Abstract
MicroRNAs (miRNAs) are small non-coding RNAs (typically consisting of 18–25 nucleotides) that negatively control expression of target genes at the post-transcriptional level. Owing to the biological significance of miRNAs, miRTarBase was developed to provide comprehensive information on experimentally validated miRNA–target interactions (MTIs). To date, the database has accumulated >13,404 validated MTIs from 11,021 articles from manual curations. In this update, a text-mining system was incorporated to enhance the recognition of MTI-related articles by adopting a scoring system. In addition, a variety of biological databases were integrated to provide information on the regulatory network of miRNAs and its expression in blood. Not only targets of miRNAs but also regulators of miRNAs are provided to users for investigating the up- and downstream regulations of miRNAs. Moreover, the number of MTIs with high-throughput experimental evidence increased remarkably (validated by CLIP-seq technology). In conclusion, these improvements promote the miRTarBase as one of the most comprehensively annotated and experimentally validated miRNA–target interaction databases. The updated version of miRTarBase is now available at http://miRTarBase.cuhk.edu.cn/.
INTRODUCTION
MicroRNAs (miRNAs) are a family of small non-coding RNAs with 18–25 nucleotides; they are transcribed from DNA sequences into primary miRNAs and then processed into precursor and mature miRNAs in animals and plants. Perfect or near-perfect complementary binding of miRNAs to their target mRNAs negatively regulates gene expression in terms of accelerating mRNA degradation or suppressing mRNA translation (1). Many investigations have reported that miRNAs play crucial roles in numerous biological processes, such cell cycle, cell proliferation, cell differentiation, apoptosis, metabolism, and cellular signaling (2,3), and various diseases. The study of miRNA–target interactions (MTIs) has been gaining a surge of interest from researchers due to the causal relationship between miRNAs and disease development. An increasing number of studies (4–6) have reported extracellular/circulating miRNAs in biological fluids, such as plasma, serum, cerebrospinal fluid, saliva, breast milk, urine, tears, colostrum and peritoneal fluid. Most importantly, dysregulated expression patterns of miRNAs may serve as potential biomarkers for disease diagnosis and prognosis.
Owing to the biological significance of miRNAs, a number of online resources have been developed for the data warehousing and functional analysis of MTIs. miRBase is the primary miRNA sequence repository that facilitates the inquiry for comprehensive miRNA nomenclature, sequence and annotation data (7). DIANA-TarBase accumulates manually curated interactions between miRNA and gene with the annotations of detailed meta-data, experimental methodologies and conditions (8). HMDD can provide functional enrichment information on miRNAs and the regulatory network of MTIs (9,10). miR2Disease can provide users disease-associated MTIs based on the information on miRNA–disease relationships obtained from research articles (11). In recent years, a variety of computational methods have been published for the identification of target binding sites of miRNAs, mainly according to the base pairing of miRNA and target sequences (12). However, these approaches developed on the basis of perfect seed pairing may result in false-positive predictions of MTIs (13). Therefore, these predicted results based on wet-lab experiments, such as real-time quantitative reverse transcription–polymerase chain reaction, enzyme-linked immunosorbent assay, immunohistochemistry and western blot, must be verified. Additionally, reporter assay is widely used in examining the physical interaction between miRNA and its target through the evidence of decreased expression level of a reporter protein (14). Given the surge of high-throughput sequencing technologies (15), the sequencing data obtained from CLIP (16), PAR-CLIP (17) and CLASH (18) can be used to explore miRNAs and their targets with expression evidence.
In post-transcriptional regulations, understanding how miRNAs downregulate target genes and transcription factors (TFs) regulate miRNAs is critical. More evidence has indicated that aberrant regulation of miRNAs by TFs can induce phenotypic variations and diseases. TransmiR is a knowledge base of comprehensive transcriptional networks between TFs and miRNAs that serve as gene dysregulators in different diseases (19). DIANA-miRGen (20) and mirTrans (21) were designed to provide annotations of cell-specific miRNA TSSs and discovery of TF-miRNA regulation networks, respectively. SomamiR is a database of somatic mutations affecting mRNAs and noncoding RNAs between miRNAs and their targets; miR2GO is a computational tool in miRNA seed regions for knowledge-based analysis of genetic variants and somatic mutations (22,23). miRNA sponges are competing endogenous RNAs (ceRNAs); miRSponge was established as an experimentally supported database that contains 599 miRNA-sponge interactions and 463 ceRNA relationships from 11 species (24).
