Abstract
Foldamers are non-natural oligomers that mimic the structural behaviour of natural peptides, proteins and nucleotides by folding into a well-defined 3D conformation in solution. Since their first description about two decades ago, numerous studies have been undertaken dealing with the design, synthesis, characterization and application of foldamers. They have huge application potential as antimicrobial, anticancer and anti-HIV agents and in materials science. Despite their importance, there is no publicly available web resource providing comprehensive information on these compounds. Here we describe FoldamerDB, an open-source, fully annotated and manually curated database of peptidic foldamers. FoldamerDB holds the information about the sequence, structure and biological activities of the foldamer entries. It contains the information on over 1319 species and 1018 activities, collected from more than 160 research papers. The web-interface is designed to be clutter-free, user-friendly and it is compatible with devices of different screen sizes. The interface allows the user to search the database, browse and filter the foldamers using multiple criteria. It also offers a detailed help page to assist new users. FoldamerDB is hoped to bridge the gap in the freely available web-based resources on foldamers and will be of interest to diverse groups of scientists from chemists to biologists. The database can be accessed at http://foldamerdb.ttk.hu/.
INTRODUCTION
Foldamers, the non-natural oligomeric compounds, represent an important class of synthetic macromolecules with a tendency to adopt well-defined three-dimensional structures (1). One of the most important representatives of self-organized biomimetic systems are peptidic foldamers including, among others, β- and γ-peptides, peptoids, oligoureas as well as aza- and aminoxy peptides (2) (Figure 1A). These non-natural compounds are highly resistant to protease and peptidase degradation and are useful in the studies of mimicking protein conformations in solution. In the last two decades, foldamer chemistry pioneered by the groups of Gellman and Seebach (1,3) has demonstrated widespread applicability of these species from nanotechnology through medical fields, to biopolymer surface recognition, catalysis and biotechnology (4–9).
Figure 1.
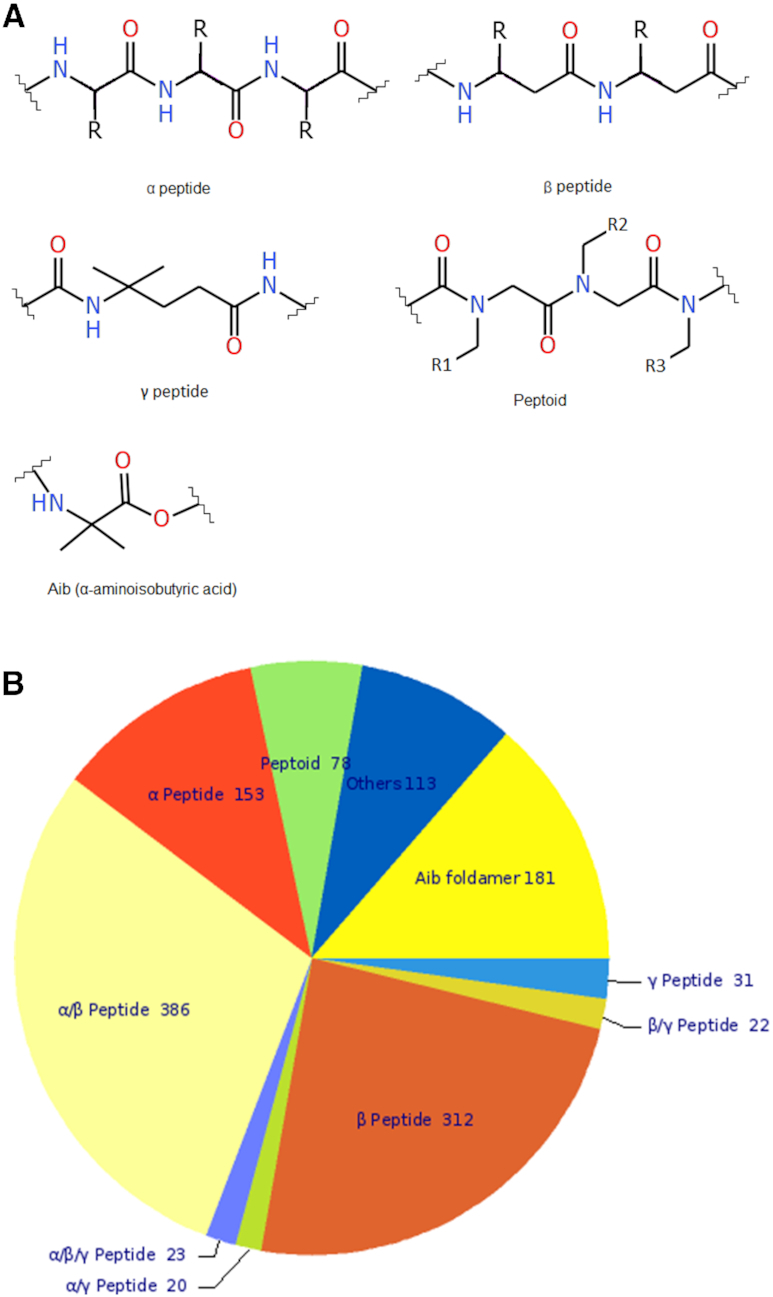
(A) Different types of peptide backbones in FoldamerDB. (B) Pie chart of distribution of peptide backbone types in FoldamerDB.
