Abstract
Background
Retinal assessment has indicated the presence of neuronal loss in neurodegenerative disorders, but its role in schizophrenia remains unclear. We sought to synthesize the available evidence considering 3 noninvasive modalities: optical coherence tomography, electroretinography, and fundus photography, and examine their diagnostic accuracy based on unpublished individual participant data, when provided by the primary study authors.
Methods
We searched MEDLINE, SCOPUS, clinicaltrials.gov, PSYNDEX, Cochrane Controlled Register of Trials (CENTRAL), WHO International Clinical Trials Registry Platform, and Google Scholar, up to October 30, 2018. Authors were contacted and invited to share anonymized participant-level data. Aggregate data were pooled using random effects models. Diagnostic accuracy meta-analysis was based on multiple cutoffs logistic generalized linear mixed modeling. This study was registered with PROSPERO, number CRD42018109344.
Results
Pooled mean differences of peripapillary retinal nerve fiber layer thickness in micrometer between 694 eyes of 432 schizophrenia patients and 609 eyes of 358 controls, from 11 case-control studies, with corresponding 95% confidence intervals (CIs) by quadrant were the following: −4.55, 95% CI: −8.28, −0.82 (superior); −6.25, 95% CI: −9.46, −3.04 (inferior); −3.18, 95% CI: −5.04, −1.31 (nasal); and −2.7, 95% CI: −4.35, −1.04 (temporal). Diagnostic accuracy, based on 4 studies, was fair to poor, unaffected by age and sex; macular area measurements performed slightly better.
Conclusion
The notion of structural and functional changes in retinal integrity of patients with schizophrenia is supported with current evidence, but diagnostic accuracy is limited. The potential prognostic, theranostic, and preventive role of retinal evaluation remains to be examined.
Keywords: optical coherence tomography/electroretinography, fundus photography, neurodegeneration, neuropsychiatry, retinal imaging, psychosis, biomarkers, optic nerve
Introduction
Schizophrenia (SZ) is a debilitating mental disorder affecting more than 20 million people worldwide.1 Currently, although SZ biomarkers are a rapidly evolving field, a structured interview by a mental health professional is still widely considered as the optimal diagnostic approach.2 In addition, there is dispute over the reliability of biomarkers in predicting or monitoring disease progression.3 Neurodegenerative diseases, such as multiple sclerosis,4 Parkinson’s disease,5 and Alzheimer’s disease6 are known to be correlated with a loss of retinal neurons.7 There is a growing body of evidence suggesting that similar changes may also be present in psychiatric disorders. Two recently published meta-analyses have shown that SZ may be correlated with loss of retinal neurons, detectable by optical coherence tomography (OCT).8,9 OCT is a noninvasive imaging method providing automated in vivo measurements of retinal sections, such as the retinal nerve fiber layer (RNFL).10
In this study, we aimed to investigate the potential role of 3 noninvasive retinal evaluation methods in SZ detection: OCT, fundus photography, and electroretinography (ERG). OCT technology differs by device model and vendor. There are 3 types of OCT devices: time-domain (TD-OCT), swept-source (SS-OCT), and spectral-domain (SD-OCT). SD-OCT and SS-OCT devices allow for more accurate measurements of retinal structures, including the ganglion cell layer (GCL) of the macula, where the cellular bodies of the optic nerve are concentrated.11 Some devices combine this layer with the adjacent inner plexiform layer (IPL), measuring GCL-IPL thickness. ERG is a noninvasive procedure that can detect retinal cell dysfunction after flash stimulation, based on analysis of response waves under high- (photopic), intermediate-, or low-luminance (scotopic) conditions.12,13
Previous OCT studies have yielded discrepant results. For instance, Silverstein et al.14 reported that decreased retinal thickness in macular area was correlated with the presence of diabetes and hypertension. Although relevant studies had measured continuous biomarkers in diseased and non-diseased groups, none of them reported outcomes of diagnostic accuracy. In order to evaluate the discriminatory ability of any of these methods and subgroup effects of age, sex, and antipsychotic medication, we analyzed individual participant data (IPD).
Methods
This study was registered with PROSPERO, number CRD42018109344. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-IPD checklist was completed (supplementary appendix 1).
Search Strategy, Selection Criteria, and Data Extraction
We performed a systematic review and meta-analysis of the literature, based on published aggregate and unpublished IPD. We independently searched the databases MEDLINE, SCOPUS, clinicaltrials.gov, PSYNDEX, Cochrane Controlled Register of Trials (CENTRAL), and WHO International Clinical Trials Registry Platform from the time of their inception to October 30, 2018, with no language restrictions. The following structured algorithm was applied for the database search: [(schizophreni* OR psychosis) AND (retinal OR retina* OR “optical coherence” OR ERG OR electroretinography)].
All observational studies (case-control, cohort, cross-sectional), reporting OCT, ERG, or fundus camera measurements in patients with a SZ diagnosis by a trained psychiatrist and at least 1 comparison group without SZ, were deemed eligible for inclusion in the systematic review. No constraints were applied regarding the age of participants or the diagnostic system used. Non-comparative studies (ie, case reports, case series), animal studies, in vitro studies, and review articles were excluded. The eligibility of results was assessed in a 3-stage process. First, the titles and abstracts were screened for their relevance to the research question. The full text and any supplementary material of potentially eligible studies was subsequently retrieved and examined independently by both authors. Any discrepancies were discussed and resolved. If the full text was unavailable, data extraction was attempted based on the abstract.
The citation indexing feature of the Google Scholar search engine was used to identify any unindexed articles published until October 30, 2018, that cited the eligible studies. A full list of the Google Scholar web addresses used during the search can be found in the supplementary appendix 2. Furthermore, we recursively screened the reference lists of the included studies for eligible entries. Authors of eligible studies, which were published within 10 years prior to search end date, were contacted via e-mail and invited to share anonymized participant-level data. A table showing all requested parameters with their standardized measurement units can be found in the supplementary appendix 3. We validated the integrity of IPD. We checked for typographic errors and implausible values, compared published summary statistics with those derived from the IPD, examined missingness, and visually assessed normality assumptions by constructing quantile-quantile plots. Authors were consulted if any anomalies were found.
Definitions and Outcomes
Peripapillary RNFL (pRNFL) thickness was reported by most OCT studies, either in quadrants or 6 sectors. Those sectors were termed as superior (S), inferior (I), nasal (N), temporal (T), and superior-temporal (ST), superior-nasal (SN), inferior-temporal (IT), inferior-nasal (IN), nasal (N), temporal (T), respectively. We analyzed pRNFL by quadrant, considering S pRNFL as the average of ST and SN pRNFL and I pRNFL as the average of IT and IN pRNFL. Total macular thickness was reported in up to 9 segments (central, inner SNIT, outer SNIT). GCL-IPL average thickness was the average of GCL thickness estimates in 6 macular sectors (ST, SN, N, T, IN, IT).
Mean differences of superior, inferior, nasal, temporal pRNFL, between the eyes of SZ patients and subjects with no diagnosed psychiatric illness were the primary outcome of aggregate data meta-analysis; the secondary outcomes were the mean difference in cubic micrometers of macular volume and the standardized mean differences of the ERG a-wave and b-wave amplitudes, under photopic and scotopic conditions. The IPD meta-analysis primarily investigated the diagnostic accuracy of OCT in SZ; the psychiatrist’s diagnosis was considered as the reference standard, and GCL-IPL average, superior, inferior, nasal, temporal pRNFL, central macular thickness measurements were the index tests. In this analysis, sensitivity and specificity at the optimal cutoff, along with the area under the summary receiver operating characteristic curve (AUSROC) estimates were the primary outcomes. Secondary outcomes were the optimal threshold per index test, across all included studies.
Quality and Risk of Bias Assessment
We evaluated the quality of all studies that provided sufficient information in the full text and any supplementary material, using the Newcastle-Ottawa Scale (NOS) for case-control and cohort studies.15 The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2)16 was used to assess the quality of all studies included in a diagnostic accuracy meta-analysis (DMA). All evaluations were performed independently by both reviewers and consensus was reached for discrepancies.
