Abstract
From clinical observations to large-scale sequencing studies, the phenotypic impact of genetic modifiers is evident. To better understand the full spectrum of the genetic contribution to human disease, concerted efforts are needed to construct a useful modifier resource for interpreting the information from sequencing data. Here, we present the PhenoModifier (https://www.biosino.org/PhenoModifier), a manually curated database that provides a comprehensive overview of human genetic modifiers. By manually curating over ten thousand published articles, 3078 records of modifier information were entered into the current version of PhenoModifier, related to 288 different disorders, 2126 genetic modifier variants and 843 distinct modifier genes. To help users probe further into the mechanism of their interested modifier genes, we extended the yeast genetic interaction data and yeast quantitative trait loci to the human and we also integrated GWAS data into the PhenoModifier to assist users in evaluating all possible phenotypes associated with a modifier allele. As the first comprehensive resource of human genetic modifiers, PhenoModifier provides a more complete spectrum of genetic factors contributing to human phenotypic variation. The portal has a broad scientific and clinical scope, spanning activities relevant to variant interpretation for research purposes as well as clinical decision making.
INTRODUCTION
A modifier gene is a gene that alters the phenotypic outcomes of a gene at another locus that in turn causes an alternation in a phenotype, frequently a genetic disease (1,2). Effects of modifiers can result from direct interaction with the target gene product, from mechanistic contribution to the same biological process and/or from functional compensation through alternative pathways (2). Genetic modifiers are considered as a frequent cause of phenotypic variability in humans (3). The phenotypic effects of modifiers include penetrance, expressivity, dominance and pleiotropy (2). Evidence for the action of modifier genes is extensive, both in humans and model organisms, and their effects on the phenotypic presentation of disease causing variants can be subtle or profound (3,4). A number of modifier variants have been reported to contribute to the final phenotype in human disorders (5–8). Assessment of a broader spectrum of genetic contributions to risk, including not only disease-causing variants but also modifier genes and protective alleles, could improve the prediction, treatment and perhaps even the prevention of human diseases.
Biomedical research has increasingly turned its focus on the understanding of the genetic basis of human disease. Exome sequencing and whole-genome sequencing (ES/WGS) are highly useful tools to identify disease-associated variants (9,10). However, one significant challenges to genetic testing is identifying pathogenic variants that are not clinically manifest due to the presence of modifier variants (11). Modifier genes convert simple genetic disorders to complex traits, hence it is important to conduct thorough research into the role of modifier variants in human disease to answer clinical questions about phenotypic variation (12).
To better understand the full spectrum of genetic contribution to disease risk, concerted efforts are needed to construct a useful modifier resource for interpreting genetic information from sequencing data. Other related data collections, such as, dbEM (13) and CEMD (14) (http://ces.b2sg.org/cemd/), only include epigenetic modifier genes whose products modulate the epigenetics or epigenomics of cancerous or normal cells. To date, there is no comprehensive database of genetic modifiers. To fill this gap, we developed the PhenoModifier, a manually curated database that provides a comprehensive overview of human genetic modifiers. We have searched the HGMD (15) and ClinVar (16) databases and found that only a few variants (∼8%) listed in PhenoModifier were collected by these two human variant databases. PhenoModifier has a broad scientific and clinical scope, spanning activities relevant to variant interpretation for research purposes as well as clinical decision making. As the first systematic database of human genetic modifiers, PhenoModifier will facilitate the elucidation of the genetic basis of human phenotypic variation.
DATA COLLECTION AND CONTENT
Data collection
Considering the important contribution of modifiers to phenotypic variation, we searched PubMed using a list of keywords, such as ‘modifier’, words that describe modifier effects, and their synonyms (Supplementary Table S1). We obtained more than ten thousand modifier related publications. Only publications that clearly stated that the studied gene(s)/variant(s) function(s) as a modifier were retained for further curation, and ∼90% of the publications were discarded by this criterion. After this preliminary screening, we carefully read the full texts and identified evidence validating the function of the reported modifier. This evidence included strength of association, results from pedigree analysis or gene activity analysis, and conclusions from review articles. Then, we extracted the variants functioning as modifiers, the evidence for its function, the affected phenotypes, the modifier effect and the species studied, and entered these data into the database. The modifier effects were allocated to the four types of phenotype effect (penetrance, expressivity, dominance, pleiotropy), and the data were further reviewed and verified by experts in the field. At last, the manually filtered data were double-checked by expert curators. The data curation and processing procedure is illustrated in Supplementary Figure S1.
