Abstract
Drug induced degradation of a target protein is a novel concept in drug discovery. Traditionally drugs modulate activity, as opposed to abundance, of their targets. Degradation inducing ligands act catalytically. Thus, one advantage of target degradation over the classical on-target mechanism is that lower drug concentration may be sufficient to cause the desired cellular effects. The first promoters of target degradation were discovered unintentionally: it turned out that some drugs ‘accidentally’ promote degradation of their target by the cellular proteolytic machinery. Elegant methods were developed to target specific proteins of interest for degradation, thus enabling the rational discovery of degradation inducers. The application of targeted degradation has so far been limited to human cells. Recently, we discovered that an antibacterial drug, the anti-tuberculosis antibiotic pyrazinamide, functions as a promotor of degradation of its bacterial target. Increasing antimicrobial resistance makes the discovery of novel antibiotics more urgent than ever. Can rational target degradation be applied for the discovery of anti-bacterials? Here, we first discuss briefly some historic examples and then recent approaches in rational target degradation for human diseases. Then, we describe how the first anti-bacterial target degradation promoter pyrazinamide triggers removal of its target. Efforts are under way to exploit this specific mechanistic knowledge for the discovery of next generation pyrazinamide. We end with the big - and open - question whether targeted protein degradation as an approach to anti-bacterial drug discovery can be generalized, similar to what has been achieved in the area of drug discovery for human diseases.
Keywords: Target degradation, PROTAC, antibacterial, pyrazinamide
1.1. Introduction
Drug discovery efforts aim to identify novel chemical entities that modulate, i.e. up- or downregulate, target protein activity (Fig. 1A). In order for drugs to exert their cellular therapeutic effects, they need to be supplied at sufficiently high concentrations and for sufficient time at the site of the disease to interfere effectively with the function of their target proteins. Achieving drug exposure at the relevant disease sites in the body can be challenging. For instance for an anti-tuberculosis (TB) drug to be efficacious, it has to be transported to non-vascularized pulmonary lesions, diffuse into necrotic and caseous foci, overcome the lipid-rich cell envelope of M. tuberculosis and finally hit its specific intracellular target for the required duration (Dartois, 2014). Recent understanding of the complexity in pharmacokinetic and on-target pharmacological requirements for a good anti-TB drug have provided some explanations for the void in new drugs in the TB drug discovery pipeline.
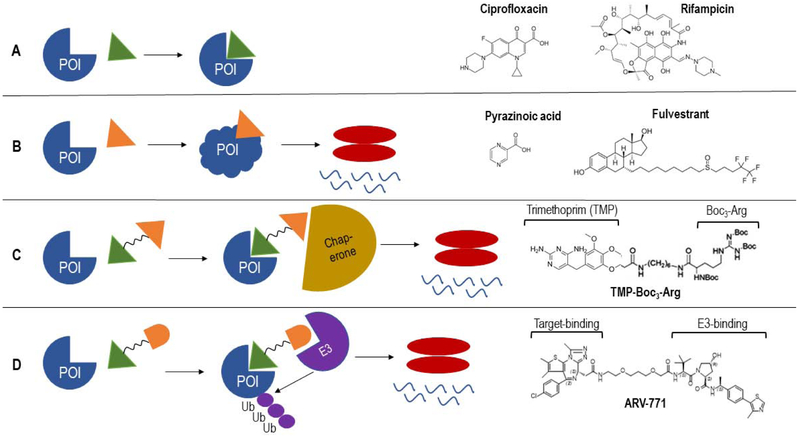
Fig. 1.