Statistical Analysis
Data management and statistical analysis were performed in R 3.5.3 environment.17 Statistical significance level (α) was set at .05. The eyes of participants were regarded as the unit of analyses. For the aggregate data meta-analysis, when IPD were not available, combined summary statistics from 3 or more studies were calculated, by pooling variances of reported right and left eye measurements. The GetData Graph Digitizer software was used to retrieve unreported data from appropriate study figures.18 Effect sizes were calculated and univariate random effects models were fitted using the metafor package.19,20 Between-study heterogeneity was appraised with the Cochran’s Q statistic21 and the Higgins’ inconsistency index (I2),22 with I2 > 50% indicating substantial heterogeneity. Four univariate generalized mixed-effects models, including more than 10 studies,23 were constructed—pRNFL superior, inferior, nasal, temporal mean differences were the dependent variables, and mean age, male percentage, NOS score, and type of OCT device used (SD vs TD) were the effect modifiers. Sensitivity analyses included leave-one-out analyses,24 publication bias assessment with Egger’s regression,25 Begg–Mazumdar rank correlation test,26 and funnel plot asymmetry evaluation with the “trim and fill” method,27 for all pRNFL quadrants. To investigate the effect of data availability bias, funnel plot asymmetry and pooled estimates of studies with IPD were compared with the results from other studies.28,29
For the IPD meta-analysis, DMA was the primary analysis. Imputed data meta-analysis was a secondary analysis, handling missing data in 2 studies14,30; missingness was less than 10% for all OCT measurements analyzed (refer to table 1, under “Measurements” column, for further details about missing measurements and supplementary appendix 3 for details about imputed data analysis methodology). Separate DMAs were done for right and left eyes. After calculating numbers of true-positive, true-negative, false-positive, and false-negative diagnosis at every possible cutoff, from each study, several generalized linear mixed-effects models of binomial family were constructed by the diagmeta package; the restricted maximum likelihood criterion was used for model selection.31,32 Cutoff values were multiplied by −1 in order to account for the negative correlation of biomarker values with test outcome.31 The nsROC package was used for nonparametric imputed data DMA33 (supplementary appendix 4). Summary ROC curves were constructed and AUSROC, specificity, sensitivity, and across-study optimal cutoffs were estimated. Heterogeneity was appraised with the residual variance of the random effects model (τ 2). We compared independent AUSROC estimates34,35 of subjects less than 40 years old vs subjects with an age ≥40 years, and male vs female subjects, and assessed diagnostic accuracy after exclusion of reported smokers and hypertensives in 2 studies—smokers were only reported by 1 study.36 Exploratory data analysis was performed for GCL-IPL average thickness measurements in cases, based on univariate, multilevel generalized linear modeling (supplementary appendix 5). Differences from published protocol are presented in supplementary appendix 6.
Table 1.
Summary of Study Characteristics
| Study (Period) | Participants (Eyes for OCT Studies) | Country | Device Type | Device Model (Device Vendor) | Measurements | Diagnostic Method | Matching Variables | Eligibility Criteria | NOS Score (S/C/E) |
|---|---|---|---|---|---|---|---|---|---|
| A. OCT studies | |||||||||
| Ascaso et al.37 (NR) | 20 (40) 10 SZ:10 controls (20 SZ: 20 control eyes) |
Spain | TD-OCT | Stratus OCT (Carl Zeiss Meditec Inc) | pRNFL: overall, by quadrant, by clock hour, macular thickness (9 segments), macular volume | Structured clinical interview50 (DSM-IV-TR) | Age, sex | Refractive error < ±2 SPH D, no known ophthalmologicala, neurological disorders | 8 (3/2/3) |
| Ascaso et al.39 (2010–2011) | 60 (120) 30 SZ:30 controls (60 SZ: 60 control eyes) |
Spain | TD-OCT | Stratus OCT (Carl Zeiss Meditec Inc) | pRNFL: overall, by quadrant and clock hour, total macular thickness (9 segments), macular volume | Structured clinical interview50 (DSM-IV) | Age, sex | Refractive error < ±2 SPH D, no known ophthalmologicala, neurological disorders, no media opacitiesb | 7 (4/2/1) |
| Celik et al.40 (NR) | 122 (122) 81 SZ: 41 controls (81 SZ: 41 control eyes) |
Turkey | SD-OCT | Spectralis OCT, version 6.0 (Heidelberg Engineering Inc) | pRNFL: 7 segments, GCL-IPL thickness: 6 segments | DSM-IV criteria | Age (18–65), sex | Refractive error ≤ −1 SPH/CYL D, no known ophthalmologicala, neurological, or systemic degenerative disorder | 8 (4/2/2) |
| Chu et al.41 (NR) | 78 (156) 38 SZ: 40 controls (76 SZ: 80 control eyes) |
UK | TD-OCT | Stratus OCT (Carl Zeiss Meditec Inc) | pRNFL: overall and by quadrant, macular volume | DIP-DM 42 | Age, sex | Normal logMAR visual acuity and Humphrey visual fields, refractive error ≤ −6 SPH D, no history of systemic disorder affecting the eyes, glaucoma, head injury, substance abuse | 4 (2/2/0) |
| Delibas et al.36 (2016–2017) | 102 (204) 63 SZ: 39 controls (126 SZ: 78 control eyes) |
Turkey | SD-OCT | Cirrus HD-OCT 4000, version 6.5 (Carl Zeiss Meditec Inc) | pRNFL: by quadrant, GCL-IPL thickness: 6 segments (IPD) | Structured clinical interview50 (DSM-IV) | Age (18–65), sex (IPD) | No psychotic attack within 6 months before recruitment, no ophthalmologicala, neurological, systemic degenerative disorders, no substance abuse within 6 months prior to enrollment, no ocular surgery, or trauma | 5 (2/2/1) |
| Joe et al.43 (NR) | 24 (48) 3 SZ:3 bipolar disorder subjects: 18 matched controls (6 SZ: 6 bipolar disorder: 36 matched control eyes) |
USA | SD-OCT | Spectralis HRA+OCT (Heidelberg Engineering Inc) |
Total macular thickness: 9 segments (IPD), macular volume: overall and 9 segments (IPD), pRNFL (IPD; 6 cases and 6 controls), choroidal thickness (aggregate data) | DI-PAD semi-structured clinical interview44 | Age, sex (IPD) | No known ophthalmologicala or neurological disorder | 5 (3/2/0) |
| Lee et al.45 (2012–2013) | 60 (60) 30 SZ: 30 controls (30 SZ: 30 control eyes) |
Malaysia | SD-OCT | Cirrus HD-OCT 4000 (Carl Zeiss Meditec Inc) | pRNFL: overall and by quadrant, total macular thickness: 9 segments, macular volume | Structured clinical interview50 (DSM-IV-TR) | Age (>18), sex, race | Normal IOP measurements, refractive error ≤ −2 SPH D, no known ophthalmological disordera, no history of syncope, CNS tumors, diabetes mellitus, ocular trauma, or ocular surgery within 3 months prior to enrollment | 8 (4/2/2) |
| Mota et al.46 (April–July 2014) | 40 (80) 20 SZ: 20 controls (40 SZ: 40 control eyes) |