Functional properties associated with genetic interactions detected in model organisms can be used to predict candidate genes that may act as genetic modifiers as well as their interacting partners (17,18). We therefore integrated genetic interaction data derived from experiments in yeast into the PhenoModifier. The data derived by Costanzo et al. (19) cover genetic interactions for 90% of all genes in yeast, and the data derived from yeast strains by Bloom et al. (20) include nearly 800 significant quantitative trait loci that contribute additively to trait variation.
The affected phenotypes collected are confined to specific diseases or phenotypes reported in the literature. To assist efficient evaluation of all possible phenotypes associated with a modifier allele, we integrated data on human genotype–phenotype associations into PhenoModifier. Genome-wide association study (GWAS) data were downloaded from the GWAS catalog (21).
To integrate data derived from studies in model organisms, we downloaded information on human–mouse gene orthologs and human–yeast gene orthologs from BioMart (22). The human and mouse protein sequences were downloaded from the InParanoid database (23).
Processing and annotation
To resolve the gene name confusions that exist in the literature, we replaced aliases with gene symbols, NCBI Entrez gene IDs and Ensembl gene IDs.
Variants are named in a variety of ways in the literature. This causes difficulties in interpretation. To resolve this issue, we converted the variant identifiers collected from the literature into mutation names following the nomenclature recommendations of the Human Genome Variation Society (HGVS) (24). We implemented several variant interpretation tools, i.e. Variant Recoder (25), SNPedia (26) and ClinVar API (16), for translating between different variant names and for identifying ambiguous descriptions and descriptions that are not in accordance with the HGVS nomenclature. PhenoModifier takes as input the HGVS descriptions at the genomic, transcript and protein levels. The genomic loci of the variants refer to the human genome build GRCh37.
Around three percent of the manually collected modifier genes were derived from studies on mice. As the PhenoModifier is focused on the human genetic modifiers, only mouse proteins with orthologs in the human genome were retained. We aligned the mouse protein sequences to the sequences of their human orthologs using BLAST (27), and the human orthologous sites of the mouse variants were thus inferred using an in-house perl script. At last, the inferred variants at the human orthologous loci were named in accordance with the HGVS recommendations (24).
Using the EMBL-EBI Ontology Lookup Service (https://www.ebi.ac.uk/ols), we normalized the target disorder names extracted from the literature in terms of standard ontologies, i.e. human disease ontology (28), human phenotype ontology (29) and other ontologies such as clinical measurement ontology, experimental factor ontology etc.
Studies in yeast have yielded large scale genetic interaction data using high-throughput methods (19,20). It has been reported that the general structure of the genetic network is conserved in eukaryotes (17,18,30), and some general principles of the genetic networks in model organisms have already been shown to extend to human genetics (31). This conservation allows us to use the extensively mapped yeast genetic interaction network as a reference to guide our study on human complex traits. To provide users with sufficient information about human modifier genes and their potential interacting partners, we extended the yeast genetic interaction data (19) to human by replacing yeast genes with their human orthologs. We grouped the genetic interaction relationship into classes as described by Costanzo et al. (19), i.e. positive and negative interactions are separated into three classes according to the thresholds of the genetic interaction score (ϵ) and the thresholds of significance, that is, lenient (P < 0.05), intermediate (P < 0.05 and |ϵ| > 0.08) and stringent (P < 0.05 and ϵ > 0.16 or ϵ < −0.12) confidence.
To provide information whether a gene contributes additively to the variation in a trait, we also extended the yeast additive loci (20) to human by replacing yeast genes with their human orthologs.
