Abstract
Schemas capture patterns across multiple experiences, accumulating information about common event structures that guide decision making in new contexts. Schemas are an important principle of leading theories of cognitive development; yet, we know little about how children and adolescents form schemas and use schematic knowledge to guide decisions. Here, we show that the ability to acquire schematic knowledge based on the temporal regularities of events increases during childhood and adolescence. Furthermore, we show that temporally mediated schematic knowledge biases reasoning decisions in an age-dependent manner. Participants with greater temporal schematic knowledge were more likely to infer that temporally related items shared other, non-temporal properties, with adults showing the greatest relationship between schema knowledge and reasoning choices. These data indicate that the mechanisms underlying schema formation and expression are not fully developed until adulthood and may reflect the ongoing maturation of hippocampus and prefrontal cortex through adolescence.
Keywords: statistical learning, temporal memory, inductive inference, cognitive maps, flexibility, generalization
Introduction
Events in our everyday lives, while never exactly the same, often share regularities. For instance, when going to a restaurant, we expect that once seated and given menus, the waitstaff will ask for our drink order first, followed by our food choice. Despite differences between individual visits to restaurants (e.g., the position of your table, the identity of your server, the drink and meal that you order), the general sequence through which events unfold is common across many trips to restaurants. Over time, these commonalities can give rise to knowledge that allows us to make predictions about what will happen when in new situations. Such knowledge about the shared features of events is a key facet of schemas (Bartlett, 1932; Piaget, 1954).
Despite ongoing debates about the precise operational definition of schemas, current perspectives generally agree that schemas represent associative information about common relationships and temporal regularities that are formed across multiple experiences (Ghosh & Gilboa, 2014; Preston, Molitor, Pudhiyidath & Schlichting, 2017; van Kesteren, Ruiter, Fernandez & Henson, 2012). Current theories further emphasize that schemas are hierarchically organized, reflecting not only commonalities across experiences, but also goal-relevant differences that predict when expectations change in different temporal or spatial contexts (Gershman, Monfils, Norman & Niv, 2017; McKenzie et al., 2014; Varga, Morton & Preston, under review). Initial schema formation thus requires extracting associative commonalities and differences that link items and contexts to specific behaviors and outcomes. Once formed, the associative relationships coded within schemas may then bias decisions about the properties of event elements according to the features they share with elements of other similar experiences. For instance, if someone asks you about the quality of your meal at the end of your restaurant visit, you might infer that the person inquiring is the manager of the restaurant.
The formation and expression of schemas are central principles of classic cognitive development theories (Kolb, 1984; Lewin, 1942; Piaget, 1954), and an important body of research demonstrates some early capacities for schema formation. From infancy, children build associative memories that incorporate both individual event elements and the order in which they are experienced (Bauer, 2007; Mandler, 1984; Nelson, 1986; Wiebe & Bauer, 2005). Young children are able to form such associative memories after a single experience (Hudson, Fivish & Kuebli, 1992) as well as with repeated exposure to an event (Bauer & Mandler 1989; Fivush & Slackman, 1996; Nelson, 1986). However, while children form knowledge about the associative properties of individual events, including their temporal order, the complexity of knowledge formation increases into adulthood.
Memory for both spatial (Lee, Wendelken, Bunge & Ghetti, 2016; Sluzenski, Newcombe & Kovacs, 2006; Lloyd, Doydum & Newcombe, 2009) and temporal associations (Lee et al., 2016; Pathman & Ghetti, 2014; Picard, Cousin, Guillery-Girard, Eustache & Piolino, 2012) improves from early childhood into adulthood. For instance, while children 11 years of age show adult like memory for individual event sequences (Lee et al., 2016), the capacity to acquire knowledge about temporal regularities across multiple events does not reach full proficiency until adulthood (Schlichting, Guarino, Schapiro, Turk-Browne & Preston, 2017). Parallel developmental studies of event narratives further show that young children are able to generate event scripts based on their past experiences, similar to adults (Nelson, Fivush, Hudson & Lucariello, 1983). However, the complexity of such generated scripts differs between children and adults, with fewer elaborative details in children’s scripts (Hudson, Fivish & Kuebli, 1992) even when controlling for differences in individual’s prior experience (Farrar & Goodman, 1990; Price & Goodman, 1990). Thus, while young children appear to form associative memories about individual events and some of their general properties, their knowledge does not encapsulate fully-fledged event schemas.
Here, we sought to expand our knowledge of schema development by testing schema formation and expression in participants aged 7 to 30 years. Our hypothesis testing framework is based on research from both the cognitive development and cognitive neuroscience domains. One prominent view (Brainerd, Holliday & Reyna, 2004; DeMaster, Coughlin & Ghetti, 2016; Lukowski, Wiebe & Bauer., 2009; Murty, Calabro & Luna., 2016; Sloutsky & Fisher, 2004) suggests that the organization of memory representations transforms across development from a rigid system that processes and stores memories individually into a more flexible system in adulthood wherein knowledge schemas represent the commonalities and differences among multiple episodes to support predictive decision making (Bartlett, 1932; Cohen & Eichenbaum, 1993; Schlichting & Preston, 2015; Tolman, 1948). Therefore, in the present paper, we test the hypothesis that the ability to form schematic knowledge of temporal associations among event elements will continue to improve into adulthood. Furthermore, we test the prediction that even when children and adolescents acquire temporal knowledge schemas, they will be less likely to flexibly express that knowledge to guide novel decision making.
In adults, schema formation and its expression have been linked to the function of the hippocampus, prefrontal cortex, and their interactions (Ghosh & Gilboa, 2014; Mack, Love & Preston, 2018; Preston & Eichenbaum, 2013; Schapiro, Rogers, Cordova, Turk-Browne, & Botvinick, 2013; Schlichting & Preston, 2015; van Kesteren et al., 2012). One central aspect of schemas is the representation of commonalities across events, which is thought to rely on hippocampal binding mechanisms (Morton, Sherrill & Preston, 2017; Schlichting & Preston, 2015). Within the domain of time, hippocampus plays a pivotal role in binding event elements according to their temporal relationships (Allen, Salz, McKenzie & Fortin, 2016; Fortin, Agster & Eichenbaum, 2002; Schapiro, Gregory, Landau, McCloskey & Turk-Browne, 2014). Neuroimaging studies in adults have shown that hippocampal representations are more similar for events that occur close together in time relative to those events that do not share temporal relationships (Ezzyat & Davachi, 2014; Hsieh, Gruber, Jenkins & Ranganath, 2014; Schapiro, Kustner & Turk-Browne, 2012). By representing the statistical co-occurrence of events across time, hippocampal representations come to reflect highly-structured temporal schemas (Schapiro, Turk-Browne, Norman & Botvinick, 2016) that support predictions about which events will follow one another (Gravina & Sederberg, 2017; Hindy, Ng & Turk-Browne., 2016; Kim, Lewis-Peacock, Norman & Turk-Browne, 2014; Stachenfeld, Botvinick & Gershman, 2017).
Prefrontal cortex has also been implicated in the representation of temporal event schemas (Schapiro et al., 2013). Patterns of activity in prefrontal cortex differentiate the order of events both at short (Jenkins & Ranganath, 2010) and long time scales (Baldassano et al., 2017; Zeithamova & Preston, 2017). Furthermore, when adults are presented with narratives of common events (e.g., eating at a restaurant, traveling through an airport) that differ in the modality of presentation as well as in their individual features (e.g., characters, specific locations), medial prefrontal cortex demonstrates schematic event patterns that generalize across narratives of the similar events, representing their common temporal structure (Baldassano, Hasson & Norman, 2018). Moreover, prefrontal cortex may be important for both representing temporal similarities, and establishing temporal boundaries in continuous experience that indicate where temporal similarities end and differences begin (Ezzyat & Davachi, 2011; Zacks, In press). Prefrontal cortex activity is sensitive to moments in time when the transition probabilities between event elements are more variable, predicting a transition to multiple possible contexts rather than a single, certain outcome (Schapiro et al., 2013). Prefrontal responses to such increased temporal uncertainty may promote the formation of temporal boundaries, perhaps by controlling the active differentiation of event elements before and after a boundary within hippocampus (Schapiro et al., 2016). Together, representation of temporal similarities and differences within hippocampus and prefrontal cortex may increase prediction accuracy when temporal schemas are expressed in new contexts.
While children show early capacities for representing temporal associations (Bauer, 2007) and regularities (Saffran, Aslin & Newport, 1996) at very young ages, the binding and differentiation mechanisms critical for temporal schema formation may not be fully developed until adulthood due to the protracted maturation of the hippocampus (Daugherty, Bender, Raz & Ofen, 2016; DeMaster, Pathman, Lee & Ghetti, 2014; Gogtay et al., 2006; Ostby et al., 2009) and prefrontal cortex (Giedd et al., 1999; Gogtay et al., 2004; Paus et al., 1999; Reiss, Abrams, Singer, Ross & Denckla, 1996). Hippocampal binding processes continue to develop into early adolescence (Geng, Redcay & Riggins, 2019; Ghetti, DeMaster, Yonelina & Bunge, 2010). Such immature hippocampal binding may limit children’s ability to extract information about temporal relationships across time, as suggested by one recent developmental study (Schlichting et al., 2017). Learning-related interactions between hippocampus and prefrontal cortex also increase into adulthood and are associated with developmental gains in memory (Menon, Boyett-Anderon & Reiss, 2005; Riggins, Geng, Blankenship & Redcay, 2016; Tang, Shafer & Ofen, 2018). In particular, prefrontal-mediated uncertainty responses that monitor the quality of memory evidence do not emerge until adolescence (Fandakova et al., 2018), which may limit formation of temporal boundaries that differentiate the statistical relationships among events. Moreover, hippocampal mechanisms that support differentiation of memory representations are thought to show a protracted developmental pattern (Keresztes et al., 2017; Ngo, Lin, Newcombe & Olson, 2019). This evidence thus suggests that while there is some capacity to learn temporal regularities at young ages, the mechanisms that support temporal schema formation are refined into adulthood as hippocampal and prefrontal structure and function mature.