The conformational pool of different types of foldamers depends on the number and nature of substituents attached to the methylene units within the backbone, the absolute configuration of substituents, the incorporation of cyclic structures, the length of the peptide backbone and on the solvent used. These features allow the formation of programmable secondary structures, e.g. various types of helices, strand-like conformations or turns (10–13). The composition of the peptide backbone and the flexible side chains allow structural diversity even for the simplest β-peptides (Figure 1A) to go beyond those of natural peptides and proteins (14,15). The majority of β-peptide foldamers adopt several helical conformations (10,16–20) stabilized by hydrogen bonds, where H14-helices (21), H12-helices (22,23) and even H10/H12 helical structures with mixed H-bond patterns appear (24). Conformational transitions of foldamers by increasing the peptide chain length or by incorporating photosensitive groups have also been reported (8). Furthermore, pleated sheets (25), hairpin turns (26,27) and higher-ordered aqueous assemblies, such as bundles (28–31), self-assembled vesicles (32), nanofibers (33) and nanospheres (34), can also be reached for these systems.
Besides peptide foldamers composed entirely of non-natural amino acids, various heterogeneous foldamers with mixed natural and non-natural amino acids, i.e. αβ, αβα, αβγ, etc., have also been developed (35,36). A number of these compounds show high potential for applicability as antimicrobial (37–39), antiviral (40) and anticancer agents (41) or in their use in gene therapy (42). As a prominent example, the phase 2 clinical trials of ‘Brilacidin’, a short arylamide foldamer, has recently been completed for the treatment of bacterial skin infections caused by S. aureus (43).
However, despite their importance and high potential for various applications, there is no publicly available database providing comprehensive information on these systems. The necessity of a focused and dedicated database for computer-based design is growing as sophisticated algorithms are being applied to advance the research in the field of antimicrobial peptides, molecular graphics, protein structure prediction, drug design and the study of drug–protein interactions (44–50). Based on these pieces of information, it is clear that a user-friendly database on foldamers would be an important resource. Here, we present ‘FoldamerDB’ a database of peptidomimetic foldamers which, to the best of our knowledge, is the first and only open resource, holding the information about the sequence, structure and biological activities of the foldamers. The current version focuses on peptidic foldamers, composed mainly of β-, γ- and mixed α/β-peptides, which have the most numerous examples. FoldamerDB will certainly bridge the gap in the freely available web-based resources on foldamers and will be of interest to diverse groups of scientists from synthetic chemists to biologists.
MATERIALS AND METHODS
Data collection and processing
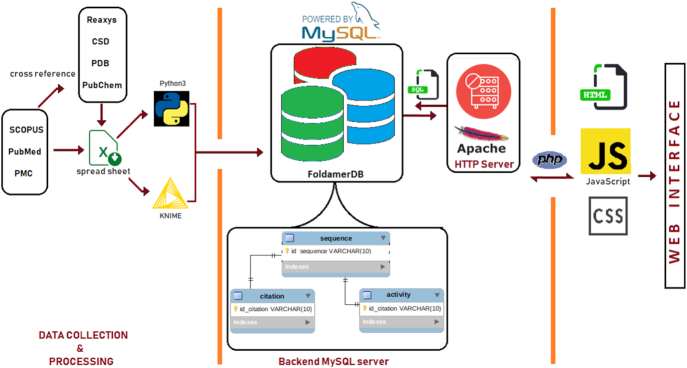
Each entry in FoldamerDB is a manually curated and annotated peptidic foldamer collected from the literature. Different combinations of keywords such as ‘foldamer’, ‘non-natural peptide’, ‘peptide’ and ‘folding’ were used to perform the search in SCOPUS, PubMed and PMC. Each literature entry was cross-referenced with the Reaxys (https://www.reaxys.com/), PubChem (51), ChEMBL (52) and NCBI databases to extract chemical and other information. The entries were also cross-referenced with CSD (Cambridge Structural Database) and PDB (Protein Data Bank) to extract the experimental structural information. A peptide was included in the database only if it was described as ‘foldamer’ or shown experimentally to fold into a specific 3D structure. Python3 (https://www.python.org/) and KNIME Analytics Platform version 3.6.2 (53) were used to process and annotate the collected data. Marvin by ChemAxon (https://chemaxon.com/products/marvin) was used to correct erroneous structures. According to the backbone type, each collected foldamer is classified into one of the following groups: α-peptide, β-peptide, γ-peptide, α/β-peptide, α/γ-peptide, α/β/γ-peptide, β/γ-peptide, Aib foldamer or peptoids (Figure 1). Note, that for machine learning, and other rational design purposes several natural α-peptides are also part of the database, as these were most often starting sequences used to be modified by non-natural insertions. Nonetheless, for simplicity, all the entries are referred to as ‘foldamers’ throughout the manuscript. Figure 2 shows the schematic workflow of data collection, processing and information flow in FoldamerDB.
