Abstract
PURPOSE
Observation is the current standard of care for smoldering multiple myeloma. We hypothesized that early intervention with lenalidomide could delay progression to symptomatic multiple myeloma.
METHODS
We conducted a randomized trial that assessed the efficacy of single-agent lenalidomide compared with observation in patients with intermediate- or high-risk smoldering multiple myeloma. Lenalidomide was administered orally at a dose of 25 mg on days 1 to 21 of a 28-day cycle. The primary end point was progression-free survival, with disease progression requiring the development of end-organ damage attributable to multiple myeloma and biochemical progression.
RESULTS
One hundred eighty-two patients were randomly assigned—92 patients to the lenalidomide arm and 90 to the observation arm. Median follow-up is 35 months. Response to therapy was observed in 50% (95% CI, 39% to 61%) of patients in the lenalidomide arm, with no responses in the observation arm. Progression-free survival was significantly longer with lenalidomide compared with observation (hazard ratio, 0.28; 95% CI, 0.12 to 0.62; P = .002). One-, 2-, and 3-year progression-free survival was 98%, 93%, and 91% for the lenalidomide arm versus 89%, 76%, and 66% for the observation arm, respectively. Only six deaths have been reported, two in the lenalidomide arm versus four in the observation arm (hazard ratio for death, 0.46; 95% CI, 0.08 to 2.53). Grade 3 or 4 nonhematologic adverse events occurred in 25 patients (28%) on lenalidomide.
CONCLUSION
Early intervention with lenalidomide in smoldering multiple myeloma significantly delays progression to symptomatic multiple myeloma and the development of end-organ damage.
INTRODUCTION
Smoldering multiple myeloma (SMM) is an asymptomatic precursor stage of multiple myeloma (MM).1,2 It is associated with a risk of progression to symptomatic MM of 10% per year,3 although patients with certain adverse prognostic factors may have a higher risk of progression of approximately 25% per year.4-6
Observation has been the current standard of care for SMM until the emergence of end-organ dysfunction meeting the criteria for clinical MM.7,8 Data from randomized trials that show the efficacy of therapy to prevent such end-organ dysfunction or improve outcome are limited. Types of early therapy can take two different approaches. First, one can take a prevention approach with low-intensity therapy directed at clonal control or, second, one can take a more intensive treatment approach for which the goal is the eradication of the malignant clone. The Spanish myeloma group assessed the combination of lenalidomide plus dexamethasone versus observation in patients with high-risk SMM.7 Although the study demonstrated improved progression-free survival (PFS) and overall survival with early intervention, it was not adopted as the standard of care for three main reasons. First, a combination regimen was used and the specific added value of lenalidomide could not be clearly isolated. Second, the study did not use modern imaging at randomization as the trial was designed before the use of magnetic resonance imaging or positron emission tomography scans were introduced as standard, more sensitive measures,9,10 leading to concerns about the possible enrollment of patients with symptomatic myeloma in this trial. Third, multiparametric flow cytometry criteria that were used to define high-risk SMM for this trial was not readily available outside of the centers that conducted the trial, which limited the generalizability of results.
To our knowledge, we conducted the largest randomized trial in SMM wherein patients received either single-agent lenalidomide or observation, which included modern imaging at the time of study entry, and used laboratory criteria that are widely available to risk classify patients. We hypothesized that the immune-modulatory effects of lenalidomide alone could prevent end-organ dysfunction without the need for corticosteroids. We also conducted quality-of-life analysis over the course of therapy in an effort to better identify the impact of early intervention in this SMM setting.
METHODS
Trial Design, Oversight, and Treatment
In this randomized, open-label, multicenter phase III trial, patients were randomly assigned with equal allocation to receive oral lenalidomide 25 mg on days 1 to 21 of every 28-day cycle or to observation. Therapy or observation was continued until disease progression, toxicity, or withdrawal for other reasons. Patients were encouraged to mobilize stem cells after four to six cycles of therapy. Patients who were assigned to lenalidomide were required to take thrombosis prophylaxis, with a minimum recommendation of aspirin at a suggested dose of 325 mg per day. Patients were stratified at randomization by time since diagnosis of SMM (≤ 1 year v > 1 year). The study protocol with the statistical analysis plan was approved by the institutional review boards of all participating institutions. Celgene provided lenalidomide but had no role in the design of the protocol, data collection, data analysis, or preparation of the manuscript.
Patients
Eligible patients had a diagnosis of asymptomatic intermediate or high-risk SMM made within the past 60 months as confirmed by both of the following within 28 days before registration: bone marrow plasmacytosis with 10% or more plasma cells—or sheets of plasma cells—and an abnormal serum free light chain (FLC) ratio (< 0.26 or > 1.65) by serum FLC assay. Original eligibility criteria, time since high-risk SMM diagnosis within 1 year, and abnormal FLC ratio of less than 0.125 or greater than 8 were revised early on in the phase III study—addendum activated in December 2013 when 21 patients had enrolled—to enhance the accrual rate. Patients who met the most recent myeloma-defining event definition of symptomatic myeloma were excluded from the trial.
End Points and Assessments
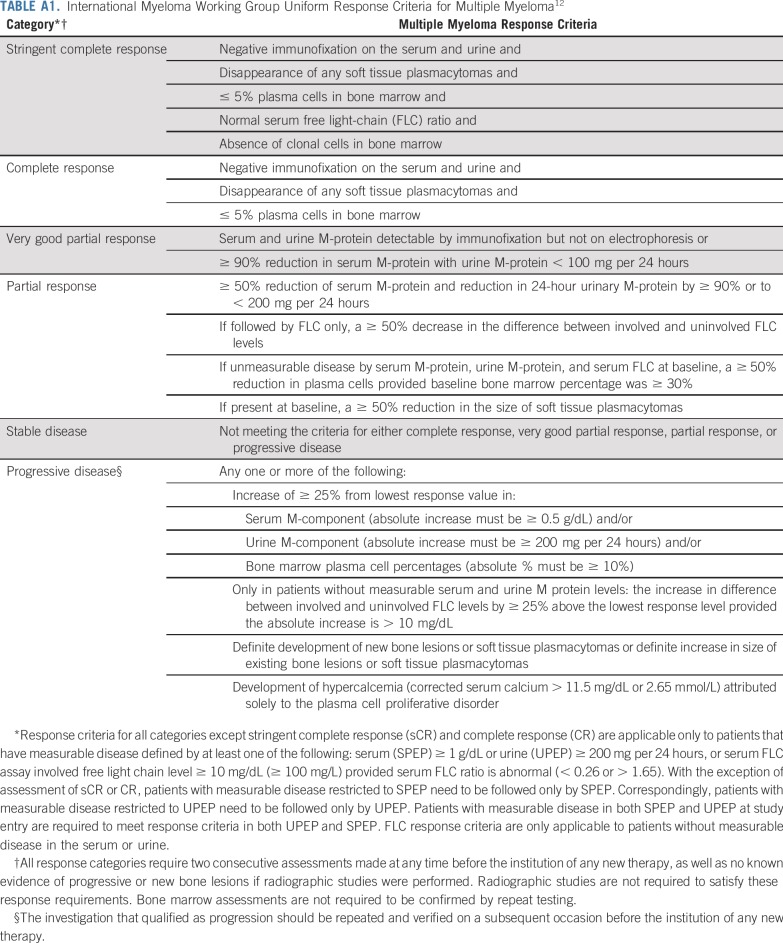
The primary end point was PFS defined as the time since randomization to the development of symptomatic MM as outlined in the American Society of Hematology/US Food and Drug Administration panel consensus.11 Accordingly, progression to symptomatic MM required the presence of biochemical disease progression as defined by the International Myeloma Working Group (IMWG) criteria for MM12 and evidence of end-organ damage felt related to the underlying clonal plasma cell proliferative disorder on the basis of the presence of one or more of the following: hypercalcemia (> 11 mg/dL), renal insufficiency (rise in serum creatinine ≥ 2 mg/dL), anemia (decrease of hemoglobin of ≥ 2 g/dL), or bone disease (development of myeloma bone lesions or soft-tissue plasmacytoma; Appendix Tables A1 and A2, online only). Response was assessed according to IMWG criteria. In patients who received lenalidomide, relative dose intensity was calculated as a percentage of full dose per protocol.
