Abstract
Ceramides are sphingolipids that modulate a variety of cellular processes via two major mechanisms: functioning as second messengers and regulating membrane biophysical properties, particularly lipid rafts, important signaling platforms. Altered sphingolipid levels have been implicated in many cardiovascular diseases, including hypertension, atherosclerosis and diabetes-related conditions, however molecular mechanisms by which ceramides impact endothelial functions remain poorly understood. In this regard, we generated mice defective of endothelial sphingolipid de novo biosynthesis by deleting the long chain subunit 2 of serine palmitoyltransferase, the first enzyme of the pathway.
Our study demonstrated that endothelial sphingolipid de novo production is necessary to regulate 1) signal transduction in response to NO agonists and, mainly via ceramides; 2) resting eNOS phosphorylation; and 3) blood pressure homeostasis. Specifically, our findings suggest a prevailing role of C16:0-ceramide in preserving vasodilation induced by tyrosine kinase and G-protein coupled receptors, except for Gq-coupled receptors, while C24:0- and C24:1-ceramides control flow-induced vasodilation. Replenishing C16:0-ceramide in vitro and in vivo reinstates endothelial cell signaling and vascular tone regulation.
This study reveals an important role of locally produced ceramides, particularly C16:0-, C24:0- and C24:1-ceramides in vascular and blood pressure homeostasis, and establishes the endothelium as a key source of plasma ceramides. Clinically, specific plasma ceramides ratios are independent predictors of major cardiovascular events. Our data also suggest that plasma ceramides might be indicative of the “diseased” state of the endothelium.
Keywords: Sphingolipid metabolism, ceramides, endothelial function, vascular tone regulation, blood pressure
Graphical Abstract
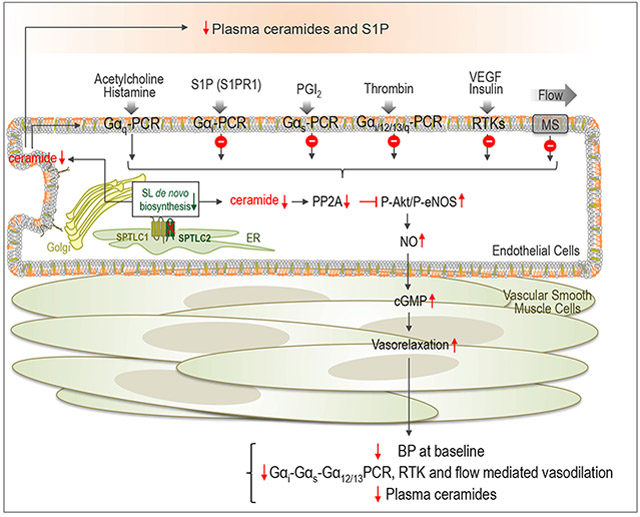
Summary:
Endothelial de novo biosynthesis of sphingolipids is necessary to preserve vascular functions and BP homeostasis
INTRODUCTION
The endothelium regulates vascular and blood pressure (BP) homeostasis by integrating moment-to-moment chemical and rheological stimuli, and the loss of this function is an early event in the pathogenesis of many cardiovascular conditions1-4.
Recently, ceramides, a subclass of sphingolipids, have been implicated in endothelial dysfunction during obesity, diabetes and their cardiovascular complications5-8.
Ceramide is the precursor of all major complex sphingolipids, important membrane components. Metabolically, ceramide is generated by the sphingolipid de novo biosynthesis as well as by the breakdown of complex sphingolipids, including sphingomyelin and glycosphingolipids9. During stress responses, the activation of either or both pathways10, 11 leads to cellular accumulation of ceramides. Additionally, ceramide can be formed by the salvage pathway12, which re-acylates sphingosine derived from sphingolipid catabolism.
The hydrophobicity of ceramide can alter biophysical membrane properties, particularly of lipid rafts, specialized signaling platforms13-15. Ceramide is also a bioactive lipid able to regulate multiple biological processes including stress responses and apoptosis, in part, by the activation of different targets, such as protein phosphatase PP2A and PP1 16, 17, protein kinase Cζ activity18 and cathepsin D19.
Recent studies suggested that specific plasma ceramide ratios are independent predictors of major cardiovascular events5, 20. During metabolic syndrome and type 2 diabetes, ceramides have been implicated in endothelial dysfunction via PP2A-mediated dephosphorylation of eNOS21, 22. While mechanisms of ceramide-mediated apoptosis have been exploited particularly in cancer, how ceramides regulate vascular functions and BP is poorly understood.
The SL de novo biosynthesis is initiated by serine palmitoyltransferase (SPT)23, 24, the first and rate-limiting enzyme. Mice lacking SPT are embryonically lethal25, emphasizing the requirement of SL for survival. Recently, we identified Nogo-B, a membrane protein of the endoplasmic reticulum, as a negative regulator of SPT activity 26. Mice lacking Nogo-B, systemically or in the endothelium, are resistant to hypertension26, inflammation27, 28 and heart failure27, suggesting that the decrease of SPT activity by Nogo-B has pathological implications.
To directly investigate the impact of the local SL de novo biosynthesis on vascular functions and BP, we generated mice lacking endothelial SPT long chain subunit 2 (Sptlc2), one of the two subunits forming SPT32, 33. Our studies demonstrate that SL de novo biosynthesis is necessary to maintain vascular and BP homeostasis. Mechanistically, the decrease of ceramides while impairs endothelial signal transduction in response to chemical and rheological stimuli, enhances basal eNOS phosphorylation state, and thus NO production. Among the most abundant endothelial ceramides, C16:0-Cer preserves endothelial receptor-mediated signaling, while C24:0-Cer and C24:1-Cer mainly control flow-mediated vasodilation. Furthermore, our study shows that plasma ceramides are dictated by the endothelium, thus circulating ceramides may be a “raconteur” of the health/diseases state of the vasculature.