Changes in the structural and functional connectivity between hippocampus and prefrontal cortex into adulthood (Simmonds, Hallquist, Asato & Luna, 2014) may further impact how schemas are later expressed to guide decision making. A central proposed function of schemas is to facilitate the extraction of common features across events to support reasoning and generalization in new contexts. In new situations, schemas help generate predictions about the appropriate actions to take based on the features of the new environment (Gershman, et al., 2017; Varga et al., under review). Schemas thus support optimal behavior by removing uncertainty about how to act in new contexts, reducing the need to use the vast amount of information available in a new environment (Varga et al., under review). In adults, the hippocampal—prefrontal circuit is essential for both acquiring schemas and applying the learned knowledge to new stimuli and contexts (Kumaran, Summerfield, Hassabis & Maguire, 2009; Peters, Fellows, & Sheldon, 2017; Spalding, Jones, Duff, Tranel & Warren, 2015; Tang et al., 2018; Tse et al., 2011; van Kesteren et al., 2012).
While generalization abilities are evident early in life (Quinn, Eimas & Rosenkratz, 1993; Rakison & Poulin-Dubois, 2002; Booth & Waxman, 2002), children are less flexible than adults in how they use prior experience to guide memory-based decisions. Children require a high degree of overlap between an original event and a test context to trigger memory retrieval (DeMaster et al., 2016). Given such rigidity of memory retrieval processes in children, these findings suggest that children will be less likely than adults to generalize knowledge across domains of experience, particularly when surface features of the tasks differ (Badger & Shapiro, 2012; Sloutsky, Deng, Fisher & Kloos, 2015; Sloutsky, Kloos & Fisher, 2007). Developmental change in the flexible expression of knowledge across tasks contexts and cognitive domains has been theoretically linked to the maturation of the hippocampus and its interactions with prefrontal cortex (Bauer, 1996; Bauer & Dow, 1994; Eichenbaum, 1997; McDonough, Mandler, McKee, & Squire, 1995; Murty et al., 2016; Tulving & Schacter, 1990). Consistent with this view, recent work using reinforcement learning tasks indicates that the ability to deploy learned information to guide new choices begins to emerge in adolescence, along with increased hippocampal—prefrontal engagement (Voss, O'Neil, Kharitonova, Briggs-Gowan & Wakschlag, 2015), and continues to develop into adulthood (Decker, Otto, Daw & Hartley, 2016; Hunt, Burk & Barnet, 2016; Kwak, Payne, Cohen & Huettel 2015). Here, we seek to expand upon this work by not only quantifying developmental differences in temporal schema acquisition, but also testing how age impacts individuals’ ability to use temporal knowledge to guide decision making a non-temporal, reasoning task. Based on previous work in cognitive development and the protracted development of hippocampal—prefrontal interactions, we predict that adults will be more likely to use schematic temporal knowledge to make reasoning decisions than either children or adolescents.
To measure age-related differences in temporal schema formation and expression, we used a modified version of the temporal community structure paradigm (Schapiro et al., 2013) in children and adolescents (7-15 years old) and adults (Fig. 1). Participants viewed a ‘parade’ of cartoon characters presented sequentially. The presentation order of the characters was determined by random walks through an underlying temporal community structure, which defined the statistical transition probabilities among each of the characters (Fig. 1a). To test whether participants’ acquired knowledge of the temporal schema, participants identified which of two sequences of characters appeared together during the parade (Fig. 1c). We further measured schema expression by having participants complete an inductive inference task. Participants learned a fact about one of the characters and had to select one of two characters to which the fact also applied (Fig. 1b). One of the options was a character from the same temporal community, while the other option was a character from a different community. We quantified the degree to which participants were biased to make inferences about the characters based on their temporal relationships to one another, as well as whether such temporal biases related to participants’ knowledge of the overarching temporal schema. Together, these measures allowed us to test our central hypotheses that: (1) temporal schema knowledge would increase with age, and (2) adults would be more likely to use temporal knowledge to guide inference decisions.
Figure 1.
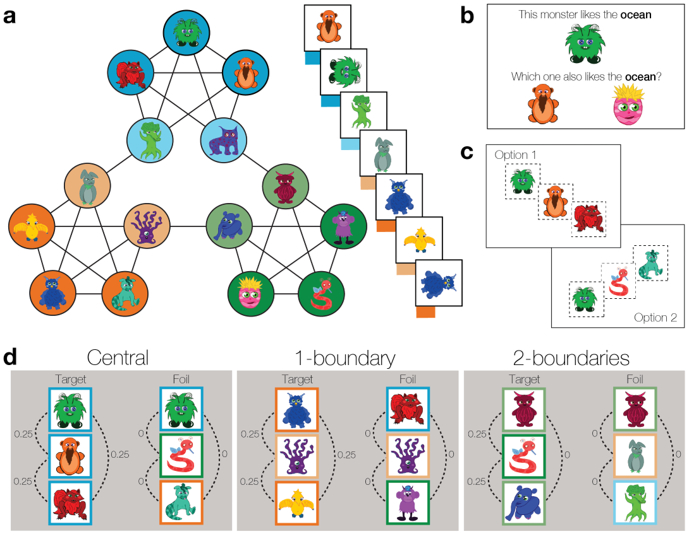
Experiment phases. a) Schematic of the temporal community structure that determined the sequence of characters during the ‘parade.’ While viewing the parade, participants made judgments about whether or not the presented character was ‘marching’ upright or had ‘fallen over.’ Unbeknownst to the participants, each of the fifteen characters was assigned to a node in the community structure (depicted by colored circles), with the lines representing the possible transitions between individual nodes in the structure. Each node is connected to four other nodes in the structure; the probability of a character being preceded or followed by a connected character in the parade sequence is equal among its four connections (0.25). This transition probability structure leads to three temporal communities (blue, green, and orange circles), with three central (dark colored circles) and two boundary (light colored circles) characters per community. Central characters are only connected to members of the same temporal community; boundary characters are connected to the three central members of the same community and one boundary character in a different temporal community. Boundary members of the same temporal community are not connected in the structure, therefore do not appear next to one another in the parade sequence. b) During the inductive inference task, one of the characters from the parade appeared at the top of the screen, and the participants were provided with information about which kind of habitat (desert, forest, or ocean) the character preferred. Participants then chose which of two characters on the bottom of the screen shared the same habitat as the cued character. One of the choices was always a member of the same temporal community and the other a member of a different temporal community. c) During the temporal knowledge task, two three-character sequences were presented on the left and right sides of the screen, and participants were asked to select the three-character sequence that contained ‘best friends’ who marched together in the parade. d) The temporal knowledge task had three trial types: central, 1-boundary, and 2-boundaries. For all trial types, the target sequence contained three characters from the same temporal community, while the foil sequence contained members of the three different temporal communities. Target sequences for central trials comprised three central members of the same community (dark borders). For 1-boundary trials, the target contained two central (dark borders) and one boundary member (light borders) of the same community. For 2-boundaries, the target contained the two boundary members (light borders) and one central member of the same community (dark border). The dotted lines represent the transition probability of any two members of the three-character sequences.
Materials and Methods
Participants.
One hundred and thirty-eight volunteers participated in the experiment across younger child (7-9 years; n = 32, 11 females), older child (10-12 years; n = 34, 19 females), adolescent (13-15 years; n = 32, 16 females), and adult (18-35 years; n = 38, 23 females) age groups. The consent/assent process was carried out using age-appropriate language in accordance with an experimental protocol approved by the Institutional Review Board at the University of Texas at Austin. Consent was obtained from the parent of participants under 18 years. All child participants and fourteen adults received monetary compensation of $10 an hour for their involvement in the study. Twenty-four additional adults participated in the study through the University of Texas Psychology Department participant pool and received two research credit hours as compensation for their involvement.
Participants were screened for psychiatric conditions using the Child Behavior Checklist (CBCL, Achenbach, 1991), which was completed by the parent/guardian of participants aged 6-17 years, or the Symptom Checklist 90-Revised for adult participants (SCL-90-R, Derogatis, 1977). From the original group of 138 participants, individuals were excluded from all subsequent analyses if they met any of the following criteria: children and adolescents with a CBCL score in the clinical range (n = 7); adults with a SCL-90-R score above the normal range (greater than 1 SD above the mean of a normative sample; n = 7); not a native English speaker (n = 4), developmental disorder diagnosis (n = 2); technical issues during data collection (n = 2). These exclusions yielded a group of 116 participants (60 females), whose data were included in the analysis: younger child (age range = 7-9 years; mean ± SEM = 8.41 ± 0.17; n = 28, 10 females), older children (age range = 10-12 years; mean ± SEM = 11.56 ± 0.16; n = 29, 16 females), adolescents (age range = 13-15 years; mean ± SEM = 13.95 ± 0.12; n = 29,14 females), and adults (age range = 18-30 years; mean ± SEM = 21.87 ± 0.72; n = 30, 20 females).
The targeted age range (7-30 years) was selected based on recent findings showing an extended developmental trajectory of temporal memory during this timeframe (Pathman & Ghetti, 2014; Picard et al., 2012; Schlichting et al., 2017) as well as corresponding evidence for changes in hippocampal—prefrontal cortex structure and function through adolescence (Daugherty et al., 2016; Giedd et al., 1999; Murty et al., 2016; Schlichting et al., 2017). The sample size was determined from our previous work using a similar paradigm in developmental (Schlichting et al., 2017) and adult samples (Pudhiyidath, Schapiro, Molitor & Preston, 2018). The age group boundaries we report were used for recruitment purposes to ensure a balanced representation of individuals across the entire developmental age range. Our analysis strategy (see below), however, treated age as a continuous variable to limit assumptions about how age impacts behavior. For instance, treating age as a continuous variable maximizes the ability to measure both linear and nonlinear developmental patterns, which may be important considering the non-linear maturation of some hippocampal subregions (Schlichting et al., 2017).