Statistical Analysis
The Kaplan-Meier method was used to characterize event-time distributions and the corresponding treatment hazard ratio (HR; lenalidomide/observation) was estimated using a stratified Cox regression model. PFS was compared between treatment groups using a stratified log-rank test in an intent-to-treat analysis including all randomized patients. Cumulative incidence (CI) estimates of second primary cancers (SPC) and progression considered death as a competing risk. Response, toxicity, SPC, and health-related quality of life (HRQoL) were evaluated in all patients starting assigned treatment. HRQoL was measured with the Physical (P) and Functional (F) domains of the Functional Assessment of Cancer Therapy-Global (FACT-G: P+F; 14 items, scored 0-56) and the FACT-Multiple Myeloma (FACT-MM;14 items scored 0-56) at registration, on treatment every 6 cycles up to 4 years and at early treatment discontinuation. Higher scores indicate better HRQoL. The mean difference in scores between arms was calculated with 95% confidence intervals with the change in FACT-G: P+F from baseline to cycle 24 the primary HRQoL endpoint. A minimally important difference (MID) of 4-6 points for between treatment group differences was pre-specified.13
The study was monitored by the ECOG-ACRIN data safety monitoring committee (DSMC). Data cutoff for this report was January 25, 2019. Additional details on the methods are provided in the Appendix (online only).
RESULTS
Patients and Treatment
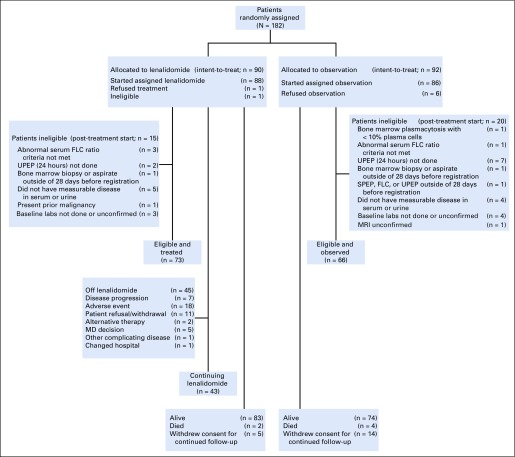
Between January 2011 and January 2013, 44 patients were enrolled in the phase II safety run in portion of the study and were treated with lenalidomide as a single agent (Appendix Fig A1, online only). Between February 2013 and July 2017, 182 patients were randomly assigned between the two treatment arms, with 90 in the lenalidomide arm and 92 in the observation arm (Fig 1).
FIG 1.
CONSORT diagram for phase III. FLC, free light chain; SPEP, serum protein electropheresis; UPEP, urine protein electropheresis.
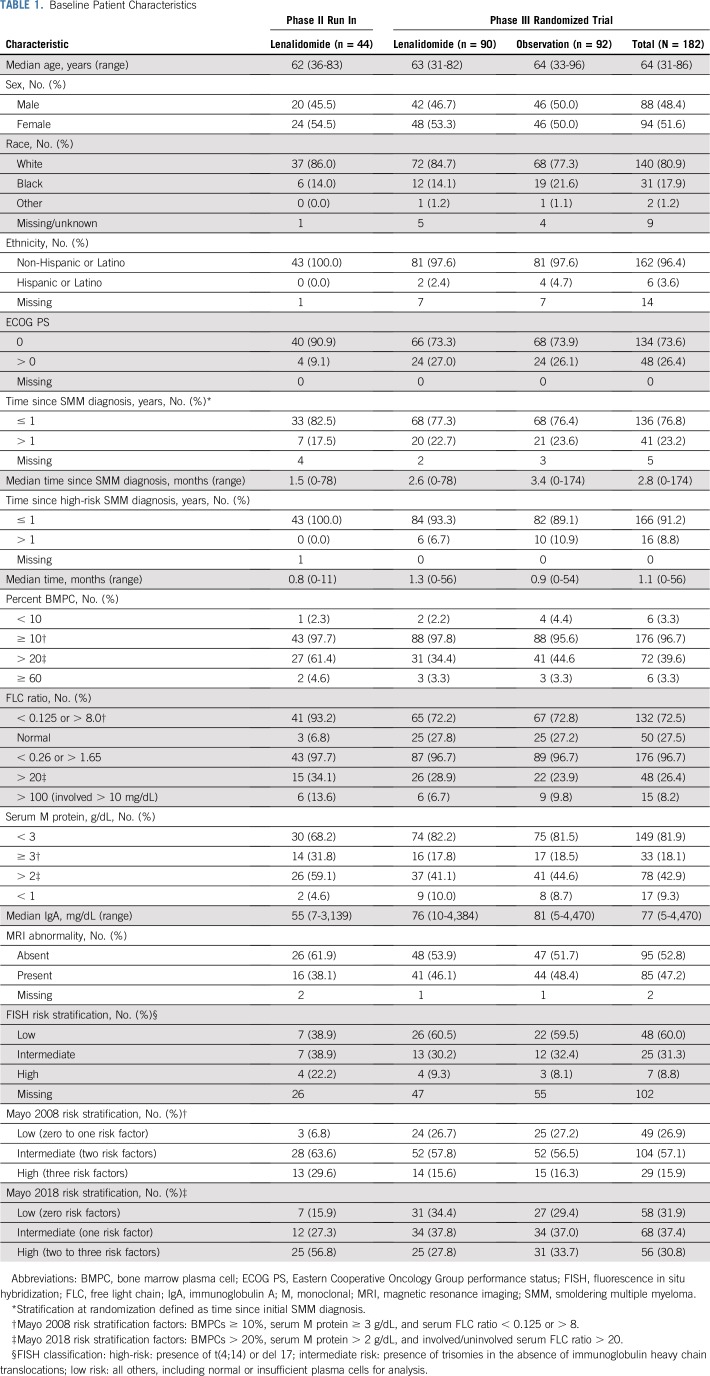
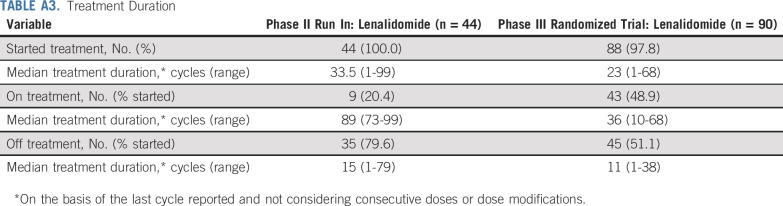
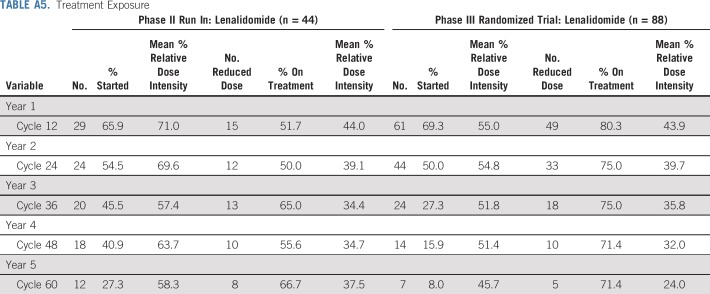
