Abstract
Little is currently known about the effects perinatal omega-3 (n-3) fatty acid deficiency on maternal-pup nurturing behaviors which have previously been shown to impact offspring neurodevelopment independent of diet. This study investigated the effects of perinatal maternal n-3 fatty acid deficiency on maternal-pup nurturing behaviors and investigated potential associations with pro-inflammatory signaling. Eight-week old virgin female Long-Evans hooded rats were randomized to a control diet containing α-linolenic acid (ALA, 18:3n-3)(CON, n=10) or an ALA-free diet (Deficient, DEF, n=11) 30 d prior to mating. On postnatal day 2 (P2) litters were culled to eight per dam. On P3, P6, and P9 dams and their litters were video recorded and maternal nurturing behaviors, including licking/grooming of pups and arched-back nursing, were scored by a blinded rater. Following weaning on P21, dam postmortem central (prefrontal cortex, PFC) and peripheral (red blood cell, RBC) fatty acid composition and central (PFC IL-1β, IL-2, IL-6, TNFα, cPLA2, COX-2 mRNA) and peripheral (plasma IL-1β, IL-2, IL-6, TNFα, CRP) pro-inflammatory biostatus assessed. DEF dams exhibited significantly lower RBC (p≤0.0001) and PFC (p≤0.0001) docosahexaenoic acid (DHA) levels compared with CON dams. Irrespective of diet dams exhibited significantly lower RBC, but not PFC, DHA levels compared with non-parous rats. DEF dams exhibited less licking/grooming (p=0.008), arched-back nursing (p≤0.0001) and blanket nursing (p=0.003), and exhibited more passive nursing (p=0.003) but not time off pups (p=0.1), compared with CON dams. PFC and plasma inflammatory measures did not differ significantly between groups. These results demonstrate that perinatal dietary n-3 fatty acid deficiency reduces maternal nurturing behavior and that this effect is not associated with enduring elevations in pro-inflammatory signaling.
Keywords: Omega-3 fatty acids, docosahexaenoic acid (DHA), maternal nurturing, inflammation
Introduction
While the adverse effects of perinatal omega-3 (n-3) fatty acid deficiency on offspring neurodevelopment has been well-characterized,1 little is currently known about its impact on maternal behavior and maternal-infant engagement. Both human2-4 and rodent5 studies have demonstrated that blood docosahexaenoic acid (DHA, 22:6n-3) levels are lower in post-partum females relative to non-pregnant females. A rodent study also demonstrated that parous dams fed an n-3 deficient diet throughout pregnancy exhibit significantly lower brain DHA levels compared with virgins fed an n-3 deficient diet.6 This reduction in maternal DHA stores coincides with fetal brain and organ DHA accrual during perinatal development.7 While postpartum depression is associated with reduced human maternal-infant engagement8,9 and lower blood DHA levels,10-14 the effects of perinatal n-3 deficiency on rat postpartum depression-like behavior have been inconsistent15,16 and the impact of perinatal n-3 deficiency on maternal-pup engagement has not been investigated.
Observational studies suggest that elevated pro-inflammatory activity is also associated with human postpartum symptoms of depression.17-20 It is relevant, therefore, that n-3 PUFAs and their bioactive metabolites have anti-inflammatory and inflammation-resolving properties, and in general mitigate pro-inflammatory signaling cascades mediated by the n-6 PUFA arachidonic acid (AA).21-23 Reductions in brain DHA levels, and associated increases in the AA/DHA ratio, similar to those observed in dams maintained on a n-3 deficient diet6 up-regulate enzymes that mediate AA-derived prostaglandin signaling, including calcium-dependent cytosolic phospholipase A2 (cPLA2) and cyclooxygenase-2 (COX-2), in male rat brain.24 Additional evidence suggests that n-3 fatty acid deficiency increases constitutive and endotoxin-induced pro-inflammatory signaling molecules, including the cytokine intereukin-6 (IL-6) and the acute phase protein C-reactive protein (CRP), in male rodents.25,26 Together these findings support a potential link between perinatal n-3 fatty acid deficiency, elevated pro-inflammatory signaling, and maternal post-partum behavior.
