Abstract
Purpose:
Black women are over-represented among premenopausal breast cancer (BC) survivors. These cases warrant genetic testing (GT), followed by risk-reducing behaviors. This study documents patterns and predictors of cancer risk management behaviors among young Black BC survivors after GT.
Methods:
Black women (N=143) with invasive BC diagnosed age ≤50 years received GT. One year post-GT, participants reported receipt of risk-reducing mastectomy, risk-reducing salpingo-oophorectomy, mammogram, breast MRI, CA125 test, and transvaginal/pelvic ultrasound. Logistic regression examined predictors of BC risk management (risk-reducing mastectomy or breast MRI) and ovarian cancer risk management (risk-reducing salpingo-oophorectomy, CA125 test, or transvaginal/pelvic ultrasound).
Results:
Sixteen participants (11%) were BRCA1/2 positive, 43 (30%) had a variant of uncertain significance, and 84 (59%) were negative. In the 12 months post-GT, no women received risk-reducing mastectomy. The majority (93%) received a mammogram, and a smaller proportion received breast MRI (33%), risk-reducing salpingo-oophorectomy (10%), CA125 test (11%), or transvaginal/pelvic ultrasound (34%). More time since BC diagnosis predicted lower likelihood of BC risk management (OR=0.54). Being a BRCA1/2 carrier (OR=4.57), greater perceived risk of recurrence (OR=8.03), and more hereditary breast and ovarian cancer knowledge (OR=1.37) predicted greater likelihood of ovarian cancer risk management.
Conclusions:
Young Black BC survivors appropriately received mammogram and ovarian cancer risk management based on BRCA1/2 test result. However, the low usage of MRI among BRCA1/2 carriers contrasts with national guidelines. Future research should examine barriers to MRI among Black BC survivors. Finally, modifiable variables predicting risk management post-GT were identified, providing implications for future interventions.
Keywords: Black, breast cancer, genetic counseling, genetic testing, health disparities, risk management
Background
Mutations in the BRCA1 and BRCA2 (BRCA1/2) genes account for 5–10% of all breast cancers and ~15% of all early onset breast cancers.1 Compared to patients without a BRCA1/2 mutation, breast cancer (BC) survivors with a BRCA1/2 mutation are at substantially elevated risk of ipsilateral and contralateral BC,2,3 as well as ovarian cancer.4 Thus, risk management for BRCA1/2 positive BC survivors is more strongly emphasized as compared to risk management for BRCA1/2 negative survivors.
National Comprehensive Cancer Network (NCCN) guidelines suggest all BC survivors, regardless of BRCA status, have annual mammograms5; however, BRCA1/2 carriers have additional surveillance and surgical options, including supplemental breast MRI.6 Prior to 2015 (the time period in which our study was conducted), NCCN guidelines for BRCA1/2 carriers also included routine ovarian cancer screening, including CA125 testing and transvaginal or pelvic ultrasound. NCCN guidelines also state BRCA1/2 carriers should have risk-reducing salpingo-oophorectomy at age 35–40 or after childbearing is complete and consider risk-reducing mastectomy.6
Thus, genetic testing (GT) for BC survivors may inform a patient about options to manage the risk of second primary cancers.6 Data from our team and others report Black women, particularly BC patients, are less likely to access GT services compared to women of other racial/ethnic groups.7,8 Thus, despite the clinical availability of GT, readily available referral criteria, and important cancer risk management implications, our understanding of the health outcomes of GT in the Black community is minimal.9–11
To fill this gap, we report on the impact of BRCA testing on BC risk management behaviors among a population-based sample of Black BC survivors diagnosed ≤50 years old. We hypothesized BRCA1/2 carriers would be more likely to report BC risk management and ovarian cancer risk management in the 12 months after GT compared to those who were non-carriers (i.e., BRCA negative or had a variant of uncertain significance [VUS]). As annual mammogram is recommended for all BC survivors, regardless of BRCA1/2 status, we hypothesized there would be no difference in receipt of mammogram by BRCA1/2 status. Finally, exploratory analyses examined whether baseline demographic, clinical, or decision-making variables would predict use of advanced BC or ovarian cancer risk management behaviors in the 12 months after GT.
Methods
Procedures and Participants
An observational, longitudinal design with three intact groups was used. Data for the present study come from participants in a parent study, which was designed to investigate genetic and lifestyle determinants of triple negative breast cancer in premenopausal Black women.12,13 Eligible participants were Black women who were: (1) diagnosed with invasive BC ≤50 years old; (2) diagnosed between 2009 and 2012; (3) living in Florida at the time of diagnosis; (4) alive at the time of recruitment; and (5) English speaking. Upon approval by the University of South Florida (104559) and the Florida Department of Health (DOH H11168) Institutional Review Boards, registry-based recruitment was initiated. The Florida Cancer Data System (FCDS), a statewide registry containing cancer incidence data, released patient contact information and available clinical and sociodemographic information on all eligible participants. The lag time between diagnosis and availability of contact information from FCDS ranged from 6–18 months.
Patients were approached using state-mandated recruitment methods of 2 mailings, 3 weeks apart, including a telephone response card giving women the option to decline or express interest in participation. If no response was received within 3 weeks of the second mailing, a study team member telephoned the participant. In those willing to participate, written informed consent was obtained via mail. Study participation included completion of a medical records release, pre- and post-test telephone-based genetic counseling, saliva sample collection for DNA extraction, and completion of study questionnaires at baseline and 12 months post-disclosure of BRCA test results.
GT included full gene sequencing and comprehensive rearrangement testing (multiplex ligation-dependent probe amplification) of the BRCA1 and BRCA2 genes. All BRCA alterations were evaluated through available clinical and research data, however a variant was classified as pathogenic if there were several lines of evidence confirming its pathogenicity through the multi-factorial model.14 All variants were searched in the literature and through the publicly available Breast Cancer Information Core (BIC) database.15 Although the role of the BIC in BRCA gene annotation has diminished in recent years, this database was representative of generally recognized pathogenic mutations at the time study GT was conducted (January 2013-January 2015).