Baseline patient and disease characteristics are listed in Table 1 for both phases of the study. Patient characteristics were well balanced between randomized arms, including classification by proposed risk models and related underlying parameters.14,15,16 Treatment duration, reason for going off treatment, and treatment exposure are listed in Appendix Tables A3, A4, and A5 (online only). Of patients who started treatment, 80% (phase II) and 51% (phase III) are off lenalidomide. Overall median treatment duration was 33.5 cycles and 23 cycles for the phase II and III studies, respectively, with patients off treatment contributing much less time. Going off treatment was primarily a result of patient withdrawal or adverse events. Among the 69% of phase III patients on treatment by cycle 12, relative dose intensity was 55% with 80% of treated patients on a reduced dose.
TABLE 1.
Baseline Patient Characteristics
Efficacy
In the phase II run in, median overall survival follow-up was 82 months (95% CI, 72 months to 84 months) and the 5-year PFS was 78% (95% CI, 65% to 93%). Seven deaths have occurred, resulting in a 5-year overall survival rate of 88% (95% CI, 78% to 98%; Appendix Fig A2, online only). Three-year cumulative incidence of progression was 10.4% (Appendix Fig A2).
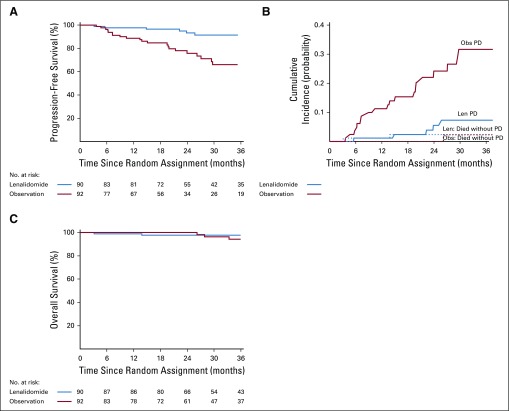
At the second planned interim analysis of PFS (July 2018 data extraction with 38% of full information available), the prespecified criteria for significance was met (one-sided stratified log-rank test P = .00025 compared with the nominal significance level of .0005) for the randomized trial. The independent DSMC recommended the release of the data. Median overall survival follow-up at the time of analysis was 35 months (95% CI, 30 months to 37 months). Response to therapy is shown in Table 2 with partial response or better rate equal to 50% (95% CI, 39% to 61%) for the lenalidomide arm. Time to response was a median of 5 months (range, 1 month to 23 months). There were no responses in the observation arm. PFS was significantly longer for lenalidomide compared with observation (treatment HR, 0.28; 95% CI, 0.12 to 0.62; P = .002). One-, 2-, and 3-year PFS was 98%, 93%, and 91% for the lenalidomide arm versus 89%, 76%, and 66% for the observation arm, respectively (Fig 2A). The basis of progression is shown in Appendix Table A6 (online only), with bone progression being the most common cause in the observation group, despite monthly follow-up for both arms. Cumulative incidence of progression at 3 years was 7.3% in the lenalidomide arm and 31.6% in the observation arm (Fig 2B). Six deaths have been reported—two in the lenalidomide arm versus four in the observation arm, with an HR for death of 0.46 (95% CI, 0.08 to 2.53; Fig 2C).
TABLE 2.
Best Response Among Lenalidomide-Treated Patients
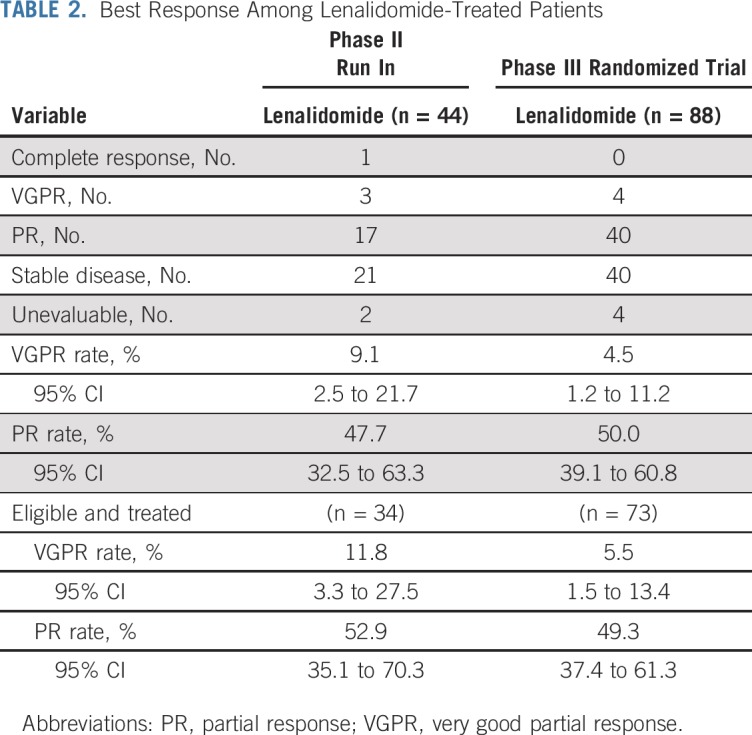
FIG 2.