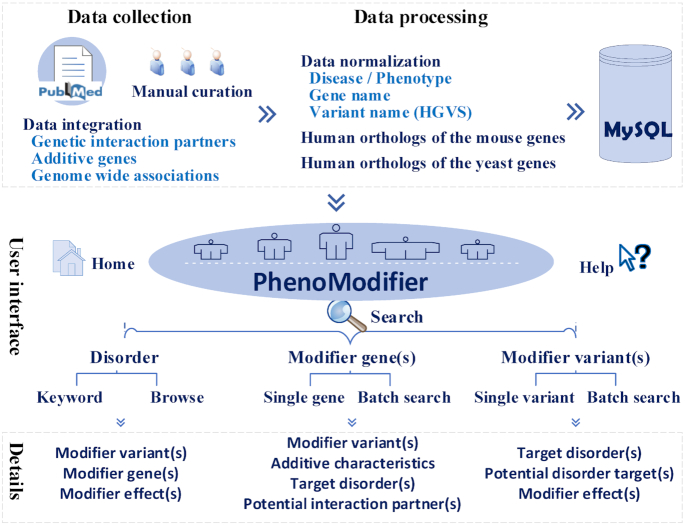
At last, the modifiers and all the related information were loaded into MySQL. Figure 1 schematically illustrates the workflow and features of the resource.
Figure 1.
Overview of data collection, annotation and database interface.
DATA ACCESS
The PhenoModifier website is available online at https://www.biosino.org/PhenoModifier and requires no registration. It provides a user-friendly interface for accessing information of modifiers. The database was built based on Tomcat 8, JDK 1.8, Bootstrap 3 and MySQL 5.5. We have tested it on Mozilla Firefox 48.0, Google Chrome 39.0 and Microsoft IE 11. The PhenoModifier resource will be updated up to four times per year, depending on the amount of newly published literature related to modifiers.
RESULTS
PhenoModifier statistics
The current version of PhenoModifier contains 3078 records of modifier information, relating to 288 different disorders, 2126 genetic modifier variants and 843 distinct modifier genes. Most of the data are collected from studies on the human. This includes 27 modifier genes (including 59 variants), which are collected from studies on mice.
To help users probe further into the mechanism of their interested modifier genes, we extended the yeast genetic interaction data (19) and yeast quantitative trait loci (20) to human. After replacing yeast genes with their human orthologs, potential interacting partners could be found for 61 modifier genes and an additional 85 modifier genes were found to be capable of contributing additively to trait variation. As single genetic modifier variants may modulate multigenic phenotypes (2), we integrated GWAS data (21) into the PhenoModifier to assist users evaluating all possible phenotypes associated with a modifier allele. Traits detected by genome-wide association study were annotated for 224 modifier variants in addition to the affected traits mined from the literature.
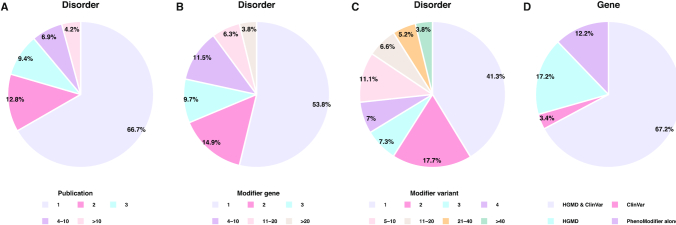
As shown in Figure 2, modifier information appears to be unevenly distributed across the 288 disorders. Though most disorders were rarely addressed in the context of genetic modifiers, 4.2% of the disorders for which modifier genes were studied in more than 10 publications (Figure 2A). Most of the disorders have few modifier genes/variants; however, there are 3.8% of the disorders for which more than 20 modifier genes have been reported (Figure 2B) and 3.8% of the disorders have more than 40 modifier variants (Figure 2C).
Figure 2.
Statistics of modifier information collected in the PhenoModifier. Percentage of disorders reported by different numbers of publications (A), related to different numbers of modifier genes (B) and including different numbers of modifier variants (C). (D) Percentage of modifier genes for which variant(s) have been annotated by the HGMD or the ClinVar database.