The primary objective of this study was to characterize the effects of perinatal maternal n-3 fatty acid deficiency on maternal-pup engagement. Primary behavioral outcomes were licking/grooming pups and arched-back nursing which have previously been shown to have an enduring adverse impact on offspring neurodevelopment.27-30 Our primary hypothesis was that dams maintained on the n-3 deficient diet would exhibit less licking/grooming and arched-back nursing compared with dams maintained on the n-3 adequate diet. The secondary objective was to determine the effects of perinatal n-3 fatty acid deficiency on pro-inflammatory signaling and to investigate associations with maternal-pup engagement.
Materials and methods
Animals and diet
Eight-week old virgin female Long-Evans hooded rats were purchased (Harlan Farms, Indianapolis, IN) and randomized to either a control diet (CON, n=10, TD.04285, Harlan-TEKLAD, Madison, WI) containing alpha-linolenic acid (ALA, 18:3n-3) or an ALA-free diet (DEF, n=11, TD.04286) containing no n-3 fatty acids. Diets were isocaloric (3.8 Kcal/g) and closely matched for all non-fat nutrients and fatty acid composition with the exception of ALA, which was absent from the DEF diet (Table 1). Females were placed on their assigned diets 30 d prior to breeding through gestation and weaning of litters on postnatal day 21 (P21). Each female was paired with a single male for one week and delivery was designated P0. This study included dams from three separate cohorts separated by approximately 8 months. Females maintained on CON (n=3) or DEF (n=3) diets that did not become pregnant served as non-parous controls for postmortem analyses. Food and water were provided ad libitum and rats were single-housed in shoebox cages with corncob bedding. Two squares of autoclaved paper towel were provided as nesting material. Dams and pups were not handled after litters were culled on P2 except for weekly cage changes by vivarium staff. Rats were maintained under standard vivarium conditions with a 12:12 hour light:dark cycle (lights on 7:00-19:00). All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and adhere to guidelines set by the National Institutes of Health.
Table 1.
Diet Compositions
| Ingredient1 | Control (TD.04285) |
Deficient (TD.04286) |
|---|---|---|
| Cornstarch | 20 | 20 |
| Sucrose | 27 | 27 |
| Dextrose | 9.9 | 9.9 |
| Maltose-dextrin | 6 | 6 |
| Cellulose | 5 | 5 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 |
| L-Cystine | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 |
| Hydrogenated coconut oil | 4.5 | 5.1 |
| Safflower | 1.9 | 1.9 |
| Flaxseed | 0.6 | 0 |
| Fish oil | 0 | 0 |
| Fatty acid composition2 | ||
| C8:0 | 3.7 | 4.1 |
| C10:0 | 3.3 | 3.7 |
| C12:0 | 38 | 32.1 |
| C14:0 | 11.5 | 12.9 |
| C16:0 | 8.8 | 9.1 |
| C18:0 | 10.7 | 11.7 |
| 16:1n-7 | nd | nd |
| 18:1n-9 | 6.7 | 5.1 |
| 18:1n-7 | nd | nd |
| 18:2n-6 | 22.5 | 21.3 |
| 20:4n-6 | nd | nd |
| 18:3n-3 | 4.9 | nd |
| 20:5n-3 | nd | nd |
| 22:5n-3 | nd | nd |
| 22:6n-3 | nd | nd |
g/100 g diet
wt % of total fatty acids
nd = not detected
Maternal behavioral analysis
Dams and their litters were video recorded for one hour per day on P3, P6 and P9 between 9:00-11:00 am. Recordings were later scored by a trained rater blinded to diet assignment. Recordings were divided into three-minute time bins (20 bins/hour recording) and the following behaviors were scored as present or absent in each time bin: (1) licking/grooming pups, (2) nursing in an arched-back posture, (3) blanket nursing, in which the dam maintains a low dorsal arch posture while nursing or is in a prone posture lying over pups, (4) passive nursing in which the dam nurses in a supine posture while lying on her back or side, and (5) dam not making physical contact with her pups (i.e., “off pups”). Representative pictures of these behaviors are presented in Figure 2. The total number of occurrences of each behavior during the 1 hour period was calculated and expressed as a percent of total number of potential occurrences for that behavior (occurrences/20 × 100 = % Frequency). Additionally, frequencies for each of the five individual behaviors were transformed into z-scores which were weighted to provide a single composite score, or “Maternal Engagement Index” (MEI), using the following formula: [(2*LG) + (2*AN) + (BN)+ (PN) + (−OP)/5] (LG = licking and grooming, AN arched-back nursing, BN = blanket nursing, PN = passive nursing, and OP = time off pups).