Time to event estimates by treatment arm in phase III: (A) progression-free survival, (B) cumulative incidence of progression, and (C) overall survival in patients with smoldering multiple myeloma. Len, lenalidomide; Obs, observation.
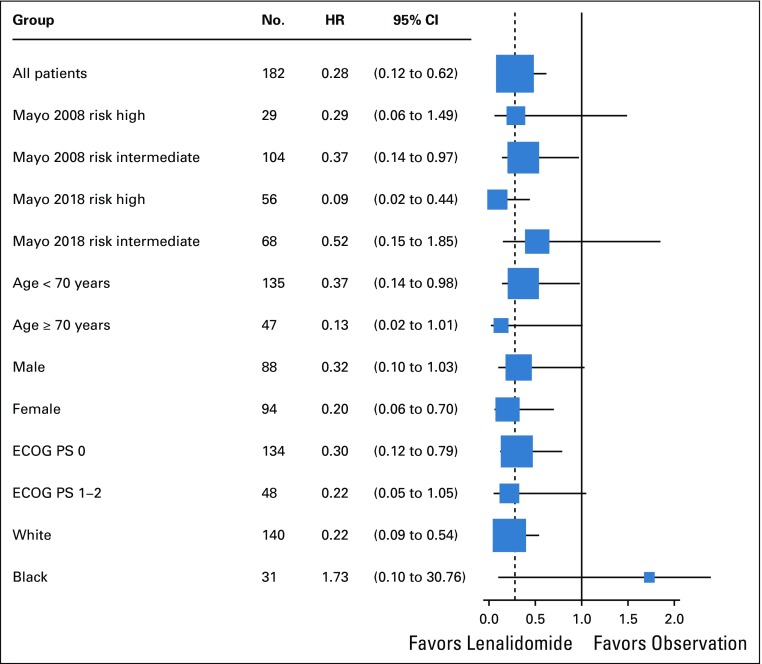
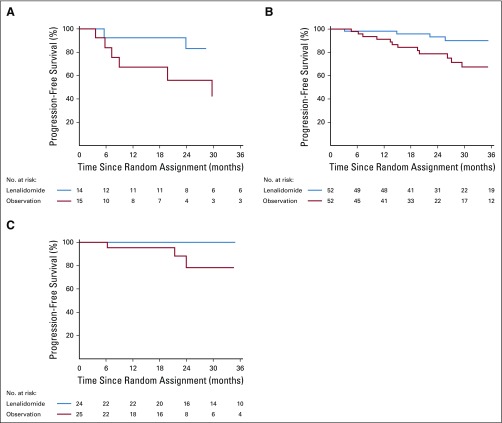
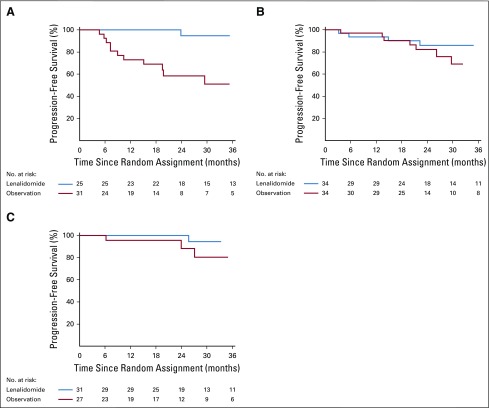
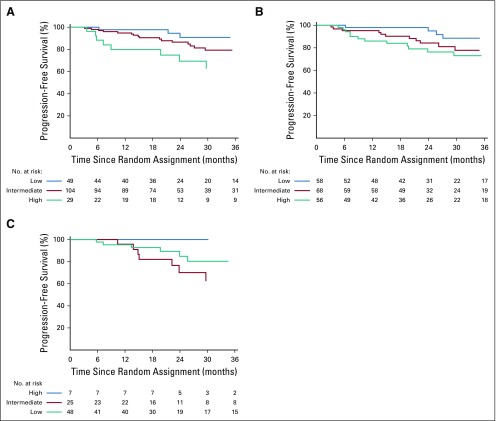
The benefit of lenalidomide was observed in most subgroups, although many subsets are relatively small. Of note, patients in all risk groups listed in Table 1 of Mayo 2008 risk stratification16 and Mayo 2018 risk stratification15 seemed to have an HR that favored early treatment (Figs 3, 4, and 5 and Appendix Fig A3, online only), but this was most pronounced in the Mayo 2018 high-risk category.
FIG 3.
Treatment hazard ratio (HR) for progression-free survival in subgroups in phase III. ECOG PS, Eastern Cooperative Oncology Group performance status.
FIG 4.
Kaplan-Meier estimates of progression-free survival by treatment arm within Mayo 2008 risk subgroups in phase III: (A) high risk, (B) intermediate risk, and (C) low risk.
FIG 5.
Kaplan-Meier estimates of progression-free survival by treatment arm within Mayo 2018 risk subgroup: (A) high risk, (B) intermediate risk, and (C) low risk.
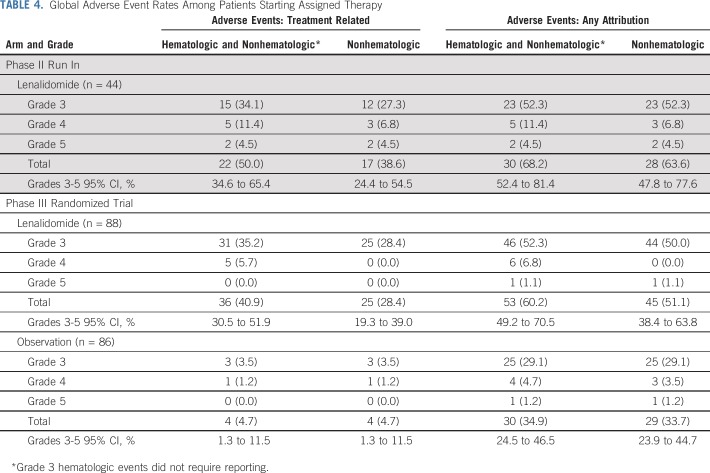
Safety
Adverse events are listed in Tables 3 and 4. In the phase II run in, 44 patients were evaluable for adverse events. Grade 3 or 4 hematologic and nonhematolgic treatment-related adverse events occurred in 20 patients (45%), with nonhematolgic adverse events occurring in 15 patients (34%). Among the phase II patients who were off treatment (n = 35), 34% (n = 12) came off therapy as a result of adverse events. One death from pulmonary embolism occurred during the study and was determined to be therapy related.