We further compared the data in PhenoModifier to those collected in the HGMD (15) and ClinVar (16) databases. We first looked at how many of the genes listed in PhenoModifier had also been collected by the other two databases. We found that variants of around 12.2% genes listed in PhenoModifier were neither included in HGMD (15) nor in ClinVar (16) (Figure 2D). When considering annotations from the variant level perspective, we found that only a few of the variants (8.4%) listed in PhenoModifier have annotations in the other two human variant databases. The data indicate that although some variants in a gene may have been collected by the HGMD or ClinVar databases, there frequently exist a number of additional variants that were only collected by the PhenoModifier database.
Web interface
The public user interface of PhenoModifier allows three types of intuitive searching for modifier associated information: querying whether a disorder may be affected by a modifier or modifiers, querying whether a gene contains any modifier variants and querying whether a variant may function as a modifier (Figure 1). The search results will return comprehensive details about the modifier-related information. The home page provides a quick search utility which can be used to search the database for all the three types of queries.
Querying on the disorder page, users can search PhenoModifier by entering a disorder name, or select a disorder item from the list. The result page contains brief information on the disorder and provides links to external resources including human disease ontology (28), OMIM (32) and human phenotype ontology database (29). The result page displays a detailed table of the modifier genes that may affect the variability of the disorder, and an extended statistical description of the genetic modifiers. By clicking on the hyperlink on a modifier gene, users can obtain more detailed information including the gene information, its potential interacting partner(s), variant(s) of the modifier gene, publication, etc. By clicking on the hyperlink of a variant, users can obtain more detailed information including variant information, target disorder(s), publication (i.e. title, abstract, PubMed ID), etc.
Querying on the gene page, users can search PhenoModifier by entering a gene name or by entering a list of genes, either gene symbol, Ensembl gene ID or NCBI gene ID. The result page contains brief information on the gene and provides links to external resources. The result page displays a detailed table of the potential partners with which the modifier gene may interact and a detailed table showing the modifier variants in the gene, publication etc. By clicking on the hyperlink on the modifier variant, users will obtain more detailed information. Batch genes query retrieves detailed information about which genes may function as modifiers.
Querying on the variant page, users can search PhenoModifier by entering a variant name or by entering a list of variants. The variant description can be at the genomic, transcript or protein level. The result page contains brief information on the variant and provides links to external resources dbSNP (33). The result page displays detailed information including target disorder, publication information (i.e. title, abstract, PubMed ID), effect type, modifier effects and specific effects stated in the original article etc. The batch variants query retrieves detailed information about which variants may function as modifiers.
The ‘search result’ pages present the number of publications that state that the gene/variant functions as a modifier as well as evidence extracted from the original article, both providing users with information about the repeatability of the annotated modifiers. The information about the modifiers as well as their phenotypic effects was summarized in the search result page. The modifier associated words were highlighted in the abstract, which can be viewed by clicking on the link of the reference ‘PubMed ID’. For a complete understanding of the impact of any gene or variant as a modifier of disease a detailed reading of the literature referenced will be needed. The search results can be directly downloaded in a JSON format file by clicking on the link ‘Download’ on the top right-hand side of the search result page.
Application of PhenoModifier
Since the Human Genome Project was completed, disease-causing mutations have been identified for many human disorders (15,16,32). However, as has been realized for a century, the same genetic mutation may have different effects on the phenotypes of different individuals, a fact that is in part due to differences in the genetic backgrounds. PhenoModifier can help users to find potential genetic modifiers and assess the phenotypic differences between individuals who inherit the same disease-causing mutation.
SUMMARY AND FUTURE PERSPECTIVES
From clinical observations to large-scale next generation sequencing studies, the phenotypic impact of genetic modifiers is evident (34,35). Knowledge of genetic modifiers is important for scientific research as well as clinical phenotyping. However, there are few systematic collections of validated modifiers. In this study we developed PhenoModifier, a database aiming at providing a comprehensive and up-to-data human genetic modifier resource to the research community as well as to clinicians. PhenoModifier is an essential supplement and improvement to the functional annotation of genetic variation in the human genome. Application of PhenoModifier in genetics tests at the Shanghai Children's Hospital has amply demonstrated its advantageousness and usefulness.
