Abstract
Knowledge of therapeutic targets and early drug candidates is useful for improved drug discovery. In particular, information about target regulators and the patented therapeutic agents facilitates research regarding druggability, systems pharmacology, new trends, molecular landscapes, and the development of drug discovery tools. To complement other databases, we constructed the Therapeutic Target Database (TTD) with expanded information about (i) target-regulating microRNAs and transcription factors, (ii) target-interacting proteins, and (iii) patented agents and their targets (structures and experimental activity values if available), which can be conveniently retrieved and is further enriched with regulatory mechanisms or biochemical classes. We also updated the TTD with the recently released International Classification of Diseases ICD-11 codes and additional sets of successful, clinical trial, and literature-reported targets that emerged since the last update. TTD is accessible at http://bidd.nus.edu.sg/group/ttd/ttd.asp. In case of possible web connectivity issues, two mirror sites of TTD are also constructed (http://db.idrblab.org/ttd/ and http://db.idrblab.net/ttd/).
INTRODUCTION
The efficiency of drug discovery depends critically on the selection of appropriate candidates of therapeutic target (1) and targeted agent (2,3). The investigation and acquired knowledge of these targets and agents are highly useful for accelerating drug discovery processes (4–6). Many open-access databases have provided comprehensive and complementary data of therapeutic targets. These include targets of different classes (7–11), drug-binding domains and targeted sites (12), target expression profiles in patients (13,14), activities of targeted agents (15–17), target-regulators (18–22), target-affiliated pathways (23,24), target–drug interaction networks (25–27), and target–disease associations (28,29). Integration of some of these data with knowledge of genetics, genomics, transcriptomics, animal models and literature enable the scoring and ranking of target–disease associations for target identification (28,29).
The targets of the TTD are validated with clinical evidence of the efficacy targets of the clinically tested drugs or with patent/literature report about the therapeutic targets of research agents. Specifically, the TTD targets are grouped into classes of successful (with at least one approved drug), clinical trial (with a clinical trial drug, but without an approved drug), patent-recorded (referenced in a patent and subsequent literature), and literature-reported targets. Differences in the target selection procedure may render TTD targets that partially differ from other databases. For instance, the TTD contains 2954 human and 465 infectious species targets, and all of the 2954 human targets, yet none of the 465 infectious species targets, are in the Open Targets Platform, which contains 27 024 targets associated with human diseases (28,29).
Some data content and access facilities, such as target regulators and patented agents, may be further improved. Knowledge of target regulators (e.g. microRNAs, transcription factors and interacting proteins) is useful for drug discovery tasks (Supplementary Table S1) such as the investigations of target druggability (6,30), systems pharmacology (31) and the discovery of multi-target and combination therapies (32,33). The structural and activity data of patented agents are useful for such tasks as the study of discovery landscapes and opportunities (34,35), as well as the development of artificial intelligence tools (36). While target regulators and patented agents can be accessed from several databases (10,11,18–22), the data access facilities are limited because no therapeutic targets or targeted-diseases are explicitly labeled, which makes it difficult for searching data with respect to therapeutic classes and disease areas.
To provide the expanded information and improved data access facilities, several major improvements were made to the Therapeutic Target Database (TTD). The first is the inclusion of two classes of target regulators, microRNAs (miRNAs) and transcription factors (TFs), for the successful (approved), clinical trial, patent-recorded and literature-reported targets in the TTD (Figure 1A). The second is the addition of the proteins directly interacting with the targets in the TTD (Figure 1A). The third is the inclusion of patented therapeutic agents and their targets searched from the patent contents and literature (Figure 1B). The fourth is the update of the recently released International Classification of Diseases codes ICD-11 for the targets in the TTD, which facilitates the access of target information using the ICD codes. The fifth is the update of the recently emerged targets and drugs since the last update (Table 1), including the previously nonincluded classes of targeted antigens of chimeric antigen receptor T-cell (CAR-T) therapy and small molecular and peptidomimetic inhibitors of immunotherapy targets. The newly added features, together with their statistics, are summarized in Supplementary Table S2.
Figure 1.
The statistics of the features newly added to the 2020 version of the TTD. (A) The inclusion of two classes of target regulators (microRNAs and transcription factors) and the addition of the proteins directly interacting with the targets; (B) the inclusion of patented therapeutic agents and their targets searched from the patent contents and literature.
Table 1.
Accumulation of drugs and their corresponding targets in the latest version and previous versions of the TTD
| 2020 | 2018 | 2016 | 2014 | 2012 | 2020 | 2018 | 2016 | 2014 | 2012 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Targets | 3419 | 3101 | 2589 | 2316 | 1981 | All Drugs | 37 316 | 29 570 | 27 165 | 20 006 | 17 816 |
| Successful | 461 | 445 | 397 | 388 | 364 | Approved | 2649 | 2544 | 2071 | 2003 | 1540 |
| Clinical Trial | 1191 | 1121 | 723 | 461 | 286 | Clinical Trial | 9465 | 8103 | 7291 | 3147 | 1423 |
| Patent-Recorded | 207 | 0 | 0 | 0 | 0 | Patented | 5,059 | 0 | 0 | 0 | 0 |
| Literature-Reported | 1560 | 1535 | 1469 | 1467 | 1331 | Experimental | 20 143 | 18 923 | 17 803 | 14 856 | 14 853 |
Patented drugs and their corresponding targets were, for the first time, collected and integrated into this version of the TTD.
