Abstract
Background and aims
Cluster roots (CRs) constitute a special root adaptation that enables plants to take up nutrients, especially phosphorus (P), from soils with low nutrient availability, including recent volcanic deposits. It is unclear, however, how CR species interact with non-cluster root-bearing (NCR) species, and how substrates’ fertility modulates potential interactions.
Methods
We experimentally assessed the net interaction between CR and NCR species using two substrates of contrasting fertility: nutrient-rich nursery mix and tephra (low P availability). We planted seedlings of two southern South American (SSA) Proteaceae, CR species and two NCR Nothofagus species in pairs (conspecifics and heterospecifics) and as singles. We analysed the effect of seedling neighbours on survival, growth performance (e.g. total biomass and leaf area) and leaf and substrate nutrient concentrations (including manganese, a proxy for P-acquisition efficiency through CR activity) using the relative interaction index.
Key Results
After three growing seasons, we found that (1) Proteaceae species had fewer CRs and lower CR biomass and grew less in the tephra than in the nursery substrate; (2) Nothofagus species did not improve their survival and growth in the presence of Proteaceae species in any substrate; (3) contrary to Nothofagus, Proteaceae species improved their growth more when planted with any neighbour (including conspecifics) than when planted alone, which was accompanied by a significant accretion of leaf P; and (4) the presence of a neighbour increased the final nitrogen and P concentrations in the nursery substrate, regardless of species identity.
Conclusions
CRs provide Proteaceae a competitive advantage over NCR species at the seedling stage, which may have important consequences for species coexistence and community structuring. The investigated SSA Proteaceae, which have not evolved in nutrient-impoverished soils, as have their relatives in south-western Australia and South Africa, improve their growth when cultivated in pairs, especially in nutrient-rich substrates.
Keywords: Competition, Embothrium coccineum, facilitation, Gevuina avellana, Nothofagus, leaf Mn, Patagonia, plant community diversity, plant–plant interactions
INTRODUCTION
Plants rely on their roots to increase stability and acquire soil water and nutrients, which are fundamental to their establishment, growth and reproduction. Roots commonly take up readily available nutrients from the soil; however, under most natural conditions nutrients are not readily available in the rhizosphere and thus plants may become nutrient-limited. Some plant species, however, exhibit special root adaptations that allow them to access nutrients that are not available to other species. Among these adaptations are cluster roots (CRs), or proteoid roots, which are brush-like rootlets common in some plant families, including the Proteaceae family (Lamont, 2003). A soil of low nutrient availability, particularly of phosphorus (P), has been found to stimulate the formation and growth of CRs, which through their exudation of organic anions (e.g. malate, citrate) and phosphatases mobilize unavailable forms of soil P (e.g. Lamont et al., 1984; Lamont, 2003; Lambers et al., 2012). In fact, the exudation rate of CRs has been found to increase under low soil P availability (Delgado et al., 2014; Avila-Valdés et al., 2019). Cluster roots allow Proteaceae species to thrive in very ancient soils that are extremely poor in nutrients, in particular P, in areas such as Western Australia (Lambers et al., 2010) and South Africa (Lamont, 1982). In contrast, southern South American (SSA) Proteaceae species thrive in rather young volcanic soils (Andisols), which, among other things, are rich in total P but poor in P availability (i.e. P is largely sorbed onto soil particles and hence not readily available; Soil Survey Staff, 1999; Borie and Rubio, 2003; Lambers et al., 2012; Piper et al., 2013). The role of the CRs in these substrates, particularly the effects of Proteaceae species on the nutrient availability and on the performance of co-occurring non-cluster root-bearing (NCR) species, remains largely unknown.
Proteaceae species with CRs are hypothesized to be significant drivers in the structuring of plant communities (Lambers et al., 2012, 2018; Piper et al., 2019). Under the species coexistence framework, CR species could potentially promote fitness differences among species in a community by either equalizing competitive fitness or increasing competitive asymmetry (Chesson, 2000). Prior work conducted in south-western Australia found that NCR species increased their growth when growing with CR species, suggesting that CR activity is beneficial for the community (Muler et al., 2014; Teste et al., 2014). Non-cluster-root species could potentially benefit from the exudation of P in the rhizosphere by CR species, a phenomenon similar to that involving nitrogen (N) fixing by, for example, legume species (e.g. Temperton et al., 2007). This potential facilitative effect of Proteaceae on NCR species would encompass a process whereby the fitness of two species is equalized; in this case, the equalizing mechanisms would act to reduce fitness differences between the species. Thus, it is possible that the survival and growth of NCR species are significantly enhanced in the presence of a facilitating CR species, which maintains its normal survival and growth. However, the role of CRs in plant–plant interactions may also involve negative or neutral interactions. For example, the process of P mining and acquisition by CRs could enable Proteaceae species to have a competitive advantage over NCR species via a drawing down of limited resources (i.e. increasing their competitive asymmetry), especially in soils of limited nutrient availability (e.g. young volcanic soils of SSA), resulting in a decrease in plant fitness of neighbouring plants. On the other hand, even if Proteaceae species do improve the nutritional status of neighbour plants species, this does not necessarily imply direct fitness benefits. For example, the presence of the SSA Proteaceae Embothrium coccineum within a nursery cushion plant species increased the leaf P concentration but also reduced the reproductive output of the cushion plant (Piper et al., 2019); similarly, E. coccineum increased the leaf protein content but not the growth rate of Sophora cassioides when they grew together (Zúñiga-Feest et al., 2018). Perhaps CRs as a trait can induce both community competition and facilitation depending on the abiotic (e.g. soil nutrient status) and biotic (e.g. neighbour identity) conditions (i.e. a context-dependent interaction).
In this study, we experimentally assessed the capacity of Proteaceae species growing in southern Chile to alter plant–plant interactions via the change in nutrient availability in the community. To accomplish this aim, we conducted an experiment where we planted seedlings of two SSA Proteaceae species (E. coccineum and Gevuina avellana) and two NCR Nothofagus species (N. betuloides and N. pumilio) either as individuals or as closely planted pairs comprising either conspecifics or heterospecifics. By planting seedlings immediately adjacent to one another, we sought to evaluate the strength of seedling interactions (positive, negative or neutral). Because plant interaction outcomes are not static but vary according to biotic and abiotic conditions (Holland and DeAngelis, 2009), we conducted the experiment using two substrates with different nutrient availability: a relatively nutrient-poor (in particular, low available P) volcanic substrate (tephra, the most common early successional substrate in the Andes of SSA) and a nutrient-rich nursery substrate. These two substrates were selected because they also represent two contrasting conditions for the formation and exudation of CRs (Zúñiga-Feest et al., 2018; Avila-Valdés et al., 2019), which likewise help to determine what the impacts of such potentially different CR functioning are for both the substrates and the plant–plant interactions. We first assessed whether the formation of CRs in Proteaceae is promoted in the tephra substrate. Second, we tested the hypothesis that Proteaceae species are able to mine P from the substrate for their own benefit, outcompeting heterospecifics, particularly in the tephra substrate. If this competition hypothesis is true, the growth and survival of Nothofagus seedlings will decrease more when planted with Proteaceae than when planted alone. Our alternative hypothesis was that Proteaceae species facilitate the survival and growth of NCR Nothofagus species, especially in the tephra substrate, potentially through Proteaceae mining of P in the rhizosphere, which is subsequently made available to the Nothofagus. If this facilitation hypothesis is true, then the Nothofagus seedlings planted with the Proteaceae seedlings will grow and survive more than when planted alone, particularly in the tephra substrate. In addition, we measured leaf mass per area (LMA) and leaf N, P and manganese (Mn) concentrations to examine the effect of the plant–plant interactions on important leaf traits and to determine relationships between plant performance responses and nutrient status.
