Abstract
PURPOSE
We hypothesized that bevacizumab will potentiate activity of pembrolizumab. We conducted a phase Ib/II, single-arm, multisite clinical trial of the combination in metastatic renal cell carcinoma (RCC).
PATIENTS AND METHODS
Patients with metastatic clear cell RCC who experienced progression after at least one systemic therapy (phase Ib) or were treatment naïve (phase II) were enrolled. In phase Ib, pembrolizumab (200 mg) and bevacizumab (10 or 15 mg/kg) were given intravenously every 3 weeks. The primary end point for phase II was overall response rate (ORR). With an 80% statistical power and a type I error probability of 0.1, 48 patients were to be accrued to detect an ORR of 42%.
RESULTS
Thirteen patients (ages 33-68 years; median, 55 years) were enrolled in the phase Ib study. No dose-limiting toxicities were reported. Pembrolizumab 200 mg and bevacizumab 15 mg/kg were chosen for phase II. Forty-eight patients (ages 42-84 years; median age, 61 years; 33 males) were accrued for the phase II study. The primary end point was met, with the ORR reaching 60.9% (95% CI, 45.4% to 74.9%), consisting of 1 complete response (CR), 2 CRs in target lesions, 25 partial responses, 18 responses of stable disease, 2 unevaluable responses. Median progression-free survival was 20.7 months (95% CI, 11.3 to 27.4 months). Median overall survival was not reached at the median follow-up of 28.3 months. The most common treatment-related grade 3 toxicities were hypertension and proteinuria. There were two grade 4 toxicities: duodenal ulcer and hyponatremia. Presence of tumor-infiltrating T cells, but not programmed death-ligand 1 expression, in tumor tissue correlated with response.
CONCLUSION
The combination of 200 mg of pembrolizumab and a 15 mg/kg dose of bevacizumab given every 3 weeks is safe and active in metastatic RCC.
INTRODUCTION
Abnormal tumor vasculature contributes to immune tolerance of tumor cells by impeding homing of cytotoxic T cells into tumor and their antitumor activity.1 Tumor environment causes accumulation and subsequent polarization of myeloid-derived suppressor cells (MDSCs),2 tumor-associated macrophages (TAMs),3 and dendritic cells4 toward immunosuppressive phenotypes. Anti-angiogenic treatment has been shown to decrease the number of MDSCs, increase the number of TAMs polarized to an immunostimulatory phenotype, and facilitate tumor infiltration by CD4+ and CD8+ T cells.5
The programmed death-1 (PD-1) receptor is expressed on activated T and B cells.6 Its major ligand, programmed death-ligand 1 (PD-L1), is expressed on a subset of macrophages but can be induced in a variety of tissues.7 When activated T cells expressing PD-1 encounter PD-L1, T-cell functions are diminished.8,9 Multiple tumor types have been shown to express PD-L1, effectively co-opting a native tolerance.10-12
Pembrolizumab (MK-3475) is a humanized monoclonal immunoglobulin G4-κ isotype antibody against PD-1 that blocks immunoregulatory signaling of the PD-1 receptor expressed by T cells.13 Single-agent pembrolizumab therapy given at 200 mg intravenously every 3 weeks for 2 years or until progression showed efficacy in treatment-naïve metastatic renal cell carcinoma (mRCC) in cohort A of KEYNOTE 427, with an overall response rate (ORR) of 33.6%.14
Bevacizumab, an anti–vascular endothelial growth factor (VEGF) antibody is approved for mRCC treatment in combination with interferon alfa-2a (IFNα-2a) on the basis of two randomized phase III studies. In the AVOREN study,15 ORR was 31% for the IFNα-2a and bevacizumab arm v 13% in the IFNα-2a arm. The addition of bevacizumab was associated with an improvement in progression-free survival (PFS) and a trend toward improvement in overall survival (OS). The CALGB 90206 trial16 showed an ORR of 25.5% for the IFNα-2a and bevacizumab arm v 13.1% in the IFNα-2a arm.
We hypothesized that the combination of bevacizumab and pembrolizumab would result in enhanced antitumor clinical activity compared with historical activity of anti-PD-1/PD-L1–blocking antibodies in mRCC.17 We conducted a phase Ib/II trial to establish first the safe dose of bevacizumab and pembrolizumab for patients with clear cell mRCC after failure of at least one systemic therapy and then, to assess efficacy and toxicity of this combination in patients with treatment-naïve mRCC.