Task design and procedures.
Participants were tested individually in a quiet testing room during a single testing session (approximately 60-90 minutes). For participants younger than 18 years, screening forms were completed by a parent while the participant completed their experimental session; adult participants took five minutes to complete the screening forms at the end of the experimental session.
Temporal community structure learning.
The stimuli were a set of fifteen cartoon characters (‘friendly monsters’) unfamiliar to participants. At the outset of the experiment, participants viewed each cartoon character twice (2s each time) in a randomized order. Participants were instructed to attend to the orientation of the characters during this initial viewing phase because they would be asked to judge which characters were rotated (‘had fallen over’) during the next phase of the experiment.
After initially viewing the cartoon characters, participants began the incidental temporal community structure learning task. In this task, participants viewed a long sequence of the cartoon characters one at a time on the screen. Participants were instructed to watch the ‘parade’ of characters and to make a button press when they believed the character was ‘marching’ correctly and another button press whenever they believed that the character had ‘fallen over.’ Unbeknownst to the participants, the presentation order unfolded as random walks through an underlying temporal community structure (Fig. 1a) that specified the transition probabilities among the characters (Schapiro et al., 2013).
Each character can be considered a node in the overall community structure, with the transition probabilities among the characters being represented as edges (Fig. 1a). Each individual character is connected to four other characters in the overall structure, meaning that each character had an equal probability (0.25) of being preceded or followed by one of its four connected characters in the sequence. This pattern of connections results in a temporal community structure with three communities, each consisting of five characters as community members. Within each community, there are three central characters and two boundary characters. Central community members were always preceded and followed by members of the same temporal community in the sequence; therefore, the transition probability of central characters to other characters within the same temporal community was 1.0 overall. Boundary characters, in contrast, could be preceded or followed by any of the central members of the same temporal community, or by a single boundary member of a different temporal community to which it was temporally associated. The overall transition probabilities of boundary characters were thus 0.75 to central members of the same community and 0.25 to the adjacent boundary character from a different temporal community. Notably, the two boundary members of the same temporal community were never seen in succession of one another, making the transition probability of two same-community boundary members 0.0.
The presentation order of the characters was determined by walks that were generated by randomly traversing among any connected node of the overall temporal community structure (Schapiro et al., 2013). For each participant, we created a unique 1500-item sequence that was divided into four learning blocks consisting of 375 items. Across participants, the average walk-length within a community (i.e., the number of times that characters from the same temporal community were presented successively) was 10 trials. On each trial, characters were presented for 1.5s, with no inter-stimulus interval. Each 375-item learning block lasted 9.5 minutes. Across participants, the assignment of each of the fifteen characters to one of the three temporal communities and to a central or boundary position within a community was randomized.
The temporal community task thus has several features consistent with our operational definition of a schema. Temporal associations must be acquired across multiple trials and cannot be learned in a single instance. Furthermore, the nature of the transition probabilities among characters imbues the structure with hierarchical features. Participants must not only extract temporal commonalities (i.e., learn which characters belong to the same communities), but they must also learn about the differences between the communities. Learning about the boundary nodes within the structure can help individuals anticipate when a shift in temporal context might occur (i.e., a transition to a new community), allowing them to correspondingly shift their predictions about which set of characters might come next in the sequence.
Importantly, during temporal structure learning, participants were not instructed about the temporal relationships among characters in the sequence; rather, they completed an orthogonal orientation detection task while viewing the sequence of characters. On each trial, participants made decisions about the orientation of the characters during the parade, indicating with a button press whether the character was ‘marching standing up’ or had ‘fallen over.’ Across the blocks, 20% of the cartoon characters were rotated at a 90-degree angle (i.e., had ‘fallen over’); the remaining characters were presented in an upright orientation. Rotated characters were randomly dispersed across the blocks. Participants were given audio feedback about their responses, with a different tone corresponding to correct and incorrect responses. At the end of each learning block, participants were given feedback on their accuracy on the orientation detection task for the immediately preceding block, which they tracked with stickers on a motivational chart. This orientation task thus directed participants’ attention to the characters during learning without explicitly directing the participants to the underlying temporal community structure. In consideration of the developmental sample tested, the orientation task used during temporal structure learning in this experiment was considerably easier than that of similar adult paradigms (Schapiro et al., 2013; Karuza, Kahn, Thompson-Schill & Bassett, 2017), which used stimuli with more ambiguous orientations. In between learning blocks, participants were given a 5-minute break, during which they complete two simple connect-the-dot drawings.
Inductive inference task.
Immediately after completing the temporal community structure learning task, participants performed an inductive inference task. During each trial of the inference task, one of the characters from the parade appeared at the top of the screen (Fig. 1b), and the participants were provided with information about which kind of habitat (desert, forest, or ocean) the character preferred (e.g., “This monster likes the ocean”). At the bottom of the screen, the participants were provided with two different characters as choices. Unbeknownst to the participants, one of the choices was always drawn from the same temporal community as the cue character, while the other option was a member of a different temporal community. Participants were given 8s to select the character that shared a preference for the same habitat as the character on the top of the screen. Participants were instructed not to rely on physical attributes to make their decision, but rather use their experiences from the experiment thus far to make their choice. Thus, this task allowed us to test whether knowledge of the temporal community structure biased inference decisions.
There were 21 inference trials in total, divided into three different cue-choice combinations. For nine inference trials, the cue character was a central member of the temporal community, and the choices were another central member of the same community or a central member of a different temporal community. For another six inference questions, a boundary character served as the cue, and the choices were a central member of the same temporal community and the boundary member of another temporal community to which the cue was temporally adjacent in the community structure. The final six inference questions also used a boundary character as the cue, but the choices consisted of the other boundary member of the same temporal community and a central member of a different temporal community. We included different question types to balance the use of central and boundary characters as cues and choices during the inference task, noting that the small number of trials per question type prevented us from examining this as an additional variable in analyses of this task.
Because there is not an objectively correct answer for this task, we calculated an overall measure of bias across all questions in the inference task. This bias score was computed by subtracting the frequency with which participants inferred that the cue shared a habitat preference with a different community member from the frequency with which they endorsed the same community member as sharing a preference with the cue. A zero bias score would thus indicate no influence of temporal knowledge on inference performance (our null hypothesis), as the proportion of choices would be evenly distributed across same and different community members. However, if temporal knowledge does influence inference decisions, we would expect to see a positive bias score, reflecting a greater tendency to infer that members of the same temporal community share other, non-temporal properties.
Temporal knowledge task.
In the last phase of the experiment, we tested participants’ ability to detect sequences of characters drawn from the same temporal community. During each trial, two three-character sequences were presented on the left and right sides of the screen in succession (Fig. 1c). The first three-character sequence was presented one item at a time (1s per character). After a 1s delay, the second three-character sequence was presented in the same manner on the other side of the screen. The presentation order of the two options was counterbalanced, with the left option being presented first on half of the trials and the right option on the other half of trials.
On each trial, participants made judgments about which group of characters were ‘best friends.’ To make their decisions, participants were instructed to think back to the parade of characters and identify ‘best friends’ as those characters that were more likely to have marched next to one another in the parade. This task thus explicitly referenced the temporal associations between the characters and asked participants to select the three-character sequence that seemed like a familiar sequence of characters from the parade. This temporal knowledge test and instructions were modified from our previous work on statistical learning in developmental samples (Schlichting et al., 2017). Participants responded by pressing a button corresponding to the side of the screen in which the more familiar sequence of characters had been presented. All participants had the option of viewing the sequences twice before making their decision.
Critically, one of the three-character sequences consisted of items from the same temporal community (target), while the other choice comprised three characters from different temporal communities (foil). There were three different test trial types during this temporal knowledge task (Fig. 1d). For central trials, the target sequence was made up of three central members of the same temporal community. The foil sequence for central trials shared one central character with the target; however, the remaining two characters were drawn from central members of different temporal communities. During 1-boundary trials, the target sequence contained two central characters and one boundary character from the same temporal community, while the foil sequence for this trial type shared the same boundary character with the target, but contained two central members from different temporal communities as the boundary item. Finally, for 2-boundaries trials, the target sequence contained the two boundary characters and one central character from the same temporal community, whereas the foil contained one of the same boundary characters as the target along with the two non-adjacent boundary characters from the other two temporal communities. Participants completed two test blocks (21 trials in each), for a total of 42 temporal knowledge trials (18 central, 12 1-boundary, and 12 2-boundaries trials). The target sequences were seen twice across the two blocks, with different foil options presented during the two test repetitions. Participants were given the option of a short break between test blocks if needed.
The critical measure of temporal knowledge was calculated as the proportion of trials for which participants selected the target sequence for each of the three trial types (central, 1-boundary, 2-boundaries) across each of the two test blocks. The temporal community structure defines the correct answer for the choice between two sequences in this task; one of the two choices always consisted of three community members, thus chance performance for this task is calculated at 0.50. For both central and 1-boundary trials, the target sequence consisted of three characters that were seen next to one another in the parade, (i.e., all characters had a 0.25 transition probability to one another), while the foil contained three characters that were never seen next to one another in the parade (i.e., all had a 0 transition probability to one another). We predicted that participants would be sensitive to the difference in the transition probabilities among elements of the target and foil sequences for these trial types and would thus be more likely to select the target sequence as a group of ‘best friends.’ In contrast, the transition probabilities that differentiate the target and foil sequences for the 2-boundaries trials are more ambiguous (Fig. 1d); thus, we predicted that participants would have greater difficulty differentiating between target and foil sequences for 2-boundaries trials.
Results
Orientation task performance during temporal community structure learning.