TARGET REGULATORS
Target-regulating MicroRNAs
MicroRNAs (miRNAs) are reported to negatively regulate the expression of ∼30% of human genes. miRNA has demonstrated profound pharmacological/pharmacokinetic implications in the regulation of therapeutic targets, drug metabolism enzymes or transporters (37). For example, miR-125b inhibits the expression of vitamin D receptor (therapeutic target of the drug calcitriol), which results in the reduced efficacy of calcitriol by a lowered target level (38); another study has shown that the overexpression of miR-24 downregulates dihydrofolate reductase (target of chemotherapeutic drug methotrexate), which induces methotrexate resistance (39). Thus, the information regarding target-regulating miRNAs is helpful for studying the regulation of targeted therapeutics in individual patients (37,40) and exploring multi-target treatment strategies (41).
Since the target-regulating miRNA data were largely dispersed in the literature, the PubMed database was systematically searched using the combination of the keywords ‘microRNA’/‘micro-RNA’/‘mi-RNA’/‘miR’/‘miRNA’/ ‘hsa-miR’/‘hsa-let’ and the names/synonyms of TTD targets. The collected data include the following: (i) the names and sequences of mature miRNAs and (ii) the experiments confirming the regulation of a studied target by certain miRNAs. The collected experiments include reporter assay, quantitative polymerase chain reaction (qPCR), in situ hybridization, northern blot, and so on (42,43). Some of the above experiments (e.g. western blot and reporter assay) are typically applied methods for discovering protein-regulating miRNAs, and some methods (e.g. qPCR, in situ hybridization and northern blot) have been frequently used to identify miRNAs coexpressed with the genes of therapeutic targets (42–45). These literature-reported regulation mechanisms are provided in the TTD.
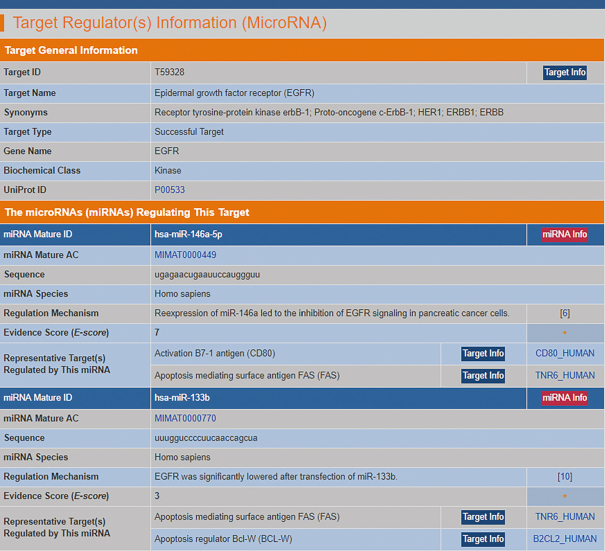
The latest version of the TTD collected 179 successful, 298 clinical trial, 62 patent-recorded and 330 literature-reported targets that are regulated by 587 miRNAs, involving 3707 miRNA and target regulation pairs. The confidence level of an experimentally observed regulation can be tentatively measured by the number of published experimental sources of evidence (46,47). Thus, an evidence score (E-score, the number of published research articles of experimental evidence) was defined for the tentative measurement of the confidence level of target-regulating miRNAs. The target-regulating miRNAs are listed with respect to their E-scores. A typical webpage providing the target-regulating miRNA is shown in Figure 2.
Figure 2.
A typical page in the TTD providing target-regulating microRNA information. The detailed information on microRNA sequences, supporting experiments, and other targets regulated by the collected microRNA. The target-regulating microRNAs are listed on the TTD webpage with respect to the E-scores, which were derived using the number of papers describing the experimental evidence. The details of each microRNA can be found by clicking the ‘miRNA Info’ button.
Target-regulating transcription factors
Transcription factors (TFs) regulate the expression of genes by controlling transcription of genetic information from DNA to messenger RNA. TFs that regulate the therapeutically relevant genes have been explored as the targets of approved drugs (48). For example, both premarin (for treating osteoporosis) and tamoxifen (for treating breast cancer) target the transcription factor estrogen receptor (ER), which regulates the expression of estrogen-responsive genes that control both osteoporosis and breast cancer, thereby manifesting their osteoporosis-preventive and anticancer effects (48). Although many TFs are important disease regulators, and thus potential therapeutic targets, many of them have been considered to be undruggable, partly owing to the large protein–protein interaction interfaces or lack of deep protein pockets for small molecule drug binding (49). Progress has been made for drug discovery against the targets previously considered to be undruggable (50). Thus, it is useful to provide the data with respect to target-regulating TFs for facilitating future efforts in targeting TFs.