Figure 2.
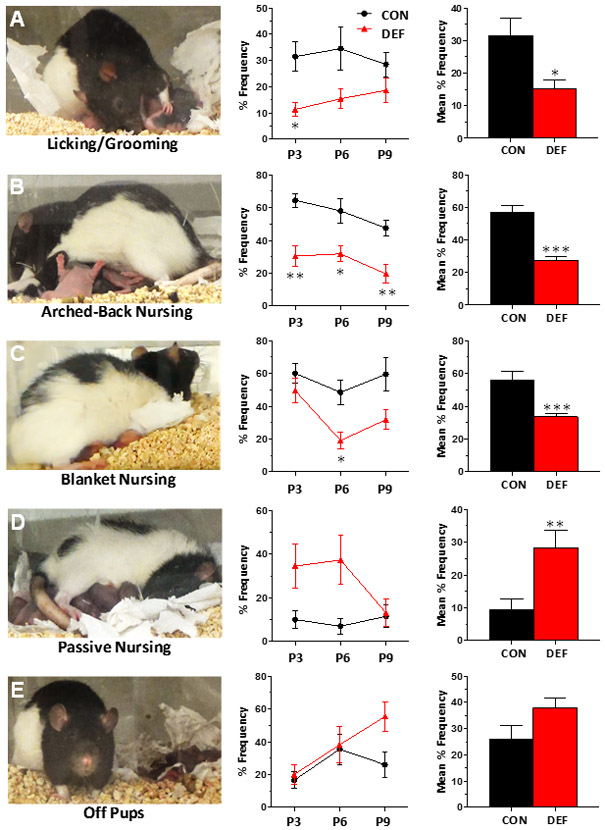
Frequency of licking/grooming (A), arched-back nursing (B), blanket nursing (C), passive nursing (D), and time off pups (E) exhibited by CON (n=10) and DEF (n=11) dams on postnatal days 3, 6 and 9. Data are expressed as group mean percent frequency ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.001 vs. CON.
Tissue collection
Following weaning on P21, parous and non-parous rats were lightly sedated with CO2 before euthanasia by decapitation. Brains were removed and washed with ice-cold saline and dissected to isolate bilateral PFC. The left PFC was used for RNA extraction and the right PFC for fatty acid analysis. Mammary tissue was also isolated from dams. Trunk blood was collected into a 1.5 ml Eppendorf tube containing 100 μl EDTA (0.5M, pH 8.0, Sigma-Aldrich Corp. St. Louis, MO) and centrifuged at 4°C for 20 min (1500×g). Plasma was isolated for cytokine/CRP assays, and red blood cells (RBCs) were washed 3 times with 0.9% NaCl. Samples were flash-frozen on dry ice and stored at −80°C.
Gas chromatography
RBC and PFC total fatty acid composition were determined with an Agilent 9820A equipped with an auto-injector (Agilent Technologies, Santa Clara, CA). The column was a DB-23 (123-2332): 30 m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with OpenLab CDS ChemStation software. Fatty acid composition is expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). Primary measures of interest were DHA, AA, and the AA/DHA ratio. Samples were processed by a technician blinded to diet assignment.
Plasma cytokines
Plasma concentrations (pg/ml) of interleukin-1 beta (IL-1β), interleukin-2 (IL-2) interleukin-6 (IL-6), tumor necrosis factor-alpha (TNFα), and CRP (ng/ml) were determined with a Luminex™ 100 IS multiplexing suspension array and flow-cytometry based analyzer (MiraiBio, South San Francisco, CA) using a LINCOplex Cytokine/Chemokine Luminex® Bead immunoassay Kit according to the manufacturer’s protocol (LINCO Research, St. Charles, MO). Samples were processed by a technician blinded to diet assignment.
