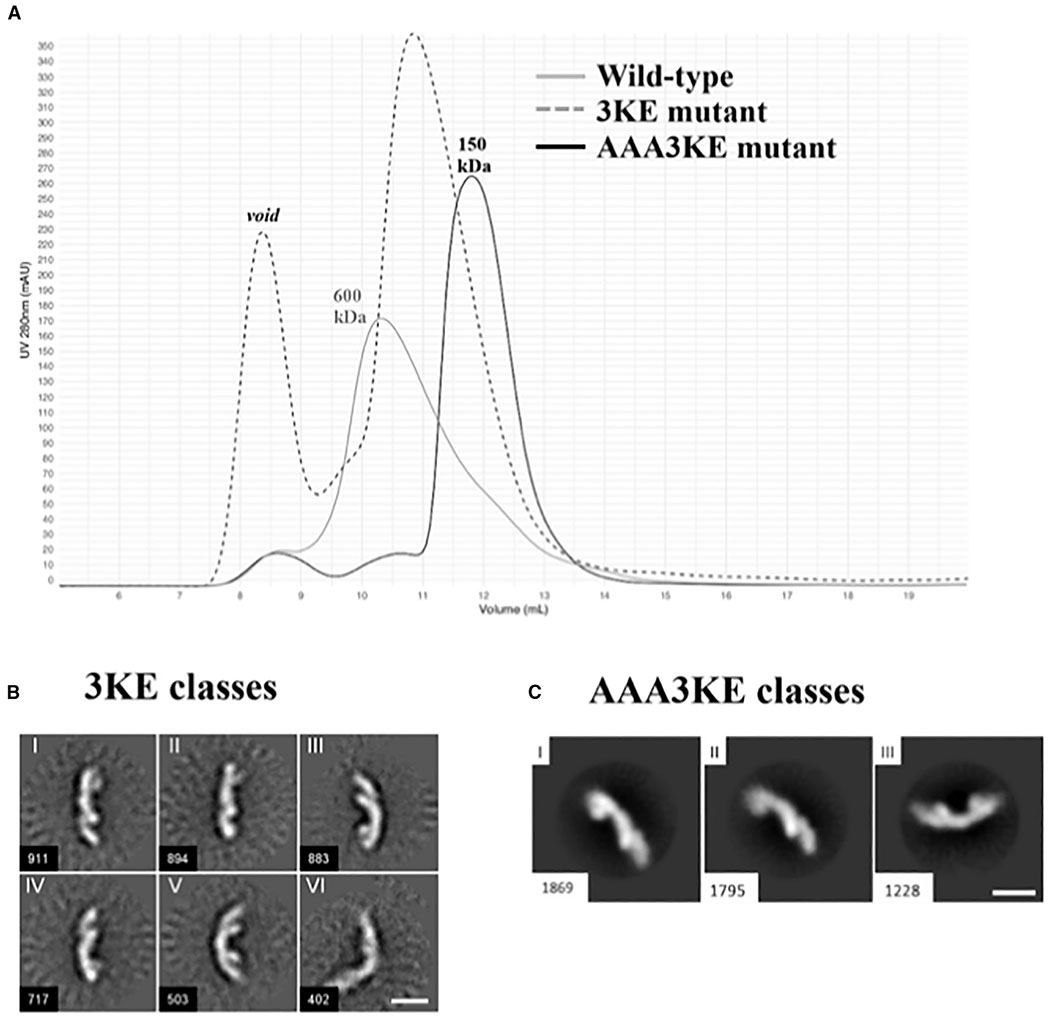
Figure 5. Mutating the Conserved VPS35 Interface Disrupts Assembly In Vitro.
(A) Gel filtration profiles of wild-type mammalian retromer (gray trace); the partial 3KE mutant (E615A/D616A/E617A; dashed trace) and the electrostatic AAA3KE mutant (E615A/D616A/E617A/K659E/K662E/K663E; black trace). The main wild-type peak elutes at a volume consistent with larger oligomeric assemblies (~600 kDa). The 3KE mutant is intermediate, while the electrostatic mutant peak elutes at a volume consistent mostly with heterotrimer (~150 kDa).
(B) Representative 2D class averages of the 3KE electrostatic mutant in negative stain. Most retromer particles exist as heterotrimers, but a population of dimers remains (class VI). Scale bar represents 10 nm.
(C) Representative 2D class averages of the AAA3KE mutant show most protein exists as heterotrimers. For both mutants, the overall 3D structure of the heterotrimer is preserved. Scale bar represents 10 nm.