Measures
Predictors included demographic characteristics, cancer-related medical factors, family cancer history, BRCA mutation status, perceived risk, and HBOC knowledge. The outcome of interest was engagement in risk management behaviors.
Demographic characteristics.
Participants reported their age, nationality, relationship status, education, income, employment status, and insurance status.
Cancer-related medical factors.
FCDS data included age at diagnosis, time since BC diagnosis, cancer stage, hormone receptor status (estrogen receptor [ER] and progesterone receptor [PR]), and human epidermal growth factor receptor 2 (HER-2) status, with information supplemented through review of medical records.
Family History.
Family cancer history was assessed by participant self-report. Family cancer history was categorized as significant for hereditary breast and ovarian cancer (HBOC) syndrome if she reported either: (1) a first or second degree relative diagnosed with BC age ≤50 years; or (2) a first or second degree relative diagnosed with ovarian cancer at any age.
BRCA Mutation Status.
Results were classified as ‘positive’ if a pathogenic/likely pathogenic was identified, negative if no pathogenic variant was identified, and as a VUS if a change in the BRCA1 or BRCA2 gene was detected, yet the resultant cancer risk was unknown. We chose to separate “negative” and “VUS” women into distinct groups, as opposed to a single “non-pathogenic” group, because there is evidence in the literature that some women make risk-reduction decisions based on VUS results.16,17 Thus, BRCA mutation status was represented as a nominal variable with three levels: negative (“0”), positive (“1”), and VUS (“2”).
Perceived Risk.
Absolute perceived risk was assessed by asking participants to indicate risk of getting BC again on a 100 point scale (0=no chance and 100=definitely get breast cancer again).18 Odds ratio (OR) per 5 point increase was estimated. Perceived risk relative to other women diagnosed after age 50 was assessed with a single item (0=“much lower” to 4=“much higher”).
HBOC Knowledge.
Women’s HBOC knowledge was assessed using a 15-item modified version of the National Center for Human Genome Research Knowledge scale including five items specific to BC survivors.19 Scores for each item were summed to create a total HBOC knowledge score (range: 0–15).
Individual Risk Management Behaviors.
Six risk management behaviors were assessed: risk-reducing mastectomy, risk-reducing salpingo-oophorectomy, mammogram, breast MRI, CA125 test, and transvaginal/pelvic ultrasound. Risk reducing surgery was assessed by women’s self-report of (1) whether they had ever received unilateral or bilateral mastectomy or salpingo-oophorectomy, and (2) what the primary reason was for the procedure. Women were classified as having risk-reducing mastectomy or risk-reducing salpingo-oophorectomy in the past 12 months if they: (1) had at-risk breast or ovarian tissue at baseline (e.g., no prior bilateral mastectomy/salpingo-oophorectomy at baseline); (2) at the 12-month follow-up, reported removal of remaining breasts/ovaries (e.g., unilateral or bilateral, as appropriate); and (3) reported the primary reason for the procedure was BC and/or ovarian cancer risk reduction. BC screening (mammogram and breast MRI) and ovarian cancer screening (CA125 test and transvaginal/pelvic ultrasound) were assessed by patients’ self-reported use of each strategy in the past 12 months. BC risk management was defined as receipt of risk-reducing mastectomy or breast MRI (0=“neither”, 1=“at least one”), while ovarian cancer risk management was defined as receipt of risk-reducing salpingo-oophorectomy or CA125 test or transvaginal/pelvic ultrasound (0=“none”, 1=“at least one”). All women (N=143) reported on risk management behaviors at the 12-month follow-up time point, regardless of GT result.
Analytic strategy
Logistic regression examined whether baseline demographic variables, clinical variables (including BRCA mutation status), or decision-making variables predicted use of any advanced risk management strategies for BC or ovarian cancer in the 12 months after GT. Potential predictor variables were first examined using univariate logistic regression. All variables were subsequently entered into multivariable logistic regression models; variables with a significance level of 0.1 remained in the model. The goodness-of-fit of the model was evaluated by Hosmer and Lemeshow’s test,20 with a non-significant χ2 (p>0.05) indicating adequate goodness-of-fit. All analyses were conducted using the statistical program SPSS (version 25, IBM), and cases with missing data were removed listwise. Power analyses were conducted for the parent study to determine adequate sample size12,13; however, post-hoc sensitivity analyses demonstrated 80% power to detect an odds ratio of 1.83 with N=143,
Analyses examining use of breast cancer risk management behaviors (RRM, mammography, and breast MRI) were conducted in the sub-sample of women who had at-risk breast tissue remaining at baseline (n=98). Analyses examining use of ovarian cancer risk management behaviors (RRO, CA125 testing, and transvaginal/pelvic ultrasound) were conducted in the sub-sample of women who had at least one intact ovary at baseline (n=140).