Owing to an increasing number of miRNA–target interaction studies being published over the past 10 years, miRTarBase (25–28) was developed to integrate MTIs and their functional roles in various biological processes. In this update, miRTarBase aims to accumulate experimentally validated MTIs, which are subsequently manually curated, based on a high-accuracy text-mining system. Furthermore, the upstream (TFs to miRNAs) and downstream (miRNAs to targets) regulations of miRNAs in different diseases have been integrated into this updated resource to enhance the functional analyses of miRNAs.
SYSTEM OVERVIEW AND DATABASE CONTENT
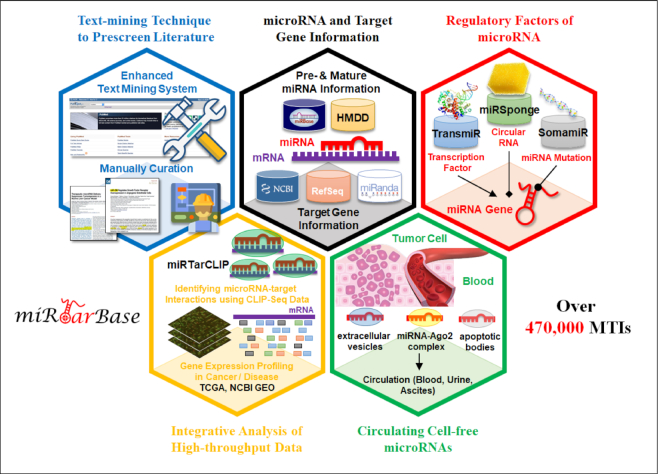
Since the first version of miRTarBase was released in 2011, the number of experimentally validated MTIs has increased drastically over the last 10 years. Meanwhile, the web interface of miRTarBase has been enhanced constantly to provide users more effective and efficient accessing experience. In recent upgrades, miRTarBase was further dedicated to provide a comprehensive MTI repository with a finer user interface. Figure 1 presents the highlighted improvements of this update. With an attempt to provide users the most comprehensive information on MTIs, a text-mining system was enhanced to search for more MTI-related articles against PubMed literature database. Our curators then manually curated these MTI-related articles to extract validated miRNA–target interactions with promising experimental evidence. In addition, many biological databases and standalone tools were integrated to increase the academic value of miRTarBase: miRNA information from miRBase (7); target gene information from National Center for Biotechnology Information (NCBI) Entrez (29) and RefSeq (30); miRNA regulators information from TransMir (19), miRSponge (24), and SomamiR (22,31); disease information from HMDD (9,10); gene and miRNA expression profiling from Gene Expression Omnibus (GEO) (32); The Cancer Genome Atlas (TCGA) (33,34); Circulating MicroRNA Expression Profiling (CMEP) (35). Table 1 displays a list of the databases that have been integrated into miRTarBase. Furthermore, in this update, users can access all relevant information on MTIs of interest through a user-friendly and interactive visualization interface.
Figure 1.
Highlighted improvements of miRTarBase 2020. As the most comprehensive resource on miRNA–target interactions, this update accumulates >470,000 manually confirmed MTIs supported with experimental evidence.
Table 1.
List of the databases that are integrated by miRTarBase
| Type | Database name |
|---|---|
| Gene and miRNA-specific databases | miRBase (7), NCBI Entrez gene (29), NCBI RefSeq (30) |
| SNP or mutation related databases | SomamiR (22,31) |
| miRNA–disease association Database | HMDD (9,10) |
| The regulation of miRNAs | TransMir (19), miRSponge (24) |
| miRNAs expression | CMEP (35), Gene Expression Omnibus (GEO) (32), The Cancer Genome Atlas (TCGA) (33,34) |
UPDATED DATABASE CONTENT AND STATISTICS
Table 2 presents the improvements and updated content of miRTarBase 2020. As indicated in the table, the number of curated articles, MTIs and species increased substantially. Up to September 2019, this update (version 8) has significantly increased the number of MTIs compared with miRTarBase 7.0. A total of 479,340 curated MTIs between 4,312 miRNAs and 23,426 target genes were collected from 11,021 research articles. In addition, this updated version has integrated additional 100 CLIP-seq data from nine independent studies that can be used to support many MTIs (Supplementary Table S1).