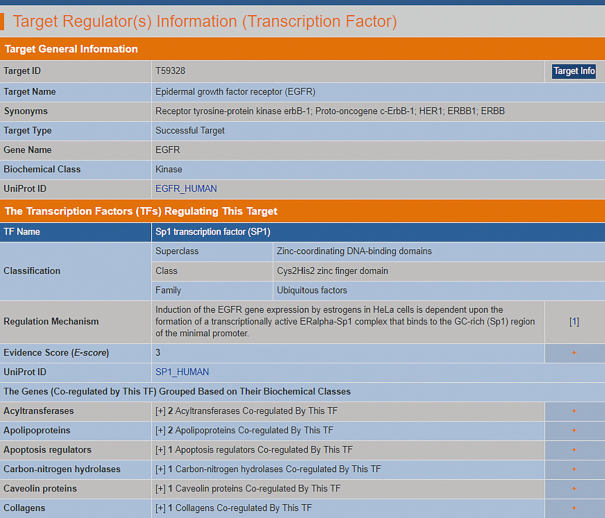
Target-regulating TF data were collected by comprehensively searching the PubMed database using the combinations of the keywords ‘transcription factor’/‘TF’/‘DNA-binding factor’/‘DNA-binding’/‘transcription regulation’/‘promoter’/‘enhancer’/‘silencer’ and names/synonyms of each therapeutic target in the TTD. In total, 55 successful, 61 clinical trial, 6 patent-recorded and 31 literature-reported targets regulated by 135 TFs were collected. These collected data include the following: (i) the names and sequences of TFs regulating each target, (ii) TF classifications (superclass, class, family and subfamily) defined by TRANSFAC (51,52) and (iii) the experiments used to confirm the regulation of a studied target by TFs. These experiments include electrophoretic mobility shift assays (53), chromatin immunoprecipitations (54), DNase footprinting assays (55), and so on. They have been used for probing the direct binding of a TF to the promoter, enhancer or silencer region of a target gene, thus leading to the initiation (56), increase (57) or repression of the transcription of the studied gene (58), respectively. These literature-reported regulation mechanisms are provided in the TTD. The target-regulating TFs are listed in the TTD with respect to their E-scores, which were derived using the numbers of papers describing the experimental evidence. Moreover, the genes coregulated by each target-regulating TF are provided in the TTD and grouped by biochemical class (Figure 3).
Figure 3.
A typical page in the TTD providing information about target-regulating transcription factors (TFs). The target-regulating TFs are listed on the TTD webpage with respect to their E-scores, which were derived using the number of papers describing the experimental evidence. The genes coregulated by each target-regulating TF are provided in the TTD and grouped using biochemical classes (GPCR, peptidase, virus penetration channel, etc.).
TARGET-INTERACTING PROTEINS
Knowledge of target-protein interactions is important for network pharmacology studies (59) and target druggability assessments (60,61). Particularly, the analysis of the human target-protein network topological properties (derived from the human target-protein interaction data) has revealed that successful targets are more highly connected to other proteins and have higher network betweenness (the number of times a studied protein appears in the shortest path between two other proteins in network divided by the total number of protein pairs) than other proteins (60); the human target-protein network topological features and human systems druggability characteristics of 89 innovative targets of the first-in-class drugs approved from 2004 to 2017 have led to a simple rule describing the clinical trial progression speed of innovative drug targets (50), which states that the human target entering clinical trials may progress more speedily through the trials if it has no violations of the following criteria: (i) two human target-protein network topological properties are within specified values (neighborhood connectivity <15 and degree <15) and (ii) three system druggability properties are within specified ranges (affiliated with <5 human signal pathways, distributed in <5 human tissues and similar to <15 human similarity proteins outside the target family) (50). The degree is the total number of interacting proteins of a given target, and neighborhood connectivity of a given target denotes the average number of human interacting proteins of its own neighbors/interacting proteins. These and other studies have shown that the target-interacting protein information is essential for facilitating the pharmacological, druggability and clinical investigations of therapeutic targets.
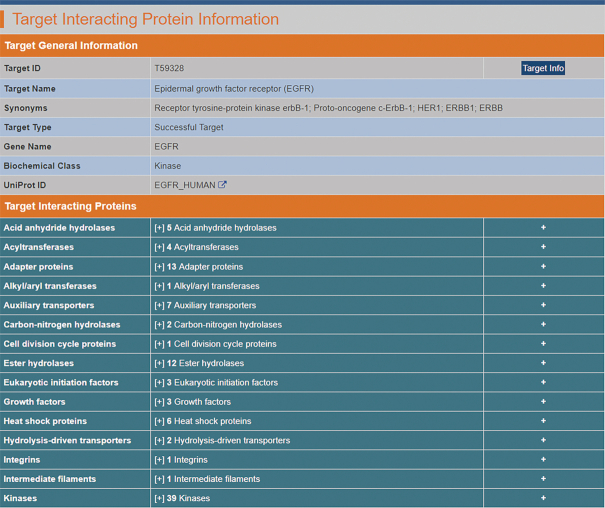
The target-interacting protein data were collected from BioGrid, which provides experimentally documented human protein physical interactions (62) based on evidence from such experimental methods as affinity capture-western blotting, colocalization studies, yeast two-hybrid screens, fluorescence resonance energy transfer, protein-fragment complementation assays, reconstituted complexes, far western blotting, proximity label-MS, and so on. Only the target-interacting proteins validated by no less than two experiments were collected, and the numbers of papers describing the experimental evidence were directly used as the evidence score (E-score). Overall, there are 139 successful, 276 clinical trial, 86 patent-recorded and 398 literature-reported targets with 2458 interacting proteins in the TTD, involving 6975 pairs of target-protein interactions. Moreover, due to the high number of target-interacting proteins (32.8% of the targets in the TTD interact with >10 proteins), it was difficult to produce a complete interaction profile of a target by simply crosslinking to other protein interaction databases. Thus, the target-interacting proteins are provided in groups of biochemical classes (GPCR, peptidase, virus penetration channel, etc.) on the webpage (Figure 4), and the target-interacting proteins within each class are listed with respect to their E-scores.
