Abstract
Purpose:
Surgical castration for metastatic prostate cancer is used less frequently than medical castration, yet costs less, requires less follow-up, and may be associated with fewer adverse effects. We sought to evaluate temporal trends and factors associated with the use of surgical castration.
Materials and Methods:
This retrospective cohort study sampled 24,805 men with newly diagnosed (de novo) metastatic prostate cancer from a national cancer registry in the United States (2004–2016). Multivariable logistic regression assessed the association between sociodemographics and surgery. Multivariable Cox regression evaluated the association between castration type and overall survival.
Results:
Overall, 5.4% of men received surgical castration. This decreased from 8.5% in 2004 to 3.5% in 2016 (Per year later: OR 0.89, 95% CI 0.87–0.91,p<0.001). Compared to Medicare, private insurance was associated with less surgery (OR 0.73, 95% CI 0.61–0.87, p<0.001) while Medicaid or no insurance was associated with more surgery (OR 1.68, 95% CI 1.34–2.11, <0.001 and OR 2.12, 95% CI 1.58–2.85, p<0.001, respectively). Regional median income >$63,000 was associated with less surgery (vs <$38,000: OR 0.61, 95% CI 0.43–0.85, p=0.004). After a median follow-up of 30 months, castration type was not associated with differences in survival (Surgical vs medical: HR 1.02, 95% CI 0.95–1.09, p=0.6).
Conclusion:
In a contemporary, real-world cohort, use of surgical castration is low and decreasing despite its potential advantages and similar survival compared to medical castration. Men with potentially limited health care access receive more surgery, perhaps reflective of a provider bias towards the perceived benefit of permanent castration.
Keywords: castration, epidemiology, orchiectomy, prostatic neoplasms, United States
Introduction
For decades, the mainstay of treatment for metastatic prostate cancer (PCa) has been to achieve castrate levels of testosterone in patients through androgen deprivation therapy (ADT).1 Men undergoing ADT most often receive medical castration—most commonly with repeat dosing of gonadotropin-releasing hormone (GnRH) analogues administered either subcutaneously or intramuscularly during a clinic visit every one to six months. Surgical castration represents another ADT option, accomplished through bilateral orchiectomy, a relatively minor, outpatient surgery. Regardless of type, ADT is associated with side effects including bone loss, metabolic syndrome, and cardiovascular events.2–4 However, recent data suggest surgical castration may carry certain advantages, including lower rates of some adverse effects,5 reduced cost over long-term follow-up,6 and, given its permanence, may minimize the effect of medication non-adherence—something associated with adverse oncologic outcomes.7
With the advent of GnRH analogues, the proportion of men with metastatic PCa managed with surgical castration decreased in the 1990’s and early 2000’s8, 9; notably, there were no reported differences in survival attributable to castration type to drive this change in practice.10 Highlighting possible non-clinical drivers of ADT type, recent work has suggested an association between surgical castration, health insurance type, and other socioeconomics factors.11 Given the aforementioned potential benefits of surgery, there is a need to better understand contemporary utilization, the drivers of differential utilization, and outcomes for castration type using national data.
We used a large, national cancer registry to identify men with newly diagnosed metastatic PCa and evaluated temporal trends and factors associated with use of surgical castration. We hypothesized use of surgical castration would be associated with non-clinical factors suggesting these elements (e.g., patient/provider preferences, sociodemographics) may be associated with treatment choices. We also sought to evaluate the association between castration type and overall survival in a contemporary, real-world cohort of men with metastatic PCa.
Methods
Data source
The data source for this study was the PCa participant user file from the National Cancer Data Base (NCDB) from 2004 to 2016. The NCDB is a hospital-based cancer registry comprised of more than 1,500 treatment facilities accredited by the American College of Surgeons and American Cancer Society’s Commission on Cancer.12 In 2015, the NCDB captured approximately 53% of all new cases of PCa diagnosed in the United States.13, 14
Patients
All men with metastatic PCa at time of diagnosis who had documented receipt of any form of hormone therapy as one of their initial forms of treatment were included (n=55,097, 100%). Men with missing sociodemographics information or treatment facility information were excluded (n=1,497, 2.7%). Men were also excluded if they lacked data on pretreatment prostate-specific antigen (PSA; n=4,547, 8.3%), clinical T stage (n=15,062, 27.3%), or Gleason grade group (n=6,527, 11.8%). Facilities were excluded if they did not contribute at least one patient every year of the study period (n=2,659, 4.8%).