Portugal | SD-OCT | Spectralis OCT (Heidelberg Engineering Inc) | pRNFL: 7 segments, total macular thickness: 9 segments, macular volume: overall and 9 segments | ICD-10 criteria | Age (>18), sex | Refractive error < −6 SPH D, IOP < 22 mm Hg, no ophthalmologicala, neurological, or degenerative systemic disorder, no substance abuse, no history of consciousness loss, no media opacitiesb | 5 (2/2/1) |
| Samani et al.47 (2014–2015) | 85 (170) 35 SZ: 50 controls (70 SZ: 100 control eyes) |
UK | SD-OCT | Leica Envisu C2300 (Leica Microsystems Inc) | pRNFL: by quadrant (IPD), total foveal macular thickness (IPD), macular thickness by retinal layer (aggregate data) | ICD-10 criteria | Age, sex, race (IPD) | Refractive error < ±6 SPH D, without diabetes mellitus, or known ophthalmological disordera, no history of substance abuse | 7 (4/2/1) |
| Silverstein et al.14 (NR) | 64 (128) 32 SZ: 32 controls (64 SZ: 64 control eyes) |
USA | SD-OCT | Cirrus HD-OCT 4000, version 8.1 (Carl Zeiss Meditec Inc) | pRNFL: overall and by quadrant (IPD), total macular thickness: foveal, 4 outer segments (missingness <5%, MAR, IPD), GCL-IPL average thickness (missingness 8%, IPD), Macular volume (IPD) Reasons for missingness: not measured, poor image quality |
Structured clinical interview50 (DSM-IV) | Age, sex (IPD) | 11 SZ: 11 controls were hypertensive, no ocular trauma, ophthalmological disordera, no history of substance abuse within 6 months prior to enrollment, no history of head trauma or loss of consciousne ss >10 min | 6 (3/2/1) |
| Topcu-Yilmaz et al.30 (2014–2015) | 95 (95) 59 SZ: 36 controls (59 SZ: 36 control eyes) |
Turkey | SD-OCT | Spectralis OCT (Heidelberg Engineering Inc.) |
Total macular thickness: 9 segments (missingness <1%, MCAR, IPD), pRNFL: 7 segments (IPD), choroidal thickness (IPD) | DSM-IV (no structured interview) | Age, sex (IPD) | Refractive error ≤ −2 SPH D, no media opacitiesb | 7 (4/2/1) |
| Yilmaz et al.48 (2014–2015) | 64 (128) 34 SZ: 30 controls(68 SZ eyes: 60 control eyes) |
Turkey | SD-OCT | Cirrus HD-OCT 400 (Carl Zeiss Meditec Inc) |
pRNFL: overall and by quadrant, total macular thickness: 9 segments, total retinal thickness | Previously diagnosed (method NR) | Age, sex | Nonsmokers, no cardiovascular disease no history of ocular surgery or trauma, no media opacitiesb | 4 (2/2/0) |
| B. ERG studies | |||||||||
| Balogh et al.49 (NR) | 46 26 SZ: 20 controls |
Hungary | ERG | Nicolet Spirit computer (Nicolet Instrument Technologies, Inc) | Repeated photopic a-wave, b-wave amplitudesc | International Neuropsychiatric Interview Plus50 (DSM-IV) | Age, sex | No history of neurological disorder, head trauma, substance abuse | 8 (3/2/3) |
| Demmin et al.51 (NR) | 50 25 SZ: 25 controls | USA | ERG | RETeval (LKC Technologies Inc) | Repeated scotopic and photopic a-wave, b-wave, photopic negative response waved amplitudes | Structured clinical interview50 (DSM-IV) | Age (18–60) | No ophthalmological disordersa, diabetes mellitus, substance abuse within 6 months prior to enrollment | 7 (4/2/1) |
| Gerbaldo et al.52 (NR) | 22 9 SZ: 13 controls |
Germany | ERG | Burian-Allen contact lens electrode, unspecified computer (vendors NR) | Repeated scotopic and photopic b-wave amplitudes | DSM-III-R criteria | Age | No detected comorbidities after history, physical and laboratory exam | 4 (3/1/0) |
| Hébert et al.53 (NR) | 255 150 SZ: 105 controls | Canada | ERG | Dawson-Trick-Litzkow electrode, Shieldex 33/9 (Statex) | Repeated scotopic and photopic a-wave, b-wave amplitudes | DSM-IV criteria | Age, sex | Cases were not refractory to treatment | 3 (1/2/0) |
| Warner et al.54 (NR) | 9 SZ: 9 controls | UK | ERG | Nicolet Spirit computer (Nicolet Instrument Technologies Inc) | Repeated scotopic and photopic a-wave, b-wave amplitude measurements | Medical records (DSM-IV) | Age, sex | No media opacitiesb, no history of ophthalmologicala or other disorders requiring treatment | 4 (2/2/0) |
| C. Fundus photography studies | |||||||||
| Meier et al.55 (2010–2012) | 27 SZ: 412 healthy subjects (54 SZ: 824 healthy eyes) |
New Zealand | Fundus camera | Canon NMR-45 with 20 D SLR backing (Canon Inc) | Retinal venular diameter, retinal arteriolar diameter (standardized)—photographs were taken when participants were 38 years old | DSM-III-R, DSM-IV criteria | None e | No pregnancy, no congenital conditions, satisfactory image quality | 9 (4/2/3) |
| Meier et al.56 (2009) | 45 SZ: 462 healthy subjects (90 SZ: 924 healthy eyes) |
Australia | Fundus camera | Nidek 3-Dx/F (Nidek Co, Ltd) | Retinal venular diameter (standardized)—photographs were taken at adolescence or early adulthood | CIDI 57 | Nonee | None reported | 7 (3/2/2) |
Note: A: OCT studies, B: ERG studies, C: Fundus photography studies. All studies, with the exception of 2 cohort studies using fundus photography by Meier et al.55 and Meier et al.56 were based on a case-control design. Controls were psychiatrically healthy individuals in all studies. Samani et al.47 also included relatives of cases in the control group. OCT, optical coherence tomography; ERG, electroretinography; TD, time domain; SD, spectral domain; SZ, schizophrenia subjects; RNFL, retinal nerve fiber layer; pRNFL, peripapillary RNFL thickness; GCL-IPL, ganglion cell layer with inner plexiform layer; SPH, optical spherical equivalent; CYL, optical cylindrical equivalent; D, diopters; logMAR, logarithm of the Minimum Angle of Resolution; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Diseases; IPD, individual participant data; NOS, Newcastle-Ottawa scale; S/C/E, selection/comparability/exposure; IOP, intraocular pressure; CKD, chronic kidney disease; DI-PAD, Diagnostic Interview for Psychosis and Affective Disorders; MCAR, missing completely at random; MAR, missing at random; DIP-DM, Diagnostic Interview for Psychosis; NR, Not reported; SLR, single-lens reflex camera; CIDI, Composite International Diagnostic Interview.
aOphthalmological disorder: any disorder that can affect the optic nerve (ie, macular degeneration, glaucoma, diabetic retinopathy, autoimmune, infectious diseases) and fixation (ie, strabismus, nystagmus).
bMedia opacity: any transparency loss in the eye’s refractive media (cornea, aqueous humor, lens, vitreous) preventing retinal evaluation.
cIn the study by Balogh et al.,49 the first set of measurements was taken at least 2 weeks after onset of medication; a second one was taken a few weeks later. Only the second set of measurements was included in quantitative synthesis.
dPhotopic negative response, reported by Demmin et al.,51 is a ganglion cell-specific ERG response wave.
eCohort studies; analysis was adjusted for covariates, which are presented in the supplementary table S5.