Overall accuracy on the orientation task was high across all participants (M = 0.95, SE = 0.0039), suggesting that participants were attentive during the sequence presentation. We used multiple linear regression to determine how performance was mediated by age, learning block, and character orientation (upright or rotated). One participant (female, age = 8 years) was excluded from the orientation task performance analysis because of a technical issue that prevented the participant’s data from being saved despite successful completion of the task. The overall model was significant (R2 = 0.41, F(15, 904) = 43.59, p < 0.001). We found significant predictors of rotation status (F(1, 904) = 27.05, p < 0.001) and an interaction between rotation status and age (b = 0.005, t(904) = 2.27, p = 0.02). Age (b = 0.002, t(904) = 1.59, p = 0.11) and block (F(3, 904) = 0.048, p = 0.99) were not significant independent predictors.
Paired t-tests revealed that participants were more accurate at identifying the orientation of upright characters (M = 0.98, SE = 0.003) than rotated characters (M = 0.86, SE = 0.003, p < 0.001). To interrogate the interaction between age and rotation status on orientation task performance, we calculated correlations between age and accuracy for upright and rotated characters separately, irrespective of learning block. We found a significant positive relationship between age and accuracy on both upright (rs = 0.58, p < 0.001) and rotated trials (rs = 0.47, p < 0.001). We considered performance further using d-prime (d’) as a measure of sensitivity. For each participant, d’ was calculated by subtracting the z-scored proportion of times participants incorrectly identified a non-rotated item as rotated (i.e., false alarm response) from the z-scored proportion of times participants correctly identified rotated trials (i.e., hit responses) across all trials. Therefore, a higher d’ was indicative of more accurate responses across the two trial types. We found that participants’ sensitivity scores correlated positively with age (rs = 0.5, p < .0001), providing additional support for age-related improvements at the orientation task (Fig. 2b).
Figure 2.
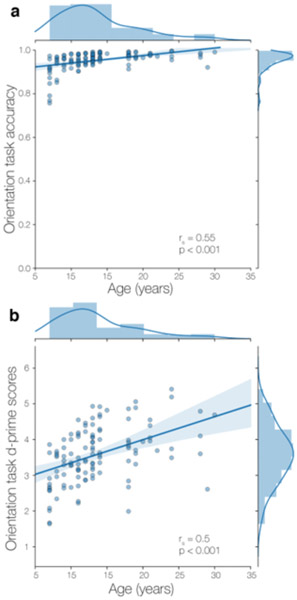
Orientation task performance during temporal community structure learning. a) Overall performance on the orientation task was high with positive improvements in performance with age. b) Participants’ d-prime performance for detecting rotated trials, further suggesting that performance on the task improved with age.
We further assessed how response times during the orientation task were mediated by age, learning block, and rotation status (upright or rotated) using multiple linear regression. The overall model was significant (R2 = 0.43, F(15, 904) = 46.90, p < 0.001), and we found a significant predictor of rotation status (F(1, 904) = 9.53, p < 0.001). A paired t-test revealed that response times for upright items (M = 0.56s, SE = 0.010) were faster than for rotated items (M = 0.67s, SE = 0.013, p < 0.001). We also observed a significant negative predictor of age (b = −0.014, t(904) = −7.39, p < 0.001), with faster response times with increasing age. Furthermore, a significant predictor of block (F(3, 904) = 3.40, p = 0.017) revealed response times decreased across the four learning blocks (first block: M = 0.65s, SE = 0.007; second block: M = 0.62s, SE = 0.007; third block: M = 0.61s, SE = 0.007; fourth block: M = 0.60s, SE = 0.007), indicating that participants got faster at detecting the orientation of the characters over the course of learning regardless of age.
Temporal knowledge task performance.
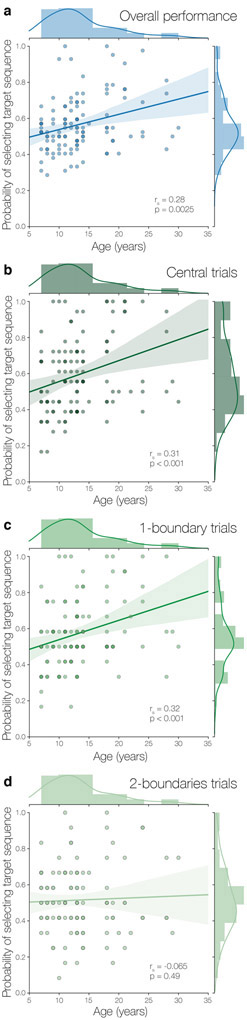
We assessed participants’ knowledge of the temporal community structure by calculating the proportion of trials for which participants selected the target sequence versus the foil sequence during the temporal knowledge task. We used multiple linear regression to determine how performance was mediated by age, trial type (central, 1-boundary, 2-boundaries), test block, and d’ scores from the structure learning task. We added d’ scores as a covariate in this model and all subsequent analyses to control for possible attentional differences during initial temporal structure learning. The overall model was significant (R2 = 0.069, F(7, 682) = 7.16, p < 0.001). We found a significant predictor of age (b = 0.019, t(682) = 3.83, p < 0.001), which indicated greater temporal knowledge with increasing age (Fig. 3a), as well as a significant predictor of trial type (F(2, 682) = 8.34, p < 0.001).
Figure 3.
Temporal knowledge task performance. a) Participants’ selection of the target sequences during the temporal knowledge test increased linearly with age, but age-related effects on performance were mediated by trial type (central, 1-boundary, 2-boundaries) as indicated by a significant interaction between age and trial type. Target sequence selection increased with age for b) central and c) 1-boundary trials. However, there was no relationship between age and performance in the d) 2-boundaries condition.
Paired t-tests revealed that participants were more accurate at selecting the target sequence for central trials (M = 0.60, SE = 0.015) and 1-boundary trials (M = 0.58, SE = 0.015) than for 2-boundaries trials (M = 0.52, SE = 0.015; p < 0.001 and p = 0.01 respectively). Furthermore, a series of one-sample t-tests confirmed that accuracy at selecting the target sequence on central and 1-boundary trials were above chance (chance = 50%, ps < 0.001); however, participants were no more likely to select the target than the foil sequence on 2-boundaries trials (p = 0.36).
The effect of trial types was further qualified by an interaction with age (F(2, 682) = 4.18, p = 0.016). To interrogate this interaction, we conducted individual correlations examining the relationships between age and the probability of selecting the target sequence for each trial type. We found a significant positive relationship between age and accuracy for both central trials (rs = 0.31, p < .001; Fig. 3b) and 1-boundary trials (rs = 0.32, p < 0.001; Fig. 3c), reflecting an increased likelihood of selecting the target sequence with increasing age. However, we found no correlation between performance on 2-boundaries trials and age (rs = −0.065, p = 0.49; Fig. 3d). Neither block (F(1, 682) = 0.59, p = 0.44) nor d’ scores from the structure learning task (b = 0.0061, t(682) = 0.49, p = 0.62) were significant predictors of performance.
We also tested whether participants’ response time when correctly selecting the target sequence was impacted by age, trial type, or test block using multiple regression. The overall model was significant (R2 = 0.13, F(6, 689) = 18.84, p < 0.001), with a significant negative predictor of age (b = −0.046, t(689) = −5.79, p < 0.001), indicating that older participants selected the target sequences more quickly than younger participants. Block (F(1, 689) = 1.75, p = 0.89) and trial type (F(2, 689) = 0.41, p = 0.67) were not significant predictors in the model.
Inductive inference performance.
We hypothesized that prior temporal experience would shape how participants made inferences about the relationships among the characters. Specifically, we wanted to quantify whether there was a greater likelihood for participants to infer that members of the same temporal community shared other, non-temporal properties than two characters from different temporal communities. To calculate whether such a temporal bias on inference existed, we computed a temporal bias score. We calculated this score by subtracting the proportion of responses that participants inferred that two characters from different temporal communities preferred the same habitat from the proportion of trials that they endorsed two same community members as sharing a habitat preference. A temporal bias score of zero would reflect no impact of temporal community structure learning on inductive inference, whereas positive bias scores would indicate an influence of temporal associations on non-temporal decision making.
Using multiple regression, we examined whether temporal bias score was mediated by age, including d’ scores from the initial structure learning task as a covariate in the model. The regression model was significant overall (R2 = 0.041, F(2, 112) = 3.41, p = 0.036); but neither age (b = 0.0055, t(112) = 1.19, p = 0.234) nor d’ scores from the structure learning task (b = 0.049, t(112) = 1.54, p = 0.127) were significant predictors of temporal bias. However, considering that age and d’ scores were highly correlated to one another (rs = 0.5, p < .0001; Fig. 2), when we examined the relationship between age and temporal bias in isolation, we found a positive relationship (rs = 0.18, p = 0.054; Fig. 4).
Figure 4.
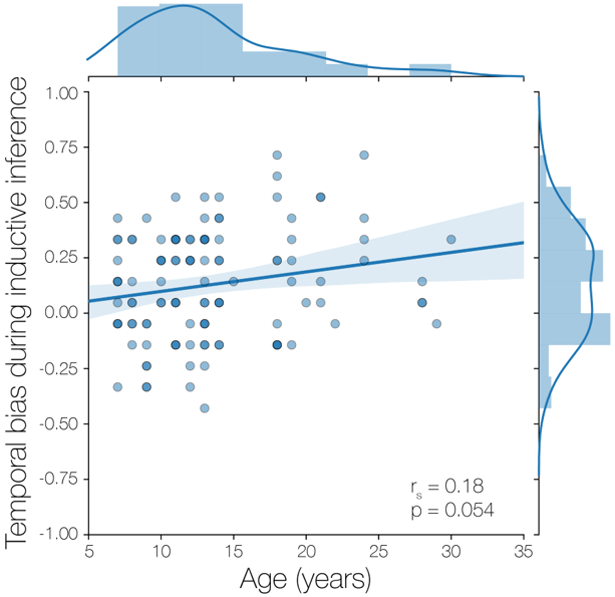
Participants’ likelihood of inferring that two members from the same temporal community or two members from different temporal communities shared habitat preferences. Temporal bias scores greater than zero reflect that a participant attributed a character from the same temporal community as sharing a habitat preference as the cued character more often than a character from a different temporal community. Multiple linear regression revealed that temporal bias scores increased with age.