Gene expression
PFC samples from n=8 rats randomly selected from each of the DEF and CON dam groups were homogenized (BioLogics Model 300 V/T ultrasonic homogenizer, Manassas, VA) in TRIzol reagent (Invitrogen, Carlsbad, CA), and total RNA isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. Total RNA was treated to remove potential DNA contamination using RNase-free DNase (Qiagen, Valencia, CA), and RNA quantified using a NanoVue spectrophotometer (GE Healthcare, Pittsburgh, PA). cDNA was prepared from 1 μg total RNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). PFC mRNA levels for target genes were amplified in quadruplicate wells of a 384 well TaqMan low density microfluidic card (Thermo Fisher, Waltham, MA) according to the manufacturer’s protocol using TaqMan Fast Advanced mater mix (Applied Biosystems, Foster City, CA) on an ABI 7900HT500 Real Time PCR System (Applied Biosystems, Foster City, CA). Pilot studies found that cyclophilin A (PPIA, Rn00690933_m1) was stable across diet groups and was therefore used as the “housekeeping” gene. Genes of interest were IL-1β (Il1b-Rn00580432_m1), IL-2 (Il2-Rn00587673_m1), IL-6 (Il6-Rn01410330_m1), TNFα (Tnf-Rn99999017_m1), cPLA2 (Pla2g4a-Rn00591916_m1), and COX-2 (Ptgs2-Rn01483828_m1). The ΔΔCt method was used to calculate gene fold change relative to the control group.31
Statistical analyses
Statistical tests were performed using IBM SPSS Statistics 23.0 (IBM Corp, Armonk, NY) and all tests were 2-tailed using alpha=0.05. Frequencies of individual maternal behaviors and MEI scores were compared using two-way repeated measures ANOVAs with Geisser-Greenhouse’s correction, with diet as the fixed factor and postnatal day (P3, P6, P9) as the repeated measure. Post hoc comparisons were made between CON and DEF dams at each time point using Sidak’s correction for multiple comparisons. For each behavior, the average frequency across postnatal days was calculated and group differences assessed with unpaired t-tests. Fatty acids and plasma CRP levels were evaluated with a two-way ANOVA, with diet and reproductive status (parous vs. non-parous) as fixed effects. Plasma cytokine and PFC gene expression levels within parous rats were assessed using unpaired t-tests. Linear regressions were performed for primary outcome variables.
Results
Maternal tissue fatty acid composition
There was a significant main effect of diet for RBC, F(1,33)=443.9, p≤0.0001)(Fig 1A) and PFC, F(1,22)=11.45, p=0.0027 (Fig. 1D) DHA levels which were significantly lower in rats fed the DEF diet compared with CON diet. Across all groups, RBC and PFC DHA levels were positively correlated (r = 0.56, p=0.0031). Arachidonic acid (AA) was elevated in DEF RBCs (main effect of diet: F(1,34)=10.18, p=0.0031 (Fig. 1B) but not PFC, F(1,22)=3.994, p=0.058 (Fig. 1E) relative to CON females. The DEF diet resulted in significant increases in the AA/DHA ratio in RBCs, F(1,33)=456.7, p≤0.0001 (Fig. 1C) and PFC, F(1,22)=12.43, p=0.0019 (Fig. 1F) relative to CON diet. There were also significant effects of reproductive status on RBC fatty acids. Parous females had significantly lower RBC DHA levels relative to non-parous females irrespective of diet, F(1,33)=112.1, p≤0.0001 (Fig. 1A). Parous females had a significantly higher RBC AA/DHA ratio relative to non-parous females, F(1,33)=134.7, p≤0.0001, and there was a significant interaction between diet and reproductive status, F(1,33)=79.52, p≤0.0001. The RBC AA/DHA ratio was greater in DEF parous females compared with DEF non-parous females (p≤0.0001), and CON parous and non-parous females did not differ significantly (p=0.32). There were no significant main effects of reproductive status for any fatty acid measures in the PFC. In mammary tissue, DHA was not detected in any of the DEF parous rats but was detectable in all CON parous rats (mean: 0.27%±0.01% total fatty acids)[data not shown].
Figure 1.

Docosahexaenoic acid (DHA)(A,D) and arachidonic acid (AA)(B,E) levels and the AA/DHA ratio (C,F) in red blood cells (RBC)(A-C) and PFC (D-F) of CON (n=10) and DEF (n=11) dams and non-parous CON (n=3) and DEF (n=3) rats on P21. Values are mg fatty acid/100 mg fatty acids (% total) or ratio and expressed as group mean ± S.E.M. *p≤0.05, **p≤0.01, ***p≤0.0001 vs. CON within reproductive status, †††p≤0.001 vs. non-parous within diet group.