PATIENTS AND METHODS
Study Design and Participants
This multicenter phase Ib/II clinical trial (BTCRC-GU14-003) of patients with metastatic, predominantly clear cell histology RCC was conducted through the BIG TEN Cancer Research Consortium. The phase Ib portion was conducted according to a standard 3 + 3 dose escalation design where if there was no dose-limiting toxicity (DLT) in first 3 patients at a bevacizumab dose of 10 mg/kg in combination with a fixed 200-mg dose of pembrolizumab, the next cohort of patients received a bevacizumab dose of 15 mg/kg in combination with the 200-mg dose of pembrolizumab. Both drugs were given intravenously in cycles of 3 weeks. Treatment was given until disease progression, unacceptable toxicity, or patient withdrawal. Once the maximum tolerated dose (MTD) of bevacizumab for the combination was identified, the cohort was expanded to 10 patients to ensure safety. Then, the phase II portion at that dose was open to accrual.
Patients were eligible for enrollment in the phase Ib portion of the trial if they had mRCC after experiencing failure of at least one prior systemic therapy. In the phase II portion of the study, eligible patients were treatment naïve. Patients were required to have measurable disease according to RECIST version 1.1 (v1.1)18 and adequate organ function within 14 days of starting therapy. The complete list of inclusion and exclusion criteria can be found in the study protocol (Data Supplement, online only).
The institutional review board at all participating centers approved the study protocol. All patients provided informed consent before study interventions were initiated.
Outcomes
The primary objective of the phase Ib portion was determining MTD, the safety, and DLTs of the combination. The primary objective of the phase II portion was objective ORR, as measured by RECIST v1.1. Secondary objectives included PFS and OS.
Bevacizumab was sourced from a commercial supply, and pembrolizumab was provided by Merck & Co. Bevacizumab and pembrolizumab were infused intravenously over approximately 30 and 60 minutes, respectively, on day 1 of every 21-day cycle and administered 15-30 minutes apart. Treatment continued until disease progression, unacceptable toxicity, withdrawal of informed consent, or patient’s death.
The study definition of DLT is provided in the study protocol (Data Supplement). The MTD was defined as the dose level below the dose that induced DLTs in at least one third of the patients. If an MTD was not determined, the highest tested dose level (200 mg pembrolizumab and 15 mg/kg bevacizumab) was defined as the recommended phase II dose.
Imaging of the chest, abdomen, and pelvis was performed every 6 weeks through cycle 9; thereafter, it was performed every 9 weeks. Response was assessed per the RECIST v1.118 and immune-related RECIST.19 Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.0).
Exploratory Studies
Expression of PD-L1 in archived diagnostic tumor tissue was determined by immunohistochemistry (IHC) using a 22C3 antibody (Qualtek Electronics, Newton, PA). Both a modified percent score (percentage of tumor and any tumor-infiltrating mononuclear inflammatory cells that had membrane staining at 1+ intensities or greater) and a modified histologic score (percentage of cells staining at either no [< 1], low [1+], moderate [2+], or high [3+] intensity) were calculated for every sample.
Tumor vascular density and CD8+ cell infiltration of archived diagnostic tumor tissue was determined by IHC at the University of Illinois at Chicago (UIC). Enumeration of circulating tumor cells (CTCs) at baseline and during treatment was performed in the laboratory of Seungpyo Hong, MD, at the UIC College of Pharmacy using CapioCyte CTC capture surfaces (Capio Biosciences, Madison, WI) with antibodies against epithelial cell adhesion molecule and epidermal growth factor receptor and rolling domains containing E-selectin, similarly to a previously described method.20
PD-L1 protein levels in serum were determined using the CHECKMARK (Martell Diagnostic Laboratories, Roseville, MN) enzyme-linked immunosorbent assay (ELISA) using paired mouse monoclonal antibodies against the extracellular domain of human PD-L1. VEGF-C at baseline and during treatment was measured by Human VEGF-C Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). Details of the method used to quantify tumor vascular density, CD8+ infiltration, CTCs, and levels of serum PD-L1 can be found in the Data Supplement.
PD-L1 expression by IHC, tumor vascular density, and CD8+ cell infiltration in tumor tissue were tested for association with the ORR, PFS, and OS using logistic regression and Cox regression models, respectively. Baseline and change in serum PD-L1 and VEGF-C levels during therapy relative to baseline were examined in relation to the best clinical response and PFS. All analyses were done using SAS 9.4 software (SAS Institute, Cary, NC).