Table 2.
Improvements and the number of miRNA–target interactions with different validation methods provided by miRTarBase 8.0
| Features | miRTarBase 7.0 | miRTarBase 8.0 |
|---|---|---|
| Release date | 2017/09/15 | 2019/09/15 |
| Known miRNA entry | miRBase v21 | miRBase v22 |
| Known Gene entry | Entrez 2017 | Entrez 2019 |
| Species | 23 | 32 |
| Curated articles | 8,510 | 11,021 |
| miRNAs | 4,076 | 4,312 |
| Target genes | 23,054 | 23,426 |
| CLIP-seq datasets | 231 | 331 |
| Curated miRNA–target interactions | 422,517 | 479,340 |
| Text-mining technique to prescreen literature | Enhanced NLP | Enhanced NLP+Scoring system |
| Download by validated miRNA–target sites | Yes | Yes |
| Browse by miRNA, gene, and disease | Yes | Yes |
| Regulation of miRNAs | No | Yes |
| Cell-free miRNA expression | No | Yes |
| MTIs Supported by strong experimental evidences | ||
| Number of MTIs validated by ‘Reporter assay’ | 9,489 | 13,922 |
| Number of MTIs validated by ‘Western blot’ | 7,258 | 12,179 |
| Number of MTIs validated by ‘qRT-PCR’ | 8,210 | 13,263 |
| Number of MTIs validated by ‘Reporter assay and Western blot’ | 6,032 | 10,257 |
| Number of MTIs validated by ‘Reporter assay or Western blot’ | 10,581 | 15,710 |
A scoring system has been incorporated into the automatic text-mining system for further manual curations to enhance the recognition of MTIs and a high score means that the article is more related to MTIs. We have also updated the version of previously integrated databases to include miRNA information, miRNA–disease associations, gene information, and mRNA sequences. Data contents provided by TransMir (19), miRSponge (24), SomamiR (22,31) and CMEP (35) were recently integrated to improve the information related to the regulation of miRNAs and the presence of cell-free miRNAs. Additionally, an enhanced viewer for displaying the TF–miRNA target relationships is embedded into the miRTarBase web interface.
Circulating miRNA expression
Circulating cell-free miRNAs as potential biomarkers of diseases have been stably presented in blood (36). Researchers have focused on the discovery, development, and evaluation of diagnostic, prognostic, and predictive biomarkers and technologies. In this update, we have integrated the data from CMEP (35) to represent the miRNA expression profiling in blood, including 10,419 samples obtained from 66 datasets. We believe that the information on circulating miRNA expression can enable biological and clinical researchers to gain biological insights into these interesting miRNAs and develop novel non-invasive biomarkers for clinical diagnosis.
Regulatory networks among miRNAs, regulators and targets
Post-transcriptional regulation mediated by miRNAs is an important control mechanism of gene expression. miRNAs have been reported to be widely detected in diverse types of cancers and other diseases (37–42). Hence, miRNAs can play an important role for diagnostics and treatment of diseases (43,44). Recent studies have been conducted to understand the mechanisms of miRNA-mediated post-transcriptional regulation (downstream regulation) by constructing miRNA–mRNA regulatory networks (45–49). Although several studies over the past decade have shown considerable changes in miRNA expression profiles in various diseases (50–55), less attention has been paid to the regulatory mechanisms of miRNA expression (upstream regulation). Constructing a comprehensive miRNA regulatory network for understanding how miRNAs are regulated is necessary to study changes in the expression level of miRNAs.