Results
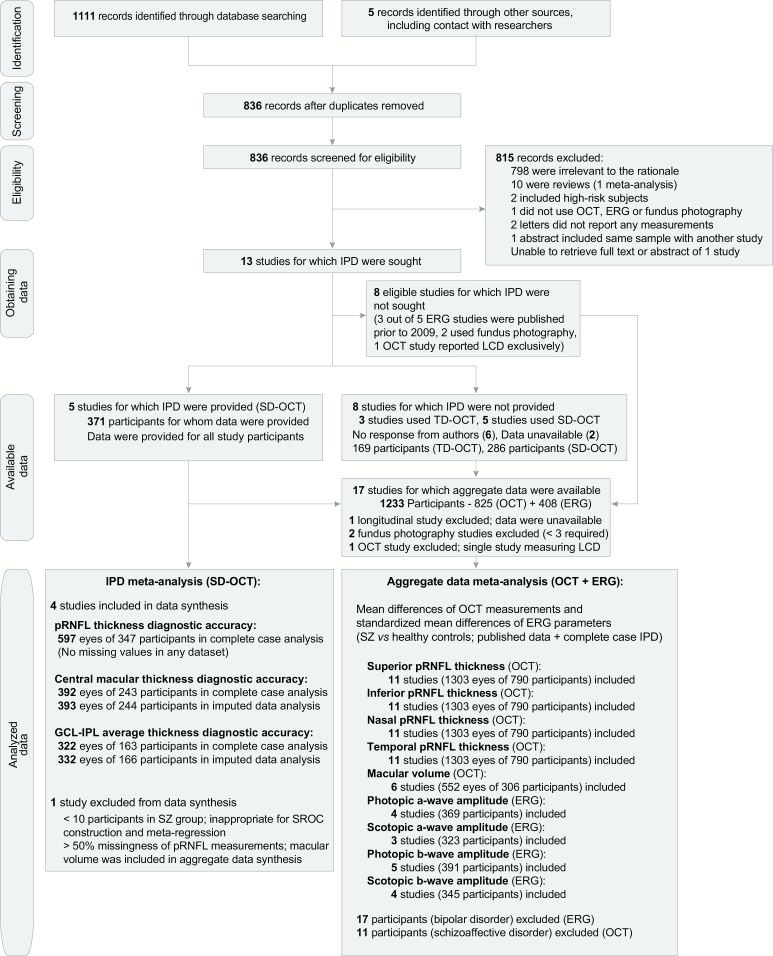
Of 1111 results from the database search and 5 additional studies found from other sources, after screening for duplicates, 815 records were excluded; 798 were irrelevant to the research question, 10 were reviews,8,58–66 2 included high-risk subjects for SZ,67,68 1 did not use an eligible ophthalmic device,69 1 was a summary overlapping with another study,70 2 were letters without any reported measurements,71,72 and the abstract of 1 study could not be retrieved.73 All eligible studies that reported ERG parameters49,51–54 were excluded from the IPD meta-analysis, because most of them49,52,54 were conducted more than 10 years before this study. We requested IPD for 13 OCT studies14,30,36,37,39–41,43,45–48,74; data of 5 SD-OCT studies were provided.14,30,36,43,47 The authors of 6 studies did not respond, whereas authors of 2 studies40,41 responded that the requested data were no longer available. No data anomalies were found. 1 study was excluded from quantitative synthesis due to the minimal number of cases; an out-of-sample evaluation of DMA results was done using IPD from this study43 (supplementary appendix 7). In total, 4 eligible studies were excluded55,56,74,75 from the aggregate data synthesis, due to lack of sufficient data. Seventeen case-control studies–12 using OCT14,30,36,37,39–41,43,45–48 (1351 eyes of 825 participants) and 5 using ERG49,51–54 (408 participants) were eventually included in the aggregate data meta-analysis; 11 subjects with schizoaffective disorder41 and 17 participants with bipolar disorder49 were excluded. 4 studies, including 597 eyes of 346 participants (42.5% of participants in eligible OCT studies, 52.7% of participants in all SD-OCT studies) were included in the IPD meta-analysis. The systematic review procedure is presented with a flow diagram38 in figure 1.
Fig. 1.
PRISMA-IPD flow diagram. IPD, individual participant data; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SZ, schizophrenia subjects; OCT, optical coherence tomography; ERG, electroretinography; TD, time-domain; SD, spectral-domain; pRNFL, peripapillary retinal nerve fiber layer; GCL-IPL, ganglion cell layer with inner plexiform layer; LCD, lamina cribrosa depth; SROC, summary receiver operating characteristic curve.
Study Characteristics and Missingness Information
Study characteristics, along with information about missing data, are summarized in table 1. Various diagnostic procedures were followed.42,44,50,57,76 The Diagnostic and Statistical Manual of Mental Disorders (DSM)77 was used as the diagnostic classification system in 13 studies, while the International Classification of Diseases (ICD)78 was used in 2 European studies. Controls were age matched in all studies. Participants with comorbidities that could affect the reported measurements were excluded in every eligible study. The Positive and Negative Syndrome Scale79 was used to assess clinical severity of SZ in 11 studies.14,30,36,37,39,40,45–47,49,51 Antipsychotic dose was reported in chlorpromazine equivalents80 in 7 studies.14,36,39,47,49,51,54 Three studies excluded participants with diagnosed diabetes mellitus (DM).45,47,51 Two studies included a subgroup of treatment-naïve patients.41,54 All 5 ERG studies reported the amplitudes of response waves under photopic conditions. Only 1 eligible study did not report ERG measurements under scotopic conditions.49 One study51 reported an additional ganglion cell-specific response wave (photopic negative response). Heterogenous pupil dilation, flash stimuli, and signal processing hardware were used. All 12 OCT studies measured pRNFL by segments; these measurements were available for less than 50% of the participants in the IPD of 1 study,43 which was eventually excluded from data synthesis. Total macular thickness was measured by segments in 7 studies,14,37,39,43,45–47 macular volume was measured in 6 studies,14,37,39,41,43,45 whereas GCL thickness in the macular area was reported in 4 studies.14,36,40,47 A Stratus TD-OCT device was used in 3 studies,37,39,41 a Cirrus SD-OCT device in 4 studies,14,36,45,48 a Spectralis SD-OCT device in 4 studies,30,40,43,46 and a handheld Leica Envisu SD-OCT device in 1 study.47 Five OCT studies36,41,43,46,48 and 3 ERG studies52–54 scored less than 6 of 9 in the NOS (supplementary table S1). A summary of data extracted from 2 cohort studies using fundus photography55,56 is presented in the supplementary table S2.
Aggregate Data Synthesis
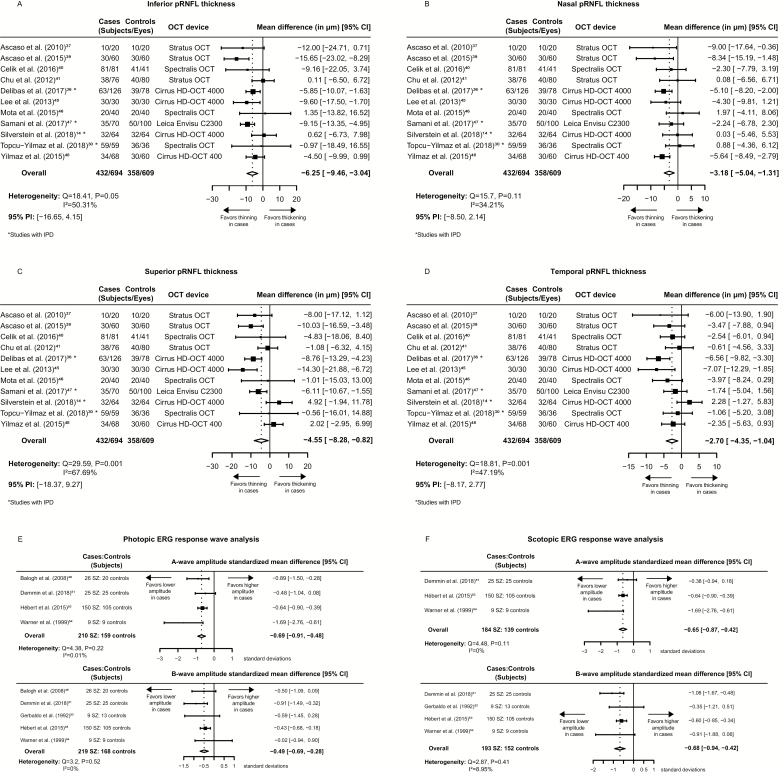
The mean differences, between cases and controls, of the pRNFL measurements by quadrant were pooled from 11 studies. The pooled estimates were −4.55 μm, 95% confidence interval (CI): [−8.28, −0.82], 95% prediction interval (PI): [−18.37, 9.27], heterogeneity: Q = 29.59 (df = 11), P = .001, I2 = 67.69% for the superior quadrant; −6.25 μm, 95% CI: [−9.46, −3.04], 95% PI: [−16.65,4.15], heterogeneity: Q = 18.41 (df = 11), P = .05, I2 = 50.31% for the inferior quadrant; −3.18 μm, 95% CI: [−5.04, −1.31], 95% PI: [−8.50, 2.14], heterogeneity: Q = 15.7 (df = 11), P = .11, I2 = 34.21% for the nasal quadrant; and −2.7 μm, 95% CI: [−4.35, −1.04], 95% PI: [−8.17, 2.77], heterogeneity: Q = 18.81 (df =11), P = .001, I2 = 47.19% for the temporal quadrant (figures 2A–D). The meta-regression analysis did show that, for a single year increase in age, nasal and inferior pRNFL thickness of cases were significantly reduced on average; the 95% CIs of model coefficients were [−1.03, −0.18] and [−1.39, −0.11] respectively, and a single-point increase of the NOS score correlated with significantly reduced superior and inferior pRNFL thickness effect size in cases; the 95% CIs of model coefficients were [−4.35, −0.07] and [−3.76, −0.57], respectively (table 2). Egger’s and Begg’s tests for publication bias were not statistically significant (refer to supplementary figure S1 for funnel plots, supplementary figure S2 for radial plots, and supplementary table S3 for statistical testing results). Macular volume mean differences were pooled from 6 studies; the overall effect size was statistically insignificant (supplementary figure S3). Standardized mean differences of ERG response waves under photopic and scotopic conditions were also pooled; a relatively small decrease of wave amplitudes in cases, along with low between-study heterogeneity, was observed (figures 2E–F). The statistical significance of pooled estimates of pRNFL thickness at the inferior, nasal, temporal quadrants, b-wave amplitudes, and a-wave amplitudes under photopic conditions was not altered by removing any of the included studies from the meta-analysis (supplementary table S4). No statistically significant differences were found in subgroup comparisons of pooled estimates from studies without IPD vs studies with IPD (supplementary table S5).