Figure 4.
A typical page in the TTD providing information about target-interacting proteins. The target-interacting proteins are provided in groups of biochemical classes (GPCR, peptidase, virus penetration channel, etc.) on a webpage, and the target-interacting proteins within each class are listed with respect to their E-scores. Detailed information of each interacting protein or target can also be found by clicking the ‘Interacting Protein Info’ or ‘Target Info’ button.
PATENTED THERAPEUTIC AGENTS AND THEIR TARGETS
Patented therapeutic agents represent special classes of bioactive molecules in the stage of early drug discovery. These agents differ from other bioactive molecules with respect to the perception of high development potential by drug developers, which makes them good indicators of drug development trends, emerging/evolving molecular landscapes and collaborative opportunities in the early developmental stage (34,35). Thus, there is a particular need for expanding the coverage of the target and drug data to cover the patented therapeutic agents and their targets. The targets of patented therapeutic agents are referred to here as the patent-recorded targets.
The patented therapeutic agents and their corresponding targets were searched and processed by the following procedure: first, all papers published during the period of 2004–2018 in Expert Opinion on Therapeutic Patents were manually reviewed; second, those key data describing the collected agents together with their targets were recorded, which included the following: (i) the title, abstract, applicants, issued ID and patent agency, (ii) potential therapeutic indications, 3D structures, and compound classes of patented agents and (iii) experimental binding activities (if available) between patented agents and their corresponding targets. In total, 5059 patented agents (belonging to 571 compound classes) included in 3145 patents issued by very diverse intellectual property authorities (World Intellectual Property Organization, United States Patent and Trademark Office, European Patent Office, National Intellectual Property Administration of China, etc.) were collected. Based on a fingerprint-based Tanimoto search, 86.7% of the patented agents collected in the TTD are different from, and 85.4% of the agents are of remote to intermediate similarity to, the patented agents in the databases with explicit target data (17). Therefore, the TTD complements the available database in collectively providing comprehensive information about the patented agents.
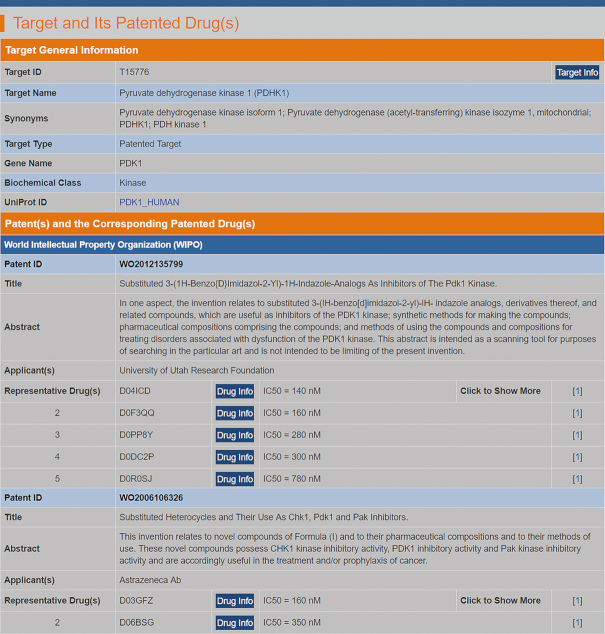
The TTD patented agents target 215 successful, 236 clinical trial and 207 patent-recorded targets. Among those patented agents, 4774 include 2D/3D structures provided in corresponding papers. These structures are manually drawn using ChemDraw (63) and can be viewed and downloaded from the TTD. Moreover, 3388 of the 5,059 agents include experimental target binding activity data and 2215 of the 5059 agents were mapped with PubChem IDs (11) according to a structural search (Tanimoto coefficient (64) between molecular fingerprints equals to one) and a subsequent visual inspection of all matches. The remaining nonmatched agents were not found in PubChem. A typical webpage of the patented agents in the TTD is shown in Figure 5.
Figure 5.
A typical page in the TTD providing information about the targets of patented therapeutic agents. The information included the patent details (patent office, title, abstract, etc.), representative patented drugs, and their corresponding activities with respect to their targets. Detailed information of each drug or target can also be found by clicking the ‘Drug Info’ or ‘Target Info’ button.
THE INTERNATIONAL CLASSIFICATION OF DISEASE ICD-11 CODES
The International Classification of Diseases (ICD) is a health statistics and diagnostics coding tool for describing human disease conditions (65), and it has been applied to other applications such as the development of artificial intelligence diagnostic tools (66). The 11th revision of ICD (ICD-11) launched in 2018 (65), which accomplished substantial improvement upon its previous version (ICD-10), with 55 000 unique codes compared with 14 400 for ICD-10. In addition to the more detailed description of the disease conditions, there are significant changes in the coding system. These include new code chapters for sexual health and traditional medicines, stroke being listed as a neurological disorder instead of a circulatory disorder, allergies being coded under immune system disease, and HIV infection being described as a chronic condition. Due to the significant changes in ICD codes, the TTD was updated with ICD-11 codes while retaining the original ICD-9 and ICD-10 codes that were introduced in previous TTD versions (7,27,67). Users can use a pull-down manual to search TTD data by means of an ICD-11 code menu.