Outcomes
The primary outcome was use of surgical castration. Based on the Facility Oncology Registry Data Standards, surgical castration in the form of orchiectomy is recorded in the NCDB under the heading “Hematologic Transplant and Endocrine Procedure” when orchiectomy is part of the first course of treatment.15 Our secondary outcome was overall survival based on castration type.
Covariates
Patient characteristics included year of diagnosis (continuous variable), age at diagnosis (continuous variable), race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or unknown/other), and Charlson comorbidity index (0, 1, or >1).16 Distance traveled to treatment facility was defined as distance from the center of the patient’s home zip code to that of the treatment facility. Patient county type was defined as metropolitan (population > 250,000) or urban/rural (population <250,000). Insurance type was defined as the primary payer, income was defined as the median household income within a patient’s home zip code, and education was defined as adult high school attainment within a patient’s home zip code.
Treatment facility characteristics included geographic location within the United States and facility type. Facility type was defined as comprehensive or academic facilities if they treated >500 cancer patients a year while community facilities treated >100 cancer patients a year. Academic facilities were those had at least four graduate medical education programs.
Finally tumor characteristics include Gleason grade group (1–5) with increasing grade representing increasing cancer aggressiveness,17 American Joint Committee on Cancer clinical T and M stage, and pretreatment PSA. Notably, PSA in the NCDB is recorded as 98 if the value is 98 ng/mL or greater, so this variable was assessed as a categorical variable.
Statistical analysis
Chi-squared and Mann-Whitney U analyses were used for univariable comparisons of categorical and continuous factors, respectively. Multivariable logistic regression was also used to evaluate the relationship between covariates and castration type. This analysis was adjusted for clustering of patients within treatment facilities (n=1,057).18 Given the number of men excluded from this analysis based on missing data on tumor characteristics, we performed a sensitivity analysis including these men with “unknown” listed as the category for their missing data. Kaplan-Meier analyses were used to estimate median overall survival while Log-rank and multivariable Cox regression were used to assess the relationship between castration type and overall survival while accounting for potential confounders with the latter. All survival analyses were conducted using RStudio, Version 1.1.463 (Boston, MA) and all others were performed using Stata 13.0 (College Station, TX) and statistical significance was determined by two-sided p<0.05. This study was deemed exempt by the Northwestern University Institutional Review Board as a retrospective review of de-identified patient data.
Results
Patient characteristics
A total of 24,805 men diagnosed with metastatic PCa were included in the final analysis. Of these, 23,461 (94.6%) were treated with medical castration and 1,344 (5.4%) were treated with surgical castration. Median age at diagnosis was 69 years and 68% of the cohort was White (Supplemental table 1). Men who received surgical castration had higher pretreatment PSA, more clinical T3–4 disease, and more comorbidities (all p<0.001). Men who underwent surgical castration were also more likely to have Medicaid or no insurance (21.0% vs 11.2%; p<0.001) and reside in zip codes with lower median household incomes and high school attainment (both p<0.001).
Trends in surgical castration
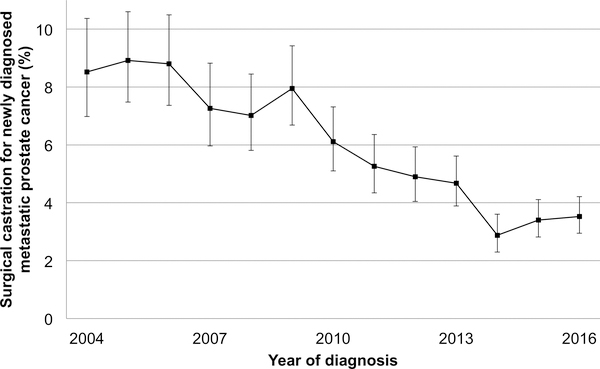
The overall rate of surgical castration was 5.4% (n=1,344). Use of surgical castration declined over time from 8.5% in 2004 to 3.5% in 2016 (Per year later OR 0.89, 95% CI 0.87–0.91,p<0.001; Figure 1 and Table 1). Patient characteristics associated with increased receipt of surgical castration included increased comorbidity index (Table 1). Higher Gleason grade group, more advanced clinical T stage, and higher serum PSA were all associated with increased use of surgery. Treatment at an academic facility was associated with decreased receipt of surgical castration compared to community facility (OR 0.68, 95% CI 0.48–0.95, p=0.026).