Furthermore, we found that response time during the inference task was age-dependent. The multiple regression model relating inference response time to age was significant overall (R2 = 0.11, F(1, 114) = 14.90, p = 0.0019), with a positive predictor of age (b = 0.052, t(114) = 3.86, p = 0.00018). The positive relationship between inference response time and age indicates that older participants took longer to make inference decisions than younger individuals.
Relationship between temporal knowledge performance and inference decisions.
To further probe how temporal relationships influenced decision making in new contexts, we examined whether participants’ knowledge of the temporal community structure predicted the degree of temporal bias observed in the inductive inference task and the degree to which this relationship was mediated by age. Using multiple regression, we tested whether the temporal bias score was predicted by temporal knowledge and age, adding d’ scores from the initial structure learning task as a covariate in the model. The overall regression model was significant (R2 = 0.14, F(4,110) = 5.83, p < 0.01); d’ scores (b = 0.043, t(110) = 1.43, p = 0.16) and temporal knowledge performance (b = −0.33, t(110) = −0.82 p = 0.42) were not significant independent predictors. Age was a marginally significant independent predictor (b = −0.030, t(110) = −1.92, p = 0.057). However, there was a significant interaction between age and the degree to which temporal knowledge performance biased inference decisions (b = 0.056, t(110) = 2.13, p = 0.034).
To interrogate this interaction, we calculated individual correlations between temporal knowledge performance and temporal bias during inference for each of the four age groups (Fig. 5): younger children (7-9 years), older children (10-12 years), adolescents (13-15 years), and adults (18-35 years). We found a positive correlation between temporal knowledge and bias during inference in adults only (rs = 0.64, p < 0.0001). None of the younger age groups showed a significant relationship between temporal knowledge and bias during inference: younger children (rs = 0.017, p = 0.93), older children (rs = 0.037, p = 0.85), and adolescents (rs= 0.055, p = 0.78). Furthermore, the correlation between temporal knowledge and bias during inference in adults was significantly different from the correlations observed in each of the other age groups (ps < 0.01).
Figure 5.
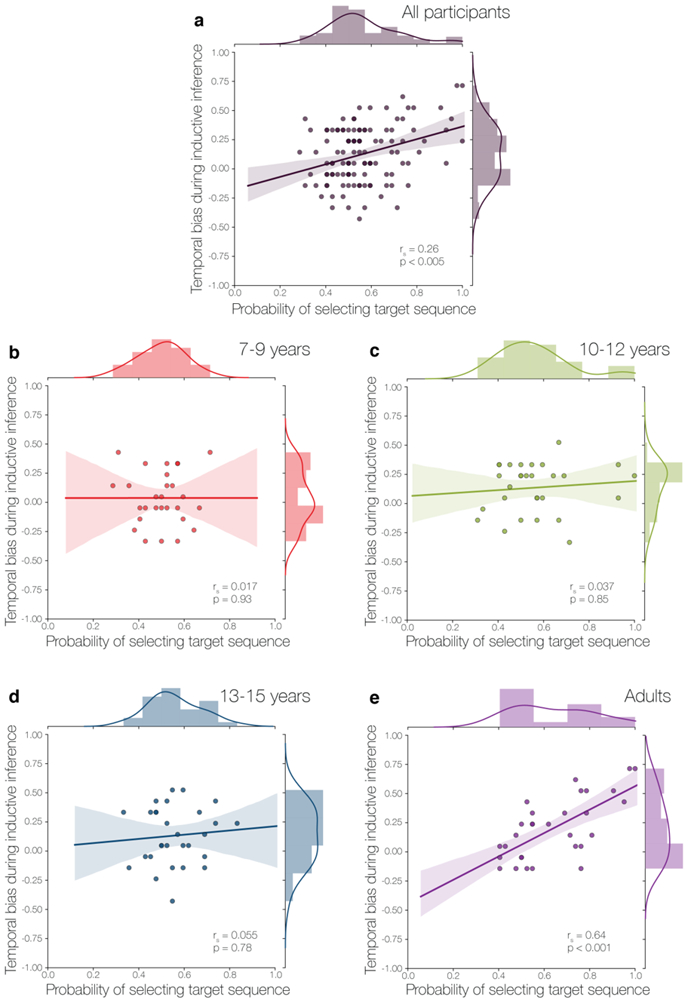
Relationship between temporal knowledge and temporal bias during inference. a) Across all participants, inference bias can be predicted from participants’ ability to identify familiar character sequences (targets) during the temporal knowledge task. However, the relationship between temporal bias during inference and temporal sequence knowledge varied with age. While b) younger children (7-9 years), c) older children (10-12 years), and d) adolescents (13-15 years) showed no relationship between temporal bias and temporal knowledge, temporal knowledge was predictive of the degree of temporal bias observed during inference in e) adults.
Discussion
In the present study, we observed age-related increases in temporal schema formation. Participants’ ability to incidentally extract knowledge about temporal statistics from a continuous stream of information improved into adulthood. Older participants were more likely to identify sequences of characters that shared temporal relationships (i.e., were members of the same temporal community) as more familiar than characters that were not seen close together in time (i.e., were characters from different temporal communities). Furthermore, older participants were more likely to use this knowledge to guide choices during inductive inference. When asked to infer which characters shared non-temporal properties (i.e., habitat preferences), older participants showed an increased bias to select members of the same temporal community. We further observed age-dependent effects on the relationship between temporal schema knowledge and temporal bias during inference. Only in the adult group was the degree of temporal bias on inference decisions directly proportional to participants’ temporal knowledge. Together, these findings indicate that the ability to form temporal schemas and apply them flexibly to inform decision making in new settings continues to improve into adulthood.
Recent theories on schemas have put considerable effort into outlining their core operational properties (Ghosh & Gilboa, 2014; McKenzie et al., 2014; Preston et al., 2017). While debate remains, current theories generally agree that schemas are hierarchical knowledge structures that are built across multiple experiences by forming links among common episodes and differentiating events that predict distinct outcomes. The temporal community structure task in the present study has features consistent with this definition as well as with other tasks from the neuroscience literature on schemas (Baraduc, Duhamel & Wirth, 2019; McKenzie et al., 2014; Tse et al., 2007; Tse et al., 2011). In this task, knowledge of the temporal transition properties among characters cannot be learned from a single exposure, but must be extracted across multiple walks through the sequence. The transition probabilities themselves define a hierarchical structure, in which the boundary nodes predict transitions to different temporal contexts (or task states) that shift expectations about what might come next in the sequence. Our data further indicate that representation of such temporal commonalities and differences in adulthood guides inference in new task contexts, another key property ascribed to schemas (Gershman et al., 2017; Varga et al., under review). The developmental differences observed in the present study indicate that the ability to extract temporal structure from the environment and deploy that knowledge flexibly to guide behavior in new settings continues to be refined through adulthood.
Statistical learning abilities are present early in life. Infants exhibit sensitivity to the structure of multimodal (Lewkowicz, 2004, 2008) and everyday event sequences (Baldwin, Baird, Saylor & Clark, 2001). They also detect regularities in syllable structure in continuous speech, discriminating triplets of syllables that are spoken together most frequently (Saffran et al., 1996). While the capacity to learn temporal regularities emerges early in development, recent findings indicate that such abilities undergo a prolonged period of refinement during childhood and adolescence. The ability to bind items to their temporal contexts continues to develop through childhood (Lee et al., 2016; Pathman & Ghetti, 2014; Picard et al., 2012). However, the ability to relate items to one another based on their shared temporal properties shows an even more protracted developmental trajectory, extending through adolescence (Lee et al., 2016). For instance, one recent study of visual statistical learning showed that while children and adolescents recognize triplet sequences whose elements always appeared successively within a continuous stream of visual input, their performance did not reach adult levels (Schlichting et al., 2017). Rather, the results revealed a linear increase in visual statistical learning from 6 years into adulthood. The present findings provide converging evidence that the ability to learn probabilistic temporal associations and organize the statistical structure of the environment into temporal schemas is refined into adulthood.
Speculatively, the refinement of temporal schema acquisition may be associated with the development of the hippocampal—prefrontal circuit that has been linked to schema representation in adults. Both rodent (Farovik et al., 2015; McKenzie et al., 2014; Place, Farovik, Brockmann & Eichenbaum, 2016; Wikenheiser & Schoenbaum, 2016) and adult human studies (Collin, Milivojevic & Doeller, 2015; Garvert, Dolan & Behrens, 2017; Schapiro et al., 2016; Schlichting, Mumford & Preston, 2015; Schlichting & Preston, 2016) indicate that hippocampus and prefrontal cortex work in concert to integrate information across multiple experiences, resulting in highly-structured representations that code the commonalities among and differences between individual events. In developmental samples, the structural maturity of hippocampus and prefrontal cortex is predictive of individuals’ ability to extract knowledge about temporal regularities and the associative commonalities across multiple events (Bauer, Duggan, Varga & Riggins, In press; Schlichting et al., 2017).
Development of the pathways connecting hippocampus and prefrontal cortex may be particularly involved in the refinement of integrative learning abilities that are critical for forming associations across time (Murty et al., 2016). In the present study, learning the transition probabilities among the characters requires integrating information from one trial to the next as well as over the course of multiple learning blocks. Maintenance of information across extended delays (Bahner et al., 2015) and reinstatement of associative predictions (Doll, Duncan, Simon, Shohamy & Daw, 2015; Zeithamova, Dominick & Preston, 2012) are both associated with increased hippocampal—prefrontal interactions in adults. Moreover, the structural integrity of the uncinate fasciculus pathway that connects hippocampus and prefrontal cortex has been linked to memory integration ability in adults (Schlichting & Preston, 2016). Notably, this pathway exhibits an extended developmental time course (Petanjek et al., 2011), with continued maturation into the third decade of life (Simmonds et al., 2014). While we did not collective brain measures in the present study, one possibility is that the immaturity of hippocampal—prefrontal connections may limit children and adolescents’ ability to track associative structure across long periods of time, such as in the present temporal community learning task. The protracted development of the uncinate fasciculus may further explain the observed variability in temporal schema learning in our adult sample, which included individuals 18-25 years of age, when the uncinate fasciculus is still developing. An important future direction of the present work will be to quantify how developmental differences in temporal schema acquisition are predicted by the structural and functional maturity of hippocampal—prefrontal connectivity.