SPECIAL CLASSES OF TARGETS OF IMMUNOTHERAPY AGENTS
New classes of immunotherapy agents have emerged. One class is CAR T-cell therapy, which is a form of immunotherapy that uses specially altered T-cells to treat cancer (68). A sample of a patient's T cells, collected from the blood, are modified to produce chimeric antigen receptors (CARs) on their surfaces. When these CAR-T cells are reinfused into patients, the new receptors on these cells enable them to bind to specific antigens on the patients’ cancer cells, thereby killing them. Knowledge regarding the target antigens of clinically used or tested CAR-T therapy is thus very useful for the study and discovery of this special class of agent. Therefore, the antigens of approved and clinical trial CAR-T therapies were systematically searched based on the following procedures: first, approved CAR-T therapies were collected from the February issue of Nature Reviews Drug Discovery (69), and the CAR-T therapies in clinical trials were obtained from the official websites or recent reports of 122 pharmaceutical companies/research institutes/hospitals; second, the clinical status of each CAR-T therapy was further validated by the ClinicalTrials.gov (70), and the corresponding disease indications and NCT numbers were confirmed; third, the target antigen of each CAR-T therapy was identified using ClinicalTrials.gov and PubMed. In total, 2 approved, 4 phase III, 166 phase II and 187 phase I CAR-T therapies for treating of 92 diseases were collected, which targeted 17 successful and 35 clinical trial targets in the TTD.
The second class of immunotherapy agents include small molecular and peptidomimetic immune checkpoint inhibitors (71). Although the monoclonal antibody immune checkpoint inhibitors are highly successful in cancer treatment, they present such problems as difficulty in penetrating into tumors and the need for slow intravenous infusion treatment (72). These problems may be partially overcome by the introduction of small molecule drugs. Successful efforts have been directed at discovering small molecular and peptidomimetic immune checkpoint inhibitors (71). Thus, PubMed was systematically searched using such combinations as the keywords ‘small molecular inhibitor’/‘small molecular agent’/‘peptidomimetic agent’/‘peptidomimetics’/‘peptidomimetic inhibitors’ and ‘immune checkpoint’. In total, 2 clinical trial, 121 patented, 2 preclinical, and 56 investigative immunotherapies for treating five classes of disease were collected.
ACCESS FACILITIES FOR THE NEWLY ADDED FEATURES
The addition of new information has made the TTD webpages too crowded, which hinders the ability of users to find the preferred information. To resolve this problem, the TTD target webpage was redesigned by categorizing the information into multiple groups: (i) target general information, (ii) drugs and their modes of actions, (iii) target regulators, (iv) target profiles in patients, (v) target affiliated pathways and (vi) target-related models and studies (Figure 6). The information in each group is itemized within a small block on the webpage, and users can access detailed information by clicking each item. The newly added features of target regulators (miRNA and TF), target-interacting proteins and the targets of patented therapeutic agents can be accessed via the ‘Target Group’ manual bar, and the small molecular and peptidomimetic inhibitors of immunotherapy targets and the patented therapeutic agents are provided under the ‘Drug Group’ manual bar. Figure 1 provides a typical page in the TTD describing the information about target-regulating miRNAs. Figure 2 shows the page illustrating the data regarding target-interacting proteins. Figure 3 exhibits the page showing the data of patented therapeutic agents together with their corresponding targets. The database platform Drupal was employed in the TTD to enhance data storage and extraction. Extensively accelerated data access and transmission were achieved via the cloud platform of Aliyun located in Silicon Valley.
Figure 6.
A redesigned TTD target webpage categorizing the information into the groups of (i) target general information, (ii) drugs and modes of action, (iii) target regulators, (iv) target profiles in patients, (v) target affiliated pathways and (vi) target-related models and studies.
CONCLUDING REMARKS
Intensive drug research and development efforts have led to extensively expanded knowledge of biological systems (73,74), disease processes (75,76) and the mechanisms of targeted therapeutics (77–79). The expanded knowledge has facilitated the successful development of new therapies (68), and it will further facilitate the discoveries of such therapeutic approaches as polypharmacology (80) and RNA therapeutics (81). For better serving the drug discovery communities, additional efforts have been directed at further enriching the TTD and other established databases with comprehensive information about drugs, targets, and their regulation.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Key R&D Program of China [2018YFC0910500]; National Natural Science Foundation of China [81872798]; Singapore Academic Research Fund grant [R-148-000-273-114]; Fundamental Research Funds for Central Universities [2018CDQYSG0007, 2018QNA7023, CDJZR14468801, 10611CDJXZ238826]; Innovation Project on Industrial Generic Key Technologies of Chongqing [cstc2015zdcy-ztzx120003]. Funding for open access charge: National Key R & D Program of China [2018YFC0910500]; National Natural Science Foundation of China [81872798].
Conflict of interest statement. None declared.