METHODS AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request
Mouse model.
We generated conditional mouse model lacking endothelial SPTLC2 subunit of SPT specifically, namely ECKO-Sptlc2. Floxed-Sptlc2 mice (Sptlc2f/f) were crossed with transgenic mice in which the VE-cadherin promoter drives expression of tamoxifen-responsive Cre (VE-Cad-CreERT2), such that tamoxifen treatment selectively deletes the loxP-flanked (‘floxed’) region of Sptlc2 in EC29. To delete Sptlc2 in EC, 7 to 8 weeks old male mice were injected intraperitoneally (i.p.) with 20 mg/kg of tamoxifen daily for 5 consecutive days. All animal experiments were approved by the Weill Cornell Institutional Animal Care and Use Committee.
Statistical analysis.
Data are expressed as mean ± SEM. Two-way ANOVA followed by Sidak’s post-test, One-way ANOVA followed by Tukey’s post-test, or Student’s t-test were used for statistical analysis as indicated. Differences were considered statistically significant when p≤0.05. All tests were two-sided. GraphPad Prism software (version 8.0, GraphPad Software, San Diego, CA) was used for all statistical analysis.
RESULTS
Endothelial sphingolipid de novo biosynthesis is an important source of local and circulating ceramide and S1P.
The endothelial deletion of Sptlc2 gene was obtained by crossing floxed Sptlc2 mice with tamoxifen inducible VE-cadherin Cre recombinase mice(Sptlc2f/f Cdh5-CreERT2; referred to as ECKO-Sptlc2). At 3 weeks post-tamoxifen treatment, the efficiency of Sptlc2 deletion in aortic EC was ≥80% (Fig. S1). Endothelial SPTLC2 expression and SPT activity were significantly reduced (Fig. 1A, B), as well as ceramides and sphingosine (Fig. 1C, D). Interestingly, whereas plasma ceramides and S1P significantly decreased (∼50% ECKO-Sptlc2 vs. controls, Fig. 1E,F), sphingomyelin levels were unchanged suggesting that the endothelium is a critical source of plasma ceramides and S1P but not sphingomyelin (Fig. S1E, F).
Figure 1. Endothelial sphingolipid de novo biosynthesis is an important source of local and circulating ceramide and S1P.
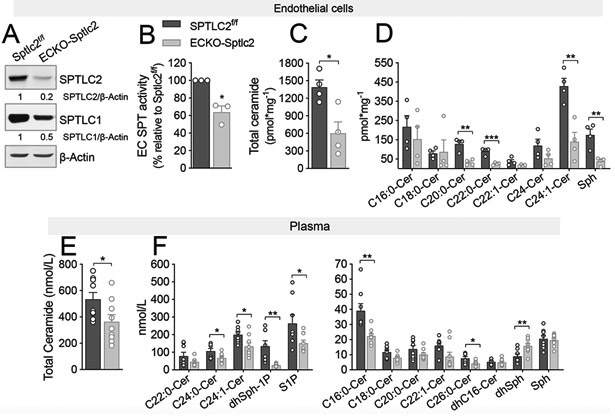
(A) WB and densitometric analysis of SPTLC1 and SPTLC2 in ECKO-Sptlc2 and Sptlc2f/f EC after 4-OHT (1μM, 72h) treatment. β-actin, loading control. (B) SPT activity in ECKO-Sptlc2 and Sptlc2f/f EC. [3H]-serine and palmitoyl-CoA were used as substrates for SPT. Sphinganine, the reaction product, was separated in TLC and quantified. (C, D) LC-MS/MS quantification of total and specific ceramides in Sptlc2f/f and ECKO-Sptlc2 after 4-OHT treatment. (A-D) N≥3 independent EC isolations/group; 4 mice/EC isolation. (E, F) LC-MS/MS analysis of ceramides, Sph and S1P in Sptlc2f/f and ECKO-Sptlc2 plasma (n≥ 9/group). dhSph-1P=Dihydro-sphingosine-1-phosphate, dhSph=dihydro-sphingosine, Sph=sphingosine. Data are expressed as mean ± SEM. * p<0.05; ** p<0.01; *** p<0.001 compared to Sptlc2f/f. Statistical significance was determined by Unpaired t-test.
Endothelial sphingolipid de novo biosynthesis is necessary to preserve vascular tone and BP homeostasis.
To study the impact of endothelial SL changes, a systematic analysis of vascular reactivity in response to NO-agonists as well as flow was performed.
Vasodilations induced by acetylcholine (Ach) and histamine, both mediated by Gαq/11-coupled receptors31, were preserved in ECKO-Sptlc2 MA vs. controls (Fig. 2A and Fig. S2A). Interestingly, although basal eNOS phosphorylation (Fig. S4), eNOS-derived NO, plasma nitrites levels, and VASP phosphorylation (Fig. 2A-C) were significantly increased in absence of SPTLC2, there were no changes in stimulated NO production (Fig. 2A), SNP-mediated relaxation (Fig. S2C), and in iNOS and nNOS levels (Fig. S2D).
Figure 2. Endothelial-derived SL control vascular tone by preserving signaling transduction to agonists and flow.