TABLE 3.
Grade 3 or Higher (≥ 5%) Adverse Events Among Lenalidomide-Treated Patients
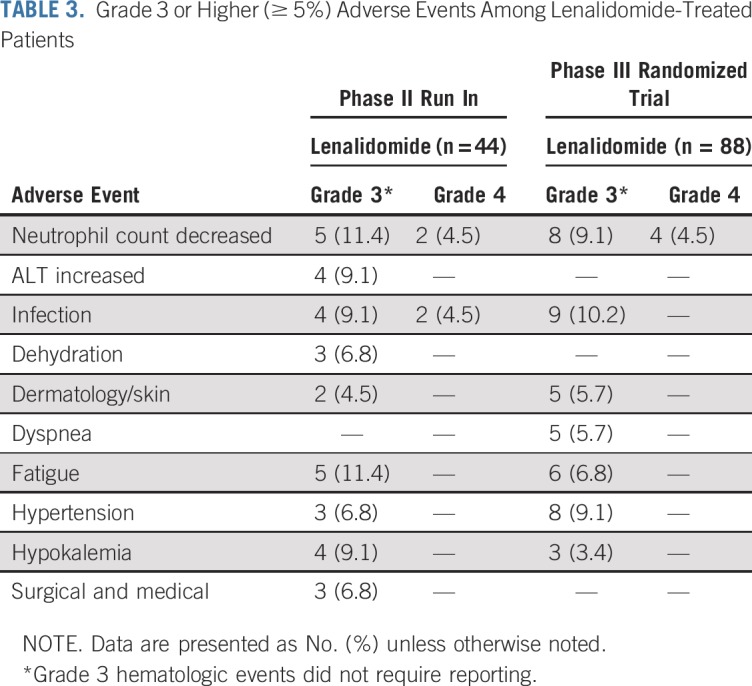
TABLE 4.
Global Adverse Event Rates Among Patients Starting Assigned Therapy
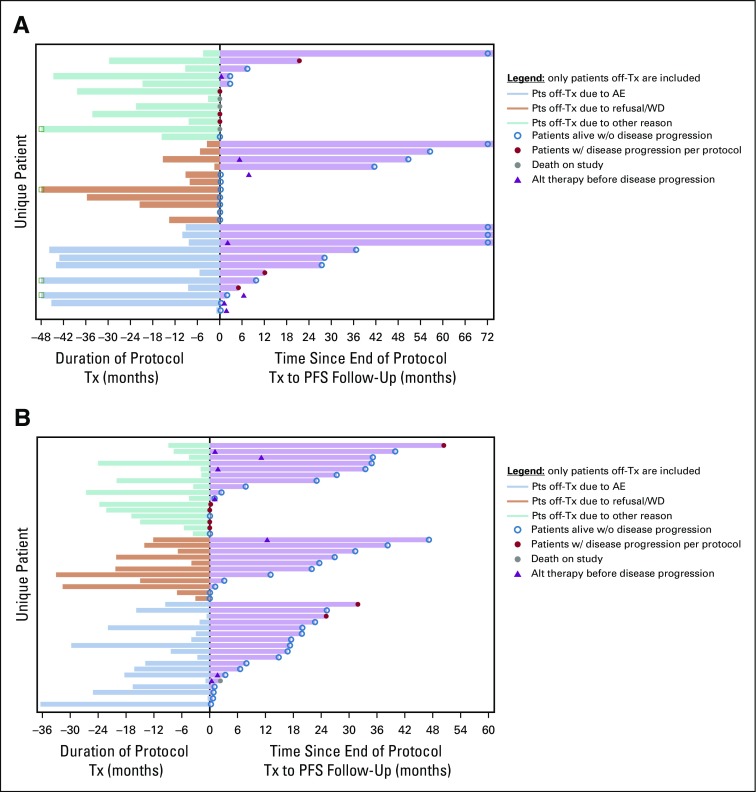
In the randomized trial, among the lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%), with nonhematologic adverse events occurring in 25 patients (28%). Among the phase III patients who were off treatment (n = 45), 40% (n = 18) came off therapy as a result of adverse events. Patients off treatment as a result of adverse events did not seem to have shortened PFS follow-up as evidenced by PFS follow-up extending well beyond the duration of delivered therapy (Appendix Fig A4, online only).
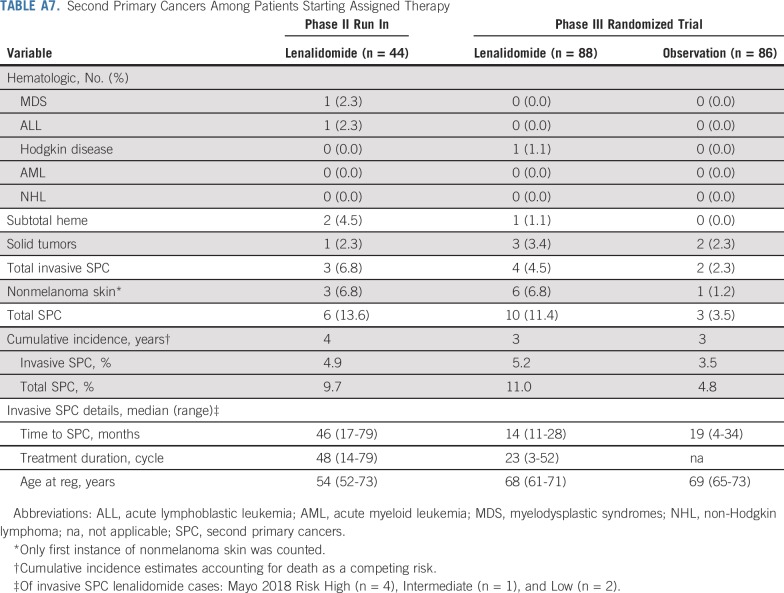
The cumulative incidence of invasive second primary cancers at 4 years was 4.9% in the phase II run in. In the randomized trial, the 3-year CI of invasive second primary cancers was 5.2% in the lenalidomide arm and 3.5% in the observation arm (Appendix Table A7, online only).