Figure 2.
Workflow of data collection and processing as well as information flow in FoldamerDB.
Database design and implementation
FoldamerDB is a relational database hosted on Apache HTTP server 2.4 with MySQL server 5.7 in the back-end. The dynamic front-end is designed using PHP 7.2, HTML5, CSS and JavaScript technologies. The Bootstrap3 and jQuery libraries are utilized to make a responsive and mobile-first front-end.
JpGraph library (https://jpgraph.net/) was used to plot charts. The 3D model of the foldamers is rendered using Jmol (http://www.jmol.org/). The information is stored using the relational database management system (RDBMS) for easy retrieval and scalability. RDBMS is a widespread database management system used extensively for popular databases with millions of data points. The information in FoldamerDB is spread over multiple tables connected to each other by the parent–child relationship.
Substructure search
JSME, a free molecule editor written in JavaScript (54), is provided for users to draw query structure. The degree of similarity between the query molecule and the database entries is assessed by the Tanimoto coefficient. The FP2 fingerprints of all FoldamerDB entries are pre-calculated and stored in the database, whereas it is calculated on the fly for the query structure. Open Babel Package, version 2.4.1 (http://openbabel.sourceforge.net/), is used for the chemical fingerprint calculation. The Tanimoto coefficient, which is a number between 0 and 1 (where 1 denote maximum similarity), is used to measure the distance between the query and hit. The Tanimoto coefficient is computed by dividing the size of the intersection (query ∩ hit) and the size of the union (query ∪ hit) of fingerprint sets of the query and the hit molecule, where 0 ≤ J(A,B) ≤ 1 and A, B are set of fingerprints of the query and hit molecules, respectively.
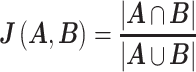 |
DATABASE OVERVIEW
Database content
FoldamerDB contains the information of over 1319 peptidic foldamers collected from approximately 160 published research articles. It provides comprehensive information about a foldamer such as 2D and 3D models, molecular properties, compound identifiers, structural information, external database IDs, biological activities, bibliography and other information (Figure 3). Links to CSD and PDB are provided to direct users to further structural information. Data are organized in several fields such as chemical diagram, chemical name, source, SMILES, InchiKey, molecular weight, molecular formula, Reaxys ID, accession number, methods of structural analysis (NMR or X-ray crystallography), NMR solvent, CCDC number, PDB ID, application, biological activity, type of foldamer and references. Additionally, various calculated properties such as LogP, number of H-bond donors and acceptors, rotatable bonds and surface area are also provided.
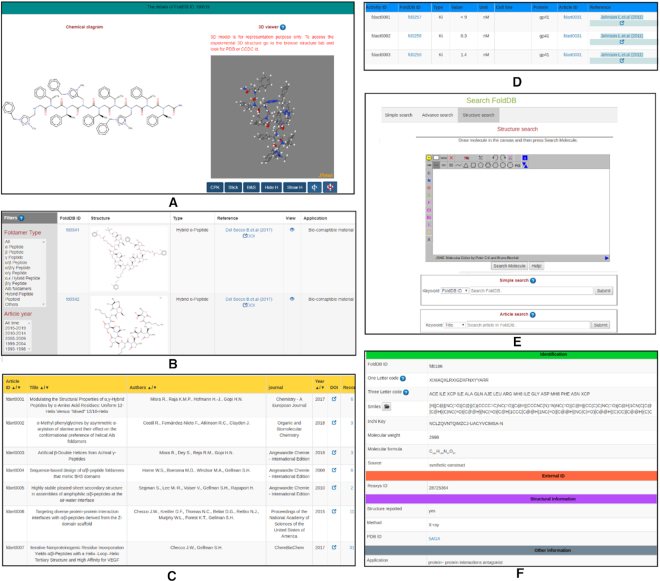
Figure 3.
Overview of different FoldamerDB webpages. Layout of: (A) structures of a foldamer entry, (B) browse foldamer table along with the filters, (C) the article page, (D) biological activities page accessible through the ‘browse activity’, (E) various search options in FoldamerDB and (F) various information blocks of a single foldamer page.