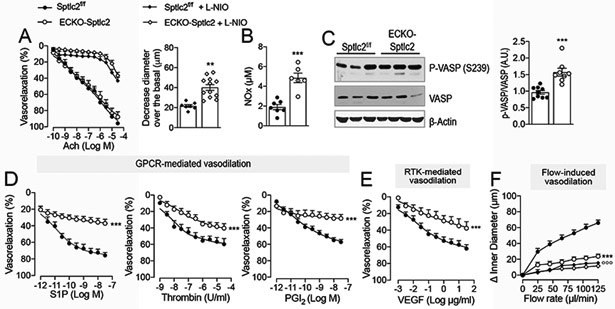
(A) Ach-mediated vasodilation of MA in absence (n≥12 mice/group, n≥22 MA/group) and presence of L-NIO (100 μM, 20 min; n≥5 mice/group n≥8 MA/group), and L-NIO-induced vasoconstriction in Sptlc2f/f and ECKO-Sptlc2 MA at baseline (n≥5 mice/group n≥8 MA/group). (B) Plasma nitrite levels (n≥6/group). (C) WB analysis for P-VASP and VASP on ECKO-Sptlc2 and Sptlc2f/f thoracic aortas (n=9 mice/group) and relative quantification. Vasodilation in response to: (D) Sphingosine-1-phosphate (S1P; n=9 mice/ group, n=9 MA/group); thrombin (n≥3 mice/group, n≥4 MA/group); prostacyclin (PGI2; n≥4 mice/group, n≥6 MA/group); (E) VEGF (n≥5 mice/group, n≥5 MA/group) and (F) flow (n≥12 mice/group, n≥15 MA/group). All data represent mean ± SEM. ** p≤0.01 and *** p≤0.001 ECKO-Sptlc2 vs. Sptlc2f/f. Statistical significance was determined by Unpaired t-test (A, B, C) or Two-way ANOVA (A, D-F).
On the contrary, the vasorelaxation in response to other agonists activating receptors other than Gq-CPR, such as S1P receptor-1 (S1P1), coupled to Gαi proteins32; protease-activated-receptor-1 (PAR1), coupled with Gα12/13, Gαq and Gαi33; and PGI2 receptor (IP)34, preferentially coupled to Gαs35 and VEGFR236, were all significantly reduced in ECKO-Sptlc2 MA compared to control (Fig. 2D,E). Insulin-mediated vasodilation37 was also reduced in Sptlc2-KO MA (Fig. S2B), suggesting that SL are generally required for receptor tyrosine kinase (RTK) signaling. Furthermore, the loss of SPTLC2 markedly suppressed flow-mediated vasodilation (Fig. 2F), whereas vasoconstriction by phenylephrine and U46619 (thromboxane A2 receptor agonist) were preserved (Fig. S2E, F). Overall, the decrease of local SL production heightens basal NO, while impairs endothelial cell signaling in response to physical and chemical stimuli, contributing to vascular tone dysregulation.
At the same time point, we measured BP by using radiotelemetry. Systolic, diastolic and mean BP, but not heart rate, were significantly reduced in ECKO-Sptlc2 compared to Sptcl2f/f (Fig. 3A-D). Furthermore, there were no differences in LF/HF ratios during the light and dark cycles, suggesting a similar sympathetic outflow in ECKO-Sptlc2 compared to Sptlc2f/f mice (Fig. 3E). These findings suggest that the increase in basal NO production might contribute, at least in part, to the lower BP.
Figure 3. Radiotelemetry measurements of BP.
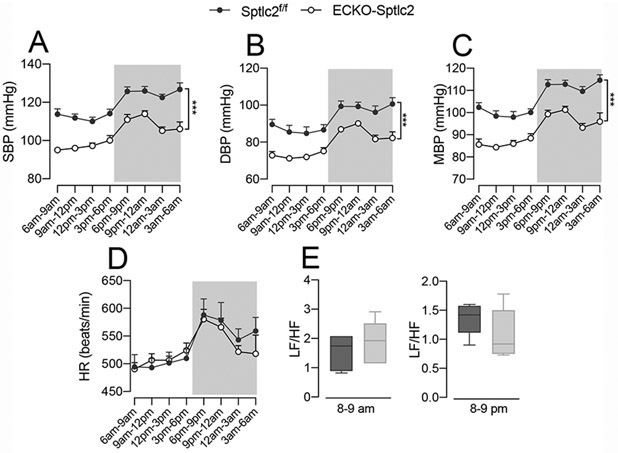
(A) Systolic, (B) diastolic and (C) mean BP, and (D) heart rate (HR) were measured by radiotelemetry for three consecutive days following the recovery from surgery (9 days) in ECKO-Sptlc2 (n=6) and Sptlc2f/f (n=6). (E) Analysis of low to high frequency (LF/HF) ratios in the same groups of mice. All data represent mean ± SEM. *** p≤0.001 ECKO-Sptlc2 vs. Sptlc2f/f mice. Statistical analysis was performed with Two-way ANOVA with Sidak post-test (A-D) and unpaired t-test (E).
Prevailing role of C16:0-Cer over C24:0- and C24:1-Cer in preserving endothelial-mediated vasodilation.
We sought to identify the biological functions of the most abundant endothelial ceramides (Fig. 1E,F), chosen based on the length of the acyl chain (C16:0-Cer vs. C24:0-Cer) and the double bond (C24:1-Cer vs C24:0-Cer). To this end, ECKO-Sptlc2 mice were treated with C16:0-, C24:0- and C24:1-Cer, at two different doses (3 and 10 mg/Kg/d for 2 days).
At low dose C16:0-Cer fully restored and enhanced VEGF-induced vasodilation of ECKO-Sptlc2 vs. control MA (Fig.4A, left panel), but not at higher dose (Fig. 4A, right panel), suggesting a bell-shape rather than linear correlation between C16:0-Cer levels and VEGF-mediated vasodilation.
Figure 4. Prevailing role of C16:0-Cer vs. C24:0- and C24:1-Cer in restoring VEGF-mediated vasodilation.