Fig. 2.
Mean differences of pRNFL measurements and standardized mean differences of ERG measurements between schizophrenia cases and controls. (A) inferior quadrant pRNFL thickness, (B) nasal quadrant pRNFL thickness, (C) superior quadrant pRNFL thickness, (D) temporal quadrant pRNFL thickness, (E) photopic ERG response wave analysis, (F) scotopic ERG response wave analysis. 95% CI, 95% confidence interval; 95% PI, 95% prediction interval; SZ, schizophrenia subjects; OCT, optical coherence tomography; ERG, electroretinography; pRNFL, peripapillary retinal nerve fiber layer; IPD, individual participant data; HD, high definition.
Table 2.
Results of Aggregate Data Meta-regression Analysis
| Study-Level Covariate | Number of Studies | Coefficient (SE) | z Statistic | P Value | 95% CI |
|---|---|---|---|---|---|
| Superior pRNFL mean difference | |||||
| Percentage of males | 11 | 0·046 (0·205) | 0·224 | .823 | −0·36, 0·445 |
| Mean patient age | 11 | −0·367 (0·461) | −0·8 | .426 | −1·27, 0·537 |
| NOS score | 11 | −2·21 (1·09) | −2·02 | .043 | −4·35, −0·07 |
| Type of OCT device | 11 | ||||
| Time-domain | 3 | Reference | |||
| Spectral-domain | 8 | 2·13 (4·36) | 0·49 | .63 | −10·68, 6·42 |
| Nasal pRNFL mean difference | |||||
| Percentage of males | 11 | −0·016 (0·1) | −0·165 | .87 | −0·21, 0·18 |
| Mean patient age | 11 | −0·61 (0·22) | −2·80 | .005 | −1·03, −0·18 |
| NOS score | 11 | −0·078 (0·69) | −0·11 | .911 | −1·28, 1·43 |
| Type of OCT device | 11 | ||||
| Time-domain | 8 | Reference | |||
| Spectral-domain | 3 | −2·58 (2·67) | −0·965 | .335 | −7·82, 2·66 |
| Inferior pRNFL mean difference | |||||
| Percentage of males | 11 | −0·12 (0·18) | −0·69 | .49 | −0·48, 0·23 |
| Mean patient age | 11 | −0·75 (0·33) | −2·31 | .02 | −1·39, −0·11 |
| NOS score | 11 | −2·16 (0·81) | −2·66 | .008 | −3·76, −0·57 |
| Type of OCT device | 11 | ||||
| Time-domain | 8 | Reference | |||
| Spectral-domain | 3 | −2·82 (3·96) | −0·71 | .477 | −10·58, 4·95 |
| Temporal pRNFL mean difference | |||||
| Percentage of males | 11 | −0·002 (0·09) | −0·017 | .986 | −0·17, 0·17 |
| Mean patient age | 11 | −0·092 (0·213) | −0·432 | .666 | −0·51, 0·326 |
| NOS score | 11 | −0·265 (0·611) | −0·434 | .664 | −1·464, 0·933 |
| Type of OCT device | 11 | ||||
| Spectral-domain | 8 | Reference | |||
| Time-domain | 3 | 0·05 (2·15) | 0·022 | .983 | −4·17, 4·27 |
Note: P values indicating statistical significance of regression coefficients are shown in bold. SE, standard error; 95% CI, 95% confidence interval of coefficient; pRNFL, peripapillary retinal nerve fiber layer thickness; NOS, Newcastle-Ottawa scale.
Participant Characteristics and Diagnostic Accuracy Evaluation Based on IPD
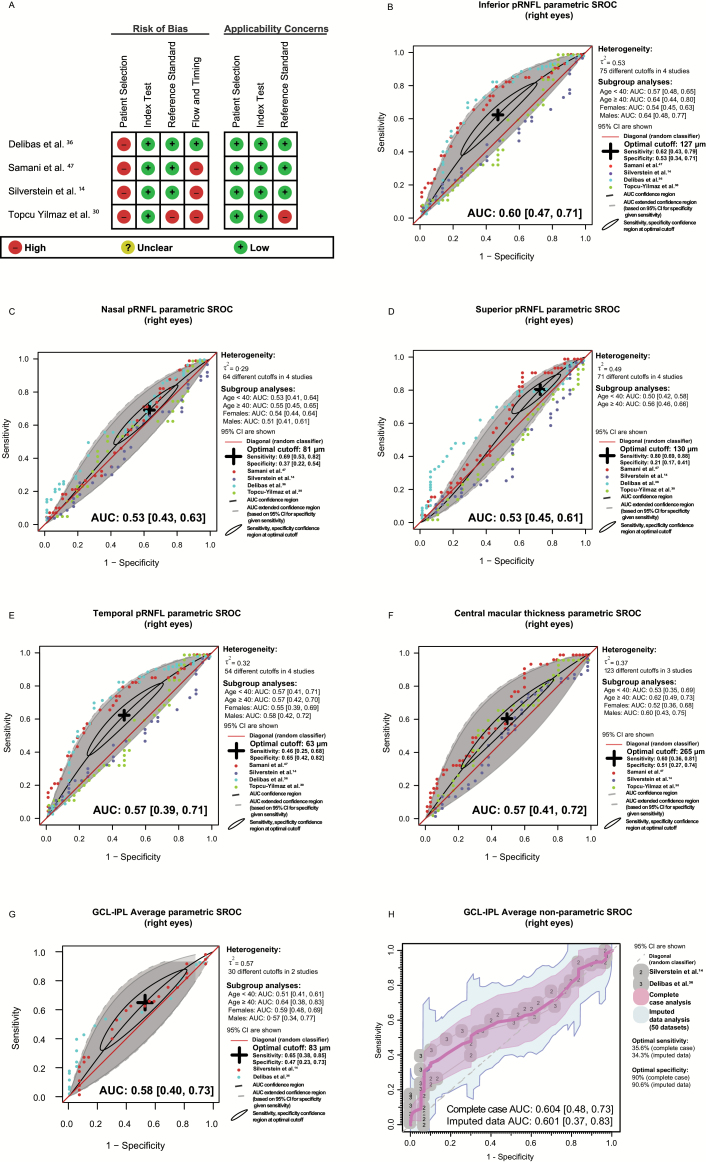
The participant characteristics of studies with IPD are presented in the supplementary table S6. QUADAS-2 quality assessment indicated that diagnostic accuracy estimates may be biased due to the case-control design of all included studies. In addition, 3 studies had not applied the reference standard to the controls14,30,47 and 1 study had not used a structured clinical interview as the reference standard,30 potentially compromising the validity of the results (figure 3A, supplementary figure S4, and supplementary appendices 8–11). Results of DMA, along with unadjusted and stratified AUSROC estimates, are shown in figures 3B–H for right eyes; refer to supplementary figure S5 for left eye results. Sensitivity and specificity estimates of right eye measurements, at optimal cutoffs, were respectively as follows: 0.80, 95% CI: 0.69, 0.88 and 0.21, 95% CI: 0.17, 0.41 (superior pRNFL); 0.62, 95% CI: 0.43, 0.79 and 0.53, 95% CI: 0.34, 0.71 (inferior pRNFL); 0.69, 95% CI: 0.53, 0.82 and 0.37, 95% CI: 0.22, 0.54 (nasal pRNFL); 0.46, 95% CI: 0.25, 0.68 and 0.65, 95% CI: 0.42, 0.82 (temporal pRNFL); 0.60, 95% CI: 0.36, 0.81 and 0.51, 95% CI: 0.27, 0.74 (central macular thickness); 0.65, 95% CI: 0.38, 0.85 and 0.47, 95% CI: 0.23, 0.73 (GCL-IPL average). Estimates based on left eye measurements were respectively as follows: 0.62, 95% CI: 0.50, 0.71 and 0.50, 95% CI: 0.38, 0.61 (superior pRNFL); 0.75, 95% CI: 0.56, 0.88 and 0.39, 95% CI: 0.21, 0.60 (inferior pRNFL); 0.60, 95% CI: 0.37, 0.79 and 0.46, 95% CI: 0.25, 0.68 (nasal pRNFL); 0.76, 95% CI: 0.62, 0.86 and 0.29, 95% CI: 0.17, 0.44 (temporal pRNFL); 0.58, 95% CI: 0.35, 0.78 and 0.56, 95% CI: 0.34, 0.77 (central macular thickness); 0.47, 95% CI: 0.20, 0.75 and 0.68, 95% CI: 0.38, 0.88 (GCL-IPL average). Inferior quadrant pRNFL thickness, central macular, and GCL-IPL average thickness at the inferior quadrant demonstrated fair discriminatory power, but were not diagnostic at the 95% confidence level. Other OCT measurements performed poorly as disease classifiers. Between-subgroup differences in the estimated AUSROCs were not statistically significant (supplementary table S7). After excluding smokers from the study by Delibas et al.36 and hypertensives, as well as participants with DM, from the study by Silverstein et al.,14 GCL-IPL average became diagnostic of SZ (supplementary figure S6). Exploration of correlations between GCL-IPL average thickness and disease-specific covariates in 2 eligible studies14,36 yielded heterogenous results (supplementary table S8).
Fig. 3.
Diagnostic accuracy meta-analysis based on right-eye individual participant data. (A) QUADAS-2 methodological quality assessment summary, (B) inferior quadrant pRNFL thickness diagnostic accuracy, (C) nasal quadrant pRNFL thickness diagnostic accuracy, (D) superior quadrant pRNFL thickness diagnostic accuracy, (E) temporal quadrant pRNFL thickness diagnostic accuracy, (F) central macular thickness diagnostic accuracy (complete case analysis), (G), GCL-IPL average thickness diagnostic accuracy (complete case analysis), (H) GCL-IPL average thickness diagnostic accuracy (imputed data analysis). Unadjusted and subgroup AUC estimates are shown. Sensitivity and specificity confidence regions at the optimal cutoff are represented with an ellipse; the center of the ellipse corresponds to their point estimates. Panel G shows the extra uncertainty due to missing data in Silverstein et al.14 study; it is based on a nonparametric analytical approach and does not take into account any cutoff information. SROC, summary receiver operating characteristic curve, AUC, area under the curve, τ 2, residual variance; pRNFL, peripapillary retinal nerve fiber layer; QUADAS-2; Quality Assessment of Diagnostic Accuracy Studies 2.