User interface layout
FoldamerDB is designed to be intuitive and user-friendly with multiple ways to navigate the database. Options are provided to browse all foldamers, articles, structures and biological activities. The rich interface for querying allows the user to easily retrieve a specific foldamer from the database. Users can perform a simple search beside the complex and chemical fingerprint-based substructure search. The web-interface is designed to be responsive and compatible with devices of different screen sizes. Following is a brief description of the main pages in FoldamerDB.
Home: Main landing page, with a brief introduction and statistical information about the database.
Search: This page has comprehensive options to perform simple, complex and substructure search (Figure 3E). The simple search allows performing a search using various fields such as internal ID (FoldDB ID), Reaxys ID, application, article title, author name and journal. Structure search, in turn, allows users to draw the query molecule and search for entries in FoldamerDB.
Browse Foldamers: This page has an interactive table spread over multiple sub-pages displaying all foldamers in FoldamerDB. Options to filter the table based on the type of backbone and year of publications are also provided (Figure 3B). Details of a single foldamer can be accessed by clicking on the FoldDB ID, which takes the user to the single foldamer view page.
Single foldamer view: This page displays both a 2D and an interactive 3D model and all information associated with a single foldamer. The animation and representation of the 3D model can be controlled using the buttons provided below (Figure 3A). Data in this page are divided into seven categories. (i) Identification (chemical name, sequence, SMILES, InchiKey, molecular weight, molecular formula, source). (ii) External IDs (Reaxys substance index, NCBI accession number). (iii) Structural data, which include information about the method used for structure determination and links to CSD or PDB. (iv) Other information viz. application, foldamer type, etc. (v) Calculated properties such as log P, number or H-bond donor or acceptor, rotatable bond and polar surface area (PSA). (vi) Biological activity along with the option to see all activities reported for other foldamers in the same reference article. (vii) Information related to citations (Figure 3F).
Browse article: List of the articles from which data are included in FoldamerDB with links to full-text options presented. The title of the article, author names, journal, year and the number of foldamers in each article is shown in the table (Figure 3C).
Browse structure: This page lists all foldamers with structure elucidation using X-ray crystallography. Only foldamers with crystal structures available in PDB or CSD are included in this list.
Browse activity: Reported biological activities of the foldamers are available on this page (Figure 3D).
Glossary: Structure and chemical name of common non-natural amino acids and foldamer building blocks are included here.
Feedback: This page holds details to give feedback and report errors. The templates for contributing new data into FoldamerDB are also available here. Data submitted by users would be reviewed by the foldamerDB team before uploading.
Analysis of foldamer types
The type of peptide backbones included and their distribution in FoldamerDB are shown in Figure 1. The most common type of foldamers is α/β-peptides, represented by 383 entries. These are followed by 312 foldamers where backbones consist of only β-amino acids. The β-amino acids could be of β2- or β3-type depending on the position of the side group on the backbone. They could also belong to cyclic β-peptides. The next most common type is Aib foldamers, which consist of one or several units of α-aminobutyric acid (Aib) residues. Since Aib is a non-proteogenic α-amino acid, we have categorised such foldamers in a separate group. There are 181 Aib foldamers in the database. Foldamers consisting of only α-amino acids constitute 156 entries, whereas peptoids are represented by 78 entries. Peptoids are special type of peptides mimicking compounds in which the side chain is attached to the N atom of the backbone rather than to the α- or β-carbon of the monomer unit (Figure 1A). The remaining foldamers are grouped into several small categories such as γ-peptide (31 entries), α/γ-peptide (20 entries), β/γ-peptide (22 entries), α/β/γ-peptide (23 entries) and others (113 entries). There are only two foldamer entries under the type α/ϵ hybrid peptide. Wherever possible, each foldamer entry is also assigned as a subtype based on the specific chemical structure of its building blocks as described in the reporting research articles.
EXAMPLES
Examples of search options in FoldamerDB are provided below:
Free text search: It allows users to perform keyword-based search on various fields of the database. Database fields included in this search are sequence (one-letter codes or three-letter codes), chemical name, molecular formula, application, solvent, type and PDB ID. A few special characters such as ‘+’ for AND, ‘-’ for NOT and ‘no operator’ for OR can be used to construct logical search queries. For example, if we search using ‘Peptoids’ as the search term, it will return all 79 foldamers of Peptoid type. In turn, if we want to exclude some peptoids from the search results, the query could be modified as ‘Peptoid-antimicrobial-antibacterial’. This will result in a narrower set of only 32 foldamers excluding the peptoids marked as antimicrobial and antibacterial. Additional information of this topic is available at http://foldamerdb.ttk.hu/help.php#foldamer_search.