MATERIALS AND METHODS
Study site and species
The experiment was conducted outdoors over three growing seasons at the El Mallín nursery (Corporación Nacional Forestal) in Puerto Aysén (45°24′ S, 72°40′ W, 30 m above sea level [m.a.s.l.]), Aysén Region, Chile. The climate is humid with a mean annual precipitation of 2034 mm that is distributed uniformly around the year; the mean summer temperature (December–February) is 12.7 °C and the mean winter temperature (June–August) is 4.4 °C (Puerto Aysén weather station, 32 m.a.s.l.; Dirección General de Aguas, 2007–2017). Embothrium coccineum and Gevuina avellana are two Proteaceae tree species that have a wide distribution in central-southern Chile. Embothrium coccineum can be found from 30° S in central Chile to 55° S in Tierra del Fuego in humid to dry sites (Rodríguez et al., 1983; Souto et al., 2009), indicating the species’ wide ecological niche (Fajardo and Piper, 2015; Fajardo et al., 2019). It occurs as a pioneer species in glacier moraines and young volcanic soils (Piper et al., 2013), but it can also grow in warm temperate rainforests (Grubb et al., 2013). Gevuina avellana has a relatively more restricted distribution (35–43° S), occurring in the Mediterranean and temperate forests as an intermediate to late successional species. Both species show a high variation in plant growth rate (Lusk and Corcuera, 2011), with E. coccineum exhibiting the fastest growth rate among sympatric species when light conditions are optimal. Neither species forms symbiotic mycorrhizal associations (Godoy et al., 1994). Nothofagus betuloides and N. pumilio (Nothofagaceae) are tree species that, like the Proteaceae species, have a comparably wide SSA distribution (Rodríguez et al., 1983). N. betuloides is an evergreen and N. pumilio is deciduous, and both present ectomycorrhizal symbiosis (Marín et al., 2018). In contrast to the 15 m height that E. coccineum and G. avellana can reach, N. betuloides and N. pumilio can reach a height of 40 m at maturity.
Experimental setting and design
In October 2013 (spring) we transplanted 2-year-old seedlings of E. coccineum, G. avellana, N. betuloides and N. pumilio into 6.3-L pots (plastic bags 20 cm in diameter and 20 cm high) as either singles or pairs. Seedlings were purchased at the El Mallín nursery and were of a similar average height at the time of the transplant: E. coccineum, 23.2 ± 2.3 cm (mean ± s.e.); G. avellana, 21.5 ± 2.1 cm; N. betuloides, 23.3 ± 3.1 cm; N. pumilio, 22.2 ± 2.8 cm (n = 288, P = 0.342). A total of 288 seedlings were planted in 168 pots. Seedlings were planted as singles, conspecific pairs or heterospecific pairs (i.e. within and between families, for example, E. coccineum with G. avellana or E. coccineum with N. pumilio). In order to stimulate root interactions, paired seedlings were planted as close to each other as possible. Pots were filled with either a nursery nutrient-rich substrate mix (3:1 mixture of clay and sand) or a volcanic tephra substrate. The nursery substrate had a relatively high nutrient availability (nitrate 6.47 ± 0.92 mg kg−1 [mean ± s.e.], ammonium 12.21 ± 0.85 mg kg−1 and phosphate 21.99 ± 0.64 mg kg−1, n = 24) with a high organic matter content of 26.01 ± 2.84 % and a pH of 4.25 ± 0.11. The tephra substrate was collected from bare-ground spots in an area with patchy vegetation that is in the vicinity of the Hudson volcano (Cajón Cofré, 46°10′ S, 72°38′ W, 550 m.a.s.l.); the tephra substrate was extracted using a shovel after the removal of any ground litter. Tephra here corresponds to fragmental material produced by the volcanic eruption of the Hudson volcano (1991); tephra is an unconsolidated material containing a large quantity of volcanic glass that has much less resistance to chemical weathering than crystalline minerals (Shoji et al., 1994; Naranjo and Stern, 1998; Schlesinger et al., 1998; Vandekerkhove et al., 2016). The tephra substrate has low nutritional levels and absence of organic matter (nitrate 1.01 ± 0.16 mg kg−1 [mean ± s.e.], ammonium 2.39 ± 0.60 mg kg−1, phosphate 7.83 ± 0.30 mg kg−1, organic matter 0.15%, pH 4.5) (Stolpe and Hepp, 2014). We note here that the available P concentration found in the nursery substrate is paralleled with the most fertile soils in which E. coccineum naturally occurs in SSA, whereas the available P concentration found in the tephra substrate is indicative of the intermediate to low levels of available P commonly found within the species’ natural distribution (Souto et al., 2009; Piper et al., 2013; Fajardo and Piper, 2015). For G. avellana, the available P concentration of the nursery substrate also represents the nutritional levels of the most fertile areas of the natural distribution of G. avellana; however, the available P concentration of the tephra substrate represents the minimal available P concentration of that distribution (Merino et al., 2016).
In the nursery, the pots were arranged in a randomized complete block design with six blocks to account for any environmental variation in the nursery. Blocks were separated from each other by 1 m, and the perimeter of the experimental area was isolated from any obstacle by 5 m; the entire experiment was located in the middle of a matrix of nursery beds used for forest tree seedling cultivation. The pots were subjected to watering during the summer months via a sprinkler system similar to that used for the other seedlings under cultivation. The experiment was regularly monitored for weeding and for cleaning in the event of plant debris interference.
Seedling survival, height and biomass and leaf nutrient concentration measurements
In late March 2016 (early autumn), all pots were transported from the nursery to the laboratory (Centro de Investigación en Ecosistemas de la Patagonia, Coyhaique, Chile), where seedlings were assessed for survival and then harvested for the determination of basal diameter, height, biomass, CRs and leaf nutrient concentrations. Survival was assessed by the appearance of the cambium in the collar at the time of harvest. Thus, a plant was considered dead if its collar cambium was dry and brown, otherwise, it was considered alive. We used the seedling biomass as a proxy for growth increment given that seedlings across species were of a similar size at the beginning of the experiment and hence we confidently assumed a similar initial biomass. Furthermore, we focused on the biomass based on the idea that the biomass of a seedling grown alone depends entirely on the species genotype and the abiotic environment, whereas the biomass of paired seedlings in the same abiotic environment could be comparatively more or less if biotic interactions are meaningful (Armas et al., 2004). Once harvested, the seedlings were separated into leaves, shoots, CRs and NCRs. For leaf area determination, all leaves of each seedling were pooled, laid flat and photographed with a reference square of known area using a Nikon Coolpix 5000 digital camera (Nikon Corporation, Tokyo, Japan); the total projected leaf area was calculated using SIGMAPROC image-processing software (Systat Software, Richmond, CA, USA). The roots were thoroughly and carefully washed with tap water, gently brushed and detached with scissors. Next, the number of CRs was recorded for each Proteaceae seedling. Cluster roots are easily recognizable in E. coccineum and even more so in G. avellana as they appear as closely spaced lateral roots along root axes; only the white, turgid CRs (Fig. 1) were counted, as CRs with these general characteristics appear to be active (Zúñiga-Feest et al., 2010). The leaves, shoots, CRs and NCRs for each seedling were then placed in labelled paper bags, dried in a forced-air stove (Memmert, Schwabach, Germany) at 70 °C for 72 h, and weighed with a scale to a precision of 0.0001 g. Next, we computed the LMA (g m−2) for each species as the oven-dried leaf mass divided by the total foliar surface area.
