Abstract
PURPOSE
A phase II study (ClinicalTrials.gov identifier: NCT00628251) showed activity of olaparib capsules versus pegylated liposomal doxorubicin in patients with germline BRCA-mutated platinum-resistant or partially platinum-sensitive relapsed ovarian cancer. We conducted a phase III trial (SOLO3) of olaparib tablets versus nonplatinum chemotherapy in patients with germline BRCA-mutated platinum-sensitive relapsed ovarian cancer who had received at least 2 prior lines of platinum-based chemotherapy.
PATIENTS AND METHODS
In this randomized, open-label trial, patients were randomly assigned 2:1 to olaparib 300 mg twice a day or physician’s choice single-agent nonplatinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). The primary end point was objective response rate (ORR) in the measurable disease analysis set assessed by blinded independent central review (BICR). The key secondary end point was progression-free survival (PFS) assessed by BICR in the intent-to-treat population.
RESULTS
Of 266 randomly assigned patients, 178 were assigned to olaparib and 88 to chemotherapy. In patients with measurable disease (olaparib, n = 151; chemotherapy, n = 72), the BICR-assessed ORR was significantly higher with olaparib than with chemotherapy (72.2% v 51.4%; odds ratio [OR], 2.53 [95% CI, 1.40 to 4.58]; P = .002). In the subgroup who had received 2 prior lines of treatment, the ORR was 84.6% with olaparib and 61.5% with chemotherapy (OR, 3.44 [95% CI, 1.42 to 8.54]). BICR-assessed PFS also significantly favored olaparib versus chemotherapy (hazard ratio, 0.62 [95% CI, 0.43 to 0.91]; P = .013; median, 13.4 v 9.2 months). Adverse events were consistent with the established safety profiles of olaparib and chemotherapy.
CONCLUSION
Olaparib resulted in statistically significant and clinically relevant improvements in ORR and PFS compared with nonplatinum chemotherapy in patients with germline BRCA-mutated platinum-sensitive relapsed ovarian cancer who had received at least 2 prior lines of platinum-based chemotherapy.
INTRODUCTION
Olaparib is a poly (ADP-ribose) polymerase (PARP) inhibitor that traps PARP onto DNA at sites of single-strand breaks, preventing their repair and generating double-strand breaks that cannot be repaired accurately in tumors harboring defects in homologous recombination repair, such as BRCA1 or BRCA2 mutations (BRCAm), leading to an accumulation of DNA damage and tumor cell death.1 In a pooled analysis2 of phase I3-5 and II6-9 trials, a durable response to olaparib capsules 400 mg twice a day administered as treatment, rather than maintenance therapy, occurred in women with heavily pretreated relapsed ovarian cancer and a germline BRCAm. The objective response rate (ORR) was 42% in the subgroup with platinum-sensitive disease who had received at least 3 prior chemotherapy regimens.2 Olaparib gained approval in the United States for treating women with germline BRCAm advanced ovarian cancer who have received 3 or more prior lines of chemotherapy,10 with a confirmatory trial mandated.
Single-agent nonplatinum chemotherapy is often used in heavily pretreated women with relapsed ovarian cancer, because a survival advantage with platinum-based chemotherapy has been shown only in first- and second-line treatment settings.11,12 Study 12 compared olaparib capsules 200 or 400 mg twice a day with pegylated liposomal doxorubicin (PLD) in patients with platinum-resistant or partially platinum-sensitive relapsed ovarian cancer and a germline BRCAm.7 Median progression-free survival (PFS; primary end point) was 8.8 versus 7.1 months with olaparib 400 mg twice a day and PLD (hazard ratio [HR], 0.86 [95% CI, 0.45 to 1.62]; P = .63), and the ORR was 31% versus 18% (P = .11).7 However, higher activity for olaparib versus PLD was observed in the subgroup of patients with partially platinum-sensitive disease (n = 34; ORR, 47% v 26%; median PFS, 9.2 v 7.4 months; data on file; Study D0810C00012). PLD seemed to have greater efficacy in Study 12 than previously reported in patients with unknown BRCAm status.13
We conducted the confirmatory phase III SOLO3 study to evaluate whether olaparib tablet monotherapy improves outcomes compared with physician’s choice single-agent nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCAm who have received at least 2 prior lines of platinum-based chemotherapy.