Results
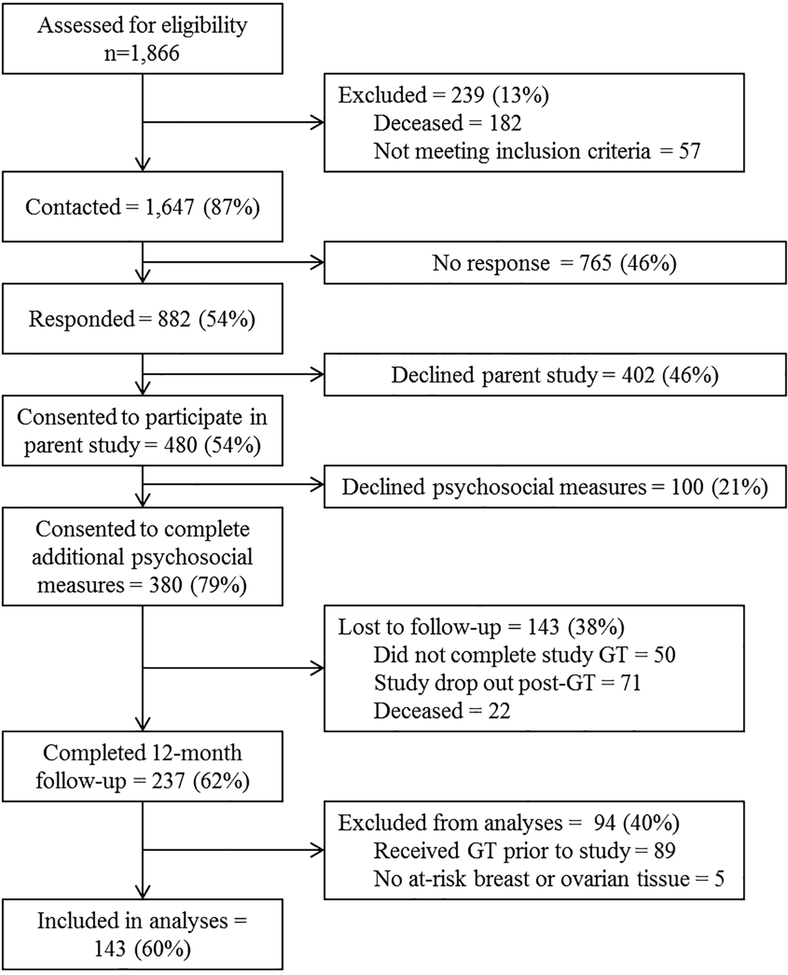
The flow of participants through the study is displayed in Figure 1. Of the 1,647 eligible Black women with BC in FCDS, we established contact with 882. Of these, 480 (54%) consented to participate in the parent study. Of the 480 parent study participants, 380 (79%) consented to participate in the current study, which entailed completing additional measures of risk management behaviors (e.g., prophylactic surgery, surveillance), psychological functioning (e.g., cancer related distress, emotional well-being), and social functioning (e.g., communication of test results) prior to genetic testing and 12 months following disclosure of GT results. Of those 380 participants, 143 (38%) were lost to follow-up (did not complete study GT [n=50], dropped out post-GT [n=71], or deceased [n=22]) and 237 (69%) completed the 12-month follow-up questionnaire. Given our focus on the behavioral impact of GT, we limited analyses to the 148 participants who did not have previous GT at the time of study enrollment. After excluding those with no at-risk breast or ovarian tissue at baseline (n=5), the final sample included 143 Black BC survivors.
Figure 1.
Study flow.
Preliminary and Descriptive Analyses.
For a complete description of the sample, see Table 1.
Table 1.
Sociodemographic, disease, and decision-making characteristics for participants (N=143).
| Mean (SD) | Range | N (%) | N Missing | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Current Age (years) | 45.09 (5.93) | 27–54 | 1 | |
| Country of Birth: % United States | 118 (82.5) | 1 | ||
| Marital Status: % single | 94 (65.7) | 1 | ||
| Education: up to GED/Diploma | 120 (83.9) | 1 | ||
| Employment Status: % employed | 74 (51.7) | 1 | ||
| Insurance Status: % insured | 119 (83.2) | 3 | ||
| Cancer-related Medical Factors | ||||
| Significant family history: % yes | 39 (27.3) | 2 | ||
| Age at Diagnosis (years) | 42.87 (6.28) | 14–50 | 1 | |
| Cancer Stage at Diagnosis | 0 | |||
| Localized | 81 (56.6) | |||
| Regional | 52 (36.4) | |||
| Distant | 4 (2.8) | |||
| Years from diagnosis to study enrollment | 2.38 (3.65) | 0.25–37.67 | 1 | |
| Years from genetic testing to follow-up | 1.26 (0.27) | 0.75–2.50 | 0 | |
| Hormone Receptor Status | ||||
| Estrogen Receptor positive: % yes | 94 (65.7) | 7 | ||
| Progesterone Receptor positive: % yes | 78 (54.5) | 11 | ||
| HER2 receptor positive: % yes | 25 (17.5) | 21 | ||
| Genetic Testing Result | 0 | |||
| Carrier | 16 (11.2) | |||
| VUS | 43 (30.1) | |||
| No mutation | 84 (58.7) | |||
| Decision-making Factors | ||||
| Perceived absolute risk of cancer recurrence (%) | 22.30 (30.32) | 0–100 | 9 | |
| Perceived risk of recurrence relative to other women over age 50 | 12 | |||
| Much lower | 47 (32.9) | |||
| A little lower | 11 (7.7) | |||
| About the same | 40 (28.0) | |||
| A little higher | 21 (14.7) | |||
| Much higher | 12 (8.4) | |||
| HBOC Knowledge | 5.06 (2.98) | 0–12 | 1 | |
All women included in these analyses received GT through the study. Most (n=84, 58.7%) were BRCA1 and BRCA2 negative and 43 (30.1%) received a VUS result. GT identified a deleterious mutation in the BRCA1 or BRCA2 gene for 16 women (11.2%); of these, 10 (63%) were BRCA1 positive and 6 (37%) were BRCA2 positive.
Use of Risk Management Behaviors.