Fig. 1.
Proteaceae species with their cluster roots (CRs). (A) Intermingled seedlings of E. coccineum (big leaves) and N. betuloides (small leaves). (B) Intermingled seedlings of G. avellana (big leaves) and N. pumilio (small leaves); note the big CRs of G. avellana. (C) Washed roots of E. coccineum; the CRs can be clearly distinguished. Photographs: Alex Fajardo.
Leaf Mn has been successfully used as a proxy for CR P acquisition efficiency in several Australian Proteaceae species (Lambers et al., 2015); thus, higher leaf Mn concentrations in either the CR Proteaceae species or NCR Nothofagus species could indicate a higher nutrient exchange. Leaf Mn was determined at the Soil and Plant Nutrient Analysis Laboratory (Universidad de Concepción, Chillán, Chile). Leaf N concentration was determined by the combustion analysis method, in which we placed 200 mg of dry, ground tissue sample in a combustion analyser (LECO TruSpec® Micro CHN, Centro de Investigación en Ecosistemas de la Patagonia, Coyhaique, Chile). Leaf P concentration was determined from 100 mg of dried ground material according to the procedure described by Murphy and Riley (1962). The extraction was done using hydrochloric–nitric acid digestion followed by fibreglass filtering with attachable disposable syringes. Leaf concentrations of N, P and Mn were expressed per unit leaf dry mass (mg g−1) and per leaf area (g m−2) (Supplementary Data Table S1).
Soil nutrient concentration determination
A sample of ~300 g of each pot’s bulk substrate was carefully separated from any root tissue and placed in a labelled resealable zip storage bag. The samples were immediately refrigerated at 3 °C for 2 d and then shipped to a soil testing laboratory (Universidad de Concepción, Chillán, Chile), where they were analysed for nitrate (NO3−, mg kg−1 soil dry weight [d.w.]), ammonium (NH4+, mg kg−1 soil d.w.) and Olsen P (a standard measure of available P). Mineral N (i.e. NH4+ and NO3−) was extracted using a 5:1 proportion of K2SO4 solution:soil, after which the extracts were analysed for ammonium and nitrate using standard colorimetric methods. Olsen P was extracted using a 20:1 proportion of pH 8.5 NaHCO3 solution:soil.
Data analysis
We first quantified plant–plant interactions by calculating the relative interaction index (RII, Armas et al., 2004) for each pot’s individuals. RII is calculated as:
where P+n represents the target individual performance (e.g. E. coccineum growth) with a neighbour of any species, and P–n the target individual performance without a neighbour (i.e. pot with a single individual). The RII can have values from –1 to 1, with negative values indicating competition and positive values facilitation (Armas et al., 2004). The RIIs were calculated for seedling survival, biomass and total leaf area as explanatory variables for plant performance and fitness. We fitted linear mixed-effects models (LMMs) using the nlme package (Pinheiro et al., 2016) in R (R Development Core Team, 2019) to analyse the effects of planting combination (i.e. conspecifics, heterospecifics of the same family, or heterospecifics of a different family) and substrate type (nursery or tephra) on the performance indicators (i.e. RIIs) of the species to understand seedling–seedling interactions. The models were fitted with block included as a random factor. Using the same statistical approach, we also computed the RIIs for leaf functional traits (LMA and leaf N, P and Mn concentrations) and bulk substrate nutrient concentrations (N and P); although we originally intended to only measure plant performance with the RIIs, we benefited from the additional information gained from assessing whether leaf functional traits and soil nutrient concentrations were altered because of the presence of neighbours. The interpretation of the RIIs in this case, instead of indicating competition or facilitation, assessed whether the value of a specific trait increases or decreases when the plant is grown with a neighbour. Finally, we estimated the number and biomass of CRs in the Proteaceae species as a function of planting combination and substrate type. For estimating the effect of planting combination and substrate type on the cluster root number and biomass, we used a generalized linear mixed-effects model (GLMM) and an LMM, respectively, with a Poisson family distribution and a log link function. We considered planting combination and substrate type as fixed effects and block as a random effect. Graphics were developed using the ggplot2 package (Wickham, 2016).
RESULTS
Seedling survival and growth based on below-ground interactions
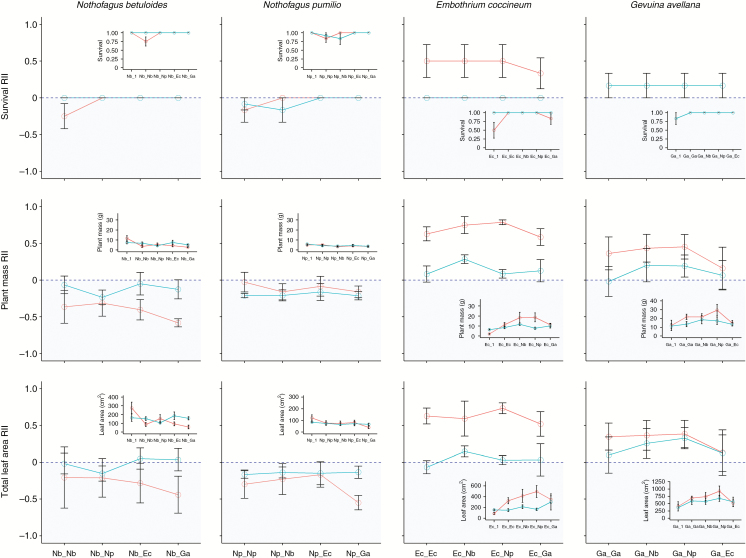
In March 2016, after three growing seasons, we found that seedling survival across species was relatively high, with most treatments having 100 % survival (Supplementary Data Table S1). While E. coccineum showed the lowest survival (50.0 %) when growing alone in the nursery substrate, G. avellana had the lowest survival (83.3 %) when growing alone in both substrates; N. betuloides (75.0 %) and N. pumilio (83.3 %) showed the lowest survival when growing with conspecifics in the nursery substrate (Supplementary Data Table S1, Fig. 2). Overall, the survival of Nothofagus species was not improved by the immediate presence of Proteaceae species in the tephra substrate, and therefore the facilitative hypothesis was not supported (Table 1, Fig. 2). In particular, we found that N. betuloides exhibited a significantly negative RII for survival when planted with a conspecific, disregarding substrate type; this result clearly indicates competition at the intraspecific level (i.e. greater survival when growing alone than with a conspecific neighbour) (Fig. 2). However, the substrate type had a strong effect on the RII for survival in E. coccineum (higher for the nursery substrate), with significantly higher survival observed when grown with any neighbour (conspecific or heterospecific) than when grown alone (Table 1, Fig. 2). Something similar happened with G. avellana for both substrate types; higher (but not significantly higher) survival was evidenced when the species was planted with a neighbour than when it was planted alone.
Fig. 2.