PATIENTS AND METHODS
Study Design and Participants
SOLO3 is a randomized, controlled, open-label, phase III trial conducted in 13 countries. Eligible patients were ≥ 18 years of age and had relapsed high-grade serous or high-grade endometrioid ovarian cancer, primary peritoneal cancer, and/or fallopian tube cancer, with at least 1 lesion (measurable and/or nonmeasurable) that could be accurately assessed at baseline by computed tomography/magnetic resonance imaging and was suitable for repeated assessment. Patients had a deleterious or suspected deleterious germline BRCAm and an Eastern Cooperative Oncology Group performance status of 0-2. Patients had received at least 2 prior lines of platinum-based chemotherapy for ovarian cancer and were partially platinum sensitive (progression 6-12 months after the end of the last platinum-based regimen) or platinum sensitive (progression > 12 months after the end of the last platinum-based regimen).
Secondary to challenges in patient recruitment resulting from the entry of PARP inhibitors to routine clinical practice, the target number of randomly assigned patients was amended from 411 to 250, and the primary end point was amended from PFS to ORR on September 29, 2017. The trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and the AstraZeneca policy of bioethics,14 under the auspices of an independent data-monitoring committee. AstraZeneca was responsible for overseeing the collection, analysis, and interpretation of the data. All patients provided written informed consent. Full eligibility criteria and the protocol are provided in the Data Supplement (online only).
Random Assignment and Procedures
Patients were randomly assigned 2:1 to olaparib tablets 300 mg twice a day or to physician’s choice of single-agent chemotherapy: PLD 50 mg/m2 on day 1 every 4 weeks; paclitaxel 80 mg/m2 on days 1, 8, 15, and 22 every 4 weeks; gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 4 weeks; or topotecan 4 mg/m2 on days 1, 8, and 15 every 4 weeks. The investigator made his/her chemotherapy choice before random assignment. Random assignment was performed using an interactive voice and Web response system, and it used a block design with stratification according to the selected chemotherapy (PLD v paclitaxel v gemcitabine v topotecan), the number of prior lines of chemotherapy for ovarian cancer (2 or 3 v ≥ 4), and the time to disease progression after the end of the last platinum-based chemotherapy regimen (6-12 months v > 12 months).
Study treatment continued until objective radiologic disease progression (Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1 criteria) or until the patient experienced unacceptable toxicity or met other discontinuation criteria (Data Supplement). After study treatment discontinuation, patients could receive the most appropriate anticancer treatment (as defined by the investigator; Data Supplement). Computed tomography or magnetic resonance imaging was performed at baseline, then at 8-week intervals for 48 weeks and then every 12 weeks. Health-related quality of life (HRQoL) was assessed using the Trial Outcome Index (TOI) of the Functional Assessment of Cancer Therapy–Ovarian Cancer questionnaire. These questionnaires were completed at baseline and at day 29 and then every 8 weeks up to week 48.
Outcomes
The primary end point was ORR as assessed by blinded independent central review (BICR) in the measurable disease analysis set (MDAS) using RECIST version 1.1. Sensitivity analysis of ORR used investigator assessment.
Secondary end points were PFS as assessed by BICR; investigator-assessed PFS; time from random assignment to second progression or death (PFS2); overall survival (OS); time from random assignment to first subsequent therapy or death (TFST); time to earliest progression by RECIST or cancer antigen-125 (CA-125) or death (Data Supplement); and time from random assignment to study treatment discontinuation or death (TDT). The HRQoL analysis evaluated the change from baseline in the TOI score for the first 48 weeks. TOI scores range from 0 to 100, with higher scores indicating better HRQoL; a difference of 10 points indicates a clinically significant difference15,16 (Data Supplement). Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
A minimum of 250 patients was needed, given the anticipation that approximately 90% of patients would have measurable disease at baseline according to BICR. At least 223 patients would give the trial > 80% power at a 2-sided significance level of 5% to show a statistically significant difference in ORR, assuming a response rate of 25% with chemotherapy and at least 45% with olaparib in patients with BICR-confirmed measurable disease at baseline. The primary ORR analysis occurred 6 months after the last patient received the treatment.
ORR was analyzed in patients with measurable disease at baseline (MDAS). PFS and all other efficacy and HRQoL data were summarized and analyzed in the intent-to-treat population (all randomly assigned patients), and safety data were summarized in the safety analysis set (all patients receiving at least 1 dose of randomly assigned treatment).
A multiple testing procedure was used to control the type I error, with ORR tested first, PFS tested if the null hypothesis for ORR was rejected, PFS2 tested if the null hypothesis for both ORR and PFS was rejected, and OS tested if the null hypothesis for ORR, PFS, and PFS2 was rejected (Data Supplement). The ORR analysis used stratified logistic regression, with the odds ratio (OR), accompanying 95% CI, and 2-sided P value calculated. The PFS, PFS2, OS, TFST, time to earliest progression by RECIST or CA-125 or death, and TDT analyses used a stratified log-rank test, with the HR, accompanying 95% CI, and 2-sided P value calculated. A linear mixed-effect model was used to calculate the change from baseline in TOI score (Data Supplement).