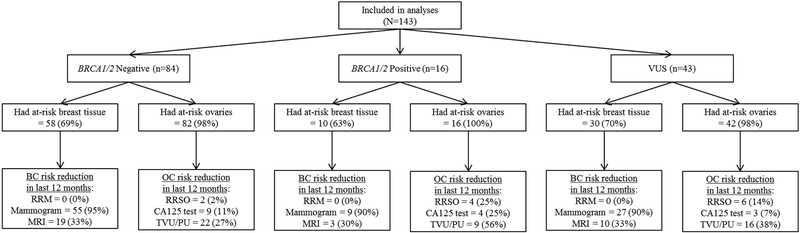
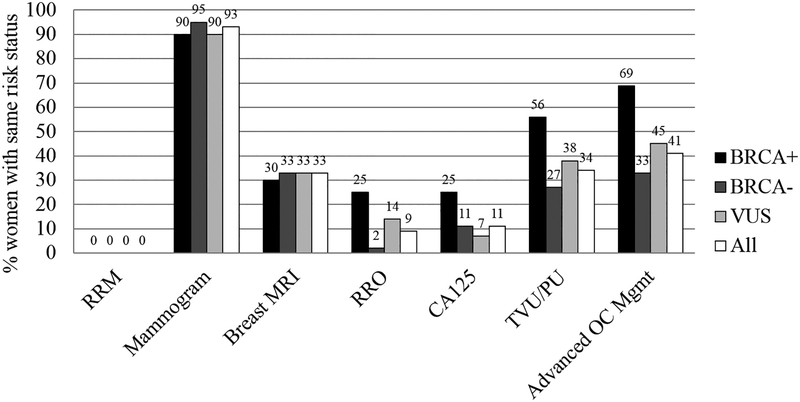
Participants’ use of risk management behaviors are presented by GT result in Figure 2 and across groups in Figure 3.
Figure 2.
Cancer risk management behaviors by genetic test result at 12-months post-genetic testing.
Figure 3.
Proportion of participants who engaged in risk management strategies by BRCA1/2 carrier status (positive, negative, or VUS).
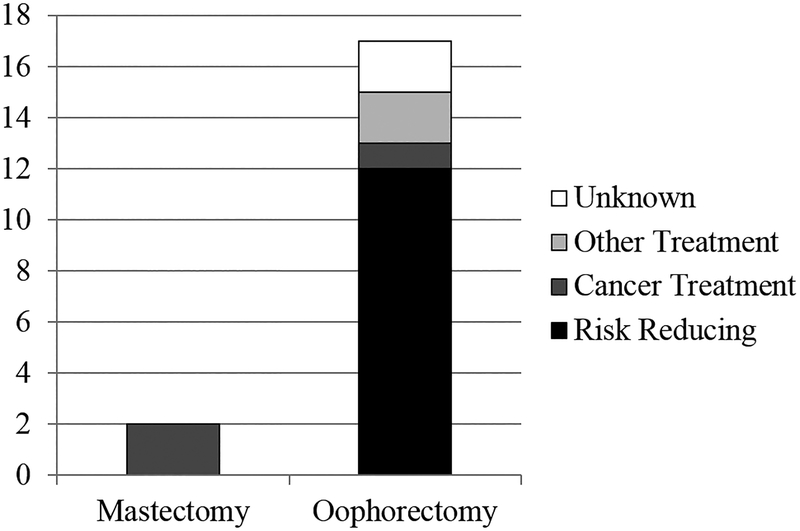
While two participants (2%) reported having mastectomy in the past 12 months, both indicated BC treatment was the primary reason (see Figure 4); thus, no women in this sample were categorized as having risk-reducing mastectomy. We had defined advanced BC screening as receipt of risk-reducing mastectomy or breast MRI a priori. However, as no women in this sample received risk-reducing mastectomy, breast MRI alone represented high-risk BC screening.
Figure 4.
Patient-reported reasons for mastectomy and salpingo-oophorectomy.
Seventeen women (12%) reported having salpingo-oophorectomy in the past 12 months; of them, 10 (59%) indicated the primary reason was to reduce risk for ovarian cancer, 1 (6%) to reduce BC risk, 1 (6%) to reduce both BC and ovarian cancer risk, 2 (12%) to treat ovarian cysts, 1 (6%) to treat ovarian cancer, and 2 (12%) did not specify a reason. Thus, a total of 12 women were categorized as having risk-reducing salpingo-oophorectomy. Of these 12 women receiving risk-reducing salpingo-oophorectomy, 2 were BRCA1/2 negative, 4 were BRCA1/2 positive, and 6 had a VUS result.
Predictors of High-Risk Management.
Results of logistic regressions are presented in Table 2. Two predictors of high-risk BC management were retained from univariate models: time since BC diagnosis (OR=0.47, 95% CI=0.24–0.93) and absolute perceived risk (OR=1.11, 95% CI=0.99–1.25). The final multivariable model for advanced BC risk management included only one predictor: time since BC diagnosis. Thus, the final multivariable model was identical to the univariate model for time since BC diagnosis and demonstrated adequate goodness-of-fit (χ2=4.12, p=0.77).
Table 2.
Results of univariate and multivariable logistic regression models examining predictors of advanced risk-reduction strategies.