-
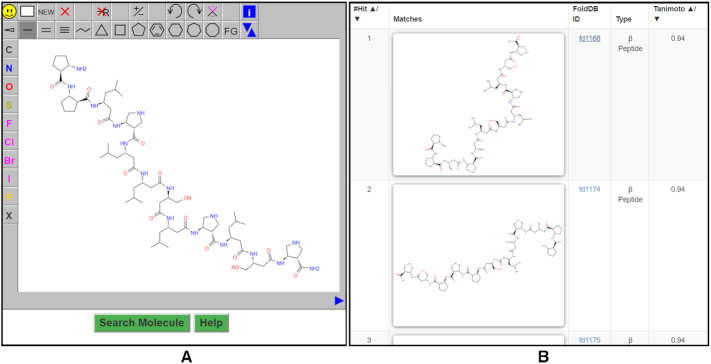
Substructure search: It allows users to draw the query structure and perform substructure search in FoldamerDB. We use the example of query molecule shown in Figure 4A, which is represented in smile format as:‘CC(C)C[C@@H](CC(=O)N[C@@H](CO)CC(=O)N[C@@H](CC(C)C)CC(=O)N[C@H]1CNC[C@@H]1C(=O)N[C@@H](CC(C)C)CC(=O)N[C@@H](CO)CC(=O)N[C@H]1CNC[C@@H]1C(N)=O)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H]1CNC[C@@H]1NC(=O)C[C@H](CC(C)C)NC(=O)[C@H]1CCC[C@@H]1NC(=O)[C@H]1CCC[C@@H]1N’
For simplified use, we can paste the smiles of our query structure into the canvas. More details about this option can be accessed from http://foldamerdb.ttk.hu/help.php#Sub-structure_Search. Once you sketched your query, press the ‘Search Molecule’ button to perform the search. Figure 4B shows the output of your structure search, where you can see the Tanimoto distance between the query and the hit molecule in the last column. Molecules with higher similarity have higher Tanimoto coefficients, closer to 1, while less similar ones have lower values, closer to 0.
Figure 4.
(A) The query molecule drawn on the JSME editor for the substructure search in FoldamerDB. (B) The search results of the query. The similarity between the query and hit molecules is measured in terms of Tanimoto index, which ranges from 0 to 1.
SUMMARY AND OUTLOOK
We have developed FoldamerDB to provide a freely available web resource on peptidic foldamers. Data were compiled by collecting the foldamers from published articles and extracting relevant information from external databases. It provides detailed information on their structure, biological activities and other significant properties. FoldamerDB currently holds 1319 foldamer entries, of which 166 are reported together with their experimental crystal structure. There are 1018 entries with biological activities reported in the literature. To the best of our knowledge, currently there is no open-source database of foldamers and FoldamerDB serves as the first database of its kind toward filling this gap. It is our hope that this web resource will allow the quick and easy use of the significant amount of structural, chemical and biological information on foldamers available and the database can be a basic tool for novel design projects involving machine learning techniques.
FUTURE WORK
FoldamerDB serves as the first milestone toward developing an integrated and comprehensive database of foldamers. Note, that the current version focuses on peptidic compounds, with the majority of the entries having mixed β-peptides, which is the largest sub-type of foldamers produced until now. However, in the future we aim to expand FoldamerDB to include information about more exotic foldamer types, such as aromatic oligoamides. For achieving this major goal as well as for keeping the database as up to date as possible, we encourage the scientific community to contribute to the FoldamerDB project and increase the number of entries. This can be done via the feedback page (http://foldamerdb.ttk.hu/feedback.php) or by contacting directly the FoldamerDB team.
DATA AVAILABILITY
FoldamerDB is an open resource database available at http://foldamerdb.ttk.hu
ACKNOWLEDGEMENTS
The authors are grateful to Dr Tünde Juhász and Gergely Kohut for their help with checking the database and fruitful discussions. B.N. is also thankful to Dr András Wacha and Ákos Bencsura for technical help and discussions.
FUNDING
Momentum programme of the Hungarian Academy of Sciences [LP2016-2]; National Competitiveness and Excellence Program [NVKP_16-1-2016-0007]; BIONANO_GINOP-2.3.2-15-2016-00017b. Funding for open access charge: Momentum programme of the Hungarian Academy of Sciences [LP2016-2].
Conflict of interest statement. None declared.