RESULTS
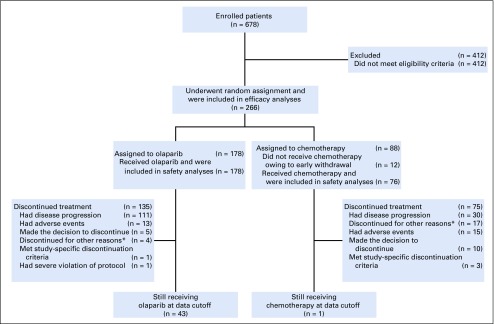
From February 24, 2015, to May 15, 2018, 266 patients underwent random assignment (Fig 1 and Data Supplement). Of the 178 patients randomly assigned to olaparib and 88 to chemotherapy (PLD, n = 47; paclitaxel, n = 20; gemcitabine, n = 13; topotecan, n = 8; intent-to-treat population), all 178 patients in the olaparib group and 76 in the chemotherapy group received treatment (safety analysis set). Twelve women (13.6%) withdrew consent before receiving chemotherapy. The primary analysis population was composed of 151 patients (84.8%) in the olaparib group and 72 (81.8%) in the chemotherapy group with measurable disease by BICR at baseline. At primary analysis data cutoff (October 10, 2018), 43 patients in the olaparib group and 1 patient in the chemotherapy group were still receiving treatment.
FIG 1.
Trial profile. The primary analysis was conducted in the measurable disease analysis set (olaparib, n = 151; placebo, n = 72). Six of the patients in the chemotherapy group did not receive chemotherapy because of early withdrawal. (*) Other reasons for discontinuation included clinical progression (n = 4, olaparib; n = 4, chemotherapy), investigator decision (n = 6, chemotherapy), and chemotherapy complete (n = 7, chemotherapy).
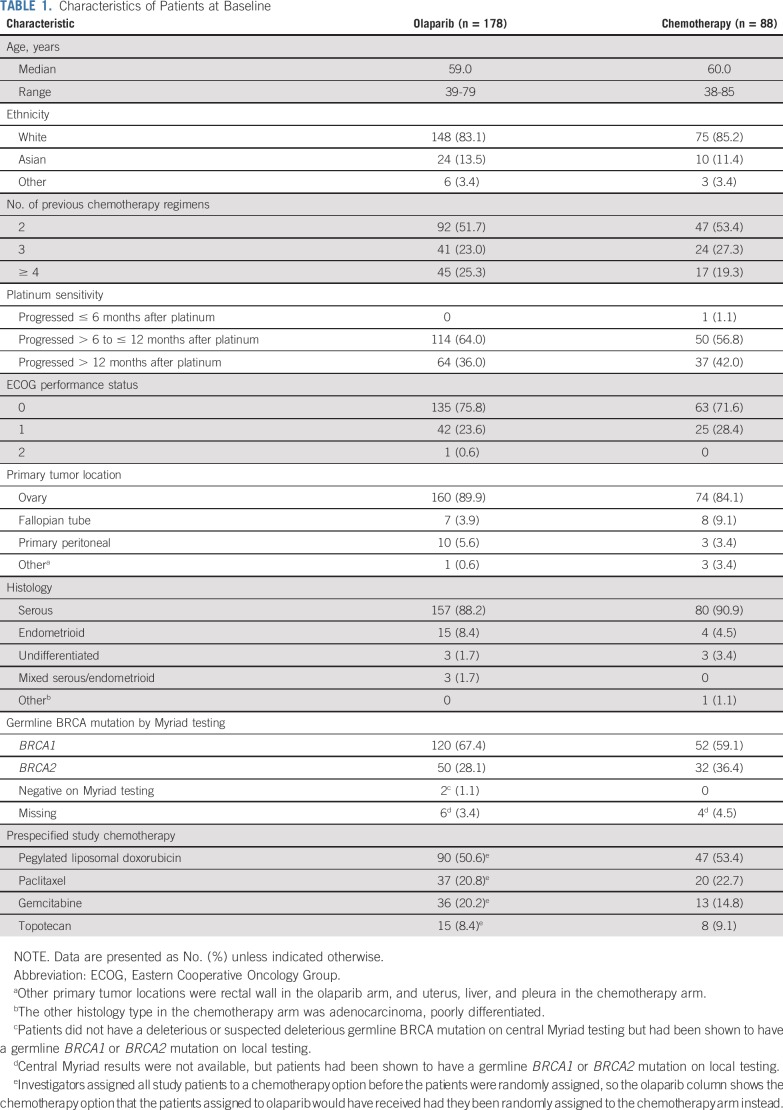
Baseline characteristics were generally well balanced between treatment arms (Table 1). At least one half of the patients had received only 2 prior lines of chemotherapy, approximately two thirds were partially platinum sensitive, and PLD was the preferred chemotherapy regimen.