Maternal nurturing behaviors
The initial mean litter size was 11±2.6 pups with a mean of 46.8%±11% male pups, and there were no group differences in litter size (p=0.32). The male/female ratio did not differ significantly between diet groups before (p=0.11) or after litters were culled (p=0.12). To minimize pups handling we did not routinely measure body weights but have found in a separate cohort that body weights of CON and DEF dams and P2 pups do not differ significantly [unpublished data]. Effects of diet on maternal nurturing behaviors are illustrated in Figure 2. Significant main effects of time were observed for arched-back nursing, blanket nursing, and time off pups, and there were no significant diet by time interactions. Relative to CON dams, DEF dams exhibited less licking/grooming pups (main effect of diet, F(1,19)=8.89, p=0.0077 (Fig. 2A), less arched-back nursing, F(1,19)=332.9, p≤0.0001 (Fig. 2B) and blanket nursing, F(1,19)=11.5, p=0.0031 (Fig. 2C), and more passive nursing F(1,19)=11.1, p=0.0035 (Fig. 2D). While DEF dams spent numerically more time off their pups, particularly on P9, this difference was not significant, F(1,19)=2.97, p=0.10 (Fig. 2E). “Maternal Engagement Index” (MEI) scores decreased over time for both CON and DEF dams, F(2,38)=3.89, p=0.032, and DEF dams had lower scores relative to CON dams, F(1,19)=30.0, p≤0.0001 (Fig. 5A,B). Average MEI scores were positively correlated with RBC DHA levels in DEF dams (r = 0.48, p=0.017)(Fig. 5D) but not in CON dams (r = 0.05, p=0.55)(Fig. 5C). MEI scores were not correlated with PFC DHA levels in DEF (r = 0.05, p=0.54) of CON dams (r = 0.00, p=0.98).
Figure 5.

Maternal Engagement Index (MEI) scores for CON (n=10) and DEF (n=11) dams on postnatal days 3, 6 and 9 (A), MEI scores averaged over days (B), and correlations between average MEI scores and RBC DHA levels within CON (C) and DEF (D) dams. Data in A and B are expressed as group mean ± S.E.M. **p<0.01, ***p<0.001 vs. CON.
Inflammatory biomarkers
Plasma IL-1β (+34%, p=0.13), IL-2 (+23%, p=0.37), IL-6 (+26, p=0.20), and TNFα (+14%, p=0.41) levels did not differ significantly between CON and DEF dams (Fig. 3). For CRP, also measured in non-parous rats, there was a significant main effect of reproductive status, F(1,32)=4.84, p=0.03 (Fig. 3E). CRP levels did not differ between parous DEF and CON rats (p=0.89) but were significantly higher in non-parous DEF (+10%, p=0.038) and parous DEF (+11%, p=0.006) rats compared with non-parous CON rats, and there was a similar trend in parous CON rats (+12%, p=0.064). CRP levels were not significantly correlated with average MEI scores (r = 0.008; p=0.74). PFC IL-1β (p=0.98), IL-2 (p=0.20), IL-6 (p=0.07), TNFα (p=0.35), cPLA2 (p=0.27), and COX-2 (p=0.75) mRNA expression did not differ significantly between CON and DEF dams (Fig. 4).
Figure 3.

Plasma concentrations (pg/ml) of pro-inflammatory cytokines IL-1β (A), IL-2 (B), IL-6 (C), and TNFα (D) in CON (n=9) and DEF (n=11) dams, and CRP concentrations (ng/ml) in parous and non-parous CON (n=3) and DEF (n=3) rats (E) on P21. Data are group mean concentration ± S.E.M. *p≤0.05, ††p≤0.001 vs. non-parous CON rats.
Figure 4.

IL-1β (A), IL-2 (B), IL-6 (C), TNFα (D), cPLA2 (E), and COX-2 (F) mRNA expression in the PFC of CON (n=8) and DEF (n=8) dams on P21. Values are group mean fold change relative to CON (ΔΔCT) ± S.E.M.