The Relative Interaction Index (RII) for survival and plant seedling biomass (g) and total leaf area (cm2) in N. betuloides (Nb), N. pumilio (Np), E. coccineum (Ec) and G. avellana (Ga) seedlings experimentally grown in a nursery (red solid line) and in a tephra substrate (turquoise broken line) and with different neighbours: conspecific pair (e.g. Ec_Ec or Nd_Nd); heterospecific pair of the same family (e.g. Ec_Ga or Nd_Np); or heterospecific pair of contrasting families (e.g. Ec_Np, Ga_Np). The RII can have values from –1 to 1, negative values indicating competition (blue sky zone) and positive values facilitation (white zone). For details of the computation of the RIIs see the Materials and Methods section. Insets show seedling survival, seedling total biomass and total leaf area of seedlings grown alone (e.g. Nb_1) or with a neighbour in the two types of substrate described above. Note that for G. avellana survival the results for both substrate types overlapped exactly (i.e. the treatments resulted in the same survival RIIs).
Table 1.
Linear mixed effects modelling to explain the variation in seedling survival and growth performance variables, derived using the Relative Interaction Index (RIII), across planting combination (PC), substrate type and their interaction. The table shows F statistics, with the associated P values in parentheses. Figures in bold indicate significant effects
| Factor | Survival | Plant mass | Total leaf area | Root mass | Height | Diameter |
|---|---|---|---|---|---|---|
| N. betuloides | ||||||
| PC | 2.14 (0.112) | 0.65 (0.588) | 0.16 (0.924) | 0.73 (0.541) | 0.18 (0.910) | 0.19 (0.902) |
| Soil | 2.03 (0.152) | 11.84 (0.002) | 4.86 (0.035) | 5.11 (0.030) | 16.91 (<0.001) | 41.87 (<0.001) |
| PC × soil | 1.92 (0.140) | 0.88 (0.463) | 0.53 (0.662) | 0.41 (0.749) | 0.26 (0.855) | 1.06 (0.377) |
| N. pumilio | ||||||
| PC | 1.03 (0.393) | 0.27 (0.848) | 0.87 (0.469) | 0.88 (0.463) | 0.54 (0.656) | 0.55 (0.650) |
| Soil | 0.11 (0.738) | 1.87 (0.181) | 3.82 (0.060) | 9.85 (0.004) | 0.01 (0.960) | 0.54 (0.466) |
| PC × soil | 0.72 (0.546) | 0.26 (0.850) | 0.91 (0.446) | 0.93 (0.437) | 0.36 (0.785) | 0.73 (0.539) |
| E. coccineum | ||||||
| PC | 0.23 (0.878) | 1.62 (0.209) | 0.37 (0.773) | 1.05 (0.390) | 0.56 (0.645) | 2.63 (0.071) |
| Soil | 27.32 (<0.001) | 57.61 (<0.001) | 29.76 (<0.001) | 26.46 (<0.001) | 86.55 (<0.001) | 45.13 (<0.001) |
| PC × soil | 0.26 (0.834) | 0.70 (0.560) | 0.42 (0.741) | 0.53 (0.664) | 0.63 (0.602) | 0.90 (0.453) |
| G. avellana | ||||||
| PC | 0.0 (1.000) | 1.06 (0.384) | 0.92 (0.446) | 0.78 (0.517) | 0.46 (0.711) | 1.07 (0.378) |
| Soil | 0.0 (1.000) | 9.06 (0.006) | 4.27 (0.048) | 7.12 (0.013) | 9.64 (0.004) | 3.56 (0.070) |
| PC × soil | 0.0 (1.000) | 0.33 (0.804) | 0.25 (0.863) | 0.38 (0.768) | 0.70 (0.560) | 1.29 (0.299) |
While the Nothofagus species grew significantly less, the Proteaceae species grew significantly more when planted with any neighbour than when planted alone in the nursery soil (Supplementary Data Table S1, Fig. 2). The Nothofagus species showed their best performance when growing alone (significantly negative RII values), especially in the nursery substrate, where, for example, N. betuloides had 11.2 g of total plant biomass and 276.0 cm2 of total leaf area (Fig. 2, Supplementary Data Table S1). The greatest total plant biomass (17.8 g) and total leaf area (932.8 cm2) were found in G. avellana when it was planted with N. pumilio in the nursery substrate (significantly positive RII values); E. coccineum also showed its highest total plant biomass (17.0 g) and total leaf area (495.9 cm2) when planted with N. pumilio in the nursery substrate (significantly positive RII values; Fig. 2). Across species, planting combination, however, did not have a significant effect on the RIIs for plant total biomass and total leaf area (Fig. 2), or for height, total root mass and collar diameter (Table 1). Substrate type did have a significant effect on the RIIs for every growth variable in all species except N. pumilio (Table 1, Fig. 2, Supplementary Data Table S1). Notably, the nursery substrate had a significant effect on the RIIs, whereby values were negative (indicating competition) for the Nothofagus species and positive (indicating potential facilitation) for the Proteaceae species (Fig. 2).
Leaf nutrient concentrations and plant nutrient pools
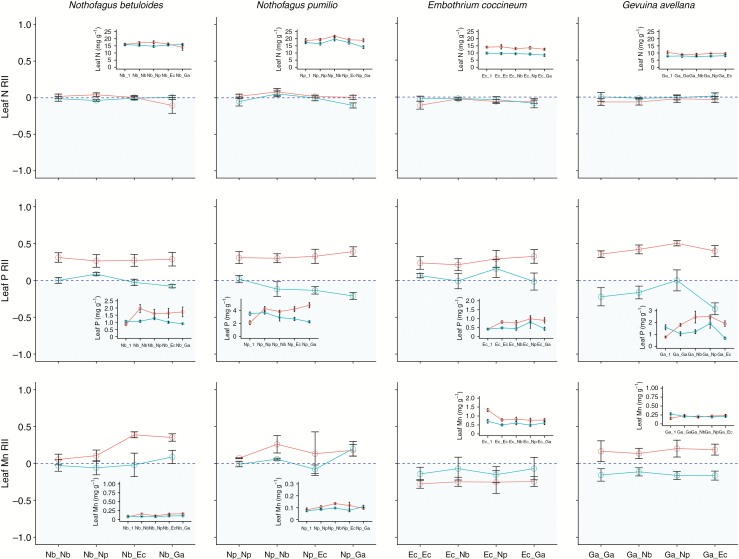
Similar to growth performance, we found that the substrate type, and not the planting combination, had a significant effect on the RII values for leaf nutrient concentrations and plant nutrient pools (Table 2, Fig. 3, Supplementary Data Table S1). Although the RIIs across species of leaf N concentrations were relatively constant among planting combinations, the substrate type had a significant effect across species on the RIIs for leaf P and Mn concentrations and plant N and P pools, all of which were higher in the nursery substrate (Table 2, Fig. 3, Supplementary Data Table S1). In particular, the RIIs across species for leaf P concentrations of seedlings planted with neighbours were significantly positive (i.e. increased) in the nursery substrate but not in the tephra substrate; thus, any seedling planted alone in the nursery substrate had a significantly lower leaf P concentration than when planted with a neighbour (Fig. 3). For G. avellana, leaf P concentrations and plant P pools were significantly higher (independent of substrate type) when it was planted with Nothofagus species than when planted with a conspecific, E. coccineum, or alone (Table 2, Fig. 3). In general, the RIIs for leaf Mn concentrations were higher in the nursery substrate for all species but E. coccineum. Nothofagus betuloides had a significantly higher leaf Mn concentration when grown with either Proteaceae species than when grown either alone or with N. pumilio, whereas N. pumilio had a significantly higher leaf Mn concentration when grown with G. avellana in both substrate types than when grown alone or with any other neighbour (Fig. 3). Gevuina avellana showed a higher Mn concentration when grown with a neighbour than when grown alone in the nursery substrate, but showed the opposite trend when grown in the tephra substrate; i.e. it had a significantly higher leaf Mn concentration when grown alone than with a neighbour (Fig. 3, Supplementary Data Table S1).