REFERENCES
- 1. Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., Karlsson A., Al-Lazikani B., Hersey A., Oprea T.I. et al.. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017; 16:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munos B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009; 8:959–968. [DOI] [PubMed] [Google Scholar]
- 3. Lee A., Lee K., Kim D.. Using reverse docking for target identification and its applications for drug discovery. Expert Opin. Drug Discov. 2016; 11:707–715. [DOI] [PubMed] [Google Scholar]
- 4. Waring M.J., Arrowsmith J., Leach A.R., Leeson P.D., Mandrell S., Owen R.M., Pairaudeau G., Pennie W.D., Pickett S.D., Wang J. et al.. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015; 14:475–486. [DOI] [PubMed] [Google Scholar]
- 5. Keseru G.M., Makara G.M.. The influence of lead discovery strategies on the properties of drug candidates. Nat. Rev. Drug Discov. 2009; 8:203–212. [DOI] [PubMed] [Google Scholar]
- 6. Zheng C.J., Han L.Y., Yap C.W., Ji Z.L., Cao Z.W., Chen Y.Z.. Therapeutic targets: progress of their exploration and investigation of their characteristics. Pharmacol. Rev. 2006; 58:259–279. [DOI] [PubMed] [Google Scholar]
- 7. Yang H., Qin C., Li Y.H., Tao L., Zhou J., Yu C.Y., Xu F., Chen Z., Zhu F., Chen Y.Z.. Therapeutic target database update 2016: enriched resource for bench to clinical drug target and targeted pathway information. Nucleic Acids Res. 2016; 44:D1069–D1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendez D., Gaulton A., Bento A.P., Chambers J., De Veij M., Felix E., Magarinos M.P., Mosquera J.F., Mutowo P., Nowotka M. et al.. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 2019; 47:D930–D940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z. et al.. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018; 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papadatos G., Davies M., Dedman N., Chambers J., Gaulton A., Siddle J., Koks R., Irvine S.A., Pettersson J., Goncharoff N. et al.. SureChEMBL: a large-scale, chemically annotated patent document database. Nucleic Acids Res. 2016; 44:D1220–D1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B. et al.. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019; 47:D1102–D1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C., Hu G., Wang K., Brylinski M., Xie L., Kurgan L.. PDID: database of molecular-level putative protein-drug interactions in the structural human proteome. Bioinformatics. 2016; 32:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Q., Zhang Y., Cui H., Chen L., Zhao Y., Lin Y., Zhang M., Xie L.. dbDEPC 3.0: the database of differentially expressed proteins in human cancer with multi-level annotation and drug indication. Database (Oxford). 2018; 2018:bay015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H., He Y., Ding G., Wang C., Xie L., Li Y.. dbDEPC: a database of differentially expressed proteins in human cancers. Nucleic Acids Res. 2010; 38:D658–D664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harding S.D., Sharman J.L., Faccenda E., Southan C., Pawson A.J., Ireland S., Gray A.J.G., Bruce L., Alexander S.P.H., Anderton S. et al.. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018; 46:D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ursu O., Holmes J., Bologa C.G., Yang J.J., Mathias S.L., Stathias V., Nguyen D.T., Schurer S., Oprea T.. DrugCentral 2018: an update. Nucleic Acids Res. 2019; 47:D963–D970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilson M.K., Liu T., Baitaluk M., Nicola G., Hwang L., Chong J.. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016; 44:D1045–D1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karagkouni D., Paraskevopoulou M.D., Chatzopoulos S., Vlachos I.S., Tastsoglou S., Kanellos I., Papadimitriou D., Kavakiotis I., Maniou S., Skoufos G. et al.. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018; 46:D239–D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H.. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou C.H., Shrestha S., Yang C.D., Chang N.W., Lin Y.L., Liao K.W., Huang W.C., Sun T.H., Tu S.J., Lee W.H. et al.. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018; 46:D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rangasamy S.B., Jana M., Roy A., Corbett G.T., Kundu M., Chandra S., Mondal S., Dasarathi S., Mufson E.J., Mishra R.K. et al.. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer's pathology. J. Clin. Invest. 2018; 128:4297–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z.P., Wu C., Miao H., Wu H.. RegNetwork: an integrated database of transcriptional and post-transcriptional regulatory networks in human and mouse. Database (Oxford). 2015; 2015:bav095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M.. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019; 47:D590–D595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim P., Cheng F., Zhao J., Zhao Z.. ccmGDB: a database for cancer cell metabolism genes. Nucleic Acids Res. 2016; 44:D959–D968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Southan C., Sharman J.L., Benson H.E., Faccenda E., Pawson A.J., Alexander S.P., Buneman O.P., Davenport A.P., McGrath J.C., Peters J.A. et al.. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016; 44:D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gohlke B.O., Nickel J., Otto R., Dunkel M., Preissner R.. CancerResource–updated database of cancer-relevant proteins, mutations and interacting drugs. Nucleic Acids Res. 2016; 44:D932–D937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y.H., Yu C.Y., Li X.X., Zhang P., Tang J., Yang Q., Fu T., Zhang X., Cui X., Tu G. et al.. Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018; 46:D1121–D1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koscielny G., An P., Carvalho-Silva D., Cham J.A., Fumis L., Gasparyan R., Hasan S., Karamanis N., Maguire M., Papa E. et al.. Open Targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 2017; 45:D985–D994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carvalho-Silva D., Pierleoni A., Pignatelli M., Ong C., Fumis L., Karamanis N., Carmona M., Faulconbridge A., Hercules A., McAuley E. et al.. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res. 2019; 47:D1056–D1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finan C., Gaulton A., Kruger F.A., Lumbers R.T., Shah T., Engmann J., Galver L., Kelley R., Karlsson A., Santos R. et al.. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 2017; 9:eaag1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao S., Iyengar R.. Systems pharmacology: network analysis to identify multiscale mechanisms of drug action. Annu. Rev. Pharmacol. Toxicol. 2012; 52:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia J., Zhu F., Ma X., Cao Z., Cao Z.W., Li Y., Li Y.X., Chen Y.Z.. Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov. 2009; 8:111–128. [DOI] [PubMed] [Google Scholar]
- 33. Csermely P., Agoston V., Pongor S.. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol. Sci. 2005; 26:178–182. [DOI] [PubMed] [Google Scholar]
- 34. Shaabani S., Huizinga H.P.S., Butera R., Kouchi A., Guzik K., Magiera-Mularz K., Holak T.A., Domling A.. A patent review on PD-1/PD-L1 antagonists: small molecules, peptides, and macrocycles (2015-2018). Expert Opin. Ther. Pat. 2018; 28:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grandjean N., Charpiot B., Pena C.A., Peitsch M.C.. Competitive intelligence and patent analysis in drug discovery. Drug Discov. Today Technol. 2005; 2:211–215. [DOI] [PubMed] [Google Scholar]
- 36. Zhavoronkov A., Ivanenkov Y.A., Aliper A., Veselov M.S., Aladinskiy V.A., Aladinskaya A.V., Terentiev V.A., Polykovskiy D.A., Kuznetsov M.D., Asadulaev A. et al.. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 2019; 37:1038–1040. [DOI] [PubMed] [Google Scholar]
- 37. Rukov J.L., Shomron N.. MicroRNA pharmacogenomics: post-transcriptional regulation of drug response. Trends Mol. Med. 2011; 17:412–423. [DOI] [PubMed] [Google Scholar]
- 38. Mohri T., Nakajima M., Takagi S., Komagata S., Yokoi T.. MicroRNA regulates human vitamin D receptor. Int. J. Cancer. 2009; 125:1328–1333. [DOI] [PubMed] [Google Scholar]
- 39. Mishra P.J., Song B., Mishra P.J., Wang Y., Humeniuk R., Banerjee D., Merlino G., Ju J., Bertino J.R.. MiR-24 tumor suppressor activity is regulated independent of p53 and through a target site polymorphism. PLoS One. 2009; 4:e8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pichler M., Calin G.A.. MicroRNAs in cancer: from developmental genes in worms to their clinical application in patients. Br. J. Cancer. 2015; 113:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uhlmann S., Mannsperger H., Zhang J.D., Horvat E.A., Schmidt C., Kublbeck M., Henjes F., Ward A., Tschulena U., Zweig K. et al.. Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Mol. Syst. Biol. 2012; 8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morquette B., Juzwik C.A., Drake S.S., Charabati M., Zhang Y., Lecuyer M.A., Galloway D.A., Dumas A., de Faria Junior O., Paradis-Isler N. et al.. MicroRNA-223 protects neurons from degeneration in experimental autoimmune encephalomyelitis. Brain. 2019; 142:2979–2995. [DOI] [PubMed] [Google Scholar]
- 43. Chandrasekaran A.R., MacIsaac M., Dey P., Levchenko O., Zhou L., Andres M., Dey B.K., Halvorsen K.. Cellular microRNA detection with miRacles: microRNA- activated conditional looping of engineered switches. Sci. Adv. 2019; 5:eaau9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Androvic P., Valihrach L., Elling J., Sjoback R., Kubista M.. Two-tailed RT-qPCR: a novel method for highly accurate miRNA quantification. Nucleic Acids Res. 2017; 45:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Franchini P., Xiong P., Fruciano C., Schneider R.F., Woltering J.M., Hulsey C.D., Meyer A.. MicroRNA gene regulation in extremely young and parallel adaptive radiations of crater lake cichlid fish. Mol. Biol. Evol. 2019; 36:2498–2511. [DOI] [PubMed] [Google Scholar]
- 46. Chatr-Aryamontri A., Oughtred R., Boucher L., Rust J., Chang C., Kolas N.K., O’Donnell L., Oster S., Theesfeld C., Sellam A. et al.. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 2017; 45:D369–D379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salwinski L., Miller C.S., Smith A.J., Pettit F.K., Bowie J.U., Eisenberg D.. The database of interacting proteins: 2004 update. Nucleic Acids Res. 2004; 32:D449–D451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heguy A., Stewart A.A., Haley J.D., Smith D.E., Foulkes J.G.. Gene expression as a target for new drug discovery. Gene Expr. 1995; 4:337–344. [PMC free article] [PubMed] [Google Scholar]
- 49. Dang C.V., Reddy E.P., Shokat K.M., Soucek L.. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer. 2017; 17:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kessler D., Gmachl M., Mantoulidis A., Martin L.J., Zoephel A., Mayer M., Gollner A., Covini D., Fischer S., Gerstberger T. et al.. Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. U.S.A. 2019; 116:15823–15829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wingender E. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief. Bioinform. 2008; 9:326–332. [DOI] [PubMed] [Google Scholar]
- 52. Matys V., Kel-Margoulis O.V., Fricke E., Liebich I., Land S., Barre-Dirrie A., Reuter I., Chekmenev D., Krull M., Hornischer K. et al.. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006; 34:D108–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawauchi J., Zhang C., Nobori K., Hashimoto Y., Adachi M.T., Noda A., Sunamori M., Kitajima S.. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J. Biol. Chem. 2002; 277:39025–39034. [DOI] [PubMed] [Google Scholar]
