Abstract
Maternal-fetal crosstalk has been implicated in long-term control of the health of offspring, including transgenerational hypertension. However, current knowledge is limited regarding maternal influences on the gut and its microbiome in blood pressure (BP) control in offspring. Therefore, the current study was designed to test the hypothesis that maternal factors influence the gut-brain axis impacting hypertension in offspring. We elected to use captopril, an antihypertensive angiotensin-converting enzyme inhibitor that possesses antibacterial properties, for the study. Pregnant female spontaneously hypertensive rats (SHR) and normotensive Wistar Kyoto (WKY) rats, were treated with captopril water (100 mg/kg/day) or sterile water throughout pregnancy and lactation. At weaning, the pups from dams drinking sterile water were continued with sterile water until 12 weeks of age. The male pups from dams drinking captopril water were divided at weaning into two groups: offspring drinking captopril water and offspring withdrawn from captopril water, then drinking sterile water until 12 weeks of age. Captopril changed gut microbiota of SHR dams, and some of these changes were reflected in their 12-week-old male offspring. These 12-week-old SHR male offspring exposed to captopril via dams demonstrated persistently decreased systolic BP, decreased number of activated microglia and neuroinflammation as well as improvement of gut inflammation and permeability. Therefore, maternal captopril treatment improves the dysregulated gut-brain axis in SHR male offspring, providing conceptual support that targeting the gut-brain axis via the mother may be a viable strategy for control of hypertension in the offspring.
Keywords: Captopril, Microbiota, Gut-Brain Axis, Transgenerational Hypertension
Graphical Abstract
Introduction
Studies from our group and others have demonstrated a