Table 2.
Linear mixed effects modelling to explain the variation in several plant nutrient trait variables, derived using the Relative Interaction Index (RII), across planting combination (PC), substrate type and their interaction The table shows F statistics, with the associated P values in parentheses. Figures in bold indicate significant effects
| factor | LMA | Leaf N | Leaf P | Leaf Mn | Plant N | Plant P |
|---|---|---|---|---|---|---|
| N. betuloides | ||||||
| PC | 0.19 (0.903) | 0.74 (0.537) | 0.57 (0.637) | 2.28 (0.128) | 0.33 (0.800) | 0.39 (0.765) |
| Soil | 0.32 (0.578) | 0.01 (0.910) | 49.45 (<0.001) | 9.39 (0.009) | 10.11 (0.003) | 1.42 (0.242) |
| PC × substrate | 0.097 (0.961) | 1.83 (0.160) | 1.00 (0.407) | 1.24 (0.337) | 1.19 (0.329) | 0.51 (0.681) |
| N. pumilio | ||||||
| PC | 0.56 (0.647) | 4.40 (0.011) | 0.32 (0.807) | 0.89 (0.484) | 0.68 (0.571) | 0.18 (0.912) |
| Soil | 3.01 (0.093) | 5.93 (0.021) | 100.70 (<0.001) | 0.14 (0.714) | 0.01 (0.969) | 14.89 (<0.001) |
| PC × substrate | 0.77 (0.517) | 0.81 (0.500) | 2.05 (0.126) | 0.44 (0.730) | 0.29 (0.832) | 0.18 (0.912) |
| E. coccineum | ||||||
| PC | 2.28 (0.106) | 0.54 (0.657) | 0.88 (0.467) | 0.38 (0.770) | 1.63 (0.210) | 1.85 (0.167) |
| Soil | 1.96 (0.175) | 0.59 (0.459) | 8.66 (0.007) | 0.68 (0.418) | 33.76 (<0.001) | 55.57 (<0.001) |
| PC × substrate | 0.26 (0.852) | 0.51 (0.677) | 0.45 (0.718) | 0.10 (0.958) | 0.64 (0.594) | 0.33 (0.801) |
| G. avellana | ||||||
| PC | 1.78 (0.175) | 0.39 (0.763) | 4.68 (0.009) | 0.01 (0.998) | 0.70 (0.559) | 3.62 (0.026) |
| Soil | 1.00 (0.327) | 1.54 (0.224) | 152.73 (<0.001) | 41.01 (<0.001) | 3.92 (0.058) | 56.31 (<0.001) |
| PC × substrate | 1.32 (0.287) | 0.20 (0.895) | 1.52 (0.231) | 0.26 (0.854) | 0.36 (0.780) | 0.15 (0.927) |
Fig. 3.
The Relative Interaction Index (RII) for leaf N, P and Mn concentrations in N. betuloides (Nb), N. pumilio (Np), E. coccineum (Ec) and G. avellana (Ga) seedlings experimentally grown in a nursery (red solid line) and in a tephra substrate (turquoise broken line) and with different neighbours: conspecific pair (e.g. Ec_Ec or Nd_Nd); heterospecific pair of the same family (e.g. Ec_Ga or Nd_Np); or heterospecific pair of contrasting families (e.g. Ec_Np, Ga_Np). The RII can have values from –1 to 1, negative values indicating a decrease (blue sky zone) and positive values an increase (white zone) in the variable value when growing with neighbours. For details of the computation of RII see the Materials and Methods section. Insets show leaf nutrient concentrations of seedlings growing alone (e.g. Nb_1) or with a neighbour in the two type of substrate described above.
Cluster roots, substrate nutrient availability and seedling interactions
When seedlings were harvested, we found that all E. coccineum and G. avellana seedlings had CRs (Fig. 1); on average, E. coccineum had more CRs and lower CR biomass per seedling than G. avellana (Table 3). Although the CR number and CR biomass per seedling did not differ between the nursery and tephra substrates in E. coccineum, in G. avellana there was significantly more CR biomass per seedling in the nursery than in the tephra substrate (Table 3).
Table 3.
Cluster root number and biomass in E. coccineum and G. avellana seedlings grown in a nursery and a tephra substrate under experimental conditions in southern Chile. The table shows F statistics for the substrate type comparison, with the associated P values in parentheses. Figures in bold indicate significant effects
| E. coccineum | G. avellana | |
|---|---|---|
| CR number | ||
| Nursery | 5.18 (0.92) | 3.40 (0.52) |
| Tephra | 4.28 (0.50) | 3.60 (0.33) |
| F | 3.18 | 0.15 |
| P | 0.075 | 0.696 |
| CR mass | ||
| Nursery | 0.50 (0.14) | 3.43 (0.51) |
| Tephra | 0.48 (0.11) | 2.11 (0.39) |
| F | 0.01 | 4.23 |
| P | 0.924 | 0.044 |
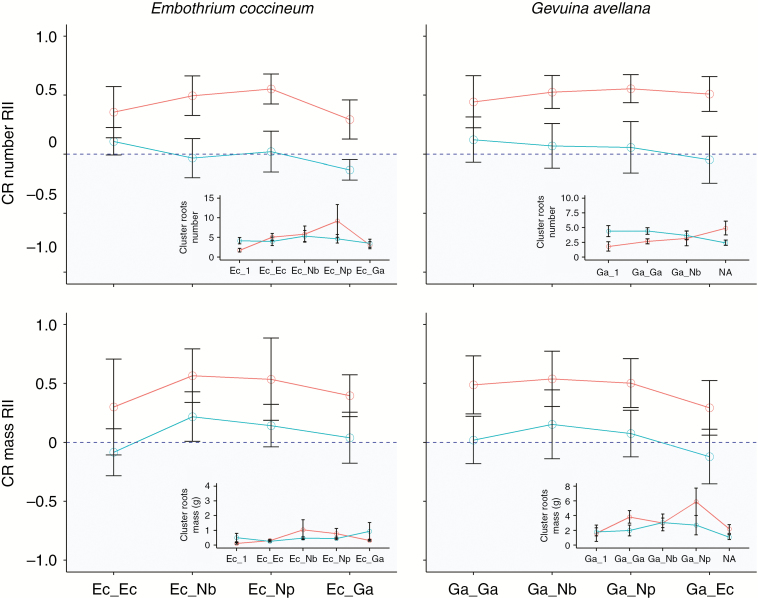
Across species, the RIIs for CR number and biomass were significantly positive when seedlings were grown with neighbours compared with when they were grown alone, especially in the nursery substrate (Fig. 4); these results indicate that CR formation was significantly promoted when the Proteaceae species were grown with neighbours in the nursery substrate. After seedling harvest, planting combination had a significant effect on both the N (F = 11.24, P = 0.001) and P (F = 13.43, P < 0.001) bulk substrate concentrations (Table 4). Across species, when seedlings were planted in pairs in the nursery substrate, the N and P bulk substrate concentrations increased by ~1.4- and ~4-fold, respectively, when compared with the bulk substrate concentrations of the single seedling pots.