REFERENCES
- 1. Gellman S.H. Foldamers: a manifesto. Acc. Chem. Res. 1998; 31:173–180. [Google Scholar]
- 2. Pasco M., Dolain C., Guichard G.. Foldamers. Supramolecular Chemistry in Water. 2019; John Wiley & Sons, Ltd; 337–374. [Google Scholar]
- 3. Seebach D., Matthews J.L.. β-Peptides: a surprise at every turn. Chem. Commun. 1997; 1997:2015–2022. [Google Scholar]
- 4. Wang P.S.P., Schepartz A.. β-Peptide bundles: design. build. analyze. biosynthesize. Chem. Commun. 2016; 52:7420–7432. [DOI] [PubMed] [Google Scholar]
- 5. Kwon S., Kim B.J., Lim H.-K., Kang K., Yoo S.H., Gong J., Yoon E., Lee J., Choi I.S., Kim H. et al.. Magnetotactic molecular architectures from self-assembly of β-peptide foldamers. Nat. Commun. 2015; 6:8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandramouli N., Ferrand Y., Lautrette G., Kauffmann B., Mackereth C.D., Laguerre M., Dubreuil D., Huc I.. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nat. Chem. 2015; 7:334–341. [DOI] [PubMed] [Google Scholar]
- 7. Kulkarni K., Hung J., Fulcher A.J., Chan A.H.P., Hong A., Forsythe J.S., Aguilar M.-I., Wise S.G., Del Borgo M.P.. β3-Tripeptides coassemble into fluorescent hydrogels for serial monitoring in vivo. ACS Biomater. Sci. Eng. 2018; 4:3843–3847. [DOI] [PubMed] [Google Scholar]
- 8. De Poli M., Zawodny W., Quinonero O., Lorch M., Webb S.J., Clayden J.. Conformational photoswitching of a synthetic peptide foldamer bound within a phospholipid bilayer. Science. 2016; 352:575–580. [DOI] [PubMed] [Google Scholar]
- 9. Checco J.W., Kreitler D.F., Thomas N.C., Belair D.G., Rettko N.J., Murphy W.L., Forest K.T., Gellman S.H.. Targeting diverse protein-protein interaction interfaces with α/β-peptides derived from the Z-domain scaffold. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:4552–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seebach D., Beck A.K., Bierbaum D.J.. The world of β‐ and γ‐peptides comprised of homologated proteinogenic amino acids and other components. Chem. Biodivers. 2004; 1:1111–1239. [DOI] [PubMed] [Google Scholar]
- 11. Goodman C.M., Choi S., Shandler S., DeGrado W.F.. Foldamers as versatile frameworks for the design and evolution of function. Nat. Chem. Biol. 2007; 3:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinek T.A., Fülöp F.. Peptidic foldamers: ramping up diversity. Chem. Soc. Rev. 2012; 41:687–702. [DOI] [PubMed] [Google Scholar]
- 13. Mándity I.M., Fülöp F.. An overview of peptide and peptoid foldamers in medicinal chemistry. Expert Opin. Drug Discov. 2015; 10:1163–1177. [DOI] [PubMed] [Google Scholar]
- 14. Beke T., Csizmadia I.G., Perczel A.. On the flexibility of β-peptides. J. Comput. Chem. 2004; 25:285–307. [DOI] [PubMed] [Google Scholar]
- 15. Beke T., Somlai C., Perczel A.. Toward a rational design of β-peptide structures. J. Comput. Chem. 2006; 27:20–38. [DOI] [PubMed] [Google Scholar]
- 16. Hetényi A., Mándity I.M., Martinek T.A., Tóth G.K., Fülöp F.. Chain-length-dependent helical motifs and self-association of β-peptides with constrained side chains. J. Am. Chem. Soc. 2005; 127:547–553. [DOI] [PubMed] [Google Scholar]
- 17. Iverson B.L. Betas are brought into the fold. Nature. 1997; 385:113–115. [DOI] [PubMed] [Google Scholar]
- 18. Abraham E., Bailey C.W., Claridge T.D.W., Davies S.G., Ling K.B., Odell B., Rees T.L., Roberts P.M., Russell A.J., Smith A.D. et al.. A systematic study of the solid state and solution phase conformational preferences of β-peptides derived from transpentacin. Tetrahedron Asymmetry. 2010; 21:1797–1815. [Google Scholar]
- 19. Abele S., Guichard G., Seebach D.. (S)-β3-Homolysine- and (S)-β3-Homoserine-Containingβ-Peptides: CD Spectra in Aqueous Solution. Helv. Chim. Acta. 1998; 81:2141–2156. [Google Scholar]
- 20. Lee M., Raguse T.L., Schinnerl M., Pomerantz W.C., Wang X., Wipf P., Gellman S.H.. Origins of the high 14-helix propensity of cyclohexyl-rigidified residues in β-peptides. Org. Lett. 2007; 9:1801–1804. [DOI] [PubMed] [Google Scholar]