Discussion
This study tested the hypothesis that dams maintained on the n-3 deficient diet would exhibit reduced licking/grooming and arched-back nursing compared with dams maintained on the n-3 adequate diet. Dams maintained on the DEF diet exhibited significantly lower erythrocyte and PFC DHA levels, and associated increases in the AA/DHA ratio, compared with dams maintained on the CON diet. In support of our hypothesis, DEF dams exhibited significantly less licking/grooming and arched-back nursing compared with CON dams. DEF dams also exhibited less blanket nursing and more passive nursing. While DEF dams spent numerically more time off their pups, particularly on P9, this difference was not statistically significant. Levels of pro-inflammatory biomarkers in plasma and PFC did not differ significantly between CON and DEF dams at weaning. MEI scores were not significantly correlated with CRP levels. Together these results demonstrate that perinatal dietary n-3 fatty acid deficiency reduces rat maternal nurturing behavior and suggest that this effect is not associated with enduring elevations in pro-inflammatory signaling.
We found that parous rats exhibited significantly lower RBC DHA levels compared with non-parous rats irrespective of diet, and that DEF parous rats had significantly lower RBC DHA levels compared with DEF non-parous rats. These findings are consistent with a previous report5 and the clinical observation that plasma phospholipid DHA levels are lower in post-partum females relative to non-pregnant females.2-4 DEF parous rats also had a significantly higher RBC AA/DHA ratio compared with DEF non-parous rats. Mammary tissue DHA was depleted in DEF dams but not CON dams. Although PFC DHA levels were significantly lower in DEF dams compared with CON dams, the main effect of reproductive status was not significant. The latter result is not consistent with the previous observation that parous rats fed an n-3 deficient diet exhibited significantly lower whole brain DHA levels (−20%) compared with virgins fed an n-3 deficient diet.6 This discrepancy may be due to study methodological differences (e.g., PFC vs. whole brain) requiring additional investigation. Together, these findings suggest maternal peripheral DHA stores are robustly reduced in response to fetal DHA accretion and that this effect is mitigated by greater perinatal dietary n-3 fatty acid intake.
The primary finding of this study is that DEF dams exhibited reduced nurturing behaviors, including licking/grooming and arched-back nursing, compared with CON dams. Previous studies have demonstrated that naturally occurring reductions in licking/grooming and arched-back nursing have an enduring adverse impact on offspring neurodevelopment compared with high licking/grooming and arched-back nursing dams maintained on the same diet.27-30 The present findings suggest that perinatal dietary n-3 fatty acid intake and DHA biostatus is an important determinant of these nurturing behaviors. Interestingly, variable behavioral responses to novelty observed in male rats maintained on the same diet were associated with natural variations in PFC DHA levels.32 It will therefore be of interest to determine whether natural variations in maternal licking/grooming and arched-back nursing are associated with natural variations in brain and/or RBC DHA levels in rats maintained on the same diet. It is also notable that pups born to low-licking and grooming dams or dams maintained on a n-3 deficient diet both exhibit elevated hypothalamic-pituitary-adrenal axis responses to stress29,33 and spatial memory deficits28,34 in adulthood. Therefore, additional studies are also warranted to decipher whether the neurodevelopmental abnormalities observed in rats born to DEF dams are due to reduced nurturing behavior and/or deficits in DHA accrual.