Examples of drugs either interfering with the activity of their target (A) or triggering degradation of their respective targets by various mechanisms (B,C,D). (A) Drug binds its specific target and alters target activity as a result of this interaction. Examples of such antibiotics are Ciprofloxacin and Rifampicin, which bind and inhibit the bacterial DNA gyrase and RNA polymerase respectively. (B) Drug binding to its target causes conformational changes of target, that results in target degradation. Pyrazinoic acid (POA) binds to its target Mycobacterium tuberculosis aspartate decarboxylase which changes its conformation and undergoes degradation via the Mtb ClpC1-P1P2 machinery (Gopal et al., 2019). Fulvestrant triggers degradation of its target Estrogen receptor ERα via the mammalian proteasome by changing its surface hydrophobicity (Wu et al., 2005). (C) Drug binds target, hydrophobic tag recruits chaperone protein which unfolds and transports the target to the cell’s degradation machinery. Example is Trimethoprim-Boc3-Arg, where Trimethoprim binds to E. coli DHFR (when expressed in mammalian cells) and the hydrophobic Boc3-Arg moiety tags it for degradation (Long et al., 2012). (D) PROTACS: bifunctional molecules with one end of the drug binding the target and the other end binding an E3 ligase which ubiquitinates target and recruits the proteasome to degrade the target. Example is ARV-771 which binds to Bromodomain and extra-terminal (BET) protein via the target-binding moiety and the E3 ligase Cereblon via the E3-binding moiety, thus resulting in degradation of BET proteins (Raina et al., 2016). POI, protein of interest.
A novel drug discovery paradigm which has gained momentum in recent years is targeted protein degradation, whereby specific drivers of human disease are selectively targeted for degradation by the cell’s own proteolytic machinery (Cromm and Crews, 2017), rather than (just) inhibiting or modulating their cellular function. This approach provides the advantage of an ‘event-driven’ pharmacology, where the molecules that facilitate target degradation can bind and induce degradation through multiple rounds, resulting in removal of greater than stoichiometric quantities of protein, as opposed to the classical ‘occupancy-driven’ pharmacology where a molecule needs to be stoichiometrically bound to the protein in order to inhibit its function (Lai and Crews, 2016). From the discovery of the first small molecule proteolysis-targeting chimaera (PROTAC) by the Crews Lab in 2008, which could bind and induce degradation of the androgen receptor (AR) to the first PROTAC molecule (ARV-110) to enter the clinic with a Phase I trial for prostate cancer earlier this year, this field has rapidly grown and gained attention from the scientific community (Mullard, 2019). While targeted protein degradation is being actively explored for different conditions ranging from cancers to neurodegenerative diseases, this concept has not been tapped upon for antibacterial discovery as yet. Our unpublished findings suggest a novel mechanism of action of pyrazinamide (PZA), a first-line anti-TB drug which was discovered for its lesion sterilizing activity in the 1950s and preliminary findings suggest that this antibiotic triggers degradation of its target aspartate decarboxylase PanD upon binding. PanD catalyzes an essential step in coenzyme A biosynthesis in M. tuberculosis (Gopal et al., 2017a; Gopal et al., 2019). Thus, pyrazinamide inhibits the production of an essential co-factor. Based on these findings and the tremendous advances made recently in the targeted degradation field for human diseases, we ask here whether targeted protein degradation can be extended to the field of anti-bacterial drug discovery. We first summarize briefly the key advances and concepts in the field, our learnings on bacterial target degradation using the (so far only) example of pyrazinamide and discuss some possibilities of capitalizing on the bacteria’s own protein degradation machinery to eliminate proteins of interest in a rational way using knowledge gained from other disease areas.
1.1.1. Molecular glues/SERDs
There have been multiple examples of compounds that were developed to inhibit specific cellular targets and were subsequently found to also induce degradation of their respective targets. For instance, the selective Estrogen receptor degraders (SERDs) such as Fulvestrant, which upon binding to their target Estrogen receptor ERα destabilize the protein by increasing surface hydrophobicity and trigger its degradation (Wittmann et al., 2007; Wu et al., 2005). This drug was found to be superior to Tamoxifen, which acts by only inhibiting the ERα signaling in the treatment of breast cancers (Anthony, 2006). Another example is Lenalidomide, used for treatment of Multiple myeloma, that acts as a ‘molecular glue’ by binding to the E3 ligase Cereblon and causing selective ubiquitination and degradation of substrates Casein kinase (CKα), Ikaros and Aiolos via the proteasome system (Krönke et al., 2014; Lu et al., 2014; Petzold et al., 2016). As the mechanisms of these molecules were found fortuitously, the challenge was to develop methods that would allow one to rationally identify chemical entities that would both specifically bind and promote degradation of a protein of interest.