- 54. Wei C.L., Wu Q., Vega V.B., Chiu K.P., Ng P., Zhang T., Shahab A., Yong H.C., Fu Y., Weng Z. et al.. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006; 124:207–219. [DOI] [PubMed] [Google Scholar]
- 55. Neish A.S., Read M.A., Thanos D., Pine R., Maniatis T., Collins T.. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol. Cell Biol. 1995; 15:2558–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gagniuc P., Ionescu-Tirgoviste C.. Gene promoters show chromosome-specificity and reveal chromosome territories in humans. BMC Genomics. 2013; 14:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pennacchio L.A., Bickmore W., Dean A., Nobrega M.A., Bejerano G.. Enhancers: five essential questions. Nat. Rev. Genet. 2013; 14:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maston G.A., Evans S.K., Green M.R.. Transcriptional regulatory elements in the human genome. Annu. Rev. Genomics Hum. Genet. 2006; 7:29–59. [DOI] [PubMed] [Google Scholar]
- 59. Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008; 4:682–690. [DOI] [PubMed] [Google Scholar]
- 60. Yao L., Rzhetsky A.. Quantitative systems-level determinants of human genes targeted by successful drugs. Genome Res. 2008; 18:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y.H., Li X.X., Hong J.J., Wang Y.X., Fu J.B., Yang H., Yu C.Y., Li F.C., Hu J., Xue W.W. et al.. Clinical trials, progression-speed differentiating features and swiftness rule of the innovative targets of first-in-class drugs. Brief. Bioinform. 2019; doi:10.1093/bib/bby130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oughtred R., Stark C., Breitkreutz B.J., Rust J., Boucher L., Chang C., Kolas N., O’Donnell L., Leung G., McAdam R. et al.. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019; 47:D529–D541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cousins K.R. Computer review of ChemDraw ultra 12.0. J. Am. Chem. Soc. 2011; 133:8388. [DOI] [PubMed] [Google Scholar]
- 64. Bajusz D., Racz A., Heberger K.. Why is Tanimoto index an appropriate choice for fingerprint-based similarity calculations. J. Cheminform. 2015; 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lancet T. ICD-11. Lancet. 2019; 393:2275. [DOI] [PubMed] [Google Scholar]
- 66. Miotto R., Li L., Kidd B.A., Dudley J.T.. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci. Rep. 2016; 6:26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu F., Shi Z., Qin C., Tao L., Liu X., Xu F., Zhang L., Song Y., Liu X., Zhang J. et al.. Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012; 40:D1128–D1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. et al.. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014; 6:224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mullard A. 2017 FDA drug approvals. Nat. Rev. Drug Discov. 2018; 17:150. [DOI] [PubMed] [Google Scholar]
- 70. Zarin D.A., Tse T., Williams R.J., Carr S.. Trial reporting in ClinicalTrials.gov - the final rule. N. Engl. J. Med. 2016; 375:1998–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang T., Wu X., Guo C., Zhang K., Xu J., Li Z., Jiang S.. Development of inhibitors of the programmed cell death-1/programmed cell death-ligand 1 signaling pathway. J. Med. Chem. 2019; 62:1715–1730. [DOI] [PubMed] [Google Scholar]
- 72. Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012; 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tomaselli D., Lucidi A., Rotili D., Mai A.. Epigenetic polypharmacology: a new frontier for epi-drug discovery. Med. Res. Rev. 2019; doi:10.1002/med.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li X.X., Yin J., Tang J., Li Y., Yang Q., Xiao Z., Zhang R., Wang Y., Hong J., Tao L. et al.. Determining the balance between drug efficacy and safety by the network and biological system profile of its therapeutic target. Front. Pharmacol. 2018; 9:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hiatt W.R., Armstrong E.J., Larson C.J., Brass E.P.. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ. Res. 2015; 116:1527–1539. [DOI] [PubMed] [Google Scholar]
- 76. Yang Q., Li B., Tang J., Cui X., Wang Y., Li X., Hu J., Chen Y., Xue W., Lou Y. et al.. Consistent gene signature of schizophrenia identified by a novel feature selection strategy from comprehensive sets of transcriptomic data. Brief. Bioinform. 2019; doi:10.1093/bib/bbz049. [DOI] [PubMed] [Google Scholar]
- 77. Johnson C.W., Lin Y.J., Reid D., Parker J., Pavlopoulos S., Dischinger P., Graveel C., Aguirre A.J., Steensma M., Haigis K.M. et al.. Isoform-specific destabilization of the active site reveals a molecular mechanism of intrinsic activation of KRas G13D. Cell Rep. 2019; 28:1538–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xue W., Yang F., Wang P., Zheng G., Chen Y., Yao X., Zhu F.. What contributes to serotonin-norepinephrine reuptake inhibitors' dual-targeting mechanism? The key role of transmembrane domain 6 in human serotonin and norepinephrine transporters revealed by molecular dynamics simulation. ACS Chem. Neurosci. 2018; 9:1128–1140. [DOI] [PubMed] [Google Scholar]
- 79. Yin J., Sun W., Li F., Hong J., Li X., Zhou Y., Lu Y., Liu M., Zhang X., Chen N. et al.. VARIDT 1.0: variability of drug transporter database. Nucleic Acids Res. 2019; doi:10.1093/nar/gkz779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kuenzi B.M., Remsing Rix L.L., Stewart P.A., Fang B., Kinose F., Bryant A.T., Boyle T.A., Koomen J.M., Haura E.B., Rix U.. Polypharmacology-based ceritinib repurposing using integrated functional proteomics. Nat. Chem. Biol. 2017; 13:1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou J., Rossi J.. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017; 16:181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.