We also investigated the effects of perinatal n-3 fatty acid deficiency and pregnancy/nursing on peripheral and central pro-inflammatory signaling. Non-parous females maintained on the DEF diet exhibited significantly higher plasma CRP levels compared with non-parous females maintained on the CON diet, a finding consistent with our previous observation in adult male rats maintained on the DEF diet throughout perinatal development.25 Interestingly, CRP levels in DEF and CON dams were numerically or significantly higher than non-parous CON rats, suggesting that pregnancy and lactation increase CRP levels irrespective of dietary n-3 fatty acid intake. However, CRP levels were not significantly correlated with MEI scores and plasma levels of CRP, IL-1β, IL-2, IL-6, and TNFα were not significantly different in CON and DEF dams. Moreover, expression of pro-inflammatory genes, including cPLA2 and COX-2 mRNA, in the PFC did not differ significantly between CON and DEF dams. The latter finding differs from previous evidence that DHA deficits in male frontal cortex (−27%) which are similar to those observed in DEF dams (−23%) were associated with increased cPLA2 and COX-2 mRNA expression,24 and potential sex differences and reproductive factors may account for this discrepancy. It also notable that IL-1β mRNA expression was not altered in DEF dams and previous findings suggest that IL-1β mRNA expression is a robust and sensitive measure of central inflammation.35 Taken together, these findings suggest that the effect of dietary n-3 fatty acid deficiency on maternal-pup engagement is not associated with enduring elevations in pro-inflammatory signaling. Interestingly, a recent clinical trial found that the antidepressant effects associated with n-3 fatty acid supplementation in moderately depressed pregnant women were also dissociable from changes in pro-inflammatory biomarkers.36
These preclinical results may take on additional significance in view of evidence that low n-3 PUFA intake and biostatus are associated with postpartum symptoms of depression10-14 which reduce maternal-infant engagement.8,9 Women with depressive mood disorders and of child-bearing potential exhibit RBC deficits,37,38 and our data suggests that initially low RBC levels increase vulnerability to perinatal DHA depletion and reduced maternal-infant engagement. Furthermore, maternal depression and lower maternal-infant engagement were found to contribute to the intergenerational transmission of emotional and behavioral dysregulation in offspring,39 and rats born to DEF dams and maintained on an n-3 deficient or n-3 adequate diet post-weaning exhibit elevated depression-like behavior in young adulthood.40 Together, these findings encourage additional investigation into the relationship between perinatal n-3 PUFA intake and biostatus and human maternal-infant engagement, particularly in women with a history of depressive mood symptoms.
The present study has a number of limitations that warrant discussion. First, maternal behaviors were assessed during a single observation window, between 9:00-11:00 am, and it is possible that assessments at different times of day or for longer periods would yield different results. Second, our assessment of central pro-inflammatory biomarkers measured only mRNA expression and did not investigate protein levels which may differ from mRNA levels. It is notable, however, that a previous study observed increases in both mRNA and protein expression of cPLA2 and COX-2 in the frontal cortex of DHA-deficient male rats.24 Third, cytokine and CRP levels were measures in plasma and other tissues, including the spleen and lymph nodes, may be more sensitive to moderate group differences. Fourth, central indices of pro-inflammatory signaling were evaluated in one brain region (PFC) which may not be representative of all brain regions. However, the PFC was selected based on previous evidence implicating it in the pathophysiology of postpartum depression,41,42 and the finding that moderate DHA deficits are associated with elevations in cPLA2 and COX-2 expression in the male rat frontal cortex.24
In conclusion, the present results demonstrate that perinatal dietary n-3 fatty acid deficiency reduces maternal nurturing behavior and that this effect is not associated with enduring elevations in systemic pro-inflammatory signaling. Other potential mechanisms, including changes in dam stress-reactivity,15,33 oxytocin signaling,43 and mesolimbic dopamine neurotransmission,44 warrant additional investigation as does the relationship between n-3 PUFA biostatus and human maternal-infant engagement.
Acknowledgments
This work was supported in part by National Institute of Health grants MH107378 to R.K.M.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.McNamara RK, Asch RH. Essentiality of omega-3 polyunsaturated fatty acids for mammalian brain development: A translational perspective. In: Watson RR and Preedy VR (Eds.), Omega-3 Fatty Acids in Brain and Neurological Health (2nd Edition), Academic Press, London, United Kingdom, pp. 3–20, 2019. [Google Scholar]
- 2.Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995;74(1):55–68. [DOI] [PubMed] [Google Scholar]
- 3.Holman RT, Johnson SB, Ogburn PL. Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc Natl Acad Sci USA. 1991;88(11):4835–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto SJ, van Houwelingen AC, Badart-Smook A, Hornstra G. The postpartum docosahexaenoic acid status of lactating and nonlactating mothers. Lipids. 1999;34 Suppl:S227. [DOI] [PubMed] [Google Scholar]