Statistical Analysis
In the phase Ib portion of the trial, a standard 3 + 3 design was used. In the phase II portion of the trial, the study end point was objective ORR (partial response [PR] or complete response [CR]) as assessed by RECIST v1.1.18 Prior studies had identified ORR with single-agent anti-PD-1 antibody of 27% in kidney cancer.17 With assumptions of 80% power to detect a 55% improvement in response to the combination over historic data on single anti-PD-1 agent activity in mRCC and a 2-sided type I error of 0.107, 48 patients were required to detect a 55% improvement to an ORR of 42%. The ORR and 95% CI were computed using SAS 9.4 software. Kaplan-Meier analysis was used to calculate PFS and OS with associated 95% CIs in both phases of the study. The effect of sex, Heng risk groups, presence of bone metastases, and prior nephrectomy status on ORR and PFS was analyzed using logistic and Cox regression models. The proportion of patients with each grade of adverse events as defined by CTCAE (version 4) was computed along with the 95% CI and reported in a tabular and descriptive manner.
RESULTS
Patient Characteristics
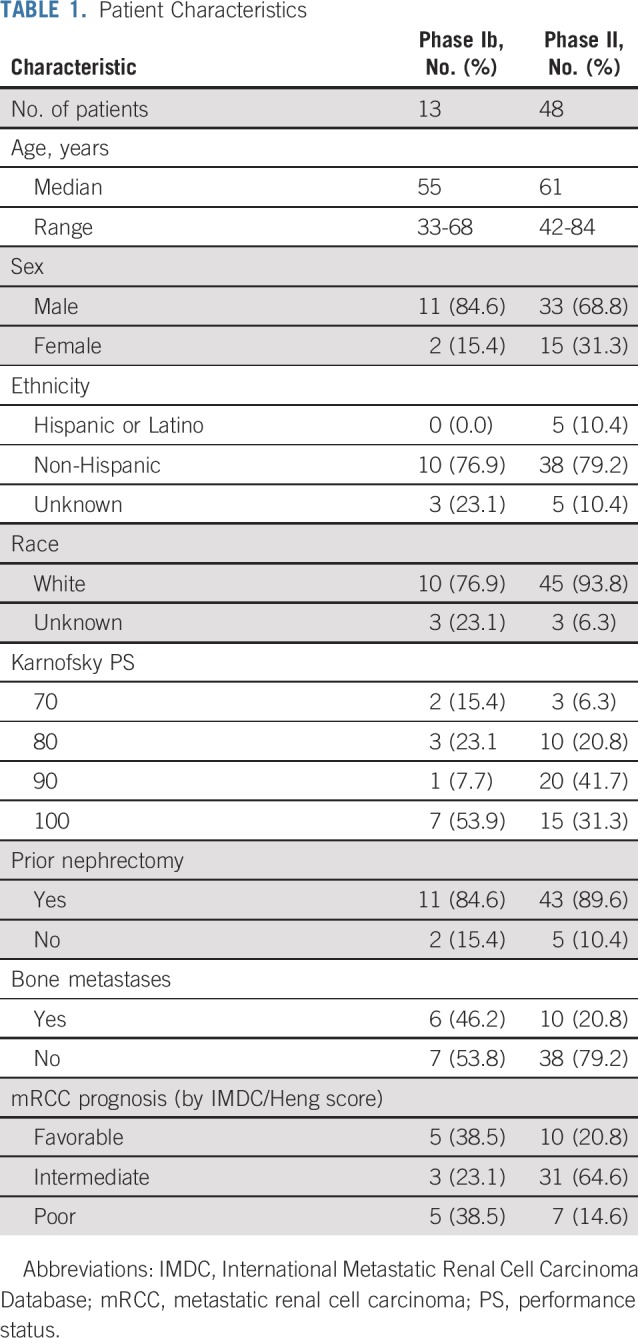
Thirteen patients (3 at 10 mg/kg and 10 at 15 mg/kg of bevacizumab) were enrolled in phase Ib, all of whom had received multiple lines of prior therapy (median age, 55 years; range, 33-68 years). Prior therapies included high-dose interleukin 2, pazopanib, axitinib, sunitinib, sorafenib, everolimus, and temsirolimus. No patient received prior immune checkpoint inhibitor (ICI) therapy. Forty-eight treatment-naïve patients were enrolled in the phase II portion of the study (median age, 61 years; range, 42-84 years). One patient did not receive any dose of either drug and was not included in the evaluation for PFS or OS. Table 1 lists the patient characteristics.