TABLE 1.
Characteristics of Patients at Baseline
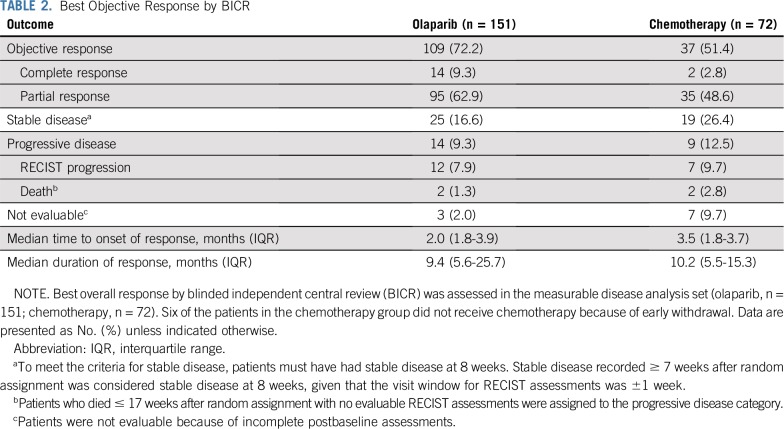
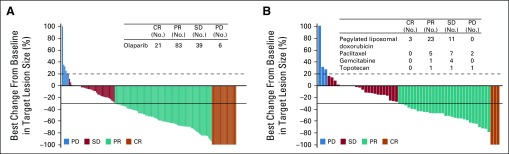
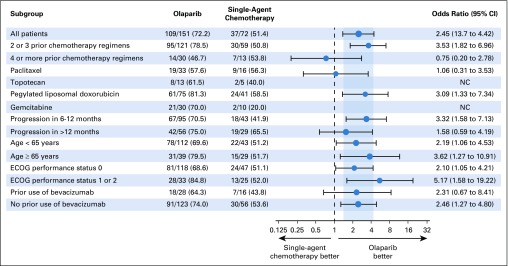
BICR-assessed ORR was significantly higher in the olaparib group than in the chemotherapy group (72.2% v 51.4%; OR 2.53 [95% CI, 1.40 to 4.58]; P = .002; Table 2 and Fig 2). ORR results were consistent in sensitivity analyses and across predefined subgroups, although patients receiving olaparib as third- or fourth-line therapy had a higher ORR than those receiving fifth-line or later olaparib (Fig 3 and Data Supplement). In a post hoc analysis, an objective response was seen in 66 (84.6%) of 78 olaparib patients and in 24 (61.5%) of 39 chemotherapy patients (OR, 3.44 [95% CI, 1.42 to 8.54]) in patients who had received 2 prior lines of treatment (52.5% of the MDAS), and in 43 (58.9%) of 73 olaparib patients and 13 (39.4%) of 33 chemotherapy patients (OR 2.21 [95% CI, 0.96 to 5.20]) in patients who had received ≥ 3 prior lines of therapy (47.5% of the MDAS). ORR was 58.5% in the PLD subgroup, 56.3% in the paclitaxel subgroup, 20.0% in the gemcitabine subgroup, and 40.0% in the topotecan subgroup (Fig 3 and Data Supplement).
TABLE 2.
Best Objective Response by BICR
FIG 2.
Best response for target lesions by patient in the olaparib and single-agent chemotherapy groups based on investigator assessment. (A) Best change from baseline in target lesion size in the olaparib group. (B) Best change from baseline in target lesion size in the single-agent chemotherapy group, with the accompanying table showing best response by chemotherapy agent. The best change from baseline in target lesion size is based on investigator assessment (RECIST version 1.1) in the measurable disease analysis set (olaparib, n = 160; chemotherapy; n = 78). Eleven patients in the olaparib group and 19 patients in the chemotherapy group were not evaluable. The dashed line represents the threshold for progressive disease (a ≥ 20% increase in the sum of diameters of target lesions), and the solid line represents the threshold for partial response (a ≥ 30% decrease in the sum of diameters of target lesions). CR, complete response; PD, disease progression, PR, partial response; SD, stable disease.
FIG 3.
Subgroup analysis of objective response rate. Objective response was assessed in the measurable disease analysis set on the basis of blinded independent central review. The analysis was performed using unadjusted logistic regression, including in all patients. For the odds ratios, the size of the circle is proportional to the number of responses, the vertical gray band represents the 95% CI for all patients, and the dashed line indicates the point of no effect. ECOG, Eastern Cooperative Oncology Group; NC, not calculated.