Discussion
The main findings of our study were that, based on available evidence from 11 case-control studies, pRNFL measurements were reduced in SZ cases, and that OCT indices had fair to poor discriminatory potential, unaffected by age and sex, in 4 matched case-control studies. Narrower PIs for inferior, nasal, and temporal pRNFL mean differences suggested that future studies of similar design will likely favor retinal thinning in these quadrants.81 Superior quadrant pRNFL was also significantly reduced. It is noteworthy that studies of higher quality reported greater mean differences of inferior and superior pRNFL thickness (table 2). However, the statistical significance of the superior quadrant mean difference was lost in the leave-one-out analysis, raising concerns about its validity. Diagnostic accuracy heterogeneity, between the 4 studies with IPD, was lower for macular area measurements (ie, GCL-IPL average thickness, foveal macular thickness). Excluding all reported hypertensives, participants with DM and smokers slightly improved GCL-IPL average diagnostic efficacy, suggesting that these factors were not responsible for the observed differences between cases and controls. Both aggregate data and IPD analyses indicated that the observed differences in pRNFL thickness were rather small to be clinically meaningful in diagnosis (supplementary table S5). Although examining variability in findings of primary studies, we found that increased age of sampled cases was correlated with greater differences in inferior and nasal quadrant pRNFL measurements from controls (table 2). Two fundus photography studies showed greater retinal vessel diameters in both young and elderly SZ patients. ERG amplitude measurements were reduced in SZ patients, in all eligible studies, and between-study heterogeneity was small. However, the small number of studies reporting ERG measurements does not allow for robust inference. Study quality was moderate to high in OCT studies and moderate to low in ERG studies, thus providing a more solid base for interpretability in the former indices. The effect of disease severity, duration, and medication on OCT measurements remains unclear.
In our study, we demonstrated that a distinct pattern of retinal changes may be emerging through 3 noninvasive modalities. Both structural and functional assessment of retinal cells indicated deficits in chronically treated SZ subjects. It is noteworthy that the GCL-IPL average thickness was the only measurement that outperformed a random test in the 2 studies that were included in IPD meta-analysis14,36; this finding is further supported by significant differences of GCL thickness in the aggregate data of 2 other OCT studies40,47 and a decreased photopic negative response in one ERG study that measured it51. The cross-sectional nature of available data does not allow for solid conclusions regarding the pathophysiology of the disease. Nevertheless, it can be hypothesized that the rate of neuronal loss in SZ subjects is higher than normally anticipated,82 regardless of medication received. This can be observed both in aggregate data and IPD: studies that included older SZ subjects reported, on average, slightly lower nasal and inferior quadrant pRNFL measurements, compared to controls, and, when IPD were available, the discriminatory ability of inferior, nasal pRNFL, and GCL-IPL average measurements was slightly improved in older subgroups, without those differences reaching statistical significance. One possible mechanism that could explain such subtle, ongoing changes in the retina is retrograde transsynaptic degeneration (RTSD).
RTSD has been described in the human visual system since late 20th century.83 It refers to progressive damage of ganglion cells secondary to synaptic dysfunction in the lateral geniculate nucleus in the thalamus, where the optic nerve fibers are connected. It has been speculated that thalamic connectivity is disrupted in SZ.84 Given the complexity and heterogeneity of the disorder, it can be hypothesized that it also indirectly affects neurons in the lateral geniculate nucleus, yet these changes are very slight, probably unrelated to the clinical severity of the disease—as suggested by exploratory analysis of IPD. However, it is interesting that retinal cell dysfunction could be detected with ERG in high-risk subjects as well.67,68 Furthermore, the only longitudinal ERG study49 found that those deficits were greater at an earlier point in the course of treatment, which may be conjectured to imply an attenuation of retinal changes after onset of treatment. It can be assumed that the rate of neuronal damage is related with the vulnerability to psychotic attack, but further studies are needed to investigate this. Another possible explanation would be that, at least in some patients, such changes may be secondary to cortical gray matter volume reduction, which has been correlated with average daily and lifetime intake of antipsychotic medication.85
Strengths and Limitations of This Study
This was the first study to evaluate the diagnostic accuracy of OCT indices in psychiatry; having access to IPD allowed more stringent analysis. We adjusted for age and sex, and we explored the effect of potential confounders (smoking, DM, hypertension) on diagnostic accuracy. Compared to 2 previously published studies,8,9 this meta-analysis did not apply any language restrictions, included more than 10 studies reporting pRNFL measurements, assessed the probability of publication bias more reliably and examined heterogeneity with meta-regression. Furthermore, we evaluated OCT measurements in the macular area (GCL-IPL, total macular thickness, macular volume).