TABLE 1.
Patient Characteristics
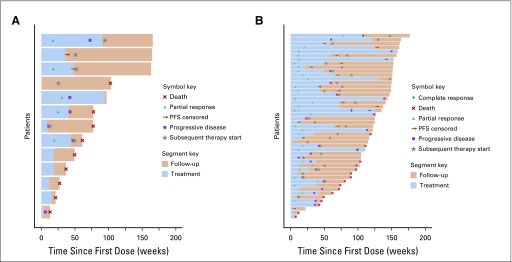
Treatment Efficacy and Duration
In the phase Ib portion of study, the safe doses of bevacizumab 15 mg/kg and pembrolizumab 200 mg every 3 weeks were established. ORR in phase Ib was 41.7% (95% CI, 15.2% to 72.3%); 1 patient had progressive disease (PD), 6 had stable disease (SD), 5 had PRs, and 1 was not evaluable. In the phase II portion, the study’s primary end point of ORR was reached at 60.9% (95% CI, 45.4% to 74.9%) with median time on treatment of 298 days (range, 21-1,113 days). Best responses in phase II were as follows: 1 CR, 2 CRs in target lesions, 25 PRs, 18 SD, and 2 unevaluable. Patients with favorable-risk disease had an ORR of 66.7%, and patients with intermediate and poor risk prognosis had an ORR of 59.5%.
Figures 1A and 1B show responses to and duration of treatment in the phase Ib and phase II studies. Neither sex nor the following factors had a significant effect on likelihood of response: Heng risk groups, presence of bone metastases, or prior nephrectomy.
FIG 1.
Swimmer plots of responses to and duration of treatment in the (A) phase Ib and (B) phase II portions of the study. PFS, progression-free survival.
Median time to response was 84 days (range, 35-544 days), and median duration of response was 832 days (95% CI, 517 to 1,049 days). Median duration of pembrolizumab and bevacizumab treatment in the phase Ib study was 6.0 months (interquartile range [IQR], 2.8-10.1 months) and 10 months (IQR, 4.6-18.8 months) in the phase II study. Overall, patients received full planned doses of both drugs; median dose was 200 mg pembrolizumab and 15 mg/kg bevacizumab per cycle in both portions of the study.
PFS and OS
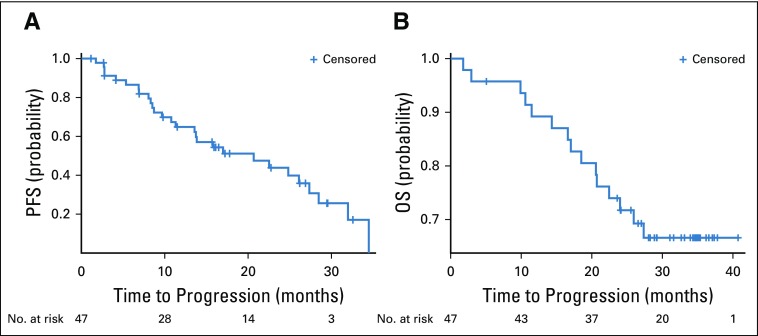
In the phase Ib study, median PFS was 9.9 months (95% CI, 4.9 to 16.7 months). Median OS was 17.9 months (95% CI, 6.3 months to upper limit not estimable). Both calculations were performed when the majority of events occurred (11 for PFS and 9 for OS).
In the phase II study, median PFS was estimated to be 20.7 months (95% CI, 11.3 to 27.4 months). Figure 2A shows the Kaplan-Meier curve for PFS. Neither sex nor Heng risk groups, presence of bone metastases, prior nephrectomy, and PD-L1 expression in tumor had a significant effect on PFS or OS. Median OS at 28.3 months was not reached (only 15 of 47 died; Fig 2B). Patients with Heng favorable-risk prognosis had a median PFS of 24.8 months (95% CI, 2.8 to 32.0 months). Patients with intermediate- and poor-risk scores had a PFS of 20.68 months (95% CI, 11.31 to 27.35 months). There was no difference in PFS between favorable- and intermediate-/poor-risk patients (hazard ratio [HR] for favorable risk, 1.15; 95% CI, 0.47 to 2.83; P = .76).