Owing to the upregulation of miRNAs expression by TFs, this update has effectively integrated the relationships between miRNAs and TFs to connect TF to the miRNA regulatory network. TransmiR is a database for TF–miRNA regulations, and it provides experimentally validated regulatory relations between TFs and miRNAs (56). The information regarding the pairs of TF–miRNA, the position of 5′ transcription start site, TF binding site, action type, tissue, species, and the evidence reference is obtained from TransmiR. Moreover, circular RNA (circRNA), another type of regulatory noncoding RNAs, has also been considered in this update. Recent studies have indicated that circRNAs can regulate gene expression by influencing mRNA translation through sponging miRNAs (57–59). The annotations of circRNAs were acquired from miRSponge, which provides experimental relationships between circRNA and miRNA (24). In particular, cancer somatic mutation in miRNAs, which can alter the interactions between miRNAs and their targets, such as mRNA, circRNA, ceRNA, and long noncoding RNAs (lncRNAs) (60), was also integrated into our repository based on the annotations of SomamiR (22,31).
Given a miRNA, both targets and their regulators are difficult to analyze based on a variety of online databases or tools. Thus, this update also aims to provide an extended platform for investigating the regulation mechanism of miRNAs by comprehensively constructing the regulatory networks among miRNAs, regulators, and targets.
ENHANCED WEB INTERFACE
As presented in Figure 2, the web interface has been re-designed to be more user-friendly, snappier, and aesthetically pleasing in this version. Users can search for the MTIs of their interest through different queries or directly browse the lists of miRNAs based on different categories: ‘by miRNA name’, ‘by target genes’, ‘by pathway’, ‘by validated method’, ‘by disease’, ‘by literature’, ‘by miRNA name’, ‘by target genes’, ‘by disease’ or ‘by species’. Users can also explore regulators, such as circRNA and TF, for the miRNAs of interest. The layout of MTI information and the regulators of miRNA expression have been rearranged to improve online readability according to user feedback. Meanwhile, the relationship between miRNAs and their regulators is illustrated with an interactive visualization of miRNA regulatory network. These improvements in web interface can promote miRTarBase as a popular online resource in miRNA research.
Figure 2.
Enhanced web interface of miRTarBase. More comprehensive information related to miRNAs, such as miRNA precursor, mature miRNA information, miRNA-associated diseases, miRNA regulators, supporting evidence, display of miRNA regulatory network, target gene information, miRNA target sites, and the expression profiles of miRNAs and their targets, are provided on the web interface of miRTarBase.
USERS OF miRTarBase
miRTarBase has been developed for 10 years; the first version, which came out in 2011, was followed by the fourth, sixth, and seventh versions in 2014, 2016 and 2017, respectively. Interestingly, the articles that have cited miRTarBase are distributed in different research areas, and they vary slightly between different versions, which may reflect the developing trend of miRNA studies (Supplementary Figure S1). Among the papers referenced to all releases of miRTarBase, ‘Biochemistry Molecular Biology’ is the most popular research field. For the miRTarBase version 1 (2011), in addition to ‘Biochemistry Molecular Biology’, the rest of the top 10 research areas include ‘Oncology’, ‘Science Technology Other Topics’, ‘Mathematical Computational Biology’, ‘Genetics Heredity’, ‘Biotechnology Applied Microbiology’, ‘Cell Biology’, ‘Research Experimental Medicine’, ‘Computer Science’ and ‘Neurosciences Neurology’. For the miRTarBase version 4 (2014) and later versions, articles in the field of ‘Oncology’ increased remarkably (ranked second continuously) compared with that in the miRTarBase version 1. For the miRTarBase version 7 (2018), articles related to ‘Pharmacology Pharmacy’ and ‘Physiology’ appeared in the top 10 research areas for the first time. However, the articles associated with ‘Computer Science’ and ‘Neurosciences Neurology’ dropped out of the top 10 fields temporarily. In addition, the number of papers cited in all versions on ‘Research Experimental Medicine’ indicated a gradual upward trend and has arisen into the top 4 areas, whereas the number of papers cited on ‘Mathematical Computational Biology’ declined regularly over time.
In summary, understanding how miRNAs downregulate target genes and TFs regulate miRNAs is critical to enhancing the functional analyses of miRNAs because aberrant regulation of miRNAs by TFs can induce phenotypic variations and diseases. Consequently, the latest update of miRTarBase addresses this issue and provides not only the targets but also the regulators of miRNAs, such as lncRNA and circRNA, to users for investigation of the up and downstream regulations of miRNAs.