Prognostic Factors and Quality-of-Life Analysis
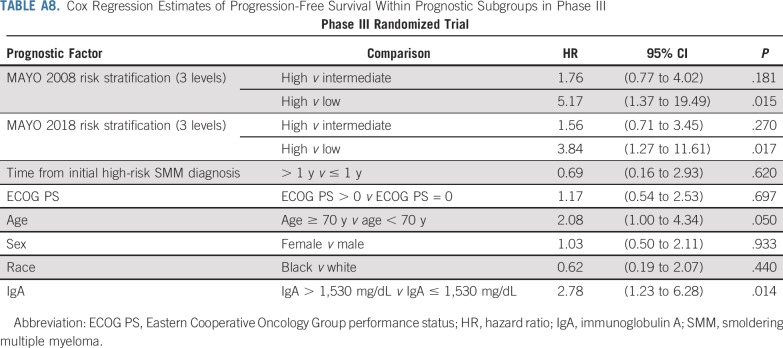
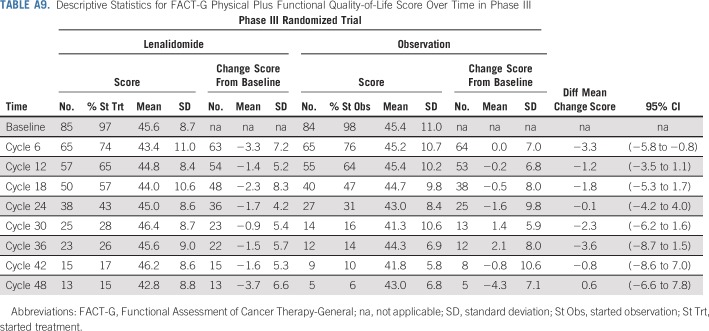
Prognosis of patients in the trial on the basis of baseline risk factors is shown in Appendix Table A8 (online only). There were too few patients with high-risk SMM by fluorescence in situ hybridization to determine the impact of high-risk cytogenetics on prognosis (Appendix Fig A5, online only). Among patients who started the assigned treatment, 97% of lenalidomide-treated patients and 98% of observation patients had baseline health-related quality-of-life data; the difference in mean change score at 24 cycles was −0.1 (95% CI, −4.2 to 4.0; Appendix Table A9, online only; Appendix Fig A6, online only).
DISCUSSION
Kyle and Greipp16 described SMM in 1980 as intermediate disease state between the premalignant monoclonal gammopathy of undetermined significance (MGUS) and symptomatic MM. Patients with SMM are at much higher risk of progression to MM or a related disorder (10% per year)3 compared with patients who have MGUS (1% per year).17 However, the paucity of effective drugs, lack of biomarkers that can identify patients who are at high risk of progression, and the absence of randomized controlled trial data that demonstrate clinical benefit justified observation alone as the standard of care.4 Early attempts to intervene in SMM to prevent end-organ damage were unsuccessful as the drugs used had high toxicity without significant benefit.18-21 In 2014, in an attempt to help patients who were at the highest risk of progression, the IMWG revised the diagnostic criteria for MM, incorporating three biomarkers that identify patients with SMM who faced an 80% risk of progression within 2 years.1 However this change affects only a small proportion of patients with SMM, and patients who fit this new definition of myeloma were not included in the current trial.
In this randomized trial, we demonstrate a significant prolongation of the time to symptomatic MM (PFS) with single-agent lenalidomide in a patient population that was well classified at baseline using modern imaging and widely available prognostic factors. Whereas we did include patients who would not be considered high risk by the current definition, we do not currently recommend that they be started on early therapy pending confirmation data. Our results mirror those previously published by the Spanish group in effect size (similar HR for early treatment v observation), which confirms the benefit of early therapy for the high-risk group.22 Too few deaths have occurred in our study to determine the impact on overall survival in our trial; however, with a similar magnitude of benefit in PFS, a survival benefit has already been demonstrated in the Spanish trial. Prevention of serious symptomatic end-organ damage—osteolytic bone lesions, acute renal failure, etc—is by itself an important goal given the longevity of patients with myeloma with modern therapy and should be recognized as an important goal of therapy in the smoldering population. Bone progression was the most common reason for progression in the trial overall but occurred far more frequently in the observation arm compared with the treatment arm (11 v three; Appendix Table A6). Hence, we believe that our results support the use of early intervention in patients with high-risk SMM—as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest—rather than continued observation.
The concept of interception, or prevention of progression, for a premalignant condition has emerged as an important concept in human cancer.23 There is a distinction between myeloma therapy—standard induction; consolidation, including stem-cell transplantation; and maintenance—versus prevention, which can take a less-intensive approach. This is especially important given data that suggest that immune control of the malignant clone may be a powerful and important regulator of progression from smoldering to symptomatic myeloma.24 Before the current study, only one trial had demonstrated benefit with a control arm—the Spanish trial—and whereas it did prove the principle that early therapy can affect outcomes without causing harm, sufficient design issues precluded widespread adoption as a standard. The current trial builds on the knowledge from that trial and here again has demonstrated benefit with lenalidomide alone in preventing the development of myeloma with associated organ damage. As such, this trial represents a paradigm shift in hematologic malignancies. We have demonstrated that early intervention with prevention does prevent the development of organ damage and symptoms, with a 72% risk reduction in more than 2 years compared with the control arm, and among the Mayo 2018 high-risk category, an HR of 0.09 that favored early intervention. Three-year PFS for the lenalidomide arm in the phase III trial is comparable to that which has been demonstrated in other smaller, phase II trials of more intensive therapy. Five-year PFS for the phase II portion of the study (78%) is also on par with that of other smaller, phase II studies that used more aggressive treatment approaches despite a short duration of therapy. Whereas other approaches using traditional myeloma therapy may ultimately be proven to be superior in the long term, they have not been tested or validated in phase III randomized trials as we currently report here. Given the data on immune regulation of progression, these therapies may be worse in the long run. This is an important distinction as we seek to answer the question of which approach—prevention or treatment—is optimal for these patients with SMM. It is also possible that, although a small fraction of patients may be cured with more aggressive therapy early, an operational cure with prevention using less intensive therapy may net similar end points without the toxicity of standard therapy. Use of surrogate end points, such as minimal residual disease negativity at early timepoints, remains unclear, and our trial suggests that control can be achieved without major responses.
Therapy with single-agent lenalidomide in our study did not have a negative impact on health-related quality of life and the toxicity profile was consistent with previous descriptions of lenalidomide-related adverse events and, in most cases, could be managed with dose modifications. Given data from the Spanish trial with limited duration therapy and our median duration of therapy of 23 months in the phase III trial, we would recommend 2 years of therapy for the highest-risk patients to limit long-term adverse events. Although only a few patients achieved deep responses—very good partial response or complete response—the effect of even limited duration of lenalidomide still offered benefit of much longer PFS. This suggests that prolonged stability is feasible in the absence of a major reduction in clonal burden. Data from a Southwest Oncology Group trial suggest that preexisting antitumor immunity is an independent predictor of the risk of progression of SMM to symptomatic MM.24 If this is correct, using such treatments as lenalidomide which enhance immune function and long-term immunologic memory can result in prolonged disease stability. Early intervention to achieve durable immunologic control is also supported by recent studies that show enrichment of stem-like memory cells in MGUS, which undergo attrition in MM.25 Conversely, it is possible that more aggressive therapy as that currently used to treat clinical MM may provide greater benefit; however, this has not yet been tested in a randomized trial. A current randomized trial by ECOG-ACRIN (ClinicalTrials.gov identifier: NCT03937635) is comparing daratumumab, lenalidomide, and dexamethasone—treatment strategy using an approved MM triplet regimen—versus lenalidomide plus dexamethasone—preventive strategy—and will provide clarity on this issue.