Figure 1:
Temporal trends in surgical castration for men with newly diagnosed metastatic prostate cancer
Error bars represent 95% confidence intervals
Table 1:
Multivariable logistic regression for factors associated with surgical castration for men with newly diagnosed metastatic prostate cancer
| Covariate | OR (95% CI) | p |
|---|---|---|
| Year of diagnosis | ||
| Per one year later | 0.89 (0.87–0.91) | <0.001 |
| Age | ||
| Per 10 year increase | 1.07 (1.00–1.15) | 0.051 |
| Race/Ethnicity | ||
| White | Reference | |
| Black | 1.09 (0.91–1.31) | 0.3 |
| Hispanic | 1.28 (0.95–1.72) | 0.107 |
| Unknown/other | 0.97 (0.72–1.31) | 0.9 |
| Gleason grade group | ||
| 5 | Reference | |
| 4 | 0.94 (0.81–1.08) | 0.4 |
| 3 | 1.15 (0.95–1.39) | 0.161 |
| 2 | 1.08 (0.86–1.37) | 0.5 |
| 1 | 0.69 (0.50–0.95) | 0.023 |
| Pretreatment PSA, ng/dl | ||
| Greater than or equal to 98 | Reference | |
| 50 to less than 98 | 0.55 (0.46–0.66) | <0.001 |
| 20 to less than 50 | 0.55 (0.46–0.66) | <0.001 |
| Less than 20 | 0.37 (0.31–0.44) | <0.001 |
| Clinical T stage | ||
| T4 | Reference | |
| T3 | 0.83 (0.71–0.97) | 0.019 |
| T2 | 0.73 (0.62–0.84) | <0.001 |
| T1 | 0.47 (0.31–0.73) | 0.001 |
| Clinical M stage | ||
| M1c | Reference | |
| M1b | 1.09 (0.89–1.33) | 0.4 |
| M1a | 0.84 (0.58–1.20) | 0.3 |
| M1NOS | 0.86 (0.68–1.07) | 0.174 |
| Comorbidities | ||
| 0 | Reference | |
| 1 | 1.40 (1.18–1.65) | <0.001 |
| >1 | 1.43 (1.14–1.79) | 0.002 |
| Geographic Location | ||
| North East | Reference | |
| North Central | 1.22 (0.89–1.66) | 0.2 |
| South | 1.40 (1.04–1.88) | 0.027 |
| West | 1.45 (1.00–2.09) | 0.048 |
| Facility Type | ||
| Community | Reference | |
| Comprehensive | 1.22 (0.89–1.67) | 0.2 |
| Academic | 0.68 (0.48–0.95) | 0.026 |
| Other | 1.35 (0.94–1.93) | 0.104 |
| Insurance Type | ||
| Medicare | Reference | |
| Private | 0.73 (0.61–0.87) | 0.001 |
| Medicaid | 1.68 (1.34–2.11) | <0.001 |
| Uninsured | 2.12 (1.58–2.85) | <0.001 |
| Other | 1.24 (0.90–1.71) | 0.180 |
| Distance traveled to treatment facility | ||
| ≤60 miles | Reference | |
| 60–120 miles | 0.75 (0.50–1.12) | 0.157 |
| >120 miles | 0.80 (0.45–1.41) | 0.4 |
| Patient’s county type | ||
| Metropolitan | Reference | |
| Urban/Rural | 1.13 (0.86–1.47) | 0.4 |
| Median income in patient’s zip code | ||
| <$38,000 | Reference | |
| $38,000–47.999 | 0.87 (0.69–1.08) | 0.2 |
| $48,000-$62,999 | 0.88 (0.69–1.14) | 0.3 |
| $63,000+ | 0.61 (0.43–0.85) | 0.004 |
| Non-high school educated in patient’s zip code | ||
| ≥21% | Reference | |
| 13–20.9% | 1.11 (0.88–1.38) | 0.4 |
| 7–12.9% | 1.11 (0.84–1.47) | 0.5 |
| <7% | 1.13 (0.83–1.54) | 0.4 |
Adjustments for clustering based on treatment facility were made. Bold indicates significance. Abbreviations: CI, confidence interval; OR, odds ratio; PSA, prostate-specific antigen.