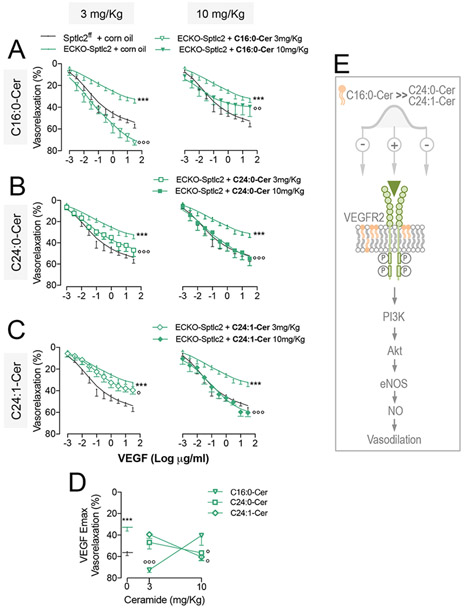
ECKO-Sptlc2 mice were treated with C16:0-Cer, C24:0-Cer, C24:1-Cer at the doses of 3 mg/Kg/d (left panels) or 10 mg/Kg/d (right panels) i.p. for 2 consecutive days. VEGF-induced vasodilation in MA from ECKO-Sptlc2 mice treated with (A) C16:0-Cer (n≥4 mice/group); (B) C24:0-Cer (n≥ 4mice/group); (C) C24:1-Cer (n≥ 4mice/group), or vehicle. (D) Maximum VEGF-induced vasodilation (Emax) of MA from ECKO-Sptlc2 mice treated with two different doses of C16:0-Cer, C24:0-Cer or C24:1-Cer compared to vehicle (indicated as 0 on X-axis). (E) Schematic carton representing the different ceramide-specific effects on VEGF-induced vasodilation. All data represent mean ± SEM. *** p≤0.001 ECKO-Sptlc2 + corn oil vs. Sptlc2f/f + corn oil; ° p≤0.05, °° p≤0.01 and °°° p≤0.001 ECKO-Sptlc2 + ceramide vs. ECKO-Sptlc2 + corn oil. Statistical significance was determined by Two-way ANOVA (A,B,C) or One-way ANOVA (D).
Only at high dose C24:0-Cer and C24:1-Cer were able to restore the vasorelaxation in response to VEGF (Fig. 4B and C). The Emax of VEGF concentration-response curve clearly shows the prevailing effects of C16:0-Cer vs. C24:0- and C24:1-Cer (Fig. 4D) also depicted in the schematic cartoon (Fig. 4E).
C16:0-Cer was also the most efficient in restoring S1P-mediated vasodilation compared to C24:0-Cer and C24:1-Cer (Fig. S3A-E). C16:0-Cer treatments restored also sphingomyelin levels in ECKO-Sptlc2 MA compared to vehicle treated (Fig. S3F). Unfortunately, despite including different branches, ceramide levels of MA were undetectable.
These data reveal specific biological functions of different ceramide species in preserving endothelial cell signaling and vasodilation.
C24:0-Cer and C24:1-Cer, but not C16:0-Cer, repristinate flow-mediated vasodilation in ECKO-Sptlc2 MA.
Low dose of C24:0-Cer and C24:1-Cer fully restored flow-mediated vasodilation in ECKO-Sptlc2 MA compared to controls (Fig. 5A, C, left panels), while at higher dose also C16:0-cer re-established vasorelaxation to flow (Fig. 5A-C, right panels). The impact of specific ceramides on flow-induced vasodilation is schematized in Fig. 5E. Membrane biophysical properties are important for a proper mechanotransduction of changes in shear stress38. Most likely ceramides with short acyl chain and/or double bond ceramide might preserve mechanotransduction properties of the endothelium by increasing membrane fluidity.
Figure 5. C24:0- and C24:1-Cer prevail over C16:0-Cer in restoring flow-mediated vasodilation in ECKO-Sptlc2 MA.
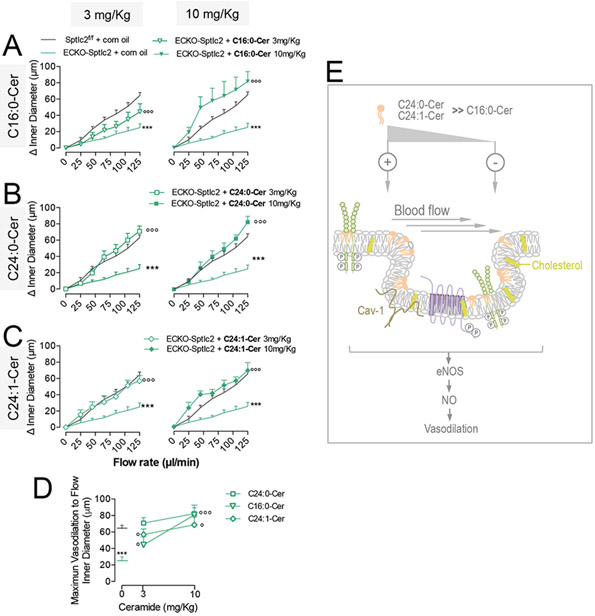
ECKO-Sptlc2 mice treated with C16:0-Cer, C24:0-Cer, C24:1-Cer at the doses of 3 mg/Kg/d (left panels) or 10 mg/Kg/d (right panels) i.p. for 2 consecutive days. Flow-induced vasodilation in MA from ECKO-Sptlc2 mice treated with (A) C16:0-Cer (n≥ 4mice/group); (B) C24:0-Cer (n≥4 mice/group); (C) C24:1-Cer (n≥4 mice/group). (D) Flow-induced maximum vasodilation (Emax) of MA from ECKO-Sptlc2 mice treated with two different doses of C16:0-Cer, C24:0-Cer or C24:1-Cer compared to corn oil as vehicle. (E) Scheme representing specific effects of different ceramides on flow-induced vasodilation. Data are expressed as mean ± SEM. *** p≤0.001 ECKO-Sptlc2 + corn oil vs. Sptlc2f/f + corn oil; ° p≤0.05 and °°° p≤0.001 ECKO-Sptlc2 + ceramide vs. ECKO-Sptlc2 + corn oil. Statistical significance was determined by Two-way ANOVA (A, B, C) or One-way ANOVA (D).