- 21. Appella D.H., Christianson L.A., Karle I.L., Powell D.R., Gellman S.H.. β-Peptide foldamers: robust helix formation in a new family of β-amino acid oligomers. J. Am. Chem. Soc. 1996; 118:13071–13072. [Google Scholar]
- 22. Seebach D., Ciceri P.E., Overhand M., Jaun B., Rigo D., Oberer L., Hommel U., Amstutz R., Widmer H.. Probing the Helical Secondary Structure of Short-Chain β-Peptides. Helv. Chim. Acta. 1996; 79:2043–2066. [Google Scholar]
- 23. Seebach D., Overhand M., Kühnle F.N.M., Martinoni B., Oberer L., Hommel U., Widmer H.. β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a β-hexapeptide in solution and its stability towards pepsin. Helv. Chim. Acta. 1996; 79:913–941. [Google Scholar]
- 24. Daura X., Gunsteren W.Fvan., Rigo D., Jaun B., Seebach D.. Studying the stability of a helical β-heptapeptide by molecular dynamics simulations. Chem. – Eur. J. 1997; 3:1410–1417. [Google Scholar]
- 25. Seebach D., Abele S., Gademann K., Jaun B.. Pleated sheets and turns ofβ-peptides with proteinogenic side chains. Angew. Chem. Int. Ed. 1999; 38:1595–1597. [DOI] [PubMed] [Google Scholar]
- 26. Daura X., Gademann K., Schäfer H., Jaun B., Seebach D., van Gunsteren W.F.. The β-peptide hairpin in solution: conformational study of a β-hexapeptide in methanol by nmr spectroscopy and md simulation. J. Am. Chem. Soc. 2001; 123:2393–2404. [DOI] [PubMed] [Google Scholar]
- 27. Cheng R.P., Gellman S.H., DeGrado W.F.. β-Peptides: from structure to function. Chem. Rev. 2001; 101:3219–3232. [DOI] [PubMed] [Google Scholar]
- 28. Wang P.S.P., Nguyen J.B., Schepartz A.. Design and high-resolution structure of a β 3 -peptide bundle catalyst. J. Am. Chem. Soc. 2014; 136:6810–6813. [DOI] [PubMed] [Google Scholar]
- 29. Daniels D.S., Petersson E.J., Qiu J.X., Schepartz A.. High-resolution structure of a beta-peptide bundle. J. Am. Chem. Soc. 2007; 129:1532–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beke T., Csizmadia I.G., Perczel A.. Theoretical study on tertiary structural elements of beta-peptides: nanotubes formed from parallel-sheet-derived assemblies of beta-peptides. J. Am. Chem. Soc. 2006; 128:5158–5167. [DOI] [PubMed] [Google Scholar]
- 31. Beke T., Czajlik A., Bálint B., Perczel A.. A theoretical comparison of self-assembling α- and β-peptide nanostructures: toward design of β-barrel frameworks. ACS Nano. 2008; 2:545–553. [DOI] [PubMed] [Google Scholar]
- 32. Mándity I.M., Fülöp L., Vass E., Tóth G.K., Martinek T.A., Fülöp F.. Building β-Peptide H10/12 foldamer helices with six-membered cyclic side-chains: fine-tuning of folding and self-assembly. Org. Lett. 2010; 12:5584–5587. [DOI] [PubMed] [Google Scholar]
- 33. Del Borgo M.P., Mechler A.I., Traore D., Forsyth C., Wilce J.A., Wilce M.C.J., Aguilar M.-I., Perlmutter P.. Supramolecular self-assembly of n -acetyl-capped β-peptides leads to nano- to macroscale fiber formation. Angew. Chem. Int. Ed. 2013; 52:8266–8270. [DOI] [PubMed] [Google Scholar]
- 34. Pizzey C.L., Pomerantz W.C., Sung B.-J., Yuwono V.M., Gellman S.H., Hartgerink J.D., Yethiraj A., Abbott N.L.. Characterization of nanofibers formed by self-assembly of beta-peptide oligomers using small angle x-ray scattering. J. Chem. Phys. 2008; 129:095103. [DOI] [PubMed] [Google Scholar]
- 35. Baldauf C., Günther R., Hofmann H.-J.. Helix Formation in α,γ- and β,γ-hybrid peptides: theoretical insights into mimicry of α- and β-peptides. J. Org. Chem. 2006; 71:1200–1208. [DOI] [PubMed] [Google Scholar]
- 36. Giuliano M.W., Maynard S.J., Almeida A.M., Guo L., Guzei I.A., Spencer L.C., Gellman S.H.. A γ-amino acid that favors 12/10-helical secondary structure in α/γ-peptides. J. Am. Chem. Soc. 2014; 136:15046–15053. [DOI] [PubMed] [Google Scholar]
- 37. Porter E.A., Wang X., Lee H.-S., Weisblum B., Gellman S.H.. Non-haemolytic β-amino-acid oligomers. Nature. 2000; 404:565–565. [DOI] [PubMed] [Google Scholar]
- 38. Raguse T.L., Porter E.A., Weisblum B., Gellman S.H.. Structure−activity studies of 14-helical antimicrobial β-peptides: probing the relationship between conformational stability and antimicrobial potency. J. Am. Chem. Soc. 2002; 124:12774–12785. [DOI] [PubMed] [Google Scholar]