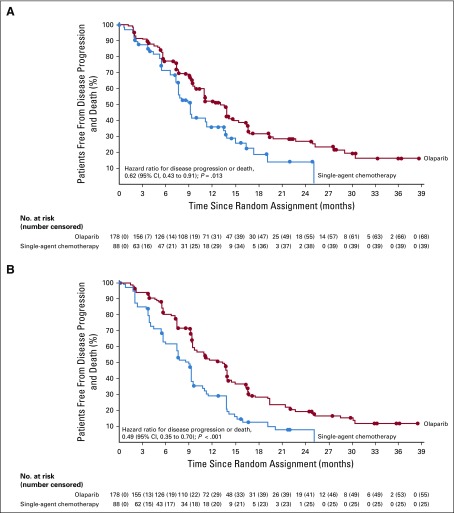
The risk of disease progression as assessed by BICR or death (proportion of patients with events [data maturity], 59.8%) was significantly reduced by 38% with olaparib versus chemotherapy (HR, 0.62 [95% CI 0.43 to 0.91]; P = .013), with a median PFS of 13.4 months for olaparib versus 9.2 months for chemotherapy (Fig 4A). The HR for investigator-assessed disease progression or death (data maturity, 69.9%) was 0.49 (95% CI, 0.35 to 0.70; P < .001), with a median PFS of 13.2 months for olaparib versus 8.5 months for chemotherapy (Fig 4B). The HR for PFS on the basis of BICR was 0.69 (95% CI, 0.48 to 1.00) in the prespecified subgroup of patients with measurable disease by BICR (Data Supplement).
FIG 4.
Kaplan-Meier estimates of progression-free survival. (A) Kaplan-Meier estimates of blinded independent central review-assessed disease progression or death in the olaparib group and the chemotherapy group. The median duration of follow-up for censored patients was 13.8 months (interquartile range [IQR], 7.51-22.08 months) in the olaparib group and 3.9 months (IQR, 0.03-12.75 months) in the chemotherapy group. (B) Kaplan-Meier estimates of investigator-assessed disease progression or death in the olaparib group and the chemotherapy group.
TFST and TDT were delayed, with a median TFST of 15.1 months for olaparib versus 10.2 months for chemotherapy (HR, 0.48 [95% CI, 0.33 to 0.71]; P < .001) and a median TDT of 13.3 months versus 5.1 months (HR, 0.17 [95% CI, 0.11 to 0.25]; P < .001; Data Supplement).
The HR for PFS2 (data maturity, 41.0%) was 0.81 (95% CI, 0.52 to 1.26; P = .35), and the median PFS2 was 23.6 months for olaparib versus 19.6 months for chemotherapy. OS data are immature.
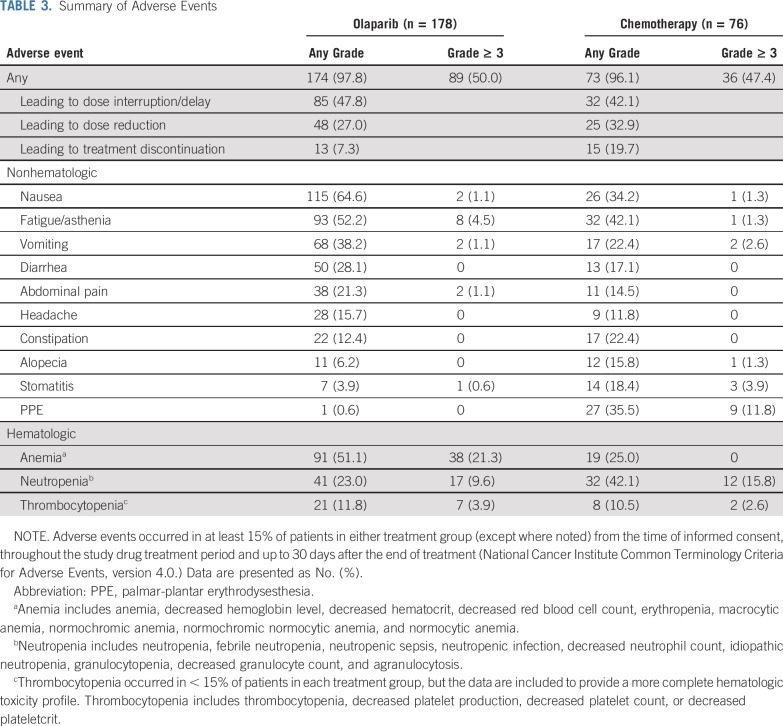
Median (interquartile range) total treatment durations were 11.3 months (7.3-19.3 months) for olaparib, 6.0 months (4.6-9.5 months) for PLD, 5.1 months (3.7-6.4 months) for paclitaxel, 3.3 months (1.3-7.3 months) for gemcitabine, and 6.2 months (3.5-8.7 months) for topotecan. The most common adverse events were nausea, fatigue/asthenia, anemia, vomiting, and diarrhea in the olaparib group and fatigue/asthenia, palmar-plantar erythrodysesthesia (PPE), nausea, neutropenia, and anemia in the chemotherapy group, and the most common grade ≥ 3 adverse events were anemia in the olaparib group and PPE and neutropenia in the chemotherapy group (Table 3).