In summary, our data, together with the results of the Spanish trial,7 support early therapy as the new standard of care in high-risk SMM on the basis of clear clinical benefit in the prevention of end-organ damage demonstrated in two independent randomized trials. Patients defined as having high-risk SMM by Mayo 2018 criteria,15 which has recently been validated by the IMWG,28 gain the greatest benefit from early therapy and are the only group for whom we recommend early intervention using lenalidomide/lenalidomide plus dexamethasone. Additional trials seeking to increase the intensity of treatment in these patients should use this less intensive approach as a comparator.
ACKNOWLEDGMENT
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group cochairs).
Appendix
Trial Design, Oversight, and Treatment
Randomization was conducted centrally using permuted blocks within stratification levels while allowing for institutional balancing. This trial also incorporated a safety Phase 2 run in prior to the start of the randomized portion of the trial. The study was coordinated by the ECOG-ACRIN Cancer Research Group.
Patients
Patients needed to have measurable levels of monoclonal protein (M-protein) at baseline defined as ≥ 1g/dL in the serum and/or ≥ 200 mg/24 hours in the urine. Baseline skeletal survey and magnetic resonance imaging of the spine and pelvis were required to exclude myeloma bone lesions or plasmacytomas. Patients were required to have ECOG performance status of 0-2, and adequate organ function. All patients were required to provide written informed consent.
Endpoints and Assessments
Disease assessments were performed at the same times for both treatment and observation arms (every cycle on treatment and in long-term follow-up) from study entry. Adverse events as described are based on the NCI Common Terminology Criteria for Adverse Events v4.0 (v5.0 since 4-1-2018). Grade 3 hematologic AEs were not required reporting. All new cases of second primary cancers (SPC; second or secondary malignancies) were required to be reported within 30 days of diagnosis throughout the entire follow-up period of 10 years. Fluorescent in situ hybridization was used to identify key SMM cytogenetic risk factors.14
Statistical Analysis
With 180 patients accrued over 45 months and an additional 9 months of follow-up to achieve full information (n=76 events), there was 96% power to detect a hazard ratio of 0.40 at a one-sided 2.5% significance level. This corresponded to a 150% improvement in median progression-free survival from 24.8 months on the observation arm to 62 months on the lenalidomide arm given exponential distribution of failure. There also was adequate power (81%) to detect a 60% reduction in the risk of death at a one-sided 2.5% significance level assuming full event information (47 deaths) at 7.5 years from activation.
FIG A1.
CONSORT diagram for phase II. FLC, free light chain; SPEP, serum protein electropheresis; UPEP, urine protein electropheresis.
FIG A2.
Time to event estimates in phase II: (A) progression-free survival, (B) cumulative incidence of progression, and (C) overall survival in patients with smoldering multiple myeloma. Len, lenalidomide.
FIG A3.
Kaplan-Meier estimates of progression-free survival by treatment arm within fluorescence in situ hybridization risk subgroups in phase III: (A) high risk, (B) intermediate risk, and (C) low risk.
FIG A4.
Swimmer’s plot patterns of treatment duration and follow-up by reason off-treatment (Tx): (A) Patients off-treatment phase II. (B) Patients off treatment phase III. AE, adverse event; PFS, progression-free survival; WD, withdrawal.
FIG A5.
Kaplan-Meier estimates of progression-free survival within prognostic subgroups in phase III: (A) Mayo 2008 risk stratification, (B) Mayo 2018 risk stratification, and (C) fluorescence in situ hybridization risk stratification.
FIG A6.
Health-related quality of life scores over time in phase III by Functional Assessment of Cancer Therapy-General (FACT-G): (A) FACT-G: Physical plus functional (P+F) well-being score (range, 0-56). (B) FACT-multiple myeloma (MM) score (range, 0-56). (C) FACT-G: Physical (P) well-being score (range, 0-28). (D) FACT-G: Functional (F) well-being score (range, 0-28).
TABLE A1.
International Myeloma Working Group Uniform Response Criteria for Multiple Myeloma12
TABLE A2.
American Society of Hematology/US Food and Drug Administration Panel Consensus11
TABLE A3.
Treatment Duration
TABLE A4.
Reason Off-Treatment
TABLE A5.
Treatment Exposure
TABLE A6.
Basis of Progression
TABLE A7.
Second Primary Cancers Among Patients Starting Assigned Therapy
TABLE A8.
Cox Regression Estimates of Progression-Free Survival Within Prognostic Subgroups in Phase III
TABLE A9.