FIG 2.
Kaplan-Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS).
Safety and Adverse Events
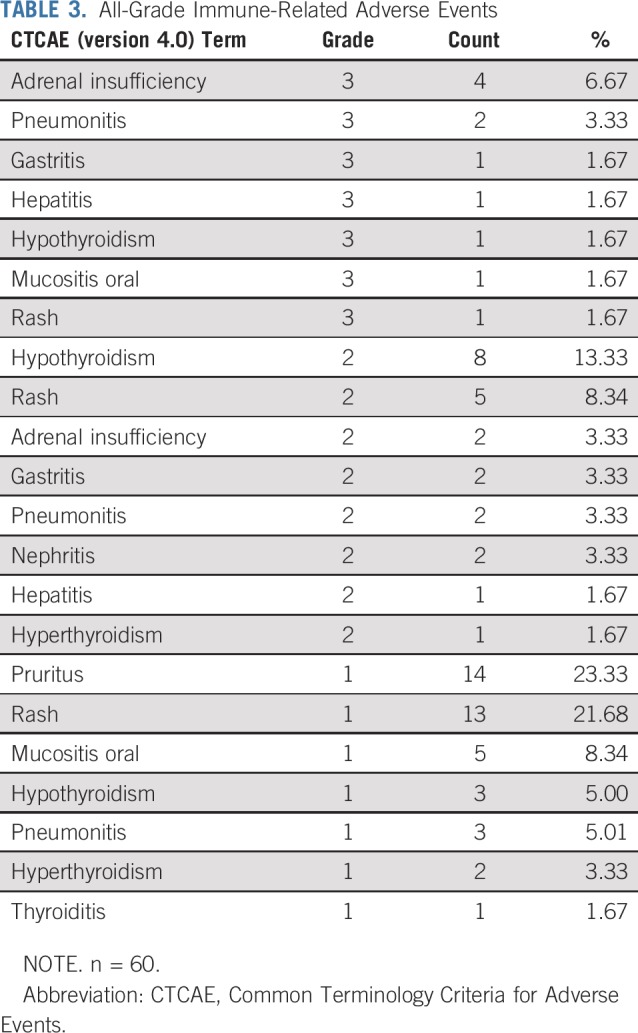
Safety was evaluated in all patients in the phase Ib and phase II portions of the study. Median number of 3-week treatment cycles was 13.5 (range, 1-48 cycles), which corresponded to 10.1 months (range, 1-36 months). Dose delays as a result of adverse events occurred in 20 patients (33.3%). The rate of treatment discontinuation because of adverse events was 33.3% (n = 20). The most common cause of treatment discontinuation was proteinuria (35%). The incidence of adverse events at least possibly related to either pembrolizumab or bevacizumab was 88.3%, and the incidence of grade 3 or 4 adverse events was 45%. The most common (> 5% incidence) treatment-related grade 3 toxicities were hypertension (25%), proteinuria (10%), adrenal insufficiency (6.7%), and pain/headaches (5.0%). There were two grade 4 toxicities (hyponatremia and duodenal ulcer). There was no grade 5 toxicities related to study treatment. Table 2 lists all the treatment-related grade 3 and 4 toxicities. There were the following grade 3 immune-related toxicities: adrenal insufficiency (6.7%); pneumonitis (3.3%); and gastritis, hepatitis, hypothyroidism, oral mucositis, and skin rash (each 1.7%; Table 3). Seventeen patients were treated with systemic steroids for immune-related toxicities. All grade > 2 adverse events in the study and adverse events that led to treatment discontinuation are listed in the Data Supplement.
TABLE 2.
Grade 3 and 4 Adverse Events Related to Treatment

TABLE 3.
All-Grade Immune-Related Adverse Events
Exploratory Studies
The analysis details of the correlation of exploratory end points with ORR, PFS, and OS are listed in the Data Supplement. There were no differences in ORR, PFS, and OS between groups, with expression of PD-L1 only in interface, in tumor only, in stroma and tumor, or without PD-L1 in stroma and tumor. Patients with tumor overexpressing PD-L1 > 0 had a trend toward better PFS after 20 months, but there was no statistical difference in overall PFS (P = .37). A higher level of tumor-infiltrating cells was associated with a trend of higher chance of response (HR, 1.80; 95% CI, 0.90 to 3.59; P = .096).