SUMMARY AND PERSPECTIVES
Since the first release of miRTarBase in 2011, experimentally validated MTIs have been continuously accumulated for nearly 10 years. Thus far, we have collected >11,000 articles with experimental evidence on MTIs. The latest release of miRTarBase 8.0 (15 September 2019) contains 479,340 curated MTIs between 4,312 miRNAs and 23,426 target genes. Previously, miRTarBase has reported disease associations of miRNAs and their targets, which can provide potential targets for disease diagnosis. To ensure the correctness of the MTI data collected from heterogeneous resources, we have repeatedly confirmed the content of the articles in miRTarBase with an improved data management system. The manual data-management interface has been optimized to enable data managers to proofread easily and accurately. In addition, we added treatment-related information on miRNAs and described the treatment association of miRNAs in particular diseases to increase the researchers' awareness of the relevance of miRNAs to treatment in the future. In conclusion, as an important biological database, miRTarBase will continually aim at updating and increasing important information related to miRNA and provide a reliable database platform for a wide range of scientific researchers.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Warshel Institute for Computational Biology funding from Shenzhen City and Longgang District; Ganghong Young Scholar Development Fund of Shenzhen Ganghong Group Co.,Ltd. ‘Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B)’ from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Conflict of interest statement. None declared.
REFERENCES
- 1. Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297. [DOI] [PubMed] [Google Scholar]
- 2. Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D.Z.. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006; 38:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shivdasani R.A. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006; 108:3646–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu H., Fan G.C.. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am. J. Cardiovasc. Dis. 2011; 1:138–149. [PMC free article] [PubMed] [Google Scholar]
- 5. Russo F., Di Bella S., Bonnici V., Lagana A., Rainaldi G., Pellegrini M., Pulvirenti A., Giugno R., Ferro A.. A knowledge base for the discovery of function, diagnostic potential and drug effects on cellular and extracellular miRNAs. BMC Genomics. 2014; 15(Suppl. 3):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H., Zhou Y., Wang Z.-Y., Yan B.-X., Zhou W.-F., Wang T.-T., Zheng M., Man X.-Y.. Exosomal microRNA profiles from serum and cerebrospinal fluid in neurosyphilis. Sex. Transm. Infect. 2019; 95:246–250. [DOI] [PubMed] [Google Scholar]
- 7. Kozomara A., Griffiths-Jones S.. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014; 42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vlachos I.S., Paraskevopoulou M.D., Karagkouni D., Georgakilas G., Vergoulis T., Kanellos I., Anastasopoulos I.L., Maniou S., Karathanou K., Kalfakakou D. et al.. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015; 43:D153–D159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Z., Shi J., Gao Y., Cui C., Zhang S., Li J., Zhou Y., Cui Q.. HMDD v3.0: a database for experimentally supported human microRNA–disease associations. Nucleic Acids Res. 2019; 47:D1013–D1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y., Qiu C., Tu J., Geng B., Yang J., Jiang T., Cui Q.. HMDD v2.0: a database for experimentally supported human microRNA and disease associations. Nucleic Acids Res. 2014; 42:D1070–D1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang Q., Wang Y., Hao Y., Juan L., Teng M., Zhang X., Li M., Wang G., Liu Y.. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2008; 37:D98–D104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajewsky N. microRNA target predictions in animals. Nat. Genet. 2006; 38:S8–S13. [DOI] [PubMed] [Google Scholar]
- 13. Didiano D., Hobert O.. Perfect seed pairing is not a generally reliable predictor for miRNA–target interactions. Nat. Struct. Mol. Biol. 2006; 13:849–851. [DOI] [PubMed] [Google Scholar]
- 14. Kuhn D.E., Martin M.M., Feldman D.S., Terry A.V. Jr., Nuovo G.J., Elton T.S.. Experimental validation of miRNA targets. Methods. 2008; 44:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y.J., Kim V., Muth D.C., Witwer K.W.. Validated MicroRNA Target Databases: An Evaluation. Drug Dev. Res. 2015; 76:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ule J., Jensen K., Mele A., Darnell R.B.. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods. 2005; 37:376–386. [DOI] [PubMed] [Google Scholar]