Descriptive Statistics for FACT-G Physical Plus Functional Quality-of-Life Score Over Time in Phase III
Supported by National Cancer Institute Grants No. CA180820, CA180794, CA180790, CA180853, CA180858, CA180864, CA189805, CA189863, CA189870, CA180888, CA180826, and CA197603 (IF QOL: CA189828), National Cancer Institute Grant No. R35-CA197603 (M.V.D.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
See accompanying Oncology Grand Rounds on page 1119
See accompanying Oncographic at doi: 10.1200/JCO.19.02273
AUTHOR CONTRIBUTIONS
Conception and design: Sagar Lonial, Susanna Jacobus, Rafael Fonseca, Matthias Weiss, Shaji Kumar, Abdulraheem M. Yacoub, Lynne I. Wagner, Madhav V. Dhodapkar, S. Vincent Rajkumar
Administrative support: Matthias Weiss, S. Vincent Rajkumar
Provision of study material or patients: Sagar Lonial, Matthias Weiss, Robert Z. Orlowski, Jonathan L. Kaufman, Jeffrey V. Matous, Robert V. Emmons, S. Vincent Rajkumar
Collection and assembly of data: Sagar Lonial, Susanna Jacobus, Matthias Weiss, Shaji Kumar, Robert Z. Orlowski, Jonathan L. Kaufman, Francis K. Buadi, Timothy O’Brien, Jeffrey V. Matous, Daniel M. Anderson, Robert V. Emmons, Anuj Mahindra, Madhav V. Dhodapkar, S. Vincent Rajkumar
Data analysis and interpretation: Sagar Lonial, Susanna Jacobus, Rafael Fonseca, Matthias Weiss, Shaji Kumar, Robert Z. Orlowski, Jonathan L. Kaufman, Abdulraheem M. Yacoub, Francis K. Buadi, Anuj Mahindra, Lynne I. Wagner, Madhav V. Dhodapkar, S. Vincent Rajkumar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sagar Lonial
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Juno Therapeutics
Research Funding: Celgene, Bristol-Myers Squibb, Takeda
Rafael Fonseca
Stock and Other Ownership Interests: Adaptive Biotechnologies
Honoraria: Celgene, Bristol-Myers Squibb, Bayer, Amgen, Janssen, Kite Pharma, Merck Sharp & Dohme, Juno Therapeutics, Takeda, AbbVie, Aduro Biotech, Sanofi
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Bayer, Amgen, Janssen, AbbVie, Kite Pharma, Merck Sharp & Dohme, Juno Therapeutics, Takeda, Aduro Biotech, Sanofi
Patents, Royalties, Other Intellectual Property: Patent for the prognostication of multiple myeloma based on genetic categorization of the disease
Travel, Accommodations, Expenses: Multiple
Other Relationship: Adaptive Biotechnologies
Shaji Kumar
Honoraria: Reddys Laboratories
Consulting or Advisory Role: Takeda (Inst), Janssen Oncology (Inst), Amgen (Inst), AbbVie (Inst), Merck (Inst), Adaptive Biotechnologies, Celgene (Inst), Genentech (Inst), Oncopeptides, Kite Pharma (Inst), Genecentrix
Research Funding: Celgene (Inst), Takeda (Inst), AbbVie (Inst), Novartis (Inst), Sanofi (Inst), Janssen Oncology (Inst), Merck (Inst), Kite Pharma (Inst), MedImmune (Inst), Genentech (Inst)
Robert Z. Orlowski
Consulting or Advisory Role: Bristol-Myers Squibb, Celgene, Janssen, Amgen, Kite Pharma, Sanofi, Takeda
Research Funding: Amgen, BioTheryX, Spectrum Pharmaceuticals
Jonathan L. Kaufman
Consulting or Advisory Role: Janssen, Takeda, Celgene, Bristol-Myers Squibb, Karyopharm Therapeutics, Incyte, TG Therapeutics, Sanofi, Amgen
Research Funding: Novartis (Inst), Merck (Inst), Celgene (Inst), Janssen (Inst)
Abdulraheem M. Yacoub
Stock and Other Ownership Interests: Hylapharm, Dynavax, Cara Therapeutics, Arderlyx
Consulting or Advisory Role: Incyte
Speakers' Bureau: Incyte, Novartis, Seattle Genetics, Agios
Jeffrey V. Matous
Honoraria: Celgene
Consulting or Advisory Role: Celgene, Pharmacyclics
Speakers' Bureau: Celgene
Research Funding: Celgene (Inst), Seattle Genetics (Inst)
Anuj Mahindra
Consulting or Advisory Role: Amgen, Genentech, Oncopeptides, Gilead Sciences
Speakers' Bureau: Sanofi, Janssen Oncology, Amgen
Research Funding: Takeda, Celgene, Janssen Oncology, Genentech, Bristol-Myers Squibb
Lynne I. Wagner
Consulting or Advisory Role: EveryFit, Janssen, Celgene
Madhav V. Dhodapkar
Consulting or Advisory Role: Genentech, Amgen, Kite Pharma, Lava Therapeutics, Janssen Oncology, Celgene
S. Vincent Rajkumar
Patents, Royalties, Other Intellectual Property: Authorship royalties from UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial IM, Landgren O. How I treat smoldering multiple myeloma. Blood. 2014;124:3380–3388. doi: 10.1182/blood-2014-08-551549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125:3069–3075. doi: 10.1182/blood-2014-09-568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry BM, Korde N, Kwok M, et al. Modeling progression risk for smoldering multiple myeloma: Results from a prospective clinical study. Leuk Lymphoma. 2013;54:2215–2218. doi: 10.3109/10428194.2013.764419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Waxman AJ. Multiple myeloma precursor disease. JAMA. 2010;304:2397–2404. doi: 10.1001/jama.2010.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mateos M-V, Hernández M-T, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369:438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 8.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1:746–754. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillengass J, Moulopoulos LA, Delorme S, et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: A study of the International Myeloma Working Group. Blood Cancer J. 2017;7:e599. doi: 10.1038/bcj.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastritis E, Moulopoulos LA, Terpos E, et al. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia. 2014;28:2402–2403. doi: 10.1038/leu.2014.230. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KC, Kyle RA, Rajkumar SV, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 12.Durie BG, Harousseau J-L, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [Erratum: Leukemia 20:2220, 2006; Leukemia 21:1134, 2007] [DOI] [PubMed] [Google Scholar]
- 13.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Gupta V, Fonseca R, et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013;27:1738–1744. doi: 10.1038/leu.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8:59. doi: 10.1038/s41408-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle RA, Greipp PR. Smoldering multiple myeloma. N Engl J Med. 1980;302:1347–1349. doi: 10.1056/NEJM198006123022405. [DOI] [PubMed] [Google Scholar]
- 18.Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjorth M, Hellquist L, Holmberg E, et al. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I: A randomized study. Eur J Haematol. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Grignani G, Gobbi PG, Formisano R, et al. A prognostic index for multiple myeloma. Br J Cancer. 1996;73:1101–1107. doi: 10.1038/bjc.1996.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riccardi A, Mora O, Tinelli C, et al. Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: A multicentre randomized study. Br J Cancer. 2000;82:1254–1260. doi: 10.1054/bjoc.1999.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witzig TE, Laumann KM, Lacy MQ, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2013;27:220–225. doi: 10.1038/leu.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateos MV, San Miguel JF. Treatment for high-risk smoldering myeloma. N Engl J Med. 2013;369:1762–1765. doi: 10.1056/NEJMc1310911. [DOI] [PubMed] [Google Scholar]
- 24.Blackburn EH. Cancer interception. Cancer Prev Res (Phila) 2011;4:787–792. doi: 10.1158/1940-6207.CAPR-11-0195. [DOI] [PubMed] [Google Scholar]
- 25.Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120) Blood. 2014;123:78–85. doi: 10.1182/blood-2013-07-515239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailur JK, McCachren SS, Doxie DB, et al. Early alterations in stem-like/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy. JCI Insight. 2019;5:127807. doi: 10.1172/jci.insight.127807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): Long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:1127–1136. doi: 10.1016/S1470-2045(16)30124-3. [DOI] [PubMed] [Google Scholar]
- 28.San Miguel JF, Mateos MV, Gonzalez V, et al. Updated risk stratification model for smoldering multiple myeloma (SMM) incorporating the revised IMWG diagnostic criteria. J Clin Oncol. 2019;37(suppl; abstr A8000) [Google Scholar]