Compared to those with Medicare insurance, private insurance was associated with lower odds of surgical castration (OR 0.73, 95% CI 0.61–0.87, p<0.001) while Medicaid or no insurance was associated with increased odds (OR 1.68, 95% CI 1.34–2.11, <0.001 and OR 2.12, 95% CI 1.58–2.85, p<0.001, respectively). Regional high school attainment was not associated with castration type while those living in regions of income >$63,000 were less likely to undergo surgical castration compared to men living in regions of income <$38,000 (OR 0.61, 95% CI 0.43–0.85, p=0.004).
Castration type and overall survival
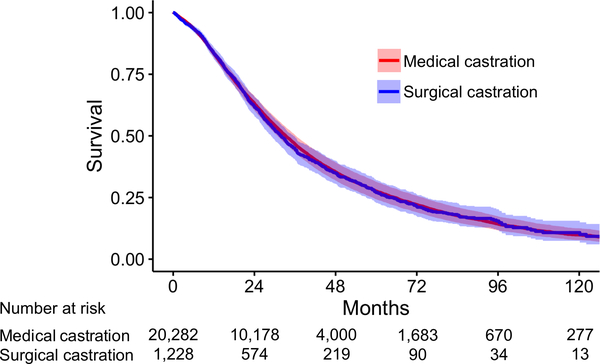
Among 21,510 men with follow-up data included in the survival analysis (surgical castration: n=1,228, 5.7%), a total of 14,376 (67%) deaths occurred during a median (Interquartile range) follow-up of 30.4 (15.5–53.9) months following diagnosis of metastatic PCa. Kaplan-Meier estimates demonstrated a median survival of 31.0 months for men who underwent medical castration and 25.5 months for men who underwent surgical castration (unadjusted Log-rank p<0.001). After adjusting for covariates, multivariable Cox regression demonstrated castration type was not significantly associated with overall survival (Surgical vs medical: HR 1.02, 95% CI 0.95–1.09, p=0.6; Figure 2 and Supplemental table 2).
Figure 2:
Adjusted overall survival based on castration type
Survival over time based on multivariable Cox regression in supplemental table 2
Sensitivity analyses
In a sensitivity analysis including all men who were excluded from the primary analysis due to missing data on tumor characteristics, a total of 45,915 were assessed. A total of 5.0% (n=2,390) underwent surgical castration in this cohort. Surgical castration decreased from 8.5% in 2004 to 2.9% in 2016 (Per 1 year later: OR 0.89, 95% CI 0.87–0.90,p<0.001). All previously significant markers of socioeconomics remained significant with similar ORs (Supplemental table 3). In 41,977 men with follow-up data, after adjusting for covariates, multivariable Cox regression demonstrated castration type was not associated with a differences in overall survival (Surgical vs medical: HR 1.00, 95% CI 0.95–1.05, p=0.9).
Discussion
In this retrospective analysis of a large cancer-registry, we assessed temporal trends and factors associated with use of surgical castration and compared overall survival by castration type in a contemporary cohort of men with de novo metastatic PCa. Overall 5.4% of patients received surgical castration which declined to 3.5% in 2016. Non-private insurance, no insurance, and lower income were associated with receipt of surgical castration. After accounting for tumor and patient characteristics, castration type was not associated with survival.
Prior work demonstrated castration type does not impact survival outcomes, however these data were from clinical trials that enrolled more than twenty years ago.10 Additionally, efficacy in a clinical trial may not always translate into real-world effectiveness in less controlled environments where non-clinical factors (e.g., socioeconomic or insurance status) can play a role.19 Data from the NCDB, with contemporary patients, suggests castration type is not associated with changes in overall survival in a cohort of men with metastatic PCa from a broad array of practice types across the United States. These findings should allay historical concerns related to oncologic outcomes based on castration type;20 however, we, along with others using various North American datasets 9, 11, 21 still show limited and declining utilization of surgical castration.