For evaluation of tumor vascular density and CD8+ cell infiltration, 41 samples from primary nephrectomy and 3 from metastases were available. Neither number of CD8+ cells per tumor area or per number of tumor cells nor tumor vascular density had any effect on ORR, PFS, or OS. There was no correlation between baseline CTC number and ORR or PFS. Enumeration of CTCs in subsequent blood draws significantly decreased in all samples compared with baseline (P = .0472), but the degree of decline did not correlate with ORR or PFS. Similarly, neither baseline soluble PD-L1 level nor change in cycle 3 predicted ORR or PFS. There was no correlation between VEGF-C at baseline or changes in VEGF-C levels in subsequent cycles of therapy with ORR, but an increase in VEGF-C in subsequent cycles marginally increased risk of progression (HR, 1.1; 95% CI, 0.98 to 1.24; P = .099) and shortened OS (HR, 1.19; 95% CI, 0.99 to 1.43; P = .062).
DISCUSSION
There were no DLTs in the phase Ib portion of the study; in both phases, therapy was well tolerated, with patients receiving a high number of cycles (median, 13.5 cycles), which corresponds to 10 months of treatment (range, 4.6-18.8 months). The incidence of grade 3 or 4 adverse events was seen in 45% of patients, which compares favorably with other combinations of ICIs and tyrosine kinase inhibitors (TKIs), where grade 3 and 4 toxicities occurred in 65%-67% of patients.21,22
The phase Ib portion of the study was done in a heavily pretreated population with multiple prior lines of therapy, yet the combination of pembrolizumab and bevacizumab had produced a substantive ORR of 41.7% (95% CI, 15.2% to 72.3%). The phase II portion of the study met its primary end point, with a high ORR of 60.9% (95% CI, 45.4% to 74.9%). The efficacy was also reflected in the median PFS of 20.7 months and in the median OS not being reached despite a median follow-up of 28.3 months.
In comparison, single-agent pembrolizumab produces an ORR of 33.6% (95% CI, 24.8% to 43.4%) in patients with RCC,14 and single bevacizumab can achieve an ORR of only 10% (95% CI, 2.9% to 24.2%).23 The ORR and PFS in the combination are comparable to other combinations of ICIs and TKIs, but the toxicity appears to be lower in this trial. Cabozantinib in combination with atezolizumab in the COSMIC-021 study in treatment-naïve patients produced an ORR of 50% (95% CI, 12% to 88%) when 40 mg of cabozantinib was used and 83% (95% CI, 36% to 100%) when 60 mg was used. Adverse events that required a dose reduction of cabozantinib occurred in 50% of patients treated with the 40-mg dose and in 100% of patients treated with the 60-mg dose. Adverse events that led to dose interruptions occurred in 50% and 67%, respectively.24 The combination of avelumab and axitinib has been reported in phase Ib25 and phase III21 studies, where patients treated with the combination had a median PFS of 13.8 months (reported in phase III but not in phase Ib). The ORR with this combination was 58% (phase Ib) and 51.4% (phase III) of patients. However, grade 3 and 4 treatment-related toxicity occurred in 58% (phase Ib) and 71.2% (phase III) of patients.
The activity of combination axitinib and pembrolizumab has been reported in phase Ib22 and phase III26 studies. Median PFS was 20.9 and 15.1 months, respectively, and ORR was 73% and 59.3%, respectively. Grade 3/4 treatment-related toxicities were seen in 65% (phase Ib) and 62.9% (phase III) of patients.
In the CheckMate 016 study of nivolumab and either pazopanib or sunitinib, treatment-related grade 3/4 adverse events and treatment discontinuations as a result of toxicity were frequent (70% and 82% and 25% and 39%, respectively). In the sunitinib and nivolumab arm, ORR was 55%, and PFS was 12.7 months; in the pazopanib and nivolumab arm, ORR was 45%, and PFS was7.2 months.27
Activity of atezolizumab and bevacizumab was tested in randomized phase II28 and III29 studies. Median PFS was 11.7 and 11.2 months, and ORR was 32% and 43%, respectively. PD-L1 expression has been reported to be associated with greater activity of atezolizumab and bevacizumab29 and that of nivolumab and ipilimumab,30 but in our study, there was no correlation between PD-L1 tissue expression and ORR or PFS.