TABLE 3.
Summary of Adverse Events
Serious adverse events occurred in 23.6% of patients in the olaparib group (most commonly anemia [2.8% of patients]) versus 18.4% of patients in the chemotherapy group (most commonly vomiting [3.9%]; Data Supplement). Myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) occurred in 4 patients (2.2%) in the olaparib group and in 3 patients (3.9%) in the chemotherapy group, and new primary malignancies occurred in 3 patients (1.7%) in the olaparib group and in no patients in the chemotherapy group (Data Supplement). Fatal adverse events occurred in 4 patients (2.2%) in the olaparib group (MDS; cardiopulmonary decompensation; AML and subarachnoid hemorrhage; and sepsis) versus 1 patient (1.3%) in the chemotherapy group (mesenteric vein thrombosis).
Adverse events were usually managed by dose modification, with fewer discontinuations seen with olaparib than with chemotherapy (Table 3 and Data Supplement). The most common adverse events leading to discontinuation were vomiting, anemia, and thrombocytopenia in the olaparib group and PPE, mucosal inflammation, peripheral neuropathy, and neutropenia in the chemotherapy group (Data Supplement).
Mean TOI scores at baseline were 73.2 in the olaparib group and 71.8 in the chemotherapy group. The overall least-squares mean change from baseline in TOI score was −2.3 with olaparib (n = 167) versus −4.8 with chemotherapy (n = 62), with a between-group difference of 2.5 (95% CI, −0.5 to 5.5; P = .108; Data Supplement).
DISCUSSION
The phase III SOLO3 study provides important prospective data regarding the efficacy of olaparib and nonplatinum chemotherapy agents in patients with heavily pretreated platinum-sensitive relapsed ovarian cancer and a germline BRCAm. Although patients with platinum-sensitive relapsed disease are likely to be retreated with platinum-based chemotherapy, benefit diminishes with third-line and later treatment,17 with no randomized data supporting an OS advantage for platinum-based chemotherapy beyond second line. The greater-than-expected activity shown by PLD in germline BRCA-mutated ovarian cancer7 raised the possibility of better treatment outcomes for heavily pretreated patients with a BRCAm. It is not surprising that most patients in SOLO3 had partially platinum-sensitive disease considering the nonplatinum chemotherapy control arm and the concept that using a nonplatinum agent to extend the platinum-free interval and potentially improve outcomes in this population was under investigation when SOLO3 was designed. Although not having a platinum agent in the comparator arm is a limitation, nonplatinum agents are acceptable options in heavily pretreated women, given that platinum agents have not demonstrated an OS benefit beyond the second-line setting.
Making go/no-go decisions during the development of PARP inhibitors has not always been straightforward.18 The development of olaparib capsules was delayed when patients in the control arm of the phase II Study 12, all of whom had germline BRCAm, responded much better than expected to PLD.7 The results of SOLO3, which to our knowledge is the first phase III trial to evaluate PARP inhibitor treatment in this setting, definitively demonstrate the advantage of the established olaparib tablet dose over nonplatinum chemotherapy. SOLO3 met its primary end point, showing a statistically significant and clinically relevant improvement in ORR in favor of olaparib. This was achieved despite the higher-than-predicted ORR seen in the chemotherapy arm.
In terms of secondary end points, PFS significantly favored olaparib versus chemotherapy in these heavily pretreated patients. Although TFST was significantly prolonged with olaparib, interim PFS2 did not significantly differ between the treatment arms. OS data are currently immature. Additional analysis of PFS2 and OS will be conducted.