| Breast: Risk-Reducing Mastectomy or Breast MRI (n=98) | Ovarian: Risk-Reducing Salpingo-oophorectomy, CA125 Test, or Transvaginal/Pelvic Ultrasound (n=140) | |||
|---|---|---|---|---|
| Predictors | Univariate | Multivariable | Univariate | Multivariable |
| Demographic Variables | ||||
| Age | 1.00 [0.90, 1.12] | 0.97 [0.92, 1.03] | ||
| Country of Birth | 0.29 [0.07, 1.30] | 1.56 [0.64, 3.76] | ||
| Education | 0.69 [0.07, 7.19] | 1.90 [0.69, 5.25] | ||
| Marital Status | 0.62 [0.18, 2.09] | 1.52 [0.74, 3.10] | ||
| Employment Status | 0.68 [0.20, 2.28] | 1.22 [0.62, 2.40] | ||
| Insurance Status | 1.04 [0.17, 6.35] | 1.37 [0.51, 3.67] | ||
| Clinical Variables | ||||
| Significant family history | 2.15 [0.40, 11.56] | 1.19 [0.56, 2.52] | ||
| Cancer Stage at Diagnosis | ||||
| Localized | (ref) | (ref) | ||
| Regional | 0.92 [0.26, 3.29] | 0.91 [0.44, 1.85] | ||
| Distant | † | 2.59 [0.23, 29.75] | ||
| Time since BC diagnosis | 0.47** [0.24, 0.93] | 0.47** [0.24, 0.93] | 0.93 [0.81, 1.08] | |
| Time since GT | 0.87 [0.13, 11.06] | 0.86 [0.24, 3.07] | ||
| Hormone Receptor Status | ||||
| ER positive | 0.62 [0.16, 2.37] | 1.92* [0.89, 4.14] | 2.42* [0.92, 1.62] | |
| PR positive | 0.67 [0.20, 2.30] | 1.58 [0.77, 3.25] | ||
| HER2 positive | 0.91 [0.22, 3.76] | 0.67 [0.27, 1.66] | ||
| BRCA1/2 carrier status | ||||
| BRCA negative | (ref) | (ref) | (ref) | |
| VUS | 3.85 [0.74, 20.13] | 1.68 [0.79, 3.61] | 2.04 [0.77, 5.37] | |
| BRCA positive | † | 4.48** [1.42, 14.20] | 6.56** [1.44, 29.85] | |
| Decision-making Variables | ||||
| Absolute perceived risk (5%) | 1.11** [0.99, 1.25] | 1.02 [0.96, 1.08] | ||
| Relative perceived risk | ||||
| Much lower | (ref) | (ref) | (ref) | |
| A little lower | 0.33 [0.04, 3.21] | 1.47 [0.39, 5.55] | 1.17 [0.25, 5.46] | |
| About the same | 0.75 [0.15, 3.84] | 0.92 [0.38, 2.25] | 0.45 [0.15, 1.32] | |
| A little higher | 0.33 [0.04, 3.21] | 1.94 [0.68, 5.51] | 1.08 [0.31, 3.75] | |
| Much higher | 2.33 [0.22, 25.25] | 4.71** [1.10, 20.15] | 9.41** [1.64, 54.05] | |
| HBOC knowledge | 1.04 [0.87, 1.24] | 1.26** [1.11, 1.43] | 1.36** [1.15, 1.62] | |
p < 0.1;
p < 0.05;
unable to be estimated
In univariate models, ovarian cancer risk management was significantly predicted by BRCA1/2 status (ORBRCA positive=4.48, 95% CI=1.42–14.20), greater HBOC knowledge (OR=1.26; 95% CI=1.11–1.43), and greater relative perceived risk (OR=4.71, 95% CI=1.10–20.15). ER status (ORpositive=1.92, 95% CI=0.89–4.14) was also retained from univariate models. The final multivariable model for advanced ovarian cancer risk management included BRCA1/2 positive status (OR=5.22, 95% CI=1.31–20.77), ER status (ORpositive=2.42, 95% CI=0.92–1.62), HBOC knowledge (OR=1.31, 95% CI=1.13–1.52), and perceived relative risk of BC recurrence (OR=9.49, 95% CI=1.83–49.05). The final multivariable model demonstrated adequate goodness-of-fit (χ2=6.54, p=0.59).
Discussion
BRCA testing has important implications for cancer risk management for high risk BC survivors.6 However, Black women are under-represented in prior research; thus, our understanding of GT health outcomes in the Black community is minimal.9–11 This is among the first papers to examine behavioral outcomes following BRCA1/2 testing in exclusively Black women. Prior studies of BRCA1/2 testing in the Black community have examined test result acceptance11 or reported only on members of a single large kindred.9 The present study utilizes a large, statewide sample and significantly extends the time period of follow-up to 12 months post-GT. As such, these data provide insight into risk management strategies utilized by Black women and what factors influence their risk-reduction behaviors.
The Black BC survivors in the present study reported rates of risk reducing behaviors – including mammography, breast MRI, CA125 testing, and transvaginal/pelvic ultrasound – similar to those previously observed.21–31 However, uptake of risk-reducing mastectomy and risk-reducing salpingo-oophorectomy in this study was significantly lower than recent studies of majority White women (risk-reducing mastectomy: 20–54%, risk-reducing salpingo-oophorectomy: 51–71%).24,32 Notably, no women in this sample received risk-reducing mastectomy following GT. This discrepancy may be due to access to care barriers33–35 such as being uncoupled from treatment. However, in the present study, 83 of women had health insurance, 73% reported having a primary care provider, and all but one reported seeing that provider in the last 12 months. Thus, future studies might incorporate qualitative methods to assess other reasons for this difference by race, such as cultural perspectives.36–46 Alternatively, this discrepancy may be attributable to the length of follow-up; if followed longer than 12-months post-GT, the present sample may demonstrate higher rates of risk-reducing mastectomy and risk-reducing salpingo-oophorectomy. Long-term studies (e.g., 5-years post-GT) of risk management behaviors in Black women post-GT are needed.
Our first hypothesis – that BRCA1/2 carriers would be more likely to report advanced BC and ovarian cancer risk management in the 12 months after GT – was only partially supported, and differences by BRCA1/2 status were only observed for ovarian cancer management. The patterns of uptake in the present study were guideline-concordant: women who were BRCA1/2 positive were more likely to have received risk-reducing salpingo-oophorectomy, CA125 test, or transvaginal/pelvic ultrasound in the past 12 months (69%), compared to women who were BRCA1/2 negative (35%) or had a VUS (42%). These findings are consistent with studies of majority White women, wherein BRCA1/2 carrier status was significantly associated with ovarian cancer risk reduction strategies.21,24 Although BRCA1/2 women were more likely to have received advanced ovarian cancer risk management, 35% of BRCA1/2 negative women received unnecessary ovarian cancer risk management in the past 12 months. This includes two BRCA1/2 negative women who received risk-reducing salpingo-oophorectomy. If based purely on the BRCA test result, this finding is problematic; however, these two women also had a family cancer history that was significant for HBOC (e.g., first or second degree relative either diagnosed with BC age ≤50 years or diagnosed with ovarian cancer at any age). Though speculative, family history may play a more significant role in risk-reduction decision-making than evidenced by the present analyses.