Fig. 4.
Neighbour effects quantified as the Relative Interaction Index (RII) on CR number and CR mass of E. coccineum (Ec) and G. avellana (Ga) experimentally planted in a nursery (red line) and a tephra substrate (turquoise line) with different identity neighbours: with a conspecific (Ec_Ec or Ga_Ga); with a heterospecific of the same family (Ec_Ga or Ga_Ec); or with N. betuloides (Nb; Ec_Nb, Ga_Nb) or N. pumilio (Np; Ec_Np, Ga_Np). Negative values of RII significantly different from zero indicate a reduction in CR number or mass when growing with a neighbour relative to being planted alone, whereas positive values stand for an increase in CR number or mass when growing with a neighbour relative to being planted alone. For details of the computation of RII see the Materials and Methods section. Insets show CR number and CR mass of E. coccineum and G. avellana.
Table 4.
Mean values (± s.e.) of nitrate (NO3−), ammonium (NH4+), total nitrogen (N) and phosphate (Olsen P) concentrations (mg kg−1) of a nursery and a tephra substrate where seedlings of Proteaceae (Prot.; E. coccineum and G. avellana) and Nothofagus (Noth.; N. betuloides and N. pumilio) species were experimentally planted for 3 years either alone (e.g. Noth_1) or in congeneric (Noth_Noth), confamiliar (Prot_Prot) or mixed (Noth_Prot) pairs
| Noth_1 | Noth_Noth | Noth_Prot | Prot_1 | Prot_Prot | |
|---|---|---|---|---|---|
| Nursery substrate | |||||
| Nitrate | 5.34 (0.79) | 3.34 (0.54) | 2.92 (0.25) | 7.59 (1.05) | 4.72 (0.81) |
| Ammonium | 12.32 (1.11) | 27.52 (1.17) | 22.04 (1.19) | 12.10 (0.59) | 22.64 (1.17) |
| Total N | 17.67 (1.60) | 30.87 (1.37) | 24.96 (1.31) | 19.67 (1.12) | 27.34 (1.85) |
| Phosphate | 21.87 (0.84) | 101.52 (4.68) | 94.68 (2.91) | 22.11 (0.43) | 98.94 (3.16) |
| Tephra substrate | |||||
| Nitrate | 0.78 (0.13) | 0.97 (0.12) | 0.96 (0.08) | 1.25 (0.18) | 1.24 (0.15) |
| Ammonium | 1.87 (0.51) | 2.58 (0.31) | 2.56 (0.27) | 2.92 (0.68) | 3.53 (0.39) |
| Total N | 2.67 (0.52) | 3.54 (0.32) | 3.51 (0.32) | 4.16 (0.72) | 4.77 (0.39) |
| Phosphate | 7.73 (0.39) | 9.98 (0.49) | 10.23 (0.27) | 7.93 (0.22) | 11.78 (0.91) |
DISCUSSION
Cluster root formation in SSA Proteaceae
Contrary to some initial expectations, we did not find that the tephra substrate stimulates CR formation and biomass in E. coccineum and G. avellana. In fact, it was the nutrient-rich conditions of the nursery substrate that stimulated a significantly greater investment in CR biomass in the G. avellana seedlings. This finding contradicts previous evidence from studies conducted in SSA Proteaceae (e.g. Zúñiga-Feest et al., 2010, 2018; Delgado et al., 2013; Avila-Valdés et al., 2019), which reported that volcanic soils and volcanic substrates (pumice) poor in P enhanced CR formation in Proteaceae species. Similarly, leaf Mn concentrations––a surrogate of P-acquisition efficiency through CR activity (Lambers et al., 2015)––were not higher in the tephra substrate but instead were similar between substrate types in G. avellana and even higher in the nursery than in the tephra substrate in the other three species (see below). It is worth mentioning that CRs of SSA Proteaceae species are hypothesized to be extremely effective at P mining, which should not necessarily induce an increase in overall biomass allocation to CR (Delgado et al., 2014). For example, Delgado et al. (2014) experimentally exposed E. coccineum seedlings to soils with a variety of P concentrations and found that CR exudation and P uptake were stimulated more than CR formation under low P concentrations. Our result that CR formation was not responsive to low substrate nutrient levels may be in line with what Delgado et al. (2014) found.
Plant interactions
Our experimental evidence indicates that the Proteaceae species had a clear competitive effect on the Nothofagus species independently of the substrate type. Both survival and growth of the Nothofagus species were significantly reduced in all cases by the contiguous presence of a neighbour, even in the presence of a conspecific or a congeneric species, which stands in contrast to more positive results when Nothofagus was planted alone. In contrast, both the survival and growth of the Proteaceae species were significantly enhanced when grown with neighbours than when grown alone, especially in the nursery substrate; the Proteaceae species, particularly E. coccineum, showed a significantly positive RII for survival when grown with a neighbour in the nursery substrate. Although Proteaceae species showed positive survival and growth RIIs when planted with Nothofagus, we cannot assert that the Nothofagus species facilitate Proteaceae species because (1) similar RIIs were observed when the Proteaceae species were planted with a conspecific or confamilial (see below), and (2) the Nothofagus species showed negative RIIs (due to intra- and interspecific competition) when planted with Proteaceae. For facilitation to occur, one species (the facilitator) must alter the environment in a way that enhances the survival, growth and reproduction of a second species (the facilitated), resulting in the benefit of at least one species and the detriment of none (Callaway, 2007). Based on the species coexistence theory (Chesson, 2000), in the absence of stabilizing mechanisms (which promote niche differences), the competitive advantage (average fitness difference) of Proteaceae over Nothofagus species would lead to the competitive exclusion of Nothofagus species. In other words, to counteract the large average fitness difference between Proteaceae and Nothofagus, in which there exists a stable coexistence of the species, (1) very strong stabilizing mechanisms must have evolved over time (e.g. the evolution of Nothofagus species as more shade-tolerant than Proteaceae, which is not the case), or (2) intraspecific competition in Proteaceae should be higher than interspecific competition, which was not the case either because Proteaceae species always survived and grew better when planted with another Proteaceae individual or species (intraspecific facilitation).
Although previous research conducted on south-western Australian Proteaceae species suggested that nutrient-uptake facilitation indeed occurs and enhances plant growth of NCR species (Muler et al., 2014; Teste et al., 2014), bolstering the existence of a community with high plant diversity (Lambers et al., 2018), our results with southern Andes Proteaceae species showed mostly the opposite. Muler et al. (2014) and Teste et al. (2014) conducted microcosm experiments using Proteaceae species from a region where soils are known to be extremely poor in both total and available P, especially when compared with the tephra substrate used in the current experiment. Overall, our results are in line with those recently reported by Zúñiga-Feest et al. (2018) of no facilitative effect of E. coccineum on an NCR species (S. cassioides [Leguminosae]) when cultivated together. Thus, the available evidence indicates that in SSA Proteaceae species CRs act to increase their competitive capacity when grown with NCR species, rather than to facilitate those species. Interestingly, we did find evidence of nutrient exchange from Proteaceae species to Nothofagus species in the nursery substrate, as the latter showed significantly higher leaf Mn concentrations when they grew with a Proteaceae species than when they grew with conspecifics or congenerics. This result adds to recent evidence that SSA Proteaceae species may indeed improve the nutritional status of neighbour plants while, at the same time, they may have no effects or even negative effects on their fitness or performance (Zúñiga-Feest et al., 2018; Piper et al., 2019). In this respect, it is probable that the fitness and performance of the NCR species become limited by resources other than P when growing with the Proteaceae species. This idea is supported by the fact that Nothofagus species growth was similarly reduced by the presence of a conspecific or a congeneric species.