In patients with tumors with a preexisting presence of effector T cells, therapy with combination ICIs and VEGF inhibitors resulted in better PFS versus sunitinib.28 This is concordant with our observation that a higher level of tumor-infiltrating immune cells at baseline was marginally associated with a higher chance of PR (odds ratio, 1.80; 95% CI, 0.90 to 3.59; P = .096).
In conclusion, the combination of pembrolizumab and bevacizumab has an acceptable toxicity profile and is active in patients with mRCC as first and subsequent lines of therapy. It could be further tested in patient populations where TKIs are not well tolerated and can cause early treatment discontinuation. With the exception of an increased number of tumor-infiltrating cells correlating with a higher likelihood of response, no predictive biomarkers in tissue or blood were identified.
ACKNOWLEDGMENT
We thank the patients who participated; the Big Ten Cancer Research Consortium for critical review of the study concept, coordinating research between clinical sites, and serving as the blood and tissue repository; Amer A. Sidani, MD, for working on the study design; Basharath Khan, MD, and the laboratories of Peter Gann, MD, ScD, and Seungpyo Hong, PhD, for help with correlative science; James P. Zacny, PhD, for formatting and editing the manuscript; and the Clinical Trials Office from the UIC Cancer Center for regulatory support, data safety review, and clinical research operations.
PRIOR PRESENTATION
Presented at 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018.
Supported by Merck & Co.
AUTHOR CONTRIBUTIONS
Conception and design: Arkadiusz Z. Dudek
Provision of study material or patients: Arkadiusz Z. Dudek, Theodore F. Logan, Eric A. Singer, Monika Joshi, Joshua M. Lang, Anas Al-Janadi, Ajjai S. Alva
Collection and assembly of data: Arkadiusz Z. Dudek, Li C. Liu, Shilpa Gupta, Theodore F. Logan, Eric A. Singer, Monika Joshi, Yousef N. Zakharia, Joshua M. Lang, James K. Schwarz, Anas Al-Janadi, Ajjai S. Alva
Data analysis and interpretation: Arkadiusz Z. Dudek, Li C. Liu, Shilpa Gupta, Theodore F. Logan, Eric A. Singer, Monika Joshi, Yousef N. Zakharia, Joshua M. Lang, Anas Al-Janadi, Ajjai S. Alva
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase Ib/II Clinical Trial of Pembrolizumab With Bevacizumab for Metastatic Renal Cell Carcinoma: BTCRC-GU14-003
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Arkadiusz Z. Dudek
Leadership: IGF Oncology, Martell Diagnostic, Squarex, TTC Oncology
Stock and Other Ownership Interests: Biothera, Vanquish Oncology, IGF Oncology
Honoraria: Biothera, Vanquish Oncology, IGF Oncology, Medtronic, TTC Oncology, Squarex, Martell Diagnostic, AstraZeneca
Consulting or Advisory Role: Vanquish Oncology, Biothera, TTC Oncology, Tychon Biosciences
Research Funding: Merck (Inst), Eli Lilly (Inst)
Shilpa Gupta
Stock and Other Ownership Interests: Nektar
Honoraria: Janssen Oncology, AstraZeneca
Consulting or Advisory Role: Genentech, Merck, Exelixis, Janssen Oncology
Speakers’ Bureau: Bristol-Myers Squibb, Janssen Oncology
Research Funding: Astellas Pharma (Inst), Medivation (Inst), Pfizer (Inst), MedImmune (Inst), Merck (Inst), Moderna Therapeutics (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst)
Theodore F. Logan
Consulting or Advisory Role: Prometheus Laboratories
Research Funding: Abbott Laboratories (Inst), Abraxis BioScience (Inst), Acceleron Pharma (Inst), Amgen (Inst), Argos Therapeutics (Inst), AstraZeneca (Inst), AVEO (Inst), BioVex (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Eli Lilly (Inst), GlaxoSmithKline (Inst), Roche (Inst), Immatics (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Synta (Inst), Threshold Pharmaceuticals (Inst), Millennium Pharmaceuticals (Inst), TRACON Pharma (Inst), Cerulean Pharma (Inst), EMD Serono (Inst), Prometheus Laboratories (Inst), Macrogenics (Inst), Peloton Therapeutics (Inst), Iovance Biotherapeutics (Inst), MedImmune (Inst), Dynavax (Inst)
Eric A. Singer
Research Funding: Astellas Pharma, Medivation
Monika Joshi
Consulting or Advisory Role: Sanofi
Research Funding: AstraZeneca (Inst), Pfizer (Inst)
Yousef N. Zakharia
Consulting or Advisory Role: Roche, Genentech, Eisai, Amgen, Castle Biosciences, Novartis, Exelixis, Pfizer, Cardinal Health, Bayer AG, Janssen Pharmaceuticals, TTC Oncology, Clovis Oncology
