a repository for circulating small noncoding RNAs in animals. Nucleic Acids Res. 2019; 47:4431–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2018; 47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backes C., Fehlmann T., Kern F., Kehl T., Lenhof H.P., Meese E., Keller A.. MiRCarta: a central repository for collecting miRNA candidates. Nucleic Acids Res. 2018; 46:D160–D167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fromm B., Domanska D., Høye E., Ovchinnikov V., Kang W., Aparicio-Puerta E., Johansen M., Flatmark K., Mathelier A., Hovig E. et al.. MirGeneDB 2.0: the metazoan microRNA complement. Nucleic Acids Res. 2019; doi:10.1093/nar/gkz885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alles J., Fehlmann T., Fischer U., Backes C., Galata V., Minet M., Hart M., Abu-Halima M., Grässer F.A., Lenhof H.P. et al.. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019; 47:3353–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rupaimoole R., Slack F.J.. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017; 16:203–221. [DOI] [PubMed] [Google Scholar]
- 9. Karagkouni D., Paraskevopoulou M.D., Chatzopoulos S., Vlachos I.S., Tastsoglou S., Kanellos I., Papadimitriou D., Kavakiotis I., Maniou S., Skoufos G. et al.. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018; 46:D239–D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu B., Li J., Cairns M.J.. Identifying miRNAs, targets and functions. Brief. Bioinformatics. 2012; 15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fehlmann T., Laufer T., Backes C., Kahramann M., Alles J., Fischer U., Minet M., Ludwig N., Kern F., Kehl T. et al.. Large-scale validation of miRNAs by disease association, evolutionary conservation and pathway activity. RNA Biol. 2019; 16:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis J.A., Saunders S.J., Mann M., Backofen R.. Combinatorial ensemble miRNA target prediction of co-regulation networks with non-prediction data. Nucleic Acids Res. 2017; 45:8745–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sticht C., De La Torre C., Parveen A., Gretz N.. miRWalk: an online resource for prediction of microRNA binding sites. PLoS ONE. 2018; 13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu J.B., Chiu C.M., Hsu S.D., Huang W.Y., Chien C.H., Lee T.Y., Huang H.D.. MiRTar: an integrated system for identifying miRNA-target interactions in human. BMC Bioinformatics. 2011; 12:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu T.P., Lee C.Y., Tsai M.H., Chiu Y.C., Hsiao C.K., Lai L.C., Chuang E.Y.. miRSystem: An Integrated System for Characterizing Enriched Functions and Pathways of MicroRNA Targets. PLoS ONE. 2012; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Backes C., Khaleeq Q.T., Meese E., Keller A.. MiEAA: MicroRNA enrichment analysis and annotation. Nucleic Acids Res. 2016; 44:W110–W116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cogswell J.P., Ward J., Taylor I.A., Waters M., Shi Y., Cannon B., Kelnar K., Kemppainen J., Brown D., Chen C. et al.. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer’s Dis.: JAD. 2008; 14:27–41. [DOI] [PubMed] [Google Scholar]
- 18. Zagganas K., Vergoulis T., Paraskevopoulou M.D., Vlachos I.S., Skiadopoulos S., Dalamagas T.. BUFET: boosting the unbiased miRNA functional enrichment analysis using bitsets. BMC Bioinformatics. 2017; 18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Y., Siklenka K., Arora S.K., Ribeiro P., Kimmins S., Xia J.. miRNet - dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016; 44:W135–W141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Preusse M., Theis F.J., Mueller N.S.. miTALOS v2: analyzing tissue specific microRNA function. PLoS ONE. 2016; 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya A., Ziebarth J.D., Cui Y.. PolymiRTS Database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res. 2013; 42:D86–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiromatzo A.O., Oliveira T.Y.K., Pereira G., Costa A.Y., Montesco C.A.E., Gras D.E., Yosetake F., Vilar J.B., Cervato M., Prado P.R.R. et al.. miRNApath: a database of miRNAs, target genes and metabolic pathways. Genet. Mol. Res.: GMR. 2007; 6:859–865. [PubMed] [Google Scholar]
- 23. Wu X., Watson M.. CORNA: testing gene lists for regulation by microRNAs. Bioinformatics. 2009; 25:832–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vlachos I.S., Zagganas K., Paraskevopoulou M.D., Georgakilas G., Karagkouni D., Vergoulis T., Dalamagas T., Hatzigeorgiou A.G.. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015; 43:W460–W466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Backes C., Kehl T., Stöckel D., Fehlmann T., Schneider L., Meese E., Lenhof H.P., Keller A.. MiRPathDB: A new dictionary on microRNAs and target pathways. Nucleic Acids Res. 2017; 45:D90–D96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Backes C., Meese E., Lenhof H.P., Keller A.. A dictionary on microRNAs and their putative target pathways. Nucleic Acids Res. 2010; 38:4476–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denham J., Gray A.J., Scott-Hamilton J., Hagstrom A.D., Murphy A.J.. Small non-coding RNAs are altered by short-term sprint interval training in men. Physiol. Rep. 2018; 6:e13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ragni E., De Luca P., Perucca Orfei C., Colombini A., Viganò M., Lugano G., Bollati V., de Girolamo L.. Insights into inflammatory Priming of adipose-derived mesenchymal stem cells: validation of extracellular vesicles-embedded miRNA reference genesas a crucial step for donor selection. Cells. 2019; 8:E369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kehl T., Backes C., Kern F., Fehlmann T., Ludwig N., Meese E., Lenhof H.P., Keller A.. About miRNAs, miRNA seeds, target genes and target pathways. Oncotarget. 2017; 8:107167–107175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chou C.H., Shrestha S., Yang C.D., Chang N.W., Lin Y.L., Liao K.W., Huang W.C., Sun T.H., Tu S.J., Lee W.H. et al.. MiRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018; 46:D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agarwal V., Bell G.W., Nam J.W., Bartel D.P.. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015; 4:doi:10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S.. MicroRNA targets in Drosophila. Genome Biol. 2003; 5:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhattacharya A., Cui Y.. MiR2GO: comparative functional analysis for microRNAs. Bioinformatics. 2015; 31:2403–2405. [DOI] [PubMed] [Google Scholar]
- 34. Stöckel D., Kehl T., Trampert P., Schneider L., Backes C., Ludwig N., Gerasch A., Kaufmann M., Gessler M., Graf N. et al.. Multi-omics enrichment analysis using the GeneTrail2 web service. Bioinformatics. 2016; 32:1502–1508. [DOI] [PubMed] [Google Scholar]
- 35. The Gene Ontology Consortium The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2018; 47:D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M.. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2018; 47:D590–D595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P., Haw R., Jassal B., Korninger F., May B. et al.. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2017; 46:D649–D655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slenter D.N., Kutmon M., Hanspers K., Riutta A., Windsor J., Nunes N., Mélius J., Cirillo E., Coort S.L., Digles D. et al.. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2017; 46:D661–D667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benjamini Y., Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodological). 1995; 57:289–300. [Google Scholar]
- 40. Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C., Rheinheimer S., Meder B., Stähler C., Meese E. et al.. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016; 44:3865–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamberg M., Backes C., Fehlmann T., Hart M., Meder B., Meese E., Keller A.. MiRTargetLink-miRNAs, genes and interaction networks. Int. J.f Mol. Sci. 2016; 17:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.