It is worth noting that Proteaceae species showed markedly higher growth rates when they grew with conspecifics or confamilial plants than when they grew alone, particularly in the nursery substrate. This result indicates that although Proteaceae did not facilitate Nothofagus, they did facilitate each other. The highest niche overlap that occurs when two conspecifics interact has traditionally led us to think that competition is the only outcome, which has dwarfed the possibility for intraspecific facilitation to occur, and yet intraspecific facilitation does happen in nature (Fajardo and McIntire, 2011; McIntire and Fajardo, 2014). In this particular case, we anticipate that the mechanism for intraspecific facilitation may be a high capacity of Proteaceae to both increase the mineralization rate and rapidly uptake nutrients from the substrate (see below). This positive interaction may be transient and once the two Proteaceae individuals grow bigger they will compete—or maybe not, and they end up being merged, as is the case for N. pumilio (McIntire and Fajardo, 2011; Fajardo et al., 2016).
Leaf, plant and soil nutrient concentrations
In general, and across species, we observed that in the nursery substrate seedlings planted with neighbours showed significantly higher leaf nutrient concentrations and plant nutrient pools, particularly of P, than seedlings planted alone and in the tephra substrate (Fig. 3, Supplementary Data Table S1). These results were surprisingly consistent with the final N and P pot bulk substrate concentrations, which in the nursery substrate were significantly higher in pots with paired seedlings, regardless of species identity, than in pots with singles (Table 4). In essence, substrate nutrient mineralization, especially P, appeared to be boosted by seedling density, which improved plant nutrition potentially due to an increase in the release of P to the rhizosphere; this would also suggest that Proteaceae were more efficient in absorbing nutrients than Nothofagus, or that Nothofagus became limited by resources other than P when they grew with a neighbour. More importantly, we found that G. avellana, and to a lesser extent E. coccineum, showed higher leaf P concentrations and plant nutrient pools when planted with Nothofagus species than when planted alone or with a conspecific. In particular, the increase in available P at the end of the experiment in conditions of seedlings planted in pairs and in the nutrient-rich nursery substrate suggests that, regardless of species, all seedlings collaborated to release more P than N: Proteaceae through their CRs and Nothofagus through their ectomycorrhizae. Thus, these results suggest the important role of Nothofagus ectomycorrhiza in the mining of soil nutrients (e.g. Marín et al., 2018) and in general of the mycorrhizal-mediated transfer of nutrients at an intra- and interspecific level (Klein et al., 2016; Lambers et al., 2018).
Two questions remain unanswered: (1) is the process of soil nutrient mining by CRs so inefficient that an abundance of P is left in the rhizosphere? and (2) is it possible to expect any positive density-dependency in plants with contrasting nutrient acquisition strategies? The first question is at the core of the idea of the potential facilitative role of Proteaceae species (i.e. for facilitation to occur the acquisition of sorbed P from soil particles must be inefficient). The response to the second question may deal with the Allee effect (Lutscher and Iljon, 2013), and with the occurrence of intraspecific facilitation (see above), in which the aggregation of individuals (i.e. an increase in density) improves the nutritional levels of plants in the population and potentially their ability to mine soil nutrients.
Conclusions
Historically, underground plant–plant interactions have been understood through the lens of competition for limited resources. Driven by the promising hypothesis that Proteaceae species, vis a vis CR nutrient-uptake facilitation, induce a positive effect on the fitness of NCR species, we established a plant interaction experiment which disproved the hypothesis. We found that Nothofagus species did not improve their survival and growth in the presence of Proteaceae species; in fact, they only increased their leaf Mn concentrations, a sign of nutrient-uptake facilitation that, however, did not enhance their survival and growth. Thus, we assert that, at least for SSA Proteaceae species, it appears all but certain that CRs do not mediate a facilitative role in community structuring. In contrast to our facilitative hypothesis, we observed that Proteaceae species improved their growth more when planted with any neighbour (i.e. any species) than when planted alone. Bolstering this result, we evidenced a significant accretion in N and P in the plant tissue of Proteaceae when planted with Nothofagus or with a conspecific, something that was mirrored in the values of nutrient concentrations found in the substrate. This increase in N and P concentrations in the substrate together with the positive effect of growing with other seedlings for Proteaceae may be explained by the rapid mineralization of the organic matter provided by the two seedlings, which, in most cases, would double that mineralized in pots with only one seedling (as all seedlings were originally of similar size). That Proteaceae species had significantly higher survival and growth when planted with conspecifics than when planted alone is congruent with other experimental evidence in the region that points to intraspecific facilitation (Fajardo and McIntire, 2011), and is applicable to developing seedling planting methods, distinct from those currently used (i.e. seedlings planted alone instead than in clusters), for restoration efforts.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: mean values of survival and several plant traits for seedlings of E. coccineum, G. avellana, N. betuloides and N. pumilio.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Jonathan Riquelme, Etienne Robert, Andrea Reyes and Juan Pablo Mora for assistance in the establishment and harvesting of the experiment. The authors also thank Alejandra Zúñiga-Feest and Hans Lambers for discussions about the interpretation of results. Finally, the authors declare that they have no conflict of interest of any kind regarding this study.
FUNDING
This work was supported by the Chilean Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) projects 1120171 and 1160329 to A.F.