Exogenous C16:0-Cer restores endothelial VEGFR2 signaling in EC lacking SPTLC2.
Mechanistic studies in vitro demonstrated that both genetic and pharmacological disruption of SPT activity impairs VEGFR2 signaling and downstream Akt-eNOS activation in murine ECKO-Stplc2 EC (Fig. S4) and HUVEC (Fig. 6A-D). It is noteworthy to mention that concentrations and durations of myriocin treatments have been carefully titrated in HUVEC (Fig. S5).
Figure 6. C16:0-cer restores VEGFR2-mediated signaling in EC treated with myriocin.
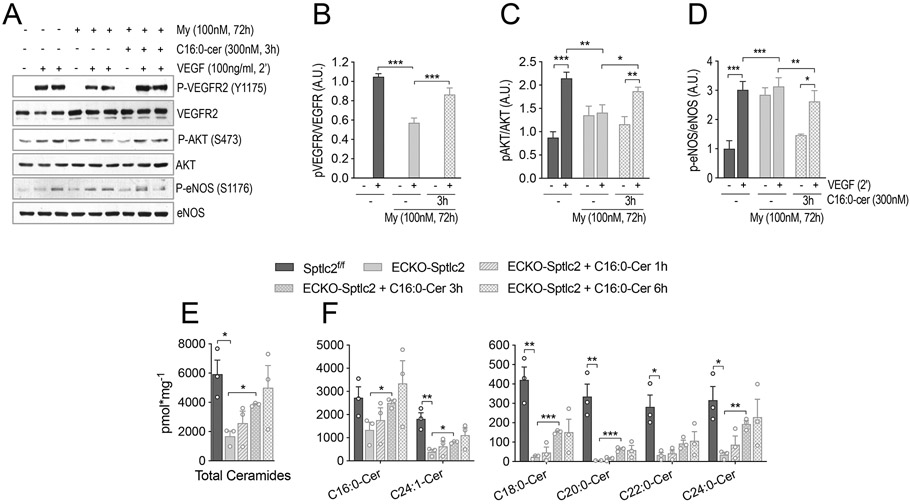
(A) WB analysis was performed on HUVEC treated with myriocin, inhibitor of SPT, or vehicle, followed by VEGF stimulation (100 ng/ml, 2 min). Some HUVEC treated with myriocin, were incubated with C16:0-Cer to restore VEGF signaling. Membranes were incubated with antibodies against P-VEGFR2 (Y1175), VEGFR2, P-AKT (S473), AKT, P-eNOS (S1176) and eNOS. (B-C) Densitometric analysis of indicated phospho/total protein ratios (n=4 independent experiments). (E,F) Sphingolipid measurements by LC-MS/MS in Sptlc2f/f and ECKO-Sptlc2 EC, treated with 300nM C16-Cer for the indicated times. (E) Total and (F) individual ceramides (3 independent EC isolations/group; 3 mice/EC isolation). Data are expressed as mean ± SEM. * p≤0.05; ** p≤0.01; *** p≤0.001. Statistical significance was determined by One-way ANOVA.
Interestingly, C16:0-Cer was able to rescue VEGFR2 signaling and Akt-eNOS phosphorylation, abrogated by myriocin treatment (Fig. 6A-D). In support of molecular signaling, exogenous C16:0-Cer was able to restore endothelial ceramide levels after 3h (Fig. 6E,F), but not hexosylceramides or sphingomyelins (Suppl. Fig. 6).
These data demonstrate that the de novo biosynthesis is necessary to maintain endothelial sphingolipid homeostasis, and reveal a prevailing role of C16:0-Cer in preserving endothelial cell signaling.
DISCUSSION
Altered sphingolipid levels are linked to hypertension, coronary artery disease, and cardiovascular complication in obesity and type 2 diabetes6, 39. Multiple clinical studies showed that specific plasma ceramides ratios, including C24:0/C16:0 and C22:0/C16:0 ceramides, independently correlate with major adverse cardiovascular events in patients with and without coronary artery diseases 5, 20, 40-43. Recently, the Mayo clinic included plasma ceramide measurements as routine to predict at risk-patients with coronary artery diseases44. However, our knowledge of the role of ceramides in the pathophysiology of blood vessels remains limited.
Recently, we discovered a novel regulatory mechanism of sphingolipid de novo biosynthesis in blood vessels. Nogo-B, a membrane protein of the endoplasmic reticulum, binds to and downregulates the activity of SPT26. In absence of systemic or endothelial Nogo, mice are protected from hypertension26, inflammation 28, and pathological cardiac hypertrophy27, mainly mediated by an increased endothelial-derived S1P and S1P1 autocrine signaling. These studies suggested that Nogo-B-dependent downregulation of SPT in the vasculature has pathological implications and raised intriguing questions. First, considering that the endothelium is “bathed” by sphingolipid-rich plasma, is the de novo production of sphingolipids necessary to maintain endothelial sphingolipid homeostasis? Does endothelial SPT downregulation promote vascular dysfunction? Are changes in plasma ceramide indicative of changes in vascular SL metabolism and functions? Which SL impact endothelial functions the most? Considering the growing evidence implicating SL, particularly ceramides, in CV diseases, these are fundamental questions to address.