However, several limitations of our study should be outlined. Adoption of a matched case-control design by all studies included in quantitative synthesis introduced bias, which precluded solid conclusions about diagnostic accuracy.86 Data availability was limited, and virtually no participants were treatment naïve, further hindering generalizability. Still, subgroup analysis did not indicate significantly different pRNFL effect estimates between studies with IPD and the remaining studies (supplementary table S4). Only 3 studies excluded participants with diagnosed DM. This condition may cause retinal thinning and can be precipitated by chronic antipsychotic administration.87 Owing to paucity of data, we did not adjust for optical spherical equivalent, race, and type of medication used. CIs calculated from imputed data analysis may be biased (supplementary appendix 3).
Implications for Future Research
All in all, it is premature to draw definite conclusions about the potential clinical utility of retinal evaluation in SZ. Even though it seems that diagnostic accuracy is limited, the role of these indices in prognostic and theranostic evaluation remains to be examined. Given the comparable findings in studies including high-risk subjects for SZ,67,68 it could be hypothesized that retinal changes may be evident early in the development of the disease, and this subgroup could be benefited from longitudinal retinal assessment—potentially allowing for risk stratification and early intervention. A comparative evaluation of retinal measurements in SZ spectrum disorders could elucidate potential differences among these entities. Longitudinal studies, with population-representative sampling and evaluation of measurement accuracy and reproducibility, are warranted; it should be noted that the potential cost of such studies may be high, given the small observed differences.
Supplementary Material
Acknowledgments
We sincerely thank Prof. Irene Gottlob (Ulverscroft Eye Unit, University of Leicester, Leicester, UK), Dr Frank Proudlock (Ulverscroft Eye Unit, University of Leicester, Leicester, UK), Dr Peter Joe (Department of Psychiatry, New York University School of Medicine, New York, NY), and Dr Pinar Topcu-Yilmaz (Department of Ophthalmology, Ankara Numune Research and Training Hospital, Ankara, Turkey) for providing data. We sincerely thank Dr Dursun Hakan Delibas (Department of Psychiatry, Izmir Bozyaka Training and Research Hospital, Izmir, Turkey) and Prof. Steven Silverstein (Department of Psychiatry, Rutgers Robert Wood Johnson Medical School, Piscataway, NJ) for providing data and contributing to the critical revision of the manuscript. We sincerely thank Dr Gerta Rücker and Dr Guido Schwarzer (Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany) for providing expert statistical advice and contributing to the critical revision of the manuscript. Both authors (Kazakos, Karageorgiou) participated in conceptualization, Karageorgiou set the rationale. Both authors performed data collection, communication with authors, curation, and had full access to the combined data. Karageorgiou prespecified methodology and performed statistical analysis based on aggregate data. Kazakos prespecified methodology, performed data management, and analysis of individual participant data. Both authors contributed in drafting the manuscript. Both authors read and approved the final version of the manuscript. We declare no competing interests.
Funding
None.
References
- 1. Diminic S, Ferrari AJ, Santomauro DF, et al. . Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belbasis L, Köhler CA, Stefanis N, et al. . Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137(2):88–97. [DOI] [PubMed] [Google Scholar]
- 3. Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: a focus on blood based diagnostics and theranostics. World J Psychiatry. 2016;6(1):102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petzold A, de Boer JF, Schippling S, et al. . Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9(9):921–932. [DOI] [PubMed] [Google Scholar]
- 5. Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004;44(24):2793–2797. [DOI] [PubMed] [Google Scholar]
- 6. Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol. 2001;112(10):1860–1867. [DOI] [PubMed] [Google Scholar]
- 7. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. [DOI] [PubMed] [Google Scholar]
- 8. Pan J, Zhou Y, Xiang Y, Yu J. Retinal nerve fiber layer thickness changes in schizophrenia: a meta-analysis of case-control studies. Psychiatry Res. 2018;270:786–791. [DOI] [PubMed] [Google Scholar]
- 9. Lizano P, Bannai D, Lutz O, Kim LA, Miller J, Keshavan M. A Meta-analysis of retinal cytoarchitectural abnormalities in schizophrenia and bipolar disorder. Schizophr Bull. 2019. doi: 10.1093/schbul/sbz029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang D, Swanson EA, Lin CP, et al. . Optical coherence tomography. Science. 1991;254(5035):1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yun SH, Tearney G, de Boer J, Bouma B. Pulsed-source and swept-source spectral-domain optical coherence tomography with reduced motion artifacts. Opt Express. 2004;12(23):5614–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marmor MF, Arden GB, Nilsson SEG, Zrenner E. Standard for clinical electroretinography: International Standardization Committee. Arch Ophthalmol. 1989;107(6):816–819. [DOI] [PubMed] [Google Scholar]
- 13. McCulloch DL, Marmor MF, Brigell MG, et al. . ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1–12. [DOI] [PubMed] [Google Scholar]
- 14. Silverstein SM, Paterno D, Cherneski L, Green S. Optical coherence tomography indices of structural retinal pathology in schizophrenia. Psychol Med. 2018;48(12):2023–2033. [DOI] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O’Connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2019. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 30, 2018.
- 16. Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 17. R Core Team. R: A Language and Environment for Statistical Computing [computer program]. Version 3.5.3. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 18. Fedorov S. GetData Graph Digitizer—Graph Digitizing Software 2019. Available at: http://getdata-graph-digitizer.com/. Accessed November 5, 2018.
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 20. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):48. [Google Scholar]
- 21. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663–1682. [DOI] [PubMed] [Google Scholar]
- 24. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–125. [DOI] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 27. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 28. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. [DOI] [PubMed] [Google Scholar]
- 29. Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14(2):134–143. [DOI] [PubMed] [Google Scholar]
- 30. Topcu-Yilmaz P, Aydin M, Cetin Ilhan B. Evaluation of retinal nerve fiber layer, macular, and choroidal thickness in schizophrenia: spectral optic coherence tomography findings. Psychiatry and Clinl Psychopharmacol. 2019;29(1):28–33. [Google Scholar]
- 31. Rücker G, Steinhauser S, Kolampally S, Schwarzer G. Diagmeta: meta-analysis of diagnostic accuracy studies with several cutpoints 2018. Available at: https://CRAN.R-project.org/package=diagmeta. Accessed November 10, 2018.