Travel, Accommodations, Expenses: NewLink Genetics
Joshua M. Lang
Stock and Other Ownership Interests: Salus Discovery
Consulting or Advisory Role: Sanofi, Immunomedics, Janssen Pharmaceuticals
Research Funding: Medivation (Inst), Agensys (Inst), GlaxoSmithKline (Inst), Immunomedics (Inst), Bristol-Myers Squibb (Inst), Janssen Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent on a technology for rare cell capture and analysis; this technology has been licensed by Salus Discovery, although no commercial products are available
Anas Al-Janadi
Honoraria: Genentech, Roche, AbbVie, Celgene, Alexion Pharmaceuticals, Takeda Pharmaceuticals, Pharmacyclics, Janssen Pharmaceuticals
Consulting or Advisory Role: Alexion Pharmaceuticals, Takeda Pharmaceuticals, Amgen
Speakers’ Bureau: Alexion Pharmaceuticals, Celgene, Genentech, Roche, AbbVie
Research Funding: Incyte (Inst), Celgene (Inst), Takeda Pharmaceuticals (Inst), MEI Pharma (Inst), Alexion Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Alexion Pharmaceuticals, Celgene, Genentech, Roche, AbbVie, Takeda Pharmaceuticals, Amgen
Ajjai S. Alva
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, Bristol-Myers Squibb
Speakers’ Bureau: AstraZeneca
Research Funding: Genentech (Inst), Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Prometheus Laboratories (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), Roche (Inst), Bayer AG (Inst), Progenics (Inst), Astellas Pharma (Inst), Arcus Biosciences (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wu NZ, Klitzman B, Dodge R, et al. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–4268. [PubMed] [Google Scholar]
- 2.Marigo I, Dolcetti L, Serafini P, et al. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JS, Landers RJ, Underwood JC, et al. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Ma C, Liu S, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128:e237–e249. doi: 10.1111/j.1365-2567.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 9.Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 10.Tang PA, Heng DY. Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep. 2013;15:98–104. doi: 10.1007/s11912-012-0284-2. [DOI] [PubMed] [Google Scholar]
- 11.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donskov F, McDermott DF, Lee JL, et al: KEYNOTE-427 cohort A: Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (ccRCC). Ann Oncol 29:viii303-viii331, 2018.
- 15.Bracarda S, Bellmunt J, Melichar B, et al. Overall survival in patients with metastatic renal cell carcinoma initially treated with bevacizumab plus interferon-α2a and subsequent therapy with tyrosine kinase inhibitors: A retrospective analysis of the phase III AVOREN trial. BJU Int. 2011;107:214–219. doi: 10.1111/j.1464-410X.2010.09707.x. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 20.Myung JH, Eblan MJ, Caster JM, et al. Multivalent binding and biomimetic cell rolling improves the sensitivity and specificity of circulating tumor cell capture. Clin Cancer Res. 2018;24:2539–2547. doi: 10.1158/1078-0432.CCR-17-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19:405–415. doi: 10.1016/S1470-2045(18)30081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal N, Vaishampayan UN, Green M, et al. Phase 1b study (COSMIC-021) of cabozantinib in combination with atezolizumab: Results of the dose-escalation stage in patients with treatment-naive advanced renal cell carcinoma.Presented atthe 17th Int Kidney Cancer Sympos Miami, FLNovember 2-3, 2018 [Google Scholar]
- 25.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): An open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19:451–460. doi: 10.1016/S1470-2045(18)30107-4. [DOI] [PubMed] [Google Scholar]
- 26.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1186/s40425-018-0420-0. Amin A, Plimack ER, Ernstoff MS, et al: Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: The CheckMate 016 study. J Immunother Cancer 6:109, 2018 [Erratum: J Immunother Cancer 7:73, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. doi: 10.1038/s41591-018-0053-3. McDermott DF, Huseni MA, Atkins MB, et al: Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [Erratum: Nat Med 24:1941, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]