- 17. Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M. Jr., Jungkamp A.C., Munschauer M. et al.. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010; 141:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helwak A., Kudla G., Dudnakova T., Tollervey D.. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013; 153:654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong Z., Cui Q., Wang J., Zhou Y.. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019; 47:D253–D258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Georgakilas G., Vlachos I.S., Zagganas K., Vergoulis T., Paraskevopoulou M.D., Kanellos I., Tsanakas P., Dellis D., Fevgas A., Dalamagas T. et al.. DIANA-miRGen v3.0: accurate characterization of microRNA promoters and their regulators. Nucleic Acids Res. 2016; 44:D190–D195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hua X., Tang R., Xu X., Wang Z., Xu Q., Chen L., Wingender E., Li J., Zhang C., Wang J.. mirTrans: a resource of transcriptional regulation on microRNAs for human cell lines. Nucleic Acids Res. 2018; 46:D168–D174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhattacharya A., Cui Y.. SomamiR 2.0: a database of cancer somatic mutations altering microRNA-ceRNA interactions. Nucleic Acids Res. 2016; 44:D1005–D1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziebarth J.D., Bhattacharya A., Cui Y.. Functional analysis of genetic variants and somatic mutations impacting microRNA-target recognition: bioinformatics resources. Methods Mol. Biol. 2019; 1970:101–120. [DOI] [PubMed] [Google Scholar]
- 24. Wang P., Zhi H., Zhang Y., Liu Y., Zhang J., Gao Y., Guo M., Ning S., Li X.. miRSponge: a manually curated database for experimentally supported miRNA sponges and ceRNAs. Database. 2015; 2015:bav098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu S.D., Lin F.M., Wu W.Y., Liang C., Huang W.C., Chan W.L., Tsai W.T., Chen G.Z., Lee C.J., Chiu C.M. et al.. miRTarBase: a database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2011; 39:D163–D169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu S.D., Tseng Y.T., Shrestha S., Lin Y.L., Khaleel A., Chou C.H., Chu C.F., Huang H.Y., Lin C.M., Ho S.Y. et al.. miRTarBase update 2014: an information resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2014; 42:D78–D85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chou C.H., Chang N.W., Shrestha S., Hsu S.D., Lin Y.L., Lee W.H., Yang C.D., Hong H.C., Wei T.Y., Tu S.J. et al.. miRTarBase 2016: updates to the experimentally validated miRNA–target interactions database. Nucleic Acids Res. 2016; 44:D239–D247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chou C.H., Shrestha S., Yang C.D., Chang N.W., Lin Y.L., Liao K.W., Huang W.C., Sun T.H., Tu S.J., Lee W.H. et al.. miRTarBase update 2018: a resource for experimentally validated microRNA–target interactions. Nucleic Acids Res. 2018; 46:D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maglott D., Ostell J., Pruitt K.D., Tatusova T.. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2011; 39:D52–D57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Leary N.A., Wright M.W., Brister J.R., Ciufo S., Haddad D., McVeigh R., Rajput B., Robbertse B., Smith-White B., Ako-Adjei D. et al.. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016; 44:D733–D745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattacharya A., Ziebarth J.D., Cui Y.. SomamiR: a database for somatic mutations impacting microRNA function in cancer. Nucleic Acids Res. 2013; 41:D977–D982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M. et al.. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013; 41:D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng M., Bragelmann J., Schultze J.L., Perner S.. Web-TCGA: an online platform for integrated analysis of molecular cancer data sets. BMC Bioinformatics. 2016; 17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomczak K., Czerwinska P., Wiznerowicz M.. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contem Onco (Pozn). 2015; 19:A68–A77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J.R., Tong C.Y., Sung T.J., Kang T.Y., Zhou X.J., Liu C.C.. CMEP: a database for circulating microRNA expression profiling. Bioinformatics. 2019; 35:3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Armand-Labit V., Pradines A.. Circulating cell-free microRNAs as clinical cancer biomarkers. Biomol. Concepts. 2017; 8:61–81. [DOI] [PubMed] [Google Scholar]