Our data clearly demonstrated that endothelial de novo biosynthesis is an important source of both plasma S1P and ceramide, but not sphingomyelins. While the endothelium45, in addition to red blood cells46, has been recognized to export S1P into the plasma with the aid of specific transporters47, 48, much less is known on the sources and mechanisms of export of other circulating sphingolipids, including ceramides. Like most cells, EC can release heterogeneous microparticles from plasma membranes into the extracellular space in response to cell activation, injury and/or apoptosis. Microparticles derived from activated endothelium present higher ceramide content49. Accordingly, we reported an upregulation of SPT activity and accumulation of ceramide into the media of TNF-α-activated EC27. Altogether, these data put forward the consideration that remodeling of plasma ceramide profile in patients, preceding major cardiovascular events5, might reflect not only changes in plasma lipoprotein composition, but also changes in endothelial-derived ceramides and vascular disease state.
S1P is a potent activator of eNOS50, mainly via S1P151. Previously, we demonstrated a crucial role for endothelial-derived S1P and autocrine S1P-S1P1-eNOS signaling in blood flow and pressure regulation51. In ECKO-Sptlc2 mice endothelial-derived S1P was markedly diminished (∼40%; Fig. 1F), while flow-mediated vasodilation was blunted (Fig. 2F), supporting a critical, but not exclusive, role for endothelial autocrine S1P-eNOS signaling in flow-regulated vascular tone. Indeed, changes in membrane biophysical properties might also be implicated in the suppression of flow-mediated vasodilation in ECKO-Sptlc2 MA.
The increase of SPT activity in EC lacking Nogo-B leads to an upregulation of S1P-S1P1-eNOS autocrine signaling and lowers BP. The downregulation of SPT activity in EC lacking Sptlc2 while triggers a general impairment of endothelial-mediated vasodilation also lowers BP. Apparently contradictory, these findings can be reconciled by considering that the genetic deletion of Nogo-B, a modulator of SPT, has a different impact on sphingolipid profile than the deletion of SPT. In the latter model, low levels of ceramides can trigger an increased activation of eNOS at baseline (Fig 2, Fig. S4), via decrease of PP2A activity, which contributes to lower systemic BP and compensates for the loss of endothelial S1P signaling. While the analysis of LF/HF ratios exclude an impact of endothelial-derived sphingolipids on sympathetic system, clearly, as BP is the result of the interplay between blood vessels, heart, kidneys and brain, other players other than blood vessels can contribute to the reset blood pressure to lower than normal value, although the loss of SPT is specific to EC.
Our data suggest an important role of ceramides as “keeper” of EC signaling, most likely by dictating optimal biophysical properties of the membranes and/or partitioning of the receptors in specialized membrane microdomains13, 52.
Specifically, while C16:0-cer exerts a prevailing role in restoring GPCR-, RTK-mediated vasodilation, C24:0- and C24:1-Cer preserve flow-mediate vascular tone regulation, suggesting that acyl chain length confers specific biological roles to ceramides in governing endothelial cell functions.
Most importantly, our data clearly demonstrate that the activation of Akt-eNOS-cGMP axis was preserved in ECKO-Sptlc2 MA (Fig. 2A-C , Fig. S2C) suggesting that the decrease in endothelial sphingolipids, although heightened Akt/eNOS basal phosphorylation, did not compromise the signal transduction of Akt-eNOS pathway.
A very interesting study of Oh P. & Schnitzer J. reported that Gq proteins preferentially segregate to caveolae, probably via interaction with caveolin-1, whereas Gs and Gi proteins locate to non-caveolar lipid rafts54. Our findings demonstrate that endogenous sphingolipids regulate GPCR-mediated signaling, except for Gq-coupled receptors, suggesting a different impact or segregation of sphingolipids in distinct specialized membrane domains. However, we do not know whether changes in endogenous sphingolipids differentially affect the lipid composition of membrane microdomains, and/or the partition of receptors and therefore their sensitivity towards ligand activation. Additional studies are needed to address these questions.
The finding that sphingolipid levels can impact specific endothelial signaling, and therefore blood flow and pressure, might have multiple clinical implications. First, sphingolipid accrual in obesity-driven type 2 diabetes has been implicated in vascular dysfunction via ceramide-activated PP2A, which in turn downregulates Akt/eNOS signaling21, 22. Although ceramide accrual has been demonstrated in aorta of hypertensive rats6, sphingolipid levels in EC during hypertension have yet to be measured. Our findings suggest that altered endothelial ceramides contribute to vascular dysfunction by impairing receptor-mediated signaling, other than disruption of the Akt/eNOS axis. Second, about 50% of prescribed drugs target GPCR. Different studies reported the important role of sphingolipid in modulating the function of different membrane proteins, including GPCR55, 56. Thus, our findings have additional clinical implications as imply that derangement in sphingolipid metabolism and levels might impact GPCR-mediated signaling, and the effects elicited by drugs targeting GPCR56.
Downregulation of SL de novo production impairs VEGFR2-mediated signaling and vasodilation following VEGF stimulation. Our data also complement the findings of Mehra V. et al. showing that C2-Cer treatment reduced basal and VEGF-activated Akt-eNOS phosphorylation and NO production, although in this study VEGFR2 phosphorylation was not assessed57.
Our data on VEGF-induced VEGFR2 phosphorylation demonstrate that ceramides preserve the initiation58 of signal transduction (Fig. 6, Fig. S4, S5).
In conclusions, our study reveal important pathophysiological implications of vascular-derived sphingolipids: 1) the de novo biosynthesis is a necessary to maintain vascular sphingolipid homeostasis and 2) regulate vascular tone and BP, mainly by preserving endothelial cell signaling and resting NO production; 3) in addition to S1P, the endothelium is a major determinant of plasma ceramide profile, clinical predictor of major cardiovascular events, 4) circulating ceramides may also be a “raconteur” of the health/diseased state of the endothelium.