Furthermore, it should be noted that CA125 testing and transvaginal/pelvic ultrasound were removed from NCCN recommendations for the management of HBOC in 2015, in the middle of data collection for this study, due to emerging evidence that annual ovarian cancer screening is not reliable to detect early-stage ovarian cancers.47 For this reason, the findings regarding CA125 testing and transvaginal/pelvic ultrasound are interesting historically, but less relevant to the current management of BRCA1/2 carriers.
In contrast with prior findings,23,24 there were no significant differences in BC management (e.g., breast MRI) by BRCA1/2 status. The lack of differences in breast MRI receipt by BRCA1/2 status, in combination with the low overall uptake of breast MRI in this sample, is concerning. In high-risk women, breast MRI significantly increases cancer detection compared to mammography alone.48 This finding prompted inclusion of MRI in addition to mammography screening for BRCA1/2 carriers in NCCN guidelines.6 However, the results presented here are inconsistent with guideline recommendations. Prior research has demonstrated barriers to BC screening in the general population exist at multiple levels, including patient-, provider-, and system-levels.49–53 Studies of breast MRI specifically have demonstrated increased use is associated with age <40, family history of BC, prior breast biopsy, and higher education.51,54 However, these studies are largely based on retrospective secondary analysis of medical record and insurance claims data in majority White (64–73%) populations. Future research should examine barriers to supplemental breast MRI among Black BC survivors following GT.
We also hypothesized there would be no difference in receipt of mammogram by BRCA1/2 status. This hypothesis was supported, but contrasts with prior findings that mammography use was higher in carriers than in non-carriers (59–92% v. 30–53%).9,22,23,31 The extremely high rates of mammography in this sample (93%) may have resulted in a ceiling effect, wherein differences between groups could not be observed. Future research should seek to identify unique features of the minority of Black BC survivors who do not receive a mammogram, despite having breast tissue at risk for recurrence.
Finally, our exploratory analyses identified clinical and decision-making variables predicting use of risk management behaviors in the 12 months after GT. Regarding BC risk management, likelihood of breast MRI decreased as time since BC diagnosis increased. As high-risk BC survivors transition back to primary care, their unique survivorship care needs may be less frequently addressed by non-oncology providers.55 This highlights the importance of long-term follow-up and the potential value of risk-reducing mastectomy, as there is no need for ongoing breast follow-up.
Advanced ovarian cancer risk management was significantly predicted by two decision-making variables: relative perceived risk and HBOC knowledge. Notably, the effects of these variables remained even when accounting for important cancer-related variables, such as BRCA1/2 status and ER status. Although annual ovarian cancer screening is no longer recommended for HBOC management, these results do provide some implications for future risk management interventions. Notably, perceived risk and HBOC knowledge are the typical targets of genetic counseling interventions.56 Thus, these types of interventions may be effectively applied to ovarian cancer risk management behaviors.
Strengths of this study include the statewide recruitment of individuals across a variety of institutions. Thus, these results are likely to be generalizable to community-based cancer survivors in Florida, rather than only those who choose to seek care at large, academic medical centers. In addition, the study sample had a relatively high retention rate, with 69% of baseline participants completing the 12-month follow-up assessment. Finally, GT was provided as part of the study, thereby standardizing information women received regarding their personal risk, HBOC, and risk management. Thus, we have greater confidence the behavioral outcomes observed were not related to differences in information provided during the process of GT, as might be the case in a naturalistic study of changes after GT provided outside of the research context.
Nonetheless, the results of the present study should be interpreted in light of some limitations. First, of the 882 women with whom we were successfully able to establish contact, only 480 (54%) consented to participate in the present study; the results may thus be subject to selection bias. Although we were unable to compare participants and non-participants on self-reported characteristics, previously published analyses compared participants to the presumed eligible individuals from the registry (n = 1191) on characteristics that were available in FCDS and found no differences in relationship status, insurance, mean age of diagnosis, stage at diagnosis, employment, or residence in a metropolitan area.57 Furthermore, although all participants consented to genetic testing as part of the study procedures, a subset (n=50) never completed GT through the study. Unfortunately, we did not collect data on why these participants did not proceed with GT; future studies are warranted to understand Black women’s reasons for not proceeding with GT. Second, risk-reducing behaviors were collected via self-report and may be subject to demand characteristics and social desirability. In addition, women self-reported reasons for mastectomy and salpingo-oophorectomy. It is unclear whether women would be aware of all of the clinical indications for these procedures (i.e., might report salpingo-oophorectomy was performed to treat ovarian cysts, but in actuality may be intended to treat cysts and reduce HBOC risk). Thus, our data may underestimate the number of risk-reducing procedures received. Future studies of risk management behaviors among Black women may include electronic medical record data in addition to patient self-report. Third, GT was provided as part of the study; although there are some benefits to this approach, GT outside of the typical clinical context has some limitations. Post-test genetic counseling was delivered via telephone, and there is evidence that phone-based genetic counseling leads to lower uptake of enhanced cancer control measures.58,59 Additionally, although a subset of participants reported that their shared their GT results with an oncologist (45%), surgeon (17%), obstetrician/gynecologist (31%), or primary care provider (36%), it is unknown if the participants’ providers counseled them regarding GT results and options for risk reduction. Fourth, the follow-up time point selected (12 months) may have limited our understanding of survivors’ risk-reduction decisions. For example, other research has found that the median time to RRM among BRCA1/2 carriers is more than two years following diagnosis of HBOC.60 Finally, we aimed to understand and highlight the demographic, clinical, and decision-making factors associated with risk-reducing behaviors. However, there may be additional predictors of risk-reducing behaviors (e.g., culture, fertility/childbearing, partner/family influence, care facility, quality of/satisfaction with care).