To ensure that PhenoModifier keeps pace with the research developments in the field, we will continue to manually curate experimentally validated data and update the database by frequent additions of new modifiers. Also, we plan to expand the connectivity of PhenoModifier to other relevant high-quality databases, i.e. ClinVar (16), OMIM (32) and GeneReviews (36). In addition, we will continue to integrate more online tools in order to increase the utility of the database.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Medical-Engineering Cross Project of Shanghai Jiao Tong University [YG2016MS33]; National Key R&D Program of China [2016YFC0901904, 2016YFC0901604, 2017YFC0907505, 2017YFC0908405, 2017YFC1201200]; Zhangjiang Special Project of National Innovation Demonstration Zone [ZJ2018-ZD-013]. Funding for open access charge: Medical-Engineering Cross Project of Shanghai Jiao Tong University [YG2016MS33]; National Key R&D Program of China [2016YFC0901904, 2016YFC0901604, 2017YFC0907505, 2017YFC0908405, 2017YFC1201200]; Zhangjiang Special Project of National Innovation Demonstration Zone [ZJ2018-ZD-013].
Conflict of interest statement. None declared.
REFERENCES
- 1. Bridges C.B. Specific modifiers of eosin eye color in Drosophila melanogaster. Journal of Experimental Zoology. 1919; 28:337–384. [Google Scholar]
- 2. Riordan J.D., Nadeau J.H.. From peas to disease: modifier genes, network resilience, and the genetics of health. Am. J. Hum. Genet. 2017; 101:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nadeau J.H. Modifier genes in mice and humans. Nat. Rev. Genet. 2001; 2:165–174. [DOI] [PubMed] [Google Scholar]
- 4. Harper A.R., Nayee S., Topol E.J.. Protective alleles and modifier variants in human health and disease. Nat. Rev. Genet. 2015; 16:689–701. [DOI] [PubMed] [Google Scholar]
- 5. Cutting G.R. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann. N.Y. Acad. Sci. 2010; 1214:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dannewitz Prosseda S., Tian X., Kuramoto K., Boehm M., Sudheendra D., Miyagawa K., Zhang F., Solow-Cordero D., Saldivar J.C., Austin E.D. et al.. Fragile Histidine Triad (FHIT), a novel modifier gene in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2019; 199:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aubart M., Gazal S., Arnaud P., Benarroch L., Gross M.S., Buratti J., Boland A., Meyer V., Zouali H., Hanna N. et al.. Association of modifiers and other genetic factors explain Marfan syndrome clinical variability. Eur. J. Hum. Genet. 2018; 26:1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang P., Chen Z., Peng Y., Cao L., Li X., Wang C., Yang H., Peng H., Shi Y., Zhou X. et al.. (CAG)n loci as genetic modifiers of age at onset in patients with spinocerebellar ataxia type 1 from mainland China. Eur. J. Neurol. 2019; 26:1130–1136. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. et al.. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013; 369:1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Below J.E., Earl D.L., Shively K.M., McMillin M.J., Smith J.D., Turner E.H., Stephan M.J., Al-Gazali L.I., Hertecant J.L., Chitayat D. et al.. Whole-Genome analysis reveals that mutations in inositol polyphosphate phosphatase-like 1 cause opsismodysplasia. Am. J. Hum. Genet. 2013; 92:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz M.B., Williams M.S., Murray M.F.. Adding protective genetic variants to clinical reporting of genomic screening results: restoring balance. JAMA. 2017; 317:1527–1528. [DOI] [PubMed] [Google Scholar]
- 12. Harper A.R., Nayee S., Topol E.J.. Protective alleles and modifier variants in human health and disease. Nat. Rev. Genet. 2015; 16:689–701. [DOI] [PubMed] [Google Scholar]
- 13. Singh Nanda J., Kumar R., Raghava G.P.S.. dbEM: A database of epigenetic modifiers curated from cancerous and normal genomes. Sci. Rep. 2016; 6:19340–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Licheva M., Bennani-Baiti I.M.. Cancer Epigenetics Modifier Database (CEMD) v0.1, Cancer Epigenetics Society. 2016; https://ces.b2sg.org/cemd/.