- 37. Adams B.D., Kasinski A.L., Slack F.J.. Aberrant regulation and function of microRNAs in cancer. Curr. Biol.: CB. 2014; 24:R762–R776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leichter A.L., Sullivan M.J., Eccles M.R., Chatterjee A.. MicroRNA expression patterns and signalling pathways in the development and progression of childhood solid tumours. Mol. Cancer. 2017; 16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hua Y., Ma X., Liu X., Yuan X., Qin H., Zhang X.. Abnormal expression of mRNA, microRNA alteration and aberrant DNA methylation patterns in rectal adenocarcinoma. PLoS One. 2017; 12:e0174461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang Z., Guo J., Xiao B., Miao Y., Huang R., Li D., Zhang Y.. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J. Gastroenterol. 2010; 45:17–23. [DOI] [PubMed] [Google Scholar]
- 41. Li P., Yin Y.L., Guo T., Sun X.Y., Ma H., Zhu M.L., Zhao F.R., Xu P., Chen Y., Wan G.R. et al.. Inhibition of Aberrant MicroRNA-133a Expression in Endothelial Cells by Statin Prevents Endothelial Dysfunction by Targeting GTP Cyclohydrolase 1 in Vivo. Circulation. 2016; 134:1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du Y., Liu M., Gao J., Li Z.. Aberrant microRNAs expression patterns in pancreatic cancer and their clinical translation. Cancer Biother. Radiopharmaceut. 2013; 28:361–369. [DOI] [PubMed] [Google Scholar]
- 43. Tufekci K.U., Oner M.G., Meuwissen R.L., Genc S.. The role of microRNAs in human diseases. Methods Mol. Biol. 2014; 1107:33–50. [DOI] [PubMed] [Google Scholar]
- 44. Ardekani A.M., Naeini M.M.. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010; 2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 45. Ryan B.M., Robles A.I., Harris C.C.. Genetic variation in microRNA networks: the implications for cancer research. Nat. Rev. Cancer. 2010; 10:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sumazin P., Yang X., Chiu H.S., Chung W.J., Iyer A., Llobet-Navas D., Rajbhandari P., Bansal M., Guarnieri P., Silva J. et al.. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011; 147:370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsang J., Zhu J., van Oudenaarden A.. MicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammals. Mol. Cell. 2007; 26:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo A.Y., Sun J., Jia P., Zhao Z.. A novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst. Biol. 2010; 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H.. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murakami Y., Yasuda T., Saigo K., Urashima T., Toyoda H., Okanoue T., Shimotohno K.. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006; 25:2537–2545. [DOI] [PubMed] [Google Scholar]
- 51. Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X. et al.. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008; 18:997–1006. [DOI] [PubMed] [Google Scholar]
- 52. Liu R., Chen X., Du Y., Yao W., Shen L., Wang C., Hu Z., Zhuang R., Ning G., Zhang C. et al.. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin. Chem. 2012; 58:610–618. [DOI] [PubMed] [Google Scholar]
- 53. Eisenberg I., Eran A., Nishino I., Moggio M., Lamperti C., Amato A.A., Lidov H.G., Kang P.B., North K.N., Mitrani-Rosenbaum S. et al.. Distinctive patterns of microRNA expression in primary muscular disorders. PNAS. 2007; 104:17016–17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hunter M.P., Ismail N., Zhang X., Aguda B.D., Lee E.J., Yu L., Xiao T., Schafer J., Lee M.L., Schmittgen T.D. et al.. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008; 3:e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li L.M., Hu Z.B., Zhou Z.X., Chen X., Liu F.Y., Zhang J.F., Shen H.B., Zhang C.Y., Zen K.. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010; 70:9798–9807. [DOI] [PubMed] [Google Scholar]
- 56. Wang J., Lu M., Qiu C., Cui Q.. TransmiR: a transcription factor-microRNA regulation database. Nucleic Acids Res. 2010; 38:D119–D122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rong D., Sun H., Li Z., Liu S., Dong C., Fu K., Tang W., Cao H.. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017; 8:73271–73281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J.. Natural RNA circles function as efficient microRNA sponges. Nature. 2013; 495:384–388. [DOI] [PubMed] [Google Scholar]
- 59. Thomson D.W., Dinger M.E.. Endogenous microRNA sponges: evidence and controversy. Nat. Rev. Genet. 2016; 17:272–283. [DOI] [PubMed] [Google Scholar]
- 60. Ziebarth J.D., Bhattacharya A., Cui Y.. Integrative analysis of somatic mutations altering microRNA targeting in cancer genomes. PLoS One. 2012; 7:e47137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.