Future extensions of the present study might further explore how performing an incidental task during temporal community structure learning influences developmental participants’ ability to learn temporal regularities. We chose to have participants perform an incidental learning task to ensure sustained attention to the character sequence during learning. Our selection of an orientation task was based on prior adult studies of temporal community structure learning (Karuza et al., 2017; Schapiro et al., 2013), which we modified to be appropriate for our youngest participants. While all participants were able to perform the orientation task well and demonstrate sustained attention, we did observe age-related differences in performance on this incidental task during temporal structure learning. However, when we controlled for such differences in attentional demand, we continued to observe age-related differences in temporal knowledge acquisition and its influence on inductive inference decisions. Our prior work in adults using computational modeling approaches shows the important role that attention plays in schema formation (Mack, Love & Preston, 2016; Mack, Preston, & Love, 2019; Mack, Love, & Preston, 2018). In the present study, it is possible that having an incidental task produced additional constraints on learning, which may have differentially impacted younger participants. However, by controlling for differences in attentional demand during learning in all of our analyses, we show that age-related differences in attention are not the sole factor underlying the developmental increases in temporal schema acquisition and expression.
One challenge for the present work is that the explicit nature of our temporal knowledge test may not be sensitive enough to detect younger participants’ knowledge of the temporal schema. Statistical learning is thought to share underlying mechanisms with other implicit learning tasks that require the extraction of associative structure from the environment (Batterink, Paller & Reber, 2019; Shohamy & Turk-Browne, 2013). Thus, an implicit marker of memory may have provided additional sensitivity to the presence of temporal knowledge at younger ages. For instance, several other studies of statistical learning have used familiarity judgements to assess temporal memory, rather than making explicit references to temporal sequences at test (Fiser & Aslin, 2002, Destrebecqz & Cleeremans, 2001; Turk-Browne, Junge & Scholl, 2005). Another possibility would be to employ eye movement measures to track memory for statistical relations as has been done in other developmental studies of statistical learning (Yu & Smith, 2011). Eye movements capture latent knowledge of associative information in both children (Pathman et al., 2014) and adults (Hannula & Ranganath, 2009). In future studies, combining eye tracking with our temporal knowledge task might provide additional sensitivity to measure the developmental emergence of temporal schema acquisition.
Despite this limitation of the temporal community learning task, our findings indicate that even when temporal knowledge is acquired, there are age-related differences in how such knowledge is deployed to guide decision making. Little work in either adult or developmental populations has directly examined how knowledge of temporal statistics biases decision making in new task contexts. One notable adult example observed a transfer of value information across pairs of images that shared a deterministic temporal relationship (Wimmer & Shohamy, 2012). The present findings extend upon that work to show that knowledge of complex probabilistic temporal statistics biases how people reason about the non-temporal relationships among event elements. Furthermore, we show that temporal biases on inductive reasoning are most prevalent in adults, indicating that generalization of temporal schema knowledge increases into adulthood.
One factor that may underlie the observed age-related differences in how temporal schema knowledge influences reasoning is the representational overlap among temporal community members. Increases in representational similarity have been proposed to underlie developmental changes in inductive generalization (Fisher, Godmin & Matlen, 2015). In adults, patterns of hippocampal and prefrontal activity elicited by members of the same temporal community are more similar than those elicited by members from different communities (Schapiro et al., 2013; Schapiro et al., 2016). Moreover, in adults, overlapping representation of two events in hippocampus facilitates inference about their unobserved relationships (Schlichting, Zeithamova & Preston, 2014). Similar findings have been observed in rodent studies, wherein increased representational overlap within hippocampus promotes generalization of learned information from one context to another (Cai et al., 2016; McKenzie et al., 2014). Maturation of the hippocampal binding (Geng et al., 2019; Ghetti et al., 2010) and differentiation (Keresztes et al., 2017; Ngo et al., 2019) mechanisms that promote representation of commonalities (through overlap) and differences (through pattern separation) may therefore underlie developmental differences in inductive generalization.
Generalization of temporal knowledge to guide reasoning decisions may further require a level of behavioral flexibility not evident until adulthood. Children require a high degree of overlap between learning and test contexts to retrieve associative knowledge (DeMaster et al., 2016). Development of hippocampal—prefrontal interactions may critically underlie the ability to express knowledge flexibly in new task contexts (Decker et al., 2016; Hunt et al., 2016; Kwak et al., 2015; Voss et al., 2015). For instance, one recent study observed age-related differences in hippocampal—prefrontal engagement that predicted developmental differences in the ability to retrieve prior knowledge to guide decision making when the task context changed (Voss et al., 2015). Notably, several older children and adolescents in our sample were able to acquire knowledge of the temporal relationships among the characters; however, in none of the developmental age groups was temporal knowledge related to their inference decisions. This finding suggests that the mechanisms that support the flexible expression of schema knowledge are not fully developed until adulthood.
An additional important question for future work using this task is the role that consolidation might play in schema acquisition and expression. Many theories of schemas highlight the important role that sleep and consolidation play in knowledge extraction (Buckner, 2010; Lewis & Durrant, 2011; Schapiro et al. 2017; Wang & Morris 2010). In particular, replay of experiences during sleep is thought to strengthen representation of commonalities that are shared across multiple experiences, while pruning idiosyncratic details from memory representations (Lewis & Durrant, 2011). Consolidation-related replay may also play an important role in emphasizing goal-relevant differences among memories that lead to the formation of hierarchical knowledge structures (Schapiro, et al., 2017; Ritvo, Turk-Browne & Norman, 2019). In the present study, all learning and testing was performed within a single experimental session; thus, the conclusions we can draw about developmental differences in performance are limited to processes related to initial schema formation. An interesting future direction would be to test age-related differences in performance after longer delays, including intervals of sleep, (Gómez, Bootzin & Nadel, 2006; Sandoval, Leclerc & Gómez, 2017). Recent work suggests that children benefit more than adults from sleep-related consolidation (Wilhelm et al., 2013), and it is possible that children’s knowledge of the temporal structure would improve over an interval of sleep. While this question is beyond the scope of the present work, future studies aimed at testing this hypothesis would provide additional important information about schema representation during development.
In summary, our findings reveal age-related differences in the initial acquisition and expression of temporal schema knowledge. The present findings contrast to some degree with a recently proposed model of memory development (Keresztes, Ngo, Lindenberger, Werkle-Bergner & Newcombe, 2018), which suggests that the mechanisms for the acquisition of statistical regularities and generalization are in place early in life while those supporting differentiation emerge late in development. Instead our findings indicate that representation of commonalities and differences both undergo extended refinement through development. Moreover, we show that there is a protracted development of generalization ability, which extends through adolescence. Very few studies have explored these behaviors in adolescence, and there is a growing appreciation that adolescence is a unique developmental time period (Casey, 2015). Our findings show that even adolescents are less likely than adults to extend their knowledge to new settings, which has important implications for conceptual knowledge acquisition, reasoning behaviors, and decision making during this developmental period. The protracted development of schema expression observed here may stem from the maturation of prefrontal control mechanisms that influence formation of hierarchical memory representations within hippocampus (Mack, Love & Preston, 2016; Murty et al., 2016). In particular, the protracted development of the anterior hippocampus (DeMaster et al., 2014; Ghetti & Bunge, 2012; Petanjek et al., 2011; Schlichting et al., 2017), which is preferentially connected to prefrontal cortex (Barbas & Blatt, 1995; Cavada, Company, Tejedor, Cruz-Rizzolo , & Reinoso-Suarez, 2000), may underlie to developmental increases in schema formation and expression that we observe through adolescence in the present study. Future neuroimaging studies using representational analysis approaches (Kriegeskorte, Mur & Bandettini, 2008) may aid in the resolution of different theoretical perspectives on knowledge acquisition and generalization during development. Such methods may help identify when distinct hippocampal and prefrontal computations supporting the representation of commonalities and differences (Schapiro et al., 2017) come online during childhood and adolescence, resulting in developmental changes in both learning and decision making.
Acknowledgements
The authors thank Susannah Cox, Nhu-Hao Hue, Benjamin Jones, Jillian Perez, Lauren Quesada, Bruce Rawlings, and Nicole Varga for assistance with participant recruitment, data collection and helpful discussion. We also thank the Children’s Research Center at the University of Texas at Austin and its affiliated staff for their support with participant recruitment.
Disclosure of interest
This work was supported by the National Institutes of Health (grants R01 MH100121 and R21 HD083785 to A.R.P. and F32 MH115585 to C.C.).
Footnotes
The authors report no conflicts of interest.