In light of clinical availability of BRCA1/2 testing, ethical practice behooves examination of the behavioral consequences of GT. The present research meets this need by characterizing the behavioral outcomes of GT in a rarely-studied at risk population: young Black BC survivors. This is an important step in leveraging genetic risk determination to reduce cancer prevention and control disparities.
Synopsis:
This study documents patterns and predictors of cancer risk management behaviors among young Black BC survivors after genetic testing. Survivors’ behaviors were guideline-concordant for mammogram and ovarian cancer risk management, but BRCA1/2 carriers under-utilized breast MRI.
Funding:
This work was supported by grants from the American Cancer Society: RSG-11-268-01-CPPB (PI: Vadaparampil), the Florida Biomedical Research Program: IBG10–34199 (PI: Pal) and the National Cancer Institute: K01 CA211789 (PI: Gonzalez), P30CA076292 (PI: Sellers) and R25 CA090314 (PI: Brandon).
Appendix A.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist.
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4–5 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 4–5 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 5 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 5–6 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 5–6 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6 |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 6–8 |
| Bias | 9 | Describe any efforts to address potential sources of bias | N/A |
| Study size | 10 | Explain how the study size was arrived at | 9 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 6–8 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 8–9 |
| (c) Explain how missing data were addressed | 9 | ||
| (e) Describe any sensitivity analyses | N/A | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 9–10 and Figure 1 |
| (c) Consider use of a flow diagram | Figure 1 | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | Table 1 |
| (c) Summarise follow-up time (eg, average and total amount) | 6 | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | 10 and Figures 2 and 3 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 11 and Table 2 |
| N/A | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | N/A |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 11–15 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 15–16 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 11–15 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 15 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 18 |
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Ferla R, Calo V, Cascio S, et al. Founder mutations in BRCA1 and BRCA2 genes. 2007;18(suppl_6): vi93–vi98. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson MP, Hartman L, Kristoffersson U, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2014;147(3):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. Journal of Clinical Oncology. 2009;27(35):5887–5892. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe KA, Lynch HT, Ghadirian P, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecologic oncology. 2005;96(1):222–226. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN). Breast Cancer Risk Reduction (Version 2.2018). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2018; https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed November 12, 2018. [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN). Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 2.2019). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2018; https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed November 12, 2018. [Google Scholar]
- 7.Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genetics in Medicine. 2011;13(4):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinney AY, Bloor LE, Mandal D, et al. The impact of receiving genetic test results on general and cancer‐specific psychologic distress among members of an African‐American kindred with a BRCA1 mutation. Cancer. 2005;104(11):2508–2516. [DOI] [PubMed] [Google Scholar]
- 10.Halbert C, Kessler L, Troxel A, Stopfer J, Domchek S. Effect of genetic counseling and testing for BRCA1 and BRCA2 mutations in African American women: a randomized trial. Public Health Genomics. 2010;13(7–8):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbert CH, Kessler L, Stopfer JE, Domchek S, Wileyto EP. Low rates of acceptance of BRCA1 and BRCA2 test results among African American women at increased risk for hereditary breast-ovarian cancer. Genetics in Medicine. 2006;8(9):576. [DOI] [PubMed] [Google Scholar]
- 12.Bonner D, Cragun D, Reynolds M, Vadaparampil ST, Pal T. Recruitment of a Population-Based Sample of Young Black Women with Breast Cancer through a State Cancer Registry. Breast J. 2016;22(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal T, Bonner D, Cragun D, et al. A high frequency of BRCA mutations in young black women with breast cancer residing in Florida. Cancer. 2015;121(23):4173–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BIC. Breast Cancer Information Core. Web-site: http://www.nhgri.nih.gov/Intramural_research/Lab_transfer/BIC/. 2015; http://www.nhgri.nih.gov/Intramural_research/Lab_transfer/BIC/. Accessed March 19, 2015.
- 16.Vos J, Otten W, van Asperen C, Jansen A, Menko F, Tibben AJPO . The counsellees’ view of an unclassified variant in BRCA1/2: recall, interpretation, and impact on life. 2008;17(8):822–830. [DOI] [PubMed] [Google Scholar]
- 17.Garcia C, Lyon L, Littell RD, Powell CBJGiM. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. 2014;16(12):896. [DOI] [PubMed] [Google Scholar]
- 18.Lipkus IM, Kuchibhatla M, McBride CM, et al. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiol Biomarkers Prev. 2000;9(9):973–975. [PubMed] [Google Scholar]
- 19.Scherr CL, Christie J, Vadaparampil ST. Breast cancer survivors’ knowledge of hereditary breast and ovarian cancer following genetic counseling: an exploration of general and survivor-specific knowledge items. Public health genomics. 2016;19(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Communications in statistics-Theory and Methods. 1980;9(10):1043–1069. [Google Scholar]
- 21.Botkin JR, Smith KR, Croyle RT, et al. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. American Journal of Medical Genetics Part A. 2003;118(3):201–209. [DOI] [PubMed] [Google Scholar]
- 22.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genetics in Medicine. 2008;10(1):19. [DOI] [PubMed] [Google Scholar]