- 32. Steinhauser S, Schumacher M, Rücker G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med Res Methodol. 2016;16(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martínez-Camblor P. Fully non-parametric receiver operating characteristic curve estimation for random-effects meta-analysis. Stat Methods Med Res. 2017;26(1):5–20. [DOI] [PubMed] [Google Scholar]
- 34. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 35. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 36. Delibas D, Karti O, Erdoğan E, Şahin T, Bilgiç Ö, Erol A. Decreases in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in schizophrenia, relation to insight: a controlled study. Anatolian J Psychiatry. 2017;19(3):264–273. [Google Scholar]
- 37. Ascaso FJ, Laura C, Quintanilla MÁ, et al. . Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: a short report. Eur J Psychiatry. 2010;24:227–235. [Google Scholar]
- 38. Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657–1665. [DOI] [PubMed] [Google Scholar]
- 39. Ascaso FJ, Rodriguez-Jimenez R, Cabezón L, et al. . Retinal nerve fiber layer and macular thickness in patients with schizophrenia: influence of recent illness episodes. Psychiatry Res. 2015;229(1–2):230–236. [DOI] [PubMed] [Google Scholar]
- 40. Celik M, Kalenderoglu A, Sevgi Karadag A, Bekir Egilmez O, Han-Almis B, Şimşek A. Decreases in ganglion cell layer and inner plexiform layer volumes correlate better with disease severity in schizophrenia patients than retinal nerve fiber layer thickness: findings from spectral optic coherence tomography. Eur Psychiatry. 2016;32:9–15. [DOI] [PubMed] [Google Scholar]
- 41. Chu EM, Kolappan M, Barnes TR, Joyce EM, Ron MA. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res. 2012;203(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34. [PubMed] [Google Scholar]
- 43. Joe P, Ahmad M, Riley G, Weissman J, Smith RT, Malaspina D. A pilot study assessing retinal pathology in psychosis using optical coherence tomography: choroidal and macular thickness. Psychiatry Res. 2018;263:158–161. [DOI] [PubMed] [Google Scholar]
- 44. Jablensky A, McGrath J, Herrman H, et al. . People living with psychotic illness: an Australian study 1997–98. Mental Health Branch, Commonwealth Department of Health and Aged Care, Canberra 1999. [Google Scholar]
- 45. Lee WW, Tajunisah I, Sharmilla K, Peyman M, Subrayan V. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(12):7785–7792. [DOI] [PubMed] [Google Scholar]
- 46. Mota M, Pego P, Klut C, et al. . Evaluation of structural changes in the retina of patients with schizophrenia. Ophthalmol Res. 2015;4(2):45–52. [Google Scholar]
- 47. Samani NN, Proudlock FA, Siram V, et al. . Retinal layer abnormalities as biomarkers of schizophrenia. Schizophr Bull. 2017;44(4):876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yılmaz U, Küçük E, Ülgen A, et al. . Retinal nerve fiber layer and macular thickness measurement in patients with schizophrenia. Eur J Ophthalmol. 2015;26(4):375–378. [DOI] [PubMed] [Google Scholar]
- 49. Balogh Z, Benedek G, Kéri S. Retinal dysfunctions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):297–300. [DOI] [PubMed] [Google Scholar]
- 50. First M, Spitzer RL, Gibbon ML, Williams J.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Patient ed. New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 51. Demmin DL, Davis Q, Roché M, Silverstein SM. Electroretinographic anomalies in schizophrenia. J Abnorm Psychol. 2018;127(4):417–428. [DOI] [PubMed] [Google Scholar]
- 52. Gerbaldo H, Demisch L, Cardinali DP. Light exposure patterns in schizophrenia. Acta Psychiatr Scand. 1992;85(1):94–95. [DOI] [PubMed] [Google Scholar]
- 53. Hébert M, Mérette C, Paccalet T, et al. . Light evoked potentials measured by electroretinogram may tap into the neurodevelopmental roots of schizophrenia. Schizophr Res. 2015;162(1):294–295. [DOI] [PubMed] [Google Scholar]
- 54. Warner R, Laugharne J, Peet M, Brown L, Rogers N. Retinal function as a marker for cell membrane omega-3 fatty acid depletion in schizophrenia: a pilot study. Biol Psychiatry. 1999;45(9):1138–1142. [DOI] [PubMed] [Google Scholar]
- 55. Meier MH, Shalev I, Moffitt TE, et al. . Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry. 2013;170(12):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meier MH, Gillespie NA, Hansell NK, et al. . Retinal microvessels reflect familial vulnerability to psychotic symptoms: a comparison of twins discordant for psychotic symptoms and controls. Schizophr Res. 2015;164(1–3):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nurnberger JI Jr, Blehar MC, Kaufmann CA, et al. . Diagnostic interview for genetic studies. rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859; discussion 863. [DOI] [PubMed] [Google Scholar]
- 58. Bernardin F, Schwan R, Lalanne L, et al. . The role of the retina in visual hallucinations: a review of the literature and implications for psychosis. Neuropsychologia. 2017;99:128–138. [DOI] [PubMed] [Google Scholar]
- 59. Chhablani PP, Ambiya V, Nair AG, Bondalapati S, Chhablani J. Retinal findings on OCT in systemic conditions. Semin Ophthalmol. 2018;33(4):525–546. [DOI] [PubMed] [Google Scholar]
- 60. Gracitelli CP, Abe RY, Diniz-Filho A, Vaz-de-Lima FB, Paranhos A Jr, Medeiros FA. Ophthalmology issues in schizophrenia. Curr Psychiatry Rep. 2015;17(5):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hosak L, Hakeem K, Raad M, Studnicka J. Is microvascular abnormality a new endophenotype in schizophrenia? Psychiatr Danub. 2015;27(3):225–229. [PubMed] [Google Scholar]
- 62. Hosak L, Sery O, Sadykov E, Studnicka J. Retinal abnormatilites as a diagnostic or prognostic marker of schizophrenia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162(3):159–164. [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez-Jimenez R, Blázquez E, Lobo A, Santos JL. Usefulness of optical coherence tomography measures as biomarkers in schizophrenia. J Neurol Neuromedicine. 2018;3:79–84. [Google Scholar]
- 64. Schönfeldt-Lecuona C, Schmidt A, Pinkhardt EH, et al. . Optical coherence tomography (OCT)–a new diagnostic tool in psychiatry?. Fortschr Neurol Psychiatr. 2014;82(10):566–571. [DOI] [PubMed] [Google Scholar]
- 65. Schmidt A, Connemann BJ, Gahr M, et al. . From imaging the brain to imaging the retina: Optical coherence tomography (OCT) in schizophrenia. Schizophr Bull. 2015;42(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn. 2015;2(2):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hébert M, Gagné A-M, Paradis M-E, et al. . Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2010;67(3):270–274. [DOI] [PubMed] [Google Scholar]
- 68. Gagné A-M, Paccalet T, Jomphe V, Lussier D, Maziade M. T29. Electroretinographic response in youths at genetic risk of schizophrenia and bipolar disorder and in normal controls: transversal and longitudinal differences and implications for the risk trajectory. Schizophr Bull. 2018;44(suppl 1):S124–S124. [Google Scholar]
- 69. Paulus W, Schwarz G, Werner A, et al. . Impairment of retinal increment thresholds in Huntington’s disease. Ann Neurol. 1993;34(4):574–578. [DOI] [PubMed] [Google Scholar]
- 70. Cabezon L, Ascaso F, Ramiro P, et al. . Optical coherence tomography: a window into the brain of schizophrenic patients. Acta Ophthalmologica. 2012;90(s249):0–0. [Google Scholar]
- 71. Gagrat D, Maggiano J, Belmaker RH. Effect of neuroleptic treatment in schizophrenia on the electroretinogram, electrooculogram, and color vision. Psychiatry Res. 1979;1(1):109. [DOI] [PubMed] [Google Scholar]
- 72. Tan CS, Lim LW, Ting DS. Assessment of choroidal and retinal thickness in psychosis. Psychiatry Res. 2018;270:1172. [DOI] [PubMed] [Google Scholar]
- 73. Schechter G, Hock P, Rodgers K, Pfefferbaum A, Marmor MF, Berger PA. Electroretinographic assessment in schizophrenia. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:746–751. [PubMed] [Google Scholar]
- 74. Ucar D, Yıldız N, Hepokur M, et al. . Retinal nerve fiber layer thickness alterations after electroconvulsive therapy in patients with mental illness. Semin Ophthalmol. 2018;33(7-8):852–857. [DOI] [PubMed] [Google Scholar]
- 75. Küçük B, Karaaslan Ö, Hacımusalar Y, Bayhan HA. Şizofreni Hastalarında Optik Koherens Tomografi ile Lamina Kribrozanın Değerlendirilmesi 2018. Available at: http://upk2018.org/sozel/sb334.pdf. Accessed October 30, 2018.
- 76. Kessler RC, Ustün TB. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13(2):93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 78. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 79. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 80. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. [DOI] [PubMed] [Google Scholar]
- 81. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87(7):899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dinkin M. Trans-synaptic retrograde degeneration in the human visual system: slow, silent, and real. Curr Neurol Neurosci Rep. 2017;17(2):16. [DOI] [PubMed] [Google Scholar]
- 84. Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry. 2016;6(12):e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Janes H, Pepe MS. Matching in studies of classification accuracy: implications for analysis, efficiency, and assessment of incremental value. Biometrics. 2008;64(1):1–9. [DOI] [PubMed] [Google Scholar]
- 87. Kessing LV, Thomsen AF, Mogensen UB, Andersen PK. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197(4):266–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.