1.1.2. Hydrophobic tagging
Hydrophobic moieties are appended onto the surface of target proteins in order to mimic partially unfolded proteins and invoke the cytosolic unfolded protein response to degrade the target (Cromm and Crews, 2017). Boc3-Arg, as a hydrophobic tag for etacrynic acid, a covalent inhibitor of Glutathione-S-transferase (GST-α1), could induce degradation of GST-α1 (Long et al., 2012). However, the utility of Boc3-Arg in therapeutics has been limited by its off-target effect on the cellular translation machinery (Coffey et al., 2016). Another strategy is to utilize bifunctional molecules with one end that binds the specific target and the other end recruiting the degradation machinery via a hydrophobic adamantyl moiety. This approach has been applied to a broader range of targets. Using this approach, it was possible to target a previously considered ‘undruggable’ pseudokinase ErbB3 via a covalent inhibitor with an adamantyl tag, resulting in ErbB3 degradation and signaling and anti-proliferative effects on pathway-dependent cell lines (Lim et al., 2015). The mechanisms of protein degradation induced by these different hydrophobic tags remains to be clearly understood.
1.1.3. PROTACs
Proteolysis-targeting chimaeras (PROTACs) are bifunctional molecules comprising of a warhead that binds to a target of interest and an E3 ligase recruiting ligand which are connected by a chemical linker. Once the target protein and the recruited E3 ligase are brought in close proximity, the target protein gets ubiquitinated and degraded by the proteasome machinery (Schneekloth et al., 2004) (Fig. 1D). In 2001, Sakamoto et al. reported the first PROTAC which consisted of the angiogenesis inhibitor ovalicin (warhead) that covalently binds to its target MetAP2, which was linked to a phosphopeptide that recruited the E3 ligase β-TRCP and thus resulted in the proteasome-mediated degradation of MetAP2 (Sakamoto et al., 2001). However, these peptide-based PROTACs display poor cellular activity due to permeability issues and immune recognition due to large molecular size. This has led to the field concentrating large efforts on the development of small molecule PROTACs. Since the discovery of small molecule E3 ligase ligands such as thalidomide and its analogs which bind Cereblon (Krönke et al., 2014), this PROTAC approach has been successfully applied to target and facilitate degradation of several different structural classes of proteins, ranging from receptor tyrosine kinases to hormone receptors to bromodomains (Bondeson et al., 2018). These small molecule PROTACs have been demonstrated to display nanomolar potencies in rapidly degrading target proteins, robustly inhibiting downstream signaling, causing in vivo tumor suppression and overcoming issues related with drug resistance due to mutations (An and Fu, 2018). Despite these convincing outcomes, these molecules are still relatively large as compared to traditional small molecules and thus may not be orally bioavailable. Newer strategies to shorten these molecules are becoming available such as Click-formed PROTACs (CLIPTACs), which utilize ‘click chemistry’ to bring the warhead and the E3-recruiting moieties together once inside the cell (Lebraud et al., 2016). With the first PROTAC molecule recently entering the clinic, the field is eagerly anticipating to learn how this approach fares in humans.
2.1. Pyrazinamide: discovery and mechanism of the first anti-bacterial inducing target degradation
The introduction of pyrazinamide (PZA) in the tuberculosis drug regimen shortened treatment from 9 to 6 months (BritishThoracicAssociation, 1982). PZA is a prodrug that is activated by a M. tuberculosis amidase to release its bioactive component pyrazinoic acid (POA) (Scorpio and Zhang, 1996). In vitro and in vivo screening to isolate spontaneous POA-resistant M. tuberculosis mutants identified missense mutations in either the aspartate decarboxylase PanD required for Coenzyme A biosynthesis or the unfoldase ClpC1, encoding a component of the caseinolytic protease ClpC1-ClpP (Gopal et al., 2017b; Gopal et al., 2016; Shi et al., 2014). Although PanD was established to be a direct cellular target, POA does not appear to directly inhibit PanD’s enzymatic activity (Gopal et al., 2017a; Gopal et al., 2019). Our ongoing studies with M. tuberculosis reporter strains suggest that POA binding to PanD accelerates ClpC1-ClpP dependent degradation of PanD. Biophysical studies indicate that POA treatment results in formation of higher order oligomers which may render PanD more susceptible to recognition and degradation by the ClpC1-ClpP complex. Thus, rather than inhibiting the biochemical activity of its target, POA appears to trigger degradation of PanD (Gopal et al., 2019) (Fig. 1B). Although a novel mechanism for an antibiotic, this is comparable to the mechanism of fortuitously discovered SERDs such as Fulvestrant, which upon binding to its target ERα, results in a structural modification that subsequently triggers target degradation via the proteasome (Wu et al., 2005) (Fig. 1B).