- 39. Epand R.F., Raguse L., Gellman S.H., Epand R.M.. Antimicrobial 14-helical β-peptides: potent bilayer disrupting agents. Biochemistry. 2004; 43:9527–9535. [DOI] [PubMed] [Google Scholar]
- 40. Corvaglia V., Carbajo D., Prabhakaran P., Ziach K., Mandal P.K., Santos V.D., Legeay C., Vogel R., Parissi V., Pourquier P. et al.. Carboxylate-functionalized foldamer inhibitors of HIV-1 integrase and Topoisomerase 1: artificial analogues of DNA mimic proteins. Nucleic Acids Res. 2019; 47:5511–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diedrich D., Moita A.J.R., Rüther A., Frieg B., Reiss G.J., Hoeppner A., Kurz T., Gohlke H., Lüdeke S., Kassack M.U. et al.. α-Aminoxy oligopeptides: synthesis, secondary structure, and cytotoxicity of a new class of anticancer foldamers. Chem. – Eur. J. 2016; 22:17600–17611. [DOI] [PubMed] [Google Scholar]
- 42. Douat C., Aisenbrey C., Antunes S., Decossas M., Lambert O., Bechinger B., Kichler A., Guichard G.. A cell-penetrating foldamer with a bioreducible linkage for intracellular delivery of DNA. Angew. Chem. Int. Ed. 2015; 54:11133–11137. [DOI] [PubMed] [Google Scholar]
- 43. Gopalakrishnan R., Frolov A.I., Knerr L., Drury W.J., Valeur E.. Therapeutic potential of foldamers: from chemical biology tools to drug candidates. J. Med. Chem. 2016; 59:9599–9621. [DOI] [PubMed] [Google Scholar]
- 44. Wang P., Hu L., Liu G., Jiang N., Chen X., Xu J., Zheng W., Li L., Tan M., Chen Z. et al.. Prediction of antimicrobial peptides based on sequence alignment and feature selection methods. PLoS ONE. 2011; 6:e18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waghu F.H., Gopi L., Barai R.S., Ramteke P., Nizami B., Idicula-Thomas S.. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 2014; 42:D1154–D1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waghu F.H., Barai R.S., Gurung P., Idicula-Thomas S.. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016; 44:D1094–D1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ross C., Nizami B., Glenister M., Sheik Amamuddy O., Atilgan A.R., Atilgan C., Tastan Bishop Ö.. MODE-TASK: large-scale protein motion tools. Bioinformatics. 2018; 34:3759–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Honarparvar B., Govender T., Maguire G.E.M., Soliman M.E.S., Kruger H.G.. Integrated approach to structure-based enzymatic drug design: molecular modeling, spectroscopy, and experimental bioactivity. Chem. Rev. 2014; 114:493–537. [DOI] [PubMed] [Google Scholar]
- 49. Nizami B., Tetko I.V., Koorbanally N.A., Honarparvar B.. QSAR models and scaffold-based analysis of non-nucleoside HIV RT inhibitors. Chemom. Intell. Lab. Syst. 2015; 148:134–144. [Google Scholar]
- 50. Nizami B., Sydow D., Wolber G., Honarparvar B.. Molecular insight on the binding of NNRTI to K103N mutated HIV-1 RT: molecular dynamics simulations and dynamic pharmacophore analysis. Mol. Biosyst. 2016; 12:3385–3395. [DOI] [PubMed] [Google Scholar]
- 51. Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B. et al.. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019; 47:D1102–D1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaulton A., Hersey A., Nowotka M., Bento A.P., Chambers J., Mendez D., Mutowo P., Atkinson F., Bellis L.J., Cibrián-Uhalte E. et al.. The ChEMBL database in 2017. Nucleic Acids Res. 2017; 45:D945–D954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Berthold M.R., Cebron N., Dill F., Gabriel T.R., Kötter T., Meinl T., Ohl P., Sieb C., Thiel K., Wiswedel B.. Preisach C, Burkhardt H, Schmidt-Thieme L, Decker R. KNIME: the konstanz information miner. Data Analysis, Machine Learning and Applications, Studies in Classification, Data Analysis, and Knowledge Organization. 2008; Berlin Heidelberg: Springer; 319–326. [Google Scholar]
- 54. Bienfait B., Ertl P.. JSME: a free molecule editor in JavaScript. J. Cheminformatics. 2013; 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
FoldamerDB is an open resource database available at http://foldamerdb.ttk.hu