Despite low use, surgical castration is also associated with better non-oncologic outcomes. In a retrospective analysis of SEER-Medicare, surgical castration was associated with lower risks of fractures (HR 0.77), peripheral arterial disease (HR 0.65), and cardiac events (HR 0.74) compared to medical castration.5 Similarly, a population-based study from Denmark suggested while medical castration was associated with increased risk of myocardial infarction (HR 1.31, 95% CI 1.16–1.49) or stroke (HR 1.19, 95% CI 1.06–1.35), surgical castration was not.22 Additionally, although both forms of ADT have been found to be similar in terms of costs within the first year of PCa treatment, 5 a previous analysis suggested surgical castration was more cost-effective per quality-adjusted life years over time, 6 reflective of the upfront costs of surgery compared to the compounded costs of additional medication dosage with GnRH analogues. The cumulative costs of medical castration will become more relevant as men with metastatic PCa live longer with recent advances in therapeutics.23
From a patient perspective, those with private or Medicare insurance might not see these added costs if the expenditures are all covered. In the NCDB, men with Medicaid or no insurance were much more likely to get surgery compared to those with Medicare insurance. Previous work has also shown use of surgery decreased as reimbursement for medical castration increased in the years prior to our study period, suggesting a financial incentive for physicians as well.24 Additionally, surgical castration’s permanence may be appealing to providers who care for patients with limited health care access and poor follow-up. Finally, although not discernable in the NCDB, it is possible those of higher socioeconomic status may more forcefully advocate for medical castration to avoid surgery.
Thus, absent differences in clinical outcomes and despite potentially improved outcomes with surgical castration, we hypothesized differential use may be due to provider or patient factors. In order to better evaluate this, we fit regression models to further understand factors associated with surgical castration use. We found even after adjusting for clinical covariates, increased receipt of surgery was noted for men without insurance (11%) or Medicaid coverage (8%) and among men from areas of lower median income.
Taken together, these findings warrant future investigation into the individual patient- and provider-level factors that affect choice of castration type. Additionally, prospective evaluation of longitudinal quality-of-life and costs in a contemporary era following either medical or surgical castration would be valuable to counsel patients on their options. If evidence continues to accumulate demonstrating similar long-term efficacy and quality-of-life along with reduced costs associated with surgery, providers should likely begin to advocate for more surgical castration. In addition, payment reform to reduce the financial incentives for medical castration may be indicated.
Our study found no difference in castration type based on patient race/ethnicity. In an analysis from a California cancer registry, Hispanic men were more likely to receive surgery compared to white men.11 Among Medicare patients who received any ADT, black men were more likely than white men to receive surgery (14% vs 7%).25 The differences in results based on race/ethnicity between our study and these previous works may be reflective of more limited study populations in the previous works (single state or elderly Medicare population) versus a national patient sample from the NCDB or other unmeasured factors.
Similarly, in the NCDB, men with more comorbidities were less likely to receive surgery. This is contrary to data from a California cancer registry which also used the Charlson comorbidity index.11 Prior work has shown the Charlson comorbidity index does not correlate well with treatment aggressiveness among men with localized disease26 which suggests future work on this topic should consider evaluation of indices which are better predictive of mortality in a general adult population or in men with PCa.27, 28
The findings of this study should be considered in the context of several limitations. First, the NCDB is not population-based; therefore, the results may not be generalizable to all practices. Second, the NCDB can only account for men who received surgical castration as a first form of treatment and cannot account for men who underwent surgical castration after a duration of medical castration. We may, as a result, underestimate use of surgical castration. Third, approximately 40% of men with metastatic PCa in the NCDB over the study period were excluded due to missing information on tumor characteristics, which may bias the reported results in indiscernible ways. However, with similar findings in our sensitivity analysis including these patients, our sense is the influence of these unknown characteristics is unlikely to change our conclusions.
Conclusion
In a national cohort of contemporary men with de novo metastatic PCa, use of surgical castration is low and decreasing despite potential advantages and similar survival compared to medical castration. Men with potentially limited healthcare access were more likely to receive surgical castration, perhaps reflective of provider preference for a permanent form of ADT for these patients. Given the potential benefits over medical castration, increasing the use of surgical castration may represent an opportunity to improve outcomes and reduce costs for men with metastatic PCa. Further work is needed to understand patient and provider level factors driving treatment decisions.