2.1.1. Next generation pyrazinamide: following the footsteps of Fulvestrant
Taking the SERD Fulvestrant as an example again, it has been possible to optimize this molecule by maximizing its ability to cause degradation of ERα (Kahraman et al., 2019) and improved outcomes were observed for breast cancer in preclinical and clinical studies by improving the oral bioavailability of the molecule while maintaining its target-degrading ability (Hamilton et al., 2018; Lai et al., 2015). Can we similarly increase the potency of POA by optimizing its affinity for its target PanD or by appending modifications on the molecule so that it is able to more efficiently recruit the ClpC1-P1P2 degradation machinery? Efforts are underway in the authors’ laboratory to increase the ‘degrader activity’ of POA (‘Target based discovery of next generation pyrazinamide’ http://grantome.com/grant/NIH/R01-AI106398-05). In a chemistry driven program using PanD-RFP reporter strains to detect enhanced degradation and POA resistant PanD mutant strains to stay on target, we aim to identify analogs with increased PanD degradation activity.
2.1.2. Targeted degradation of proteins of interest as novel approach for anti-bacterials?
The approach employed to develop a next generation pyrazinamide is specific to the drug-target pair pyrazinamide-PanD, and to mycobacteria. Can we find general methods to discover small molecules that promote degradation of specific desired bacterial proteins? In other words, can we apply a ‘PROTAC-like’ approach to anti-bacterial drug discovery? In an anti-viral discovery effort, a recent study employed this approach to conjugate the reversible inhibitor of the Hepatitis C virus (HCV) protease (Telaprevir) to an E3 ligase-recruiting ligand, resulting in a molecule capable of both inhibiting and degrading the HCV protease. Furthermore, this approach could overcome the issue of resistance to traditional enzyme inhibitors like Telaprevir (de Wispelaere et al., 2019). In order to translate this approach for an anti-bacterial, we would need well-validated recruiters of bacterial degradation machineries. In the absence of these recruiters, one could consider alternate approaches such as hydrophobic tagging of target binders/inhibitors. As a ‘proof-of-concept study’, Trimethoprim, a widely used antibiotic with specific inhibition of bacterial DHFR over mammalian DHFR, was tagged to a hydrophobic Boc3-Arg moiety. This resulted in degradation of E. coli DHFR, when expressed in mammalian cells (Long et al., 2012) (Fig. 1C). Whether this approach would work to degrade bacterial targets inside the bacterial cell using the bacterial protein degradation machinery is yet to be determined. Considering the post-antibiotic era we are facing and the meager anti-bacterial pipelines, there is a need for novel approaches in the field. The very impressive advances in the field of targeted protein degradation in the area of human diseases, together with the renaissance in prokaryotic cell biology give reasons to be optimistic. Development of rational and general approaches for the discovery of antibiotics that induce degradation of bacterial targets may be a possibility.
ACKNOWLEDGEMENTS
We would like to thank Wassihun Aragaw and Veronique Dartois, Hackensack Meridian Health Center for Discovery and Innovation, Malcolm Cole and Courtney Aldrich, University of Minnesota, and Gerhard Grueber, Nanyang Technological University, Singapore, for discussions. T.D. is grateful for his Toh Chin Chye Visiting Professorship at the National University of Singapore.