LITERATURE CITED
- Armas C, Ordiales R, Pugnaire FI. 2004. Measuring plant interactions: a new comparative index. Ecology 85: 2682–2686. [Google Scholar]
- Avila-Valdés A, Piper FI, Zúñiga-Feest A. 2019. Cluster root formation and function vary in two species with contrasting geographic ranges. Plant and Soil 440: 25–38. [Google Scholar]
- Borie F, Rubio R. 2003. Total and organic phosphorus in Chilean volcanic soils. Gayana Botanica 60: 69–73. [Google Scholar]
- Callaway RM. 2007. Positive interactions and interdependence in plant communities. Dordrecht: Springer. [Google Scholar]
- Chesson P. 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31: 343–366. [Google Scholar]
- Delgado M, Zúñiga-Feest A, Alvear M, Borie F. 2013. The effect of phosphorus on cluster-root formation and functioning of Embothrium coccineum (R. et J. Forst.). Plant and Soil 373: 765–773. [Google Scholar]
- Delgado M, Suriyagoda L, Zúñiga-Feest A, Borie F, Lambers H. 2014. Divergent functioning of Proteaceae species: the South American Embothrium coccineum displays a combination of adaptive traits to survive in high-phosphorus soils. Functional Ecology 28: 1356–1366. [Google Scholar]
- Fajardo A, McIntire EJB. 2011. Under strong niche overlap conspecifics do not compete but help each other to survive: facilitation at the intraspecific level. Journal of Ecology 99: 642–650. [Google Scholar]
- Fajardo A, Piper FI. 2015. High foliar nutrient concentrations and resorption efficiency in Embothrium coccineum (Proteaceae) in southern Chile. American Journal of Botany 102: 208–216. [DOI] [PubMed] [Google Scholar]
- Fajardo A, McIntire EJB, Olson ME. 2019. When short stature is an asset in trees. Trends in Ecology and Evolution 34: 195–199. [DOI] [PubMed] [Google Scholar]
- Fajardo A, Torres-Díaz C, Till-Bottraud I. 2016. Disturbance and density-dependent processes (competition and facilitation) influence the fine-scale genetic structure of a tree species population. Annals of Botany 117: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy R, Romero R, Carrillo R. 1994. Estatus microtrófico de la flora vascular en bosques de coníferas nativas del sur de Chile. Revista Chilena de Historia Natural 67: 209–220. [Google Scholar]
- Grubb PJ, Bellingham PJ, Kohyama TS, Piper FI, Valido A. 2013. Disturbance regimes, gap-demanding trees and seed mass related to tree height in warm temperate rain forests worldwide. Biological Reviews 88: 701–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JN, DeAngelis DL. 2009. Consumer-resource theory predicts dynamic transitions between outcomes of interspecific interactions. Ecology Letters 12: 1357–1366. [DOI] [PubMed] [Google Scholar]
- Klein T, Siegwolf RTW, Körner C. 2016. Belowground carbon trade among tall trees in a temperate forest. Science 352: 342–344. [DOI] [PubMed] [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. 2010. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 334: 11–31. [Google Scholar]
- Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A. 2012. Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Annals of Botany 110: 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL. 2015. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends in Plant Science 20: 83–90. [DOI] [PubMed] [Google Scholar]
- Lambers H, Albornoz F, Kotula L, et al. . 2018. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant and Soil 424: 11–33. [Google Scholar]
- Lamont B. 1982. Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. Botanical Review 48: 597–689. [Google Scholar]
- Lamont BB. 2003. Structure, ecology and physiology of root clusters – a review. Plant and Soil 248: 1–19. [Google Scholar]
- Lamont BB, Brown G, Mitchell DT. 1984. Structure, environmental effects on their formation and function of proteoid roots in Leucadendron laureolum (Proteaceae). New Phytologist 97: 381–390. [Google Scholar]
- Lusk CH, Corcuera LJ. 2011. Effects of light availabilty and growth rate on leaf lifespan of four temperate rainforest Proteaceae. Revista Chilena de Historia Natural 84: 269–277. [Google Scholar]
- Lutscher F, Iljon T. 2013. Competition, facilitation and the Allee effect. Oikos 122: 621–631. [Google Scholar]
- Marín C, Valenzuela E, Godoy R, Palfner G. 2018. Diversity and growth-effects of ectomycorrhizal fungi of a Nothofaguspumilio forest in the Andes of Southern Chile. Boletín Micológico 33: 9–20. [Google Scholar]
- McIntire EJB, Fajardo A. 2011. Facilitation within species: a possible origin of group selected superorganisms. American Naturalist 178: 88–97. [DOI] [PubMed] [Google Scholar]
- McIntire EJB, Fajardo A. 2014. Facilitation as a ubiquitous driver of biodiversity. New Phytologist 201: 403–416. [DOI] [PubMed] [Google Scholar]
- Merino C, Godoy R, Matus F. 2016. Soil enzymes and biological activity at different levels of organic matter stability. Journal of Soil Science and Plant Nutrition 16: 14–30. [Google Scholar]
- Muler AL, Oliveira RS, Lambers H, Veneklaas EJ. 2014. Does cluster-root activity benefit nutrient uptake and growth of co-existing species? Oecologia 174: 23–31. [DOI] [PubMed] [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. [Google Scholar]
- Naranjo JA, Stern CR. 1998. Holocene explosive activity of Hudson Volcano, southern Andes. Bulletin of Volcanology 59: 291–306. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R-Core-team. 2016. nlme: linear and nonlinear mixed effects models. R package Version 3.1-128 http://CRAN.R-project.org/package=nlme. [Google Scholar]
- Piper FI, Baeza G, Zúñiga-Feest A, Fajardo A. 2013. Soil nitrogen, and not phosphorus, promotes cluster-root formation in a South American Proteaceae, Embothrium coccineum. American Journal of Botany 100: 2328–2338. [DOI] [PubMed] [Google Scholar]
- Piper FI, Fajardo A, Baeza G, Cavieres LA. 2019. The association between a nurse cushion plant and a cluster-root bearing tree species alters the plant community structure. Journal of Ecology. In press. [Google Scholar]
- R Development Core Team. 2019. R: A language and environment for statistical computing. Version,3.6.0. Vienna: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- Rodríguez R, Matthei O, Quezada M. 1983. Flora arbórea de Chile. Concepción: Editorial de la Universidad de Concepción, Chile. [Google Scholar]
- Schlesinger WH, Bruijnzeel LA, Bush MB, et al. . 1998. The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Krakatau, Indonesia. Biogeochemistry 40: 37–55. [Google Scholar]
- Shoji I, Nanzyo M, Dahlgren RA. 1994. Volcanic ash soils, Vol. 21 Amsterdam: Elsevier Science Publisher. [Google Scholar]
- Soil Survey Staff. 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. Handbook 436. Washington: Natural Resources Conservation Service, U.S. Department of Agriculture. [Google Scholar]
- Souto CP, Premoli AC, Reich PB. 2009. Complex bioclimatic and soil gradients shape leaf trait variation in Embothrium coccineum (Proteaceae) among austral forests in Patagonia. Revista Chilena de Historia Natural 82: 209–222. [Google Scholar]
- Stolpe NB, Hepp C. 2014. Caracterización taxonómica de los suelos de los valles de interés agropecuario de la Región de Aysén. Boletín INIA 299. Coyhaique: Instituto de Investigaciones Agropecuarias, Chile. [Google Scholar]
- Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N. 2007. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151: 190–205. [DOI] [PubMed] [Google Scholar]
- Teste FP, Veneklaas EJ, Dixon KW, Lambers H. 2014. Complementary plant nutrient-acquisition strategies promote growth of neighbour species. Functional Ecology 28: 819–828. [Google Scholar]
- Vandekerkhove E, Bertrand S, Reid B, Bartels A, Charlier B. 2016. Sources of dissolved silica to the fjords of northern Patagonia (44–48°S): the importance of volcanic ash soil distribution and weathering. Eart Surface Processes and Landforms 41: 499–512. [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer. [Google Scholar]
- Zúñiga-Feest A, Delgado M, Alberdi M. 2010. The effect of phosphorus on growth and cluster-root formation in the Chilean Proteaceae: Embothrium coccineum (R. et J. Forst.). Plant and Soil 334: 113–121. [Google Scholar]
- Zúñiga-Feest A, Muñoz G, Bustos-Salazar A, et al. . 2018. The nitrogen fixing specie Sophora cassioides (Fabaceae) is nutritionally favored and their rhizosphere bacteria modified when is co-cultivated with the cluster root forming Embothrium coccineum (Proteaceae). Journal of Soil Science and Plant Nutrition 18: 597–616. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.