Either accrual or depletion of SL has pathological implications, suggesting that SL needs to be tightly maintained within a narrow physiological window to preserve the “health state”. Our study not only identifies biological functions of specific ceramide species in endothelial cell signaling, but also paves the way to a better understanding as to how sphingolipid derangement could contribute to endothelial dysfunction seen in metabolic syndrome and other cardiovascular diseases.
PERSPECTIVES
In recent years the importance of ceramides in cardiovascular and metabolic diseases has gained much attention. Multiple clinical studies established plasma ceramide as independent predictor of cardiovascular events in at risk-patients with coronary artery diseases. While, both accrual and depletion of SL has pathological implications, suggesting the need of a tight regulation to preserve the “health state”, the impact of sphingolipids, particularly ceramides, on vascular functions and BP remains poorly understood.
Our study revealed a critical role of sphingolipid de novo biosynthesis in preserving endothelial cell functions and BP. Specifically, endothelial-derived ceramides emerge as critical regulator of receptor-mediated signaling, mechanotransduction and eNOS to preserve vascular and BP homeostasis.
Clinical implications of our findings extend to pathologies where ceramide is altered, including cardiovascular and metabolic diseases. Our study not only identifies biological functions of specific ceramide species in endothelial cell signaling, but also paves the way to a better understanding as to how sphingolipid derangement, particularly ceramide, could contribute to endothelial dysfunction seen in metabolic syndrome and other cardiovascular diseases, and could impact the therapeutic approach to treat those conditions.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Impaired endothelial de novo sphingolipid biosynthesis is sufficient to trigger vascular dysfunction and lower blood pressure.
Plasma ceramide profile, predictor of major cardiovascular events, is dictated by the endothelium and might be a “raconteur” of the endothelial health/disease state
Among SL, ceramides are necessary to preserves specific receptor-mediated signals and mechanotransduction pathways
What is relevant?
Altered sphingolipid homeostasis is causative of vascular dysfunction. Targeting sphingolipid metabolism to restore homeostasis may provide a therapeutic approach for hypertension and other related cardiovascular conditions underlined by sphingolipid derangements
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant R01 HL126913 to A. Di Lorenzo, National Institute of Neurological Disorders and Stroke of National Institutes of Health grant R21 NS104512 to A. Di Lorenzo; by the Ph.D. Program “Molecular Medicine” of the University of Graz (Austria) and Austrian Marshall Plan Scholarship to I. Del Gaudio.
Non-standard abbreviations and acronyms
- Ach
Acetylcholine
- BP
Blood Pressure
- Cer
Ceramide
- GPCR
G-protein Coupled Receptors
- EC
Endothelial Cell
- MA
Mesenteric Artery
- My
Myriocin
- NO
Nitric Oxide
- PE
Phenylephrine
- RTK
Tyrosine Kinase Receptor
- SL
Sphingolipid
- SM
Sphingomyelin
- SNP
Sodium Nitroprussiate
- SPT
Serine Palmitoyltransferase
- VSMC
Vascular Smooth Muscle Cell
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844 [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudzinski DM, Michel T. Life history of enos: Partners and pathways. Cardiovasc Res. 2007;75:247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dorr M, Vasan RS, Schaffer JE. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, van den Born BJ, Wijesinghe DS, Chalfant CE, MacAleese L, Eijkel GB, Heeren RM, Alewijnse AE, Peters SL. Hypertension is associated with marked alterations in sphingolipid biology: A potential role for ceramide. PLoS One. 2011;6:e21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornemann T, Worgall TS. Sphingolipids and atherosclerosis. Atherosclerosis. 2013;226:16–28 [DOI] [PubMed] [Google Scholar]
- 8.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–550 [DOI] [PubMed] [Google Scholar]
- 9.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. The Journal of biological chemistry. 2002;277:25847–25850 [DOI] [PubMed] [Google Scholar]
- 10.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: Relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728 [DOI] [PubMed] [Google Scholar]
- 11.Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. The Journal of biological chemistry. 2000;275:9078–9084 [DOI] [PubMed] [Google Scholar]
- 12.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: Physical foundations and biological effects. J Cell Physiol. 2000;184:285–300 [DOI] [PubMed] [Google Scholar]
- 14.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39 [DOI] [PubMed] [Google Scholar]
- 15.Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim Biophys Acta. 2011;1808:2753–2760 [DOI] [PubMed] [Google Scholar]
- 16.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2a. The Journal of biological chemistry. 1993;268:15523–15530 [PubMed] [Google Scholar]
- 17.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2a. Activation is stereospecific and regulated by phosphatidic acid. The Journal of biological chemistry. 1999;274:20313–20317 [DOI] [PubMed] [Google Scholar]
- 18.Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J. Protein kinase c zeta isoform is critical for kappa b-dependent promoter activation by sphingomyelinase. The Journal of biological chemistry. 1994;269:19200–19202 [PubMed] [Google Scholar]
- 19.Heinrich M, Wickel M, Winoto-Morbach S, Schneider-Brachert W, Weber T, Brunner J, Saftig P, Peters C, Kronke M, Schutze S. Ceramide as an activator lipid of cathepsin d. Adv Exp Med Biol. 2000;477:305–315 [DOI] [PubMed] [Google Scholar]