Supplementary Material
Acknowledgments:
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Funding/Support: This work was supported in part by the National Institutes of Health grant 5U01CA196390 and the Prostate Cancer Foundation (EMS) as well as the 2019 Urology Care Foundation Residency Research Award Program and the Russell Scott, Jr., MD Urology Research Fund (ABW).
Role of Funders/Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of interests: The authors have no conflicts of interest
References
- 1.Kunath F, Grobe HR, Rucker G et al. : Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev: CD009266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier A, Ghosh S, McGlynn B et al. : Prostate cancer, androgen deprivation therapy, obesity, the metabolic syndrome, type 2 diabetes, and cardiovascular disease: a review. Am J Clin Oncol, 35: 504, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Lee WC, Brandman J et al. : Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol, 23: 7897, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Tsai HK, D’Amico AV, Sadetsky N et al. : Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst, 99: 1516, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Choueiri TK, Hamnvik OP et al. : Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy. JAMA Oncol, 2: 500, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Bayoumi AM, Brown AD, Garber AM: Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. Journal of the National Cancer Institute, 92: 1731, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Partridge AH, Avorn J, Wang PS et al. : Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst, 94: 652, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Thomsen FB, Sandin F, Garmo H et al. : Gonadotropin-releasing Hormone Agonists, Orchiectomy, and Risk of Cardiovascular Disease: Semi-ecologic, Nationwide, Population-based Study. Eur Urol, 72: 920, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Krahn M, Bremner KE, Tomlinson G et al. : Androgen deprivation therapy in prostate cancer: are rising concerns leading to falling use? BJU Int, 108: 1588, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Seidenfeld J, Samson DJ, Hasselblad V et al. : Single-therapy androgen suppression in men with advanced prostate cancer: A systematic review and meta-analysis. Annals of Internal Medicine, 132: 566, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Borno HT, Lichtensztajn DY, Gomez SL et al. : Differential use of medical versus surgical androgen deprivation therapy for patients with metastatic prostate cancer. Cancer, 125: 453, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boffa DJ, Rosen JE, Mallin K et al. : Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol, 3: 1722, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin, 65: 5, 2015 [DOI] [PubMed] [Google Scholar]
- 14.American College of Surgeons. NCDB Benchmark Reports. Available at: http://oliver.facs.org/BMPub/index.cfm. Accessed June 1, 2019
- 15.Commision on Cancer: Facility Oncology Registry Data Standards Revised for 2016. Pages 306 and 412, 2016 ed, p. 306 and 412 [Google Scholar]
- 16.Berglund A, Garmo H, Tishelman C et al. : Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol, 185: 833, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Epstein JI, Amin MB, Reuter VE et al. : Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol, 41: e1, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Panageas KS, Schrag D, Riedel E et al. : The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med, 139: 658, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Unger JM, Barlow WE, Martin DP et al. : Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst, 106: dju002, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill DC: Treatment of Metastatic Prostate-Cancer - Factors That Influence Treatment Selection and Methods to Increase Acceptance of Orchiectomy. Urology, 32: 408, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Shahinian VB, Kuo YF, Gilbert SM: Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med, 363: 1822, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Jespersen CG, Norgaard M, Borre M: Androgen-deprivation Therapy in Treatment of Prostate Cancer and Risk of Myocardial Infarction and Stroke: A Nationwide Danish Population-based Cohort Study. European Urology, 65: 704, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Fizazi K, Tran N, Fein L et al. : Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol, 20: 686, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Weight CJ, Klein EA, Jones JS: Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer, 112: 2195, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Beebe-Dimmer JL, Ruterbusch JJ, Cooney KA et al. : Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: A SEER-Medicare investigation. Cancer Med, 8: 3325, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daskivich TJ, Chamie K, Kwan L et al. : Matching tumor risk with aggressiveness of treatment in men with multiple comorbidities and early-stage prostate cancer. Cancer, 119: 3446, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC, Walraven C: The mortality risk score and the ADG score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care, 49: 940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daskivich TJ, Kwan L, Dash A et al. : An Age Adjusted Comorbidity Index to Predict Long-Term, Other Cause Mortality in Men with Prostate Cancer. J Urol, 194: 73, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.