Phase II trials investigating other PARP inhibitors in patients with platinum-sensitive relapsed ovarian cancer and a BRCAm demonstrated an ORR of 66% with rucaparib in women who had received at least 2 prior lines of chemotherapy (integrated analysis19 of Study 1020 and ARIEL221) and 39% with niraparib in women who had received at least 3 prior lines of chemotherapy (QUADRA22). In patients with relapsed ovarian cancer and a BRCAm, regardless of platinum sensitivity, the ORR was 68% with third-line rucaparib therapy and 45% with fourth-line or later rucaparib therapy.19
In terms of placing SOLO3 in context with olaparib maintenance therapy trials,23,24 the results of SOLO1, a phase III trial in patients with newly diagnosed BRCA-mutated advanced ovarian cancer, indicate that the early introduction of olaparib offers the greatest benefit, including the potential for cure. SOLO1 showed the largest absolute gain in PFS achieved to date with any PARP inhibitor in any ovarian cancer setting: median PFS was approximately 36 months longer with maintenance olaparib than with placebo.23 Maintenance olaparib also significantly prolonged PFS versus placebo in patients with BRCA-mutated platinum-sensitive relapsed ovarian cancer in the phase III SOLO2 trial,24 with the phase II Study 19 trial showing that the benefit of maintenance olaparib extended to patients with relapsed disease without a BRCAm.25,26
Thus, platinum-based chemotherapy followed by maintenance olaparib remains the first choice of treatment of patients with newly diagnosed BRCA-mutated advanced ovarian cancer and for patients with platinum-sensitive relapsed ovarian cancer regardless of BRCA status. SOLO3 provides an alternative chemotherapy-free treatment option for patients with platinum-sensitive relapsed ovarian cancer and a BRCAm who have received at least 2 prior lines of chemotherapy. In this setting, the population of patients with BRCA-mutated cancer who have not received prior PARP inhibitor therapy is expected to diminish over time as patients are identified and treated with PARP inhibitors earlier in their disease. The role of olaparib in treating patients with relapsed disease who have received prior PARP inhibitor therapy remains to be established.
The tolerability profiles of olaparib and chemotherapy in SOLO3 were consistent with those in previous studies.13,23,24,26-28 The incidence of severe and serious adverse events seemed similar in the olaparib and chemotherapy groups, although women receiving chemotherapy were more than twice as likely than those receiving olaparib to discontinue treatment because of an adverse event; the nature of these adverse events differed between the treatment arms.
There is an established increased risk of MDS/AML in women with ovarian cancer who have received platinum agents.29 The incidence of MDS/AML in the olaparib group was similar to that seen in the chemotherapy group and was consistent with that previously reported with olaparib23,24,30 and other PARP inhibitors.31,32 Two of the 3 patients in the chemotherapy group who developed MDS/AML had received a PARP inhibitor as a subsequent line of therapy (Data Supplement). There was no clinically meaningful change in HRQoL after olaparib monotherapy, and no clinically or statistically significant difference between olaparib and chemotherapy in HRQoL.
A limitation of SOLO3 is its open-label design. It was not feasible to conduct a blinded study, given the different routes and schedules of administration and tolerability profiles of the study drugs. To ensure this trial was robust, the primary ORR analysis was based on BICR. Other potential limitations are the changes in sample size and primary end point, which reflected increasingly slow patient recruitment because of the exclusion of patients who had received prior PARP inhibitor therapy.
In conclusion, olaparib resulted in statistically significant and clinically relevant improvements in ORR and PFS compared with nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCAm who had received at least 2 prior lines of platinum-based chemotherapy.
ACKNOWLEDGMENT
This study is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp (MSD), a subsidiary of Merck & Co, Kenilworth, NJ. We thank all the women who participated in this study, their families, and the investigators, and Teodora Goranova (AstraZeneca) for her contribution to the provision of the BRCA data. Medical writing assistance was provided by Gillian Keating, MBChB, from Mudskipper Business, funded by AstraZeneca and MSD.
Presented in an oral presentation at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported by AstraZeneca as part of an alliance between AstraZeneca and Merck Sharp & Dohme, a subsidiary of Merck, Kenilworth, NJ.