- 20.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, Marz W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Juni P, Rodondi N, Raber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygard O, Mach F, Sinisalo J, Luscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond ldl-cholesterol. Eur Heart J. 2016;37:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD. Ceramide mediates vascular dysfunction in diet-induced obesity by pp2a-mediated dephosphorylation of the enos-akt complex. Diabetes. 2012;61:1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD. Ceramide-initiated protein phosphatase 2a activation contributes to arterial dysfunction in vivo. Diabetes. 2015;64:3914–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buede R, Rinker-Schaffer C, Pinto WJ, Lester RL, Dickson RC. Cloning and characterization of lcb1, a saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. Journal of bacteriology. 1991;173:4325–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagiec MM, Baltisberger JA, Wells GB, Lester RL, Dickson RC. The lcb2 gene of saccharomyces and the related lcb1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc Natl Acad Sci U S A. 1994;91:7899–7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-coa transferase (spt) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51 [DOI] [PubMed] [Google Scholar]
- 26.Cantalupo A, Zhang Y, Kothiya M, Galvani S, Obinata H, Bucci M, Giordano FJ, Jiang XC, Hla T, Di Lorenzo A. Nogo-b regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat Med. 2015;21:1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Huang Y, Cantalupo A, Azevedo PS, Siragusa M, Bielawski J, Giordano FJ, Di Lorenzo A. Endothelial nogo-b regulates sphingolipid biosynthesis to promote pathological cardiac hypertrophy during chronic pressure overload. JCI Insight. 2016;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Lorenzo A, Manes TD, Davalos A, Wright PL, Sessa WC. Endothelial reticulon-4b (nogo-b) regulates icam-1-mediated leukocyte transmigration and acute inflammation. Blood. 2011;117:2284–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-b2 controls vegf-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486 [DOI] [PubMed] [Google Scholar]
- 30.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics. 2001;5:89–97 [DOI] [PubMed] [Google Scholar]
- 31.Hill SJ. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990;42:45–83 [PubMed] [Google Scholar]
- 32.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors edg-1, edg-3, and h218/edg-5 to the g(i), g(q), and g(12) families of heterotrimeric g proteins. The Journal of biological chemistry. 1999;274:27351–27358 [DOI] [PubMed] [Google Scholar]
- 33.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimokawa H, Flavahan NA, Lorenz RR, Vanhoutte PM. Prostacyclin releases endothelium-derived relaxing factor and potentiates its action in coronary arteries of the pig. Br J Pharmacol. 1988;95:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Wu J, Ruan KH. Solution structure of the first intracellular loop of prostacyclin receptor and implication of its interaction with the c-terminal segment of g alpha s protein. Biochemistry. 2006;45:1734–1744 [DOI] [PubMed] [Google Scholar]
- 36.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces edrf-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–592 [DOI] [PubMed] [Google Scholar]
- 37.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto K, Ando J. Endothelial cell and model membranes respond to shear stress by rapidly decreasing the order of their lipid phases. J Cell Sci. 2013;126:1227–1234 [DOI] [PubMed] [Google Scholar]
- 39.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594 [DOI] [PubMed] [Google Scholar]
- 40.de Carvalho LP, Tan SH, Ow GS, Tang Z, Ching J, Kovalik JP, Poh SC, Chin CT, Richards AM, Martinez EC, Troughton RW, Fong AY, Yan BP, Seneviratna A, Sorokin V, Summers SA, Kuznetsov VA, Chan MY. Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Transl Sci. 2018;3:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides. Arterioscler Thromb Vasc Biol. 2018;38:1933–1939 [DOI] [PubMed] [Google Scholar]
- 42.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, Gouni-Berthold I, Berthold HK, Kleber ME, Laaksonen R, Marz W. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and pcsk9 deficiency. J Clin Endocrinol Metab. 2014;99:E45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, Oemrawsingh R, Serruys P, van Geuns RJ, Boersma E, Laaksonen R, Kardys I. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res. 2018;59:1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westra B Ceramides, plasma [a test in focus]. 2016
- 45.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298 [DOI] [PubMed] [Google Scholar]
- 47.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527 [DOI] [PubMed] [Google Scholar]
- 48.Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM, Torta F, Cazenave-Gassiot A, Matsumura T, Kim S, Toh SES, Suda T, Silver DL, Wenk MR, Nguyen LN. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature. 2017;550:524–528 [DOI] [PubMed] [Google Scholar]
- 49.Serban KA, Rezania S, Petrusca DN, Poirier C, Cao D, Justice MJ, Patel M, Tsvetkova I, Kamocki K, Mikosz A, Schweitzer KS, Jacobson S, Cardoso A, Carlesso N, Hubbard WC, Kechris K, Dragnea B, Berdyshev EV, McClintock J, Petrache I. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci Rep. 2016;6:31596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of akt and map kinase pathways by edg and bradykinin receptors in vascular endothelial cells. The Journal of biological chemistry. 2001;276:12420–12426 [DOI] [PubMed] [Google Scholar]
- 51.Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T, Di Lorenzo A. S1pr1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension. 2017;70:426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goni FM, Alonso A. Biophysics of sphingolipids i. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–1921 [DOI] [PubMed] [Google Scholar]
- 53.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411 [DOI] [PubMed] [Google Scholar]
- 54.Oh P, Schnitzer JE. Segregation of heterotrimeric g proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas g(i) and g(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjorkholm P, Ernst AM, Hacke M, Wieland F, Brugger B, von Heijne G. Identification of novel sphingolipid-binding motifs in mammalian membrane proteins. Biochim Biophys Acta. 2014;1838:2066–2070 [DOI] [PubMed] [Google Scholar]
- 56.Jafurulla M, Chattopadhyay A. Sphingolipids in the function of g protein-coupled receptors. European journal of pharmacology. 2015;763:241–246 [DOI] [PubMed] [Google Scholar]
- 57.Mehra VC, Jackson E, Zhang XM, Jiang XC, Dobrucki LW, Yu J, Bernatchez P, Sinusas AJ, Shulman GI, Sessa WC, Yarovinsky TO, Bender JR. Ceramide-activated phosphatase mediates fatty acid-induced endothelial vegf resistance and impaired angiogenesis. Am J Pathol. 2014;184:1562–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