References:
- Achenbach TM (1991). Manual for the Child Behavior Checklist/4-18 and 1991 profile. Burlington, VT: Department of Psychiatry, University of Vermont. [Google Scholar]
- Allen TA, Salz DM, McKenzie S, & Fortin NJ (2016). Nonspatial sequence coding in CA1 neurons. J Neurosci, 36(5), 1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger JR & Shapiro LR (2012). Evidence of a transition from perceptual to category induction in 3- to 9-year-old children. J Exp Child Psychol, 113, 131–146. [DOI] [PubMed] [Google Scholar]
- Bahner F, Demanuele C, Schweiger J, Gerchen MF, Zamoscik V, Ueltzhoffer K, . . . Meyer-Lindenberg A (2015). Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacology, 40(7), 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Chen J, Zadbood A, Pillow JW, Hasson U, & Norman KA (2017). Discovering event structure in continuous narrative perception and memory. Neuron, 95(3), 709–721 e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Hasson U, & Norman KA (2018). Representation of real-world event schemas during narrative perception. J Neurosci, 38(45), 9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA, Baird JA, Saylor MM, & Clark MA (2001). Infants parse dynamic action. Child Dev, 72(3), 708–717. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Duhamel JR, & Wirth S (2019). Schema cells in the macaque hippocampus. Science, 363(6427), 635–639. [DOI] [PubMed] [Google Scholar]
- Barbas H, & Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus, 5(6), 511–533. [DOI] [PubMed] [Google Scholar]
- Bartlett FC (1932). Remembering: A study in experimental and social psychology. Cambridge, England: Cambridge University Press. [Google Scholar]
- Batterink LJ, Paller KA, & Reber PJ (2019). Understanding the neural bases of implicit and statistical learning. Top Cogn Sci, 11, 482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ (1996). What do infants recall of their lives? Memory for specific events by one- to two-year-olds. Am Psychol, 51(1), 29–41. [DOI] [PubMed] [Google Scholar]
- Bauer PJ (2007). Remembering the times of our lives: Memory in infancy and beyond. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Bauer PJ, & Dow GA (1994). Episodic memory in 16- and 20-month-old children: Specifics are generalized but not forgotten. Dev Psychol, 30(3), 403–417. [Google Scholar]
- Bauer PJ, Dugan JA, Varga NL, & Riggins T (In press). Relations between neural structures and children’s self-derivation of new knowledge through memory integration. Dev Cogn Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, & Mandler JM (1989). One thing follows another: Effects of temporal structure on 1- to 2-year-olds’ recall of events. Dev Psychol, 25(2), 197–206. [Google Scholar]
- Booth AE, & Waxman S (2002). Object names and object functions serve as cues to categories for infants. Dev Psychol, 38(6), 948–957. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Holliday RE, & Reyna VF (2004). Behavioral measurement of remembering phenomenologies: So simple a child can do it. Child Dev, 75(2), 505–522. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2010). The role of the hippocampus in prediction and imagination. Annu Rev Psychol, 61, 27–48. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, . . . Silva AJ (2016). A shared neural ensemble links distinct contextual memories encoded close in time. Nature, 534(7605), 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, & Reinoso-Suarez F (2000). The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex, 10(3), 220–242. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, & Eichenbaum HE (1993). Memory, Amnesia, and the Hippocampal System. Cambridge, MA: The MIT Press. [Google Scholar]
- Collin SH, Milivojevic B, & Doeller CF (2015). Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci, 18(11), 1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Raz N, & Ofen N (2016). Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus, 26(2), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JH, Otto AR, Daw ND, & Hartley CA (2016). From creatures of habit to goal-directed learners: Tracking the developmental emergence of model-based reinforcement learning. Psychol Sci, 27(6), 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Coughlin C, & Ghetti S (2016). Retrieval flexibility and reinstatement in the developing hippocampus. Hippocampus, 26(4), 492–501. [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, & Ghetti S (2014). Structural development of the hippocampus and episodic memory: Developmental differences along the anterior/posterior axis. Cereb Cortex, 24(11), 3036–3045. [DOI] [PubMed] [Google Scholar]
- Derogatis LR (1977). SCL-90-R: Administration, scoring and procedures: Manual 1. Baltimore, MD: Clinical Psychometric Research. [Google Scholar]
- Destrebecqz A, & Cleeremans A (2001). Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychon Bull Rev, 8(2), 343–350. [DOI] [PubMed] [Google Scholar]
- Doll BB, Duncan KD, Simon DA, Shohamy D, & Daw ND (2015). Model-based choices involve prospective neural activity. Nat Neurosci, 18(5), 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (1997). Declarative memory: Insights from cognitive neurobiology. Annu Rev Psychol, 48(1), 547–572. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, & Davachi L (2011). What constitutes an episode in episodic memory? Psychol Sci, 22(2), 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, & Davachi L (2014). Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron, 81(5), 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandakova Y, Bunge SA, Wendelken C, Desautels P, Hunter L, Lee JK, & Ghetti S (2018). The importance of knowing when you don't remember: Neural signaling of retrieval failure predicts memory improvement over time. Cereb Cortex, 28(1), 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Place RJ, McKenzie S, Porter B, Munro CE, & Eichenbaum H (2015). Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. J Neurosci, 35(21), 8333–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar MJ, & Goodman GS (1992). Developmental changes in event memory. Child Dev, 63(1), 173–187. [PubMed] [Google Scholar]
- Fiser J, & Aslin RN (2002). Statistical learning of higher-order temporal structure from visual shape sequences. J Exp Psychol Learn Mem Cogn, 28(3), 458–467. [DOI] [PubMed] [Google Scholar]
- Fisher AV, Godwin KE, & Matlen BJ (2015). Development of inductive generalization with familiar categories. Psychon Bull Rev, 22(5), 1149–1173. [DOI] [PubMed] [Google Scholar]
- Fivush F, & Slackman E (1986). The acquisition and development of scripts In Nelson K (Ed.), Event knowledge: Structure and Function in Development (pp. 71–96). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Fortin NJ, Agster KL, & Eichenbaum HB (2002). Critical role of the hippocampus in memory for sequences of events. Nat Neurosci, 5(5), 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvert MM, Dolan RJ, & Behrens TE (2017). A map of abstract relational knowledge in the human hippocampal-entorhinal cortex. Elife, 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F, Redcay E, & Riggins T (2019). The influence of age and performance on hippocampal function and the encoding of contextual information in early childhood. Neuroimage, 195, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Monfils MH, Norman KA, & Niv Y (2017). The computational nature of memory modification. Elife, 6, 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Bunge SA (2012). Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci, 2(4), 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, & Bunge SA (2010). Developmental differences in medial temporal lobe function during memory encoding. J Neurosci, 30(28), 9548–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh VE, & Gilboa A (2014). What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia, 53, 104–114. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, . . . Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci, 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, . . . Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, . . . Thompson PM (2006). Dynamic mapping of normal human hippocampal development. Hippocampus, 16(8), 664–672. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, Nadel L (2006). Naps promote abstraction in language-learning infants. Psychol Sci, 17(8), 670–4. [DOI] [PubMed] [Google Scholar]
- Gravina MT, & Sederberg PB (2017). The neural architecture of prediction over a continuum of spatiotemporal scales. Curr Opin in Behav Sci, 17, 194–202. [Google Scholar]
- Hannula DE, & Ranganath C (2009). The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron, 63(5), 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindy NC, Ng FY, & Turk-Browne NB (2016). Linking pattern completion in the hippocampus to predictive coding in visual cortex. Nat Neurosci, 19(5), 665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, & Ranganath C (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron, 81(5), 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JA, Fivush R, & Kuebli J (1992). Scripts and episodes: The development of event memory. Appl Cogn Psychol, 6, 483–505. [Google Scholar]
- Hunt PS, Burk JA, & Barnet RC (2016). Adolescent transitions in reflexive and non-reflexive behavior: Review of fear conditioning and impulse control in rodent models. Neurosci Biobehav Rev, 70, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, & Ranganath C (2010). Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci, 30(46), 15558–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuza EA, Kahn AE, Thompson-Schill SL, & Bassett DS (2017). Process reveals structure: How a network is traversed mediates expectations about its architecture. Sci Rep, 7(1), 12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Bender AR, Bodammer NC, Lindenberger U, Shing YL, & Werkle-Bergner M (2017). Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc Natl Acad Sci U S A, 114(34), 9212–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Ngo CT, Lindenberger U, Werkle-Bergner M, & Newcombe NS (2018). Hippocampal maturation drives memory from generalization to specificity. Trends Cogn Sci, 22(8), 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Lewis-Peacock JA, Norman KA, & Turk-Browne NB (2014). Pruning of memories by context-based prediction error. Proc Natl Acad Sci U S A, 111(24), 8997–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb DA (1984). Experiential learning: Experience as the Source of Learning and Development (Vol. 1). Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Kriegeskorte N, Mur M, & Bandettini P (2008). Representational similarity analysis - connecting the branches of systems neuroscience. Front Syst Neurosci, 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, & Maguire EA (2009). Tracking the emergence of conceptual knowledge during human decision making. Neuron, 63(6), 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Payne JW, Cohen AL, & Huettel SA (2015). The rational adolescent: Strategic information processing during decision making revealed by eye tracking. Cogn Dev, 36, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Wendelken C, Bunge SA, & Ghetti S (2016). A time and place for everything: Developmental differences in the building blocks of episodic memory. Child Dev, 87(1), 194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin K (1942). Field theory and learning In Nelson HB (Ed.), The Forty-first Yearbook of the National Society for the Study of Education: Part II, The Psychology of Learning. (pp. 215–242). Chicago, IL: University of Chicago. [Google Scholar]
- Lewis PA, Durrant SJ (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci, 15, 343–351. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ (2004). Perception of serial order in infants. Dev Sci, 7(2), 175–184. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ (2008). Perception of dynamic and static audiovisual sequences in 3- and 4-month-old infants. Child Dev, 79(5), 1538–1554. [DOI] [PubMed] [Google Scholar]
- Lloyd ME, Doydum AO, & Newcombe NS (2009). Memory binding in early childhood: Evidence for a retrieval deficit. Child Dev, 80(5), 1321–1328. [DOI] [PubMed] [Google Scholar]