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
AUTHOR CONTRIBUTIONS
Conception and design: Richard T. Penson, Jae-Weon Kim, Sang Young Ryu, Tsveta Milenkova, Elizabeth S. Lowe
Provision of study material or patients: Ricardo Villalobos Valencia, David Cibula, Nicoletta Colombo, Charles A. Leath III, Mariusz Bidziński, Jae-Weon Kim, Joo Hyun Nam, Radoslaw Madry, Carlos Hernández, Paulo A.R. Mora, Sang Young Ryu, Giovanni Scambia
Collection and assembly of data: Richard T. Penson, Ricardo Villalobos Valencia, David Cibula, Nicoletta Colombo, Charles A. Leath III, Mariusz Bidziński, Jae-Weon Kim, Joo Hyun Nam, Radoslaw Madry, Carlos Hernández, Paulo A.R. Mora, Sang Young Ryu, Elizabeth S. Lowe, Giovanni Scambia
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard T. Penson
Honoraria: AbbVie, AstraZeneca, Clovis Oncology, Eisai, Genentech/Roche, Janssen Oncology (J&J), Mersana Therapeutics, Newlink Genetics, Sutro Biopharma, Tesaro, Vascular Biogenics
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Baxalta, Care4ward, Clovis Oncology, Eisai, Genentech, Merck, Mersana, Tesaro, Vascular Biogenics, Sutro, Janssen Oncology
Research Funding: Array BioPharma (Inst), AstraZeneca (Inst), Cerulean Pharma (Inst), Eisai (Inst), Genentech (Inst), Regeneron (Inst), Sanofi (Inst), Tesaro (Inst), Vascular Biogenics (Inst)
Patents, Royalties, Other Intellectual Property: BMJ, Blackwell Publishing, UpToDate
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, Genentech, Tesaro, Vascular Biogenics
Other Relationship: AbbVie
Ricardo Villalobos Valencia
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Asofarma
Speakers' Bureau: MSD Oncology, AstraZeneca, Roche, Amgen, Merck, Pfizer
Research Funding: Roche/Genentech, AstraZeneca, Lilly, Amgen, Merck Sharp & Dohme, Merck, Advaxis, Pfizer
Travel, Accommodations, Expenses: Roche, Pfizer, MSD Oncology, Asofarma
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche
Nicoletta Colombo
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, PharmaMar
Consulting or Advisory Role: Roche/Genentech, PharmaMar, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Takeda, Tesaro, BioCad, GSK
Charles A. Leath III
Honoraria: Mateon Therapeutics
Consulting or Advisory Role: Celsion, Unleash Immuno Oncolytics, AbbVie, Clovis Oncology, Eisai
Research Funding: Novartis (Inst), AstraZeneca (Inst), Celsion (Inst), Plexxikon (Inst), Mateon Therapeutics (Inst), Tesaro (Inst), Merck (Inst), Roche/Genentech (Inst), AbbVie (Inst), Syros Pharmaceuticals (Inst)
Radoslaw Madry
Consulting or Advisory Role: GSK, AstraZeneca
Speakers' Bureau: Roche, AstraZeneca, GSK
Travel, Accommodations, Expenses: Roche, AstraZeneca
Tsveta Milenkova
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Elizabeth S. Lowe
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Laura Barker
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety. Ann Oncol. 2016;27:1013–1019. doi: 10.1093/annonc/mdw133. [DOI] [PubMed] [Google Scholar]
- 3.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 4.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 5.Mateo J, Moreno V, Gupta A, et al. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol. 2016;11:401–415. doi: 10.1007/s11523-016-0435-8. [DOI] [PubMed] [Google Scholar]
- 6.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 8.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s006lbl.pdf AstraZeneca: Highlights of prescribing information: LYNPARZA (olaparib) tablets, for oral use.
- 11.Bruchim I, Jarchowsky-Dolberg O, Fishman A. Advanced (>second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2013;166:94–98. doi: 10.1016/j.ejogrb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 13.Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 14. AstraZeneca: Global policy: Bioethics. https://www.astrazeneca.com/content/dam/az/PDF/Sustainability/code-of-ethics-2018/AZ%20Code%20of%20Ethics%20-%20English.pdf.
- 15.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 16.Osoba D, Bezjak A, Brundage M, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41:280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Villalobos VM, Wang YC, Sikic BI. Reannotation and analysis of clinical and chemotherapy outcomes in the ovarian data set from The Cancer Genome Atlas. JCO Clin Cancer Inform. 2018;2:1–16. doi: 10.1200/CCI.17.00096. [DOI] [PubMed] [Google Scholar]
- 18.Domchek SM, Mitchell G, Lindeman GJ, et al. Challenges to the development of new agents for molecularly defined patient subsets: Lessons from BRCA1/2-associated breast cancer. J Clin Oncol. 2011;29:4224–4226. doi: 10.1200/JCO.2011.36.8134. [DOI] [PubMed] [Google Scholar]
- 19.Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147:267–275. doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Kristeleit R, Shapiro GI, Burris HA, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23:4095–4106. doi: 10.1158/1078-0432.CCR-16-2796. [DOI] [PubMed] [Google Scholar]
- 21.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 22.Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 23.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 24.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 25.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 26.Friedlander M, Matulonis U, Gourley C, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018;119:1075–1085. doi: 10.1038/s41416-018-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 28.ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–2193. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 29.Morton LM, Dores GM, Schonfeld SJ, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5:318–325. doi: 10.1001/jamaoncol.2018.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579–1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]
- 31.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]