- 15. Stenson P.D., Mort M., Ball E.V., Evans K., Hayden M., Heywood S., Hussain M., Phillips A.D., Cooper D.N.. The human gene mutation database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017; 136:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landrum M.J., Lee J.M., Benson M., Brown G.R., Chao C., Chitipiralla S., Gu B., Hart J., Hoffman D., Jang W. et al.. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018; 46:D1062–D1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koch E.N., Costanzo M., Bellay J., Deshpande R., Chatfield-Reed K., Chua G., D’Urso G., Andrews B.J., Boone C., Myers C.L.. Conserved rules govern genetic interaction degree across species. Genome Biol. 2012; 13:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mackay T.F. Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nat. Rev. Genet. 2014; 15:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costanzo M., VanderSluis B., Koch E.N., Baryshnikova A., Pons C., Tan G., Wang W., Usaj M., Hanchard J., Lee S.D. et al.. A global genetic interaction network maps a wiring diagram of cellular function. Science. 2016; 353:aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bloom J.S., Kotenko I., Sadhu M.J., Treusch S., Albert F.W., Kruglyak L.. Genetic interactions contribute less than additive effects to quantitative trait variation in yeast. Nat. Commun. 2015; 6:8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., McMahon A., Morales J., Mountjoy E., Sollis E. et al.. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019; 47:D1005–D1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brazma A., Kasprzyk A., De Moor B., Davis S., Durinck S., Huber W., Moreau Y.. BioMart and bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics. 2005; 21:3439–3440. [DOI] [PubMed] [Google Scholar]
- 23. Sonnhammer E.L.L., Östlund G.. InParanoid 8: orthology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res. 2015; 43:D234–D239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. den Dunnen J.T., Dalgleish R., Maglott D.R., Hart R.K., Greenblatt M.S., McGowan-Jordan J., Roux A.-F., Smith T., Antonarakis S.E., Taschner P.E.M.. HGVS recommendations for the description of sequence variants: 2016 update. Hum. Mutat. 2016; 37:564–569. [DOI] [PubMed] [Google Scholar]
- 25. Hunt S.E., McLaren W., Gil L., Thormann A., Schuilenburg H., Sheppard D., Parton A., Armean I.M., Trevanion S.J., Flicek P. et al.. Ensembl variation resources. Database. 2018; 2018:bay119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cariaso M., Lennon G.. SNPedia: a wiki supporting personal genome annotation, interpretation and analysis. Nucleic Acids Res. 2012; 40:D1308–D1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L.. BLAST+: architecture and applications. BMC Bioinformatics. 2009; 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schriml L.M., Mitraka E., Munro J., Tauber B., Schor M., Nickle L., Felix V., Jeng L., Bearer C., Lichenstein R. et al.. Human disease ontology 2018 update: classification, content and workflow expansion. Nucleic Acids Res. 2019; 47:D955–D962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohler S., Carmody L., Vasilevsky N., Jacobsen J.O.B., Danis D., Gourdine J.P., Gargano M., Harris N.L., Matentzoglu N., McMurry J.A. et al.. Expansion of the Human Phenotype Ontology (HPO) knowledge base and resources. Nucleic Acids Res. 2019; 47:D1018–D1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehner B., Crombie C., Tischler J., Fortunato A., Fraser A.G.. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 2006; 38:896–903. [DOI] [PubMed] [Google Scholar]
- 31. Wong S.L., Zhang L.V., Tong A.H., Li Z., Goldberg D.S., King O.D., Lesage G., Vidal M., Andrews B., Bussey H. et al.. Combining biological networks to predict genetic interactions. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amberger J.S., Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A.. OMIM.org: online mendelian inheritance in man (omim(r)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015; 43:D789–D798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherry S.T., Ward M.H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K.. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001; 29:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marmor M., Hertzmark K., Thomas S.M., Halkitis P.N., Vogler M.. Resistance to HIV infection. J. Urban Health. 2006; 83:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Debette S., Kamatani Y., Metso T.M., Kloss M., Chauhan G., Engelter S.T., Pezzini A., Thijs V., Markus H.S., Dichgans M. et al.. Common variation in PHACTR1 is associated with susceptibility to cervical artery dissection. Nat. Genet. 2015; 47:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A.. GeneReviews. 2018; Seattle: University of Washington. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.