- Lukowski AF, Wiebe SA, & Bauer PJ (2009). Going beyond the specifics: Generalization of single actions, but not temporal order, at 9 months. Infant Behav Dev, 32(3), 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Love BC, & Preston AR (2016). Dynamic updating of hippocampal object representations reflects new conceptual knowledge. Proc Natl Acad Sci U S A, 113(46), 13203–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Love BC, & Preston AR (2018). Building concepts one episode at a time: The hippocampus and concept formation. Neurosci Lett, 680, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Preston AR, & Love BC (2019). Ventromedial prefrontal cortex compression during concept learning. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler JM (1984). Stories, scripts, and scenes: Aspects of schema theory (Vol. 10). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Mcdonough L, Mandler JM, Mckee RD, & Squire LR (1995). The deferred imitation task as a nonverbal measure of declarative memory. Proc Natl Acad Sci USA, 92(16), 7580–7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Riviere PD, & Eichenbaum H (2014). Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron, 83(1), 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, & Reiss AL (2005). Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Res Cogn Brain Res, 25(1), 379–385. [DOI] [PubMed] [Google Scholar]
- Morton NW, Sherrill KR, & Preston AR (2017). Memory integration constructs maps of space, time, and concepts. Curr Opin Behav Sci, 17, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Calabro F, & Luna B (2016). The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev, 70, 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K (1986). Event knowledge: Structure and function in development. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Nelson KA, Fivush R, Hudson JA, & Lucariello J (1983). Scripts and the development of memory In Chi MTH (Ed.), Contributions to Human Development: Trends in Memory Development Research (pp. 52–70). Basel: Kargar. [Google Scholar]
- Ngo CT, Lin Y, Newcombe NS, & Olson IR (2019). Building up and wearing down episodic memory: Mnemonic discrimination and relational binding. J Exp Psychol Gen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, & Walhovd KB (2009). Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci, 29(38), 11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathman T, & Ghetti S (2014). The eyes know time: A novel paradigm to reveal the development of temporal memory. Child Dev, 85(2), 792–807. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, . . . Evans AC (1999). Structural maturation of neural pathways in children and adolescents: in vivo study. Science, 283(5409), 1908–1911. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, & Kostovic I (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A, 108(32), 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SL, Fellows LK, & Sheldon S (2017). The ventromedial frontal lobe contributes to forming effective solutions to real-world problems. J Cogn Neurosci, 29(6), 991–1001. [DOI] [PubMed] [Google Scholar]
- Piaget J (1954). The construction of reality in the child. New York: Basic. [Google Scholar]
- Picard L, Cousin S, Guillery-Girard B, Eustache F, & Piolino P (2012). How do the different components of episodic memory develop? Role of executive functions and short-term feature-binding abilities. Child Dev, 83(3), 1037–1050. [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, & Eichenbaum H (2016). Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci, 19(8), 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Curr Biol, 23(17), R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Molitor RJ, Pudhiyidath A, & Schlichting ML (2017). Schemas In Eichenbaum H (Ed.), Memory Systems, Vol. 3 of Learning and Memory: A Comprehensive Reference, 2nd edition, Byrne JH (ed.) (pp. 125–132). Oxford: Academic Press. [Google Scholar]
- Price DWW, & Goodman GS (1990). Visiting the wizard: Children’s memory for a recurring event. Child Dev, 61(3), 664–680. [PubMed] [Google Scholar]
- Pudhiyidath A, Schapiro AC, Molitor RJ, & Preston AR (2018). Hippocampal representations of temporal statistics predict subsequent reasoning. Program No. 335.02. Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2018. Online. [Google Scholar]
- Quinn PC, Eimas PD, & Rosenkrantz SL (1993). Evidence for representations of perceptually similar natural categories by 3-month-old and 4-month-old infants. Perception, 22(4), 463–475. [DOI] [PubMed] [Google Scholar]
- Rakison DH, & Poulin-dubois D (2002). You do this way and I’ll got that way: Developmental changed in infants’ detection of correlations among static and dynamic features in motion events. Child Dev, 73(3), 682–699. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, & Denckla MB (1996). Brain development, gender and IQ in children. A volumetric imaging study. Brain, 119 (Pt 5), 1763–1774. [DOI] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, & Redcay E (2016). Hippocampal functional connectivity and episodic memory in early childhood. Dev Cogn Neurosci, 19, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo VJH, Turk-Browne NB, & Norman KA (2019). Nonmonotonic plasticity: How memory retrieval drives learning. Trends Cogn Sci, 23(9), 726–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, & Newport EL (1996). Statistical learning by 8-month-old infants. Science, 274(5294), 1926–1928. [DOI] [PubMed] [Google Scholar]
- Sandoval M, Leclerc JA, Gómez RL. (2017). Words to sleep on: Naps facilitate verb generalization in habitually and nonhabitually napping preschoolers. Child Dev, 88(5), 1615–1628. [DOI] [PubMed] [Google Scholar]
- Schapiro AC, Gregory E, Landau B, McCloskey M, & Turk-Browne NB (2014). The necessity of the medial temporal lobe for statistical learning. J Cogn Neurosci, 26(8), 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV, & Turk-Browne NB (2012). Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr Biol, 22(17), 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, McDevitt EA, Chen L, Norman KA, Mednick SD, & Rogers TT (2017). Sleep benefits memory for semantic category structure while preserving exemplar-specific information. Sci Rep, 7(14869), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Rogers TT, Cordova NI, Turk-Browne NB, & Botvinick MM (2013). Neural representations of events arise from temporal community structure. Nat Neurosci, 16(4), 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Turk-Browne NB, Botvinick MM, & Norman KA (2017). Complementary learning systems within the hippocampus: A neural network modelling approach to reconciling episodic memory with statistical learning. Philos Trans R Soc Lond B Biol Sci, 372(1711). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Turk-Browne NB, Norman KA, & Botvinick MM (2016). Statistical learning of temporal community structure in the hippocampus. Hippocampus, 26(1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Guarino KF, Schapiro AC, Turk-Browne NB, & Preston AR (2017). Hippocampal structure predicts statistical learning and associative inference abilities during development. J Cogn Neurosci, 29(1), 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Mumford JA, & Preston AR (2015). Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun, 6, 8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, & Preston AR (2015). Memory integration: Neural mechanisms and implications for behavior. Curr Opin Behavioral Sciences, 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, & Preston AR (2016). Hippocampal-medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiol Learn Mem, 134, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Zeithamova D, & Preston AR (2014). CA1 subfield contributions to memory integration and inference. Hippocampus, 24(10), 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, & Turk-Browne NB (2013). Mechanisms for widespread hippocampal involvement in cognition. J Exp Psychol Gen, 142(4), 1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, & Luna B (2014). Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage, 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloutsky VM, Deng SW, Fisher AV & Kloos H (2015). Conceptual influences on induction: A case for a late onset. Cogn Psychol 82, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloutsky VM, & Fisher AV (2004). When development and learning decrease memory. Evidence against category-based induction in children. Psychol Sci, 15(8), 553–558. [DOI] [PubMed] [Google Scholar]
- Sloutsky VM, Kloos H & Fisher AV (2007). When looks are everything: Appearance similarity versus kind information in early induction. Psychol Sci 18, 179–185. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, & Kovacs SL (2006). Binding, relational memory, and recall of naturalistic events: A developmental perspective. J Exp Psychol Learn Mem and Cogn, 32(1), 89–100. [DOI] [PubMed] [Google Scholar]
- Spalding KN, Jones SH, Duff MC, Tranel D, & Warren DE (2015). Investigating the neural correlates of schemas: Ventromedial prefrontal cortex is necessary for normal schematic influence on memory. J Neurosci, 35(47), 15746–15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld KL, Botvinick MM, & Gershman SJ (2017). The hippocampus as a predictive map. Nat Neurosci, 20(11), 1643–1653. [DOI] [PubMed] [Google Scholar]
- Tang L, Shafer AT, & Ofen N (2018). Prefrontal cortex contributions to the development of memory formation. Cereb Cortex, 28(9), 3295–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC (1948). Cognitive maps in rats and men. Psychol Rev, 55(4), 189–208. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, & Morris RG (2007). Schemas and memory consolidation. Science, 316(5821), 76–82. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, & Morris RG (2011). Schema-dependent gene activation and memory encoding in neocortex. Science, 333(6044), 891–895. [DOI] [PubMed] [Google Scholar]
- Tulving E, & Schacter DL (1990). Priming and memory systems. Science, 247(4940), 301–306. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Jungé JA, & Scholl BJ (2005). The automaticity of visual statistical learning. J Exp Psychol Gen 134(4), 552–564. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Ruiter DJ, Fernandez G, & Henson RN (2012). How schema and novelty augment memory formation. Trends Neurosci, 35(4), 211–219. [DOI] [PubMed] [Google Scholar]
- Varga NL, Morton NW, & Preston AR (2019). Schema, inference, and memory In Kahana MJ, & Wagner AD (eds.), Handbook on Human Memory. Oxford University Press. [Google Scholar]
- Voss JL, O'Neil JT, Kharitonova M, Briggs-Gowan MJ, & Wakschlag LS (2015). Adolescent development of context-dependent stimulus-reward association memory and its neural correlates. Front Hum Neurosci, 9, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, & Morris RGM (2010). Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Ann Rev Psychol, 61, 49–79. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, & Bauer PJ (2005). Interference from additional props in an elicited imitation task: When in sight, firmly in mind. J Cogn Dev, 6(3), 325–363. [Google Scholar]
- Wikenheiser AM, & Schoenbaum G (2016). Over the river, through the woods: Cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci, 17(8), 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Rose M, Imhof KI, Rasch B, Büchel C, & Born J (2013). The sleeping child outplays the adult’s capacity to convert implicit into explicit knowledge. Nat Neurosci, 16(4), 391–393. [DOI] [PubMed] [Google Scholar]
- Wimmer GE, & Shohamy D (2012). Preference by association: how memory mechanisms in the hippocampus bias decisions. Science, 338(6104), 270–273. [DOI] [PubMed] [Google Scholar]
- Yu C, & Smith LB (2011). What you learn is what you see: Using eye movements to study infant cross-situational word learning. Dev Sci, 14(2), 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM (In press). Event perception and memory. Annu Rev Psychol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, & Preston AR (2012). Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron, 75(1), 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, & Preston AR (2017). Temporal proximity promotes integration of overlapping events. J Cogn Neurosci, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]