FUNDING
Research reported in this publication is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number 2R01AI106398-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS:
- PZA
pyrazinamide
- POA
pyrazinoic acid
- PROTAC
proteolysis targeting chimaeras
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An S and Fu L, 2018. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs, EBioMedicine. 36, 553–562. 10.1016/j.ebiom.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony H, 2006. Pure oestrogen antagonists for the treatment of advanced breast cancer, Endocrine-Related Cancer Endocr Relat Cancer. 13, 689–706. 10.1677/erc.1.00846. [DOI] [PubMed] [Google Scholar]
- Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S, Wang J, Hamman BD, Ishchenko A and Crews CM, 2018. Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead, Cell Chemical Biology. 25, 78–87.e5. 10.1016/j.chembiol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BritishThoracicAssociation, 1982. A Controlled Trial of Six Months Chemotherapy in Pulmonary Tuberculosis, American Review of Respiratory Disease. 126, 460–462. 10.1164/arrd.1982.126.3.460. [DOI] [PubMed] [Google Scholar]
- Coffey RT, Shi Y, Long MJC, Marr MT and Hedstrom L, 2016. Ubiquilin-mediated Small Molecule Inhibition of Mammalian Target of Rapamycin Complex 1 (mTORC1) Signaling, Journal of Biological Chemistry. 291, 5221–5233. 10.1074/jbc.M115.691584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromm PM and Crews CM, 2017. Targeted Protein Degradation: from Chemical Biology to Drug Discovery, Cell Chemical Biology. 24, 1181–1190. 10.1016/j.chembiol.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartois V, 2014. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells, Nature Reviews Microbiology. 12, 159 10.1038/nrmicro3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wispelaere M, Du G, Donovan KA, Zhang T, Eleuteri NA, Yuan JC, Kalabathula J, Nowak RP, Fischer ES, Gray NS and Yang PL, 2019. Small molecule degraders of the hepatitis C virus protease reduce susceptibility to resistance mutations, Nature Communications. 10, 3468 10.1038/s41467-019-11429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P, Nartey W, Ragunathan P, Sarathy J, Kaya F, Yee M, Setzer C, Manimekalai MSS, Dartois V, Grüber G and Dick T, 2017a. Pyrazinoic Acid Inhibits Mycobacterial Coenzyme A Biosynthesis by Binding to Aspartate Decarboxylase PanD, ACS Infectious Diseases. 3, 807–819. 10.1021/acsinfecdis.7b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P, Sarathy J, Yee M, Ragunathan P, Shin J, Bhushan S, Zhu J, Akopian T, Kandror O, Lim TK, Gengenbacher M, Lin Q, Rubin EJ, Grüber G and Dick T, 2019. Pyrazinamide triggers degradation of its target aspartate decarboxylase, bioRxiv. 674416 10.1101/674416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P, Tasneen R, Yee M, Lanoix J-P, Sarathy J, Rasic G, Li L, Dartois V, Nuermberger E and Dick T, 2017b. In Vivo-Selected Pyrazinoic Acid-Resistant Mycobacterium tuberculosis Strains Harbor Missense Mutations in the Aspartate Decarboxylase PanD and the Unfoldase ClpC1, ACS infectious diseases. 3, 492–501. 10.1021/acsinfecdis.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P, Yee M, Sarathy J, Low JL, Sarathy JP, Kaya F, Dartois V, Gengenbacher M and Dick T, 2016. Pyrazinamide Resistance Is Caused by Two Distinct Mechanisms: Prevention of Coenzyme A Depletion and Loss of Virulence Factor Synthesis, ACS Infectious Diseases. 2, 616–626. 10.1021/acsinfecdis.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EP, Patel MR, Armstrong AC, Baird RD, Jhaveri K, Hoch M, Klinowska T, Lindemann JPO, Morgan SR, Schiavon G, Weir HM and Im S-A, 2018. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2− Advanced Breast Cancer, Clinical Cancer Research. 24, 3510–3518. 10.1158/1078-0432.Ccr-17-3102. [DOI] [PubMed] [Google Scholar]