- 23.Beery TA, Williams JK. Risk reduction and health promotion behaviors following genetic testing for adult-onset disorders. Genetic testing. 2007;11(2):111–123. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz MD, Isaacs C, Graves KD, et al. Long‐term outcomes of BRCA1/BRCA2 testing: Risk reduction and surveillance. Cancer. 2012;118(2):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalfe KA, Birenbaum‐Carmeli D, Lubinski J, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. International journal of cancer. 2008;122(9):2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beattie MS, Crawford B, Lin F, Vittinghoff E, Ziegler J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genetic testing and molecular biomarkers. 2009;13(1):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradbury AR, Ibe CN, Dignam JJ, et al. Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Genetics in Medicine. 2008;10(3):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1-and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. Journal of Clinical Oncology. 2008;26(8):1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metcalfe KA, Lubinski J, Ghadirian P, et al. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. Journal of Clinical Oncology. 2008;26(7):1093–1097. [DOI] [PubMed] [Google Scholar]
- 30.Friebel TM, Domchek SM, Neuhausen SL, et al. Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clinical breast cancer. 2007;7(11):875–882. [DOI] [PubMed] [Google Scholar]
- 31.Watson M, Foster C, Eeles R, et al. Psychosocial impact of breast/ovarian (BRCA 1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. British journal of cancer. 2004;91(10):1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia C, Wendt J, Lyon L, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecologic oncology. 2014;132(2):428–433. [DOI] [PubMed] [Google Scholar]
- 33.Hall MJ, Olopade OI. Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006;24(14):2197–2203. [DOI] [PubMed] [Google Scholar]
- 34.Oloparde OI. Genetics in clinical cancer care: A promise unfulfilled among minority populations. Cancer Epidemiol Biomarkers Prev. 2004;13(11):1683–1686. [PubMed] [Google Scholar]
- 35.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast‐ovarian cancer. Cancer. 2009;115(10):2222–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Education and Counseling. 2003;51(3):217–227. [DOI] [PubMed] [Google Scholar]
- 37.Hughes C, Fasaye G-A, LaSalle VH, Finch C. Sociocultural influences on participation in genetic risk assessment and testing among African American women. Patient Education and Counseling. 2003;51(2):107–114. [DOI] [PubMed] [Google Scholar]
- 38.Guidry JJ, Matthews‐Juarez P, Copeland VA. Barriers to breast cancer control for African‐American women: the interdependence of culture and psychosocial issues. Cancer. 2003;97(S1):318–323. [DOI] [PubMed] [Google Scholar]
- 39.Furr LA. Perceptions of genetics research as harmful to society: differences among samples of African-Americans and European-Americans. Genetic testing. 2002;6(1):25–30. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed IE, Williams KS, Tamburrino M, Wryobeck J, Carter S. Understanding locally advanced breast cancer: what influences a woman’s decision to delay treatment? Preventive medicine. 2005;41(2):399–405. [DOI] [PubMed] [Google Scholar]
- 41.Spurlock WR, Cullins LS. Cancer fatalism and breast cancer screening in African American women. ABNF Journal. 2006;17 (1). [PubMed] [Google Scholar]
- 42.Kessler L, Collier A, Brewster K, et al. Attitudes about genetic testing and genetic testing intentions in African American women at increased risk for hereditary breast cancer. Genetics in Medicine. 2005;7(4):230. [DOI] [PubMed] [Google Scholar]
- 43.Edwards TA, Thompson HS, Kwate NOA, et al. Association between temporal orientation and attitudes about BRCA1/2 testing among women of African descent with family histories of breast cancer. Patient Education and Counseling. 2008;72(2):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meiser B, Eisenbruch M, Barlow-Stewart K, Tucker K, Steel Z, Goldstein D. Cultural aspects of cancer genetics: setting a research agenda. Journal of Medical Genetics. 2001;38(7):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt CL, Caplan L, Schulz E, Blake V, Southward VL, Buckner AV. Development and validation of measures of religious involvement and the cancer experience among African Americans. Journal of health psychology. 2009;14(4):525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moorman PG, Barrett NJ, Wang F, et al. Effect of Cultural, Folk, and Religious Beliefs and Practices on Delays in Diagnosis of Ovarian Cancer in African American Women. Journal of Women’s Health. 2018;28(4):444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodward E, Sleightholme H, Considine A, Williamson S, McHugo J, Cruger D. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high‐risk and population risk women is ineffective. British Journal of Obstetrics and Gynecology. 2007;114(12):1500–1509. [DOI] [PubMed] [Google Scholar]
- 48.Lehman CD. Role of MRI in screening women at high risk for breast cancer. J Magn Reson Imaging. 2006;24(5):964–970. [DOI] [PubMed] [Google Scholar]
- 49.Gramling R, Nash J, Siren K, Eaton C, Culpepper L. Family physician self-efficacy with screening for inherited cancer risk. Ann Fam Med. 2004;2(2):130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gramling R, Clarke J, Simmons E. Racial distribution of patient population and family physician endorsed importance of screening patients for inherited predisposition to cancer. J Health Care Poor Underserved. 2009;20(1):50–54. [DOI] [PubMed] [Google Scholar]
- 51.Haas JS, Hill DA, Wellman RD, et al. Disparities in the use of screening magnetic resonance imaging of the breast in community practice by race, ethnicity, and socioeconomic status. Cancer. 2016;122(4):611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onega T, Hubbard R, Hill D, et al. Geographic access to breast imaging for US women. Journal of the American College of Radiology. 2014;11(9):874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George SA. Barriers to breast cancer screening: an integrative review. Health care for women international. 2000;21(1):53–65. [DOI] [PubMed] [Google Scholar]
- 54.Miles R, Wan F, Onega TL, et al. Underutilization of Supplemental Magnetic Resonance Imaging Screening Among Patients at High Breast Cancer Risk. Journal of Women’s Health. 2018;27(6):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Research Council. From cancer patient to cancer survivor: Lost in transition. National Academies Press;2005. 0309095956. [Google Scholar]
- 56.Biesecker BB. Goals of genetic counseling. Clinical genetics. 2001;60(5):323–330. [DOI] [PubMed] [Google Scholar]
- 57.Bonner D, Cragun D, Reynolds M, Vadaparampil ST, Pal TJTbj. Recruitment of a Population‐Based Sample of Young Black Women with Breast Cancer through a State Cancer Registry. 2016;22(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinney AY, Steffen LE, Brumbach BH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow-up. 2016;34(24):2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinney AY, Butler KM, Schwartz MD, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. 2014;106(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wevers M, Schmidt M, Engelhardt E, et al. Timing of risk reducing mastectomy in breast cancer patients carrying a BRCA1/2 mutation: retrospective data from the Dutch HEBON study. 2015;14(3):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]