- 5.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J Nutr. 2007;137(11):2425–2430. [DOI] [PubMed] [Google Scholar]
- 6.Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in N-3 polyunsaturated fatty acids. Biol Psychiatry. 2006;60(9):987–990. [DOI] [PubMed] [Google Scholar]
- 7.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31(8):859–865. [DOI] [PubMed] [Google Scholar]
- 8.Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67(5):2512–2526. [PubMed] [Google Scholar]
- 9.Reck C, Hunt A, Fuchs T, Weiss R, Noon A, Moehler E, Downing G, Tronick EZ, Mundt C. Interactive regulation of affect in postpartum depressed mothers and their infants: an overview. Psychopathology. 2004;37(6):272–280. [DOI] [PubMed] [Google Scholar]
- 10.Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69(1-3):15–29. [DOI] [PubMed] [Google Scholar]
- 11.Hoge A, Tabar V, Donneau AF, Dardenne N, Degée S, Timmermans M, Nisolle M, Guillaume M, Castronovo V. Imbalance between omega-6 and omega-3 polyunsaturated fatty acids in early pregnancy is predictive of postpartum depression in a Belgian cohort. Nutrients. 2019;11(4). E876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin PY, Chang CH, Chong MF, Chen H, Su KP. Polyunsaturated fatty acids in perinatal depression: A systematic review and meta-analysis. Biol Psychiatry. 2017;82(8):560–569. [DOI] [PubMed] [Google Scholar]
- 13.Markhus MW, Skotheim S, Graff IE, Frøyland L, Braarud HC, Stormark KM, Malde MK. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One. 2013;8(7):e67617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69(4):237–243. [DOI] [PubMed] [Google Scholar]
- 15.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33(9):1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang M, Liu Y, Wang L, Li H, Cai H, Zhang M, Dang R, Xue Y, Wu Y. An Ω−3 fatty acid-deficient diet during gestation induces depressive-like behavior in rats: the role of the hypothalamo-pituitary-adrenal (HPA) system. Food Funct. 2018;9(6):3481–3488. [DOI] [PubMed] [Google Scholar]
- 17.Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. 2008;10(2):128–133. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol. 2012;94(2):202–209. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Zhang Y, Gao Y, Zhang Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. 2016;243:43–48. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R, Cox J, Bosmans E. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25(2):121–137. [DOI] [PubMed] [Google Scholar]
- 21.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100(4):1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6(6):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589–597. [PubMed] [Google Scholar]
- 24.Rao JS, Ertley RN, DeMar JC Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12(2):151–157. [DOI] [PubMed] [Google Scholar]
- 25.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukot Essent Fatty Acids. 2010;83(4-6):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mingam R, Moranis A, Bluthé RM, De Smedt-Peyrusse V, Kelley KW, Guesnet P, Lavialle M, Dantzer R, Layé S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95(9):5335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. [DOI] [PubMed] [Google Scholar]
- 30.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vancassel S, Blondeau C, Lallemand S, Cador M, Linard A, Lavialle M, Dellu-Hagedorn F. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behav Brain Res. 2007;180(2):119–126. [DOI] [PubMed] [Google Scholar]
- 33.Chen HF, Su HM. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. Nutr Biochem. 2013;24(1):70–80. [DOI] [PubMed] [Google Scholar]
- 34.Moriguchi T, Greiner RS, Salem N Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75(6):2563–2573. [DOI] [PubMed] [Google Scholar]
- 35.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127(3):378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishi D, Su KP, Usuda K, Chang JP, Hamazaki K, Ishima T, Sano Y, Ito H, Isaka K, Tachibana Y, Tanigaki S, Suzuki T, Hashimoto K, Matsuoka YJ. Plasma estradiol levels and antidepressant effects of omega-3 fatty acids in pregnant women. Brain Behav Immun. 2019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126(1-2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara RK, Jandacek R, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, Strakowski SM, DelBello MP. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: a candidate prodromal risk biomarker. Early Interv Psychiatry. 2016;10(3):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouvette-Turcot AA, Fleming AS, Unternaehrer E, Gonzalez A, Atkinson L, Gaudreau H, Steiner M, Meaney MJ. Maternal symptoms of depression and sensitivity mediate the relation between maternal history of early adversity and her child temperament: The inheritance of circumstance. Dev Psychopathol. 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Weiser MJ, Wynalda K, Salem N Jr, Butt CM. Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats. J Lipid Res. 2015;56(1):151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167(11):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEwen AM, Burgess DT, Hanstock CC, Seres P, Khalili P, Newman SC, Baker GB, Mitchell ND, Khudabux-Der J, Allen PS, LeMelledo JM. Increased glutamate levels in the medial prefrontal cortex in patients with postpartum depression. Neuropsychopharmacology. 2012;37(11):2428–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]