- Kahraman M, Govek SP, Nagasawa JY, Lai A, Bonnefous C, Douglas K, Sensintaffar J, Liu N, Lee K, Aparicio A, Kaufman J, Qian J, Shao G, Prudente R, Joseph JD, Darimont B, Brigham D, Heyman R, Rix PJ, Hager JH and Smith ND, 2019. Maximizing ER-α Degradation Maximizes Activity in a Tamoxifen-Resistant Breast Cancer Model: Identification of GDC-0927, ACS Medicinal Chemistry Letters. 10, 50–55. 10.1021/acsmedchemlett.8b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA and Ebert BL, 2014. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells, Science (New York, N.Y.). 343, 301–305. 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Kahraman M, Govek S, Nagasawa J, Bonnefous C, Julien J, Douglas K, Sensintaffar J, Lu N, Lee K.-j., Aparicio A, Kaufman J, Qian J, Shao G, Prudente R, Moon MJ, Joseph JD, Darimont B, Brigham D, Grillot K, Heyman R, Rix PJ, Hager JH and Smith ND, 2015. Identification of GDC-0810 (ARN-810), an Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) that Demonstrates Robust Activity in Tamoxifen-Resistant Breast Cancer Xenografts, Journal of Medicinal Chemistry. 58, 4888–4904. 10.1021/acs.jmedchem.5b00054. [DOI] [PubMed] [Google Scholar]
- Lai AC and Crews CM, 2016. Induced protein degradation: an emerging drug discovery paradigm, Nature Reviews Drug Discovery. 16, 101 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebraud H, Wright DJ, Johnson CN and Heightman TD, 2016. Protein Degradation by In-Cell Self-Assembly of Proteolysis Targeting Chimeras, ACS Central Science. 2, 927–934. 10.1021/acscentsci.6b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Xie T, Westover KD, Ficarro SB, Tae HS, Gurbani D, Sim T, Marto JA, Jänne PA, Crews CM and Gray NS, 2015. Development of small molecules targeting the pseudokinase Her3, Bioorganic & Medicinal Chemistry Letters. 25, 3382–3389. 10.1016/j.bmcl.2015.04.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Marcus J.C., Gollapalli Deviprasad R. and Hedstrom L, 2012. Inhibitor Mediated Protein Degradation, Chemistry & Biology. 19, 629–637. 10.1016/j.chembiol.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong K-K, Bradner JE and Kaelin WG Jr., 2014. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins, Science (New York, N.Y.). 343, 305–309. 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A, 2019. First targeted protein degrader hits the clinic, Nature Reviews Drug Discovery. 18, 237–239. 10.1038/d41573-019-00043-6. [DOI] [PubMed] [Google Scholar]
- Petzold G, Fischer ES and Thomä NH, 2016. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase, Nature. 532, 127 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AMK, Wang J, Chen X, Dong H, Siu K, Winkler JD, Crew AP, Crews CM and Coleman KG, 2016. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer, Proceedings of the National Academy of Sciences. 113, 7124 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM and Deshaies RJ, 2001. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation, Proceedings of the National Academy of Sciences. 98, 8554 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneekloth JS, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K and Crews CM, 2004. Chemical Genetic Control of Protein Levels: Selective in Vivo Targeted Degradation, Journal of the American Chemical Society. 126, 3748–3754. 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- Scorpio A and Zhang Y, 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus, Nature Medicine. 2, 662–667. 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen J, Feng J, Cui P, Zhang S, Weng X, Zhang W and Zhang Y, 2014. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis, Emerging microbes & infections. 3, e58–e58. 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BM, Sherk A and McDonnell DP, 2007. Definition of Functionally Important Mechanistic Differences among Selective Estrogen Receptor Down-regulators, Cancer Research. 67, 9549 10.1158/0008-5472.CAN-07-1590. [DOI] [PubMed] [Google Scholar]
- Wu Y-L, Yang X, Ren Z, McDonnell DP, Norris JD, Willson TM and Greene GL, 2005. Structural Basis for an Unexpected Mode of SERM-Mediated ER Antagonism, Molecular Cell. 18, 413–424. 10.1016/j.molcel.2005.04.014. [DOI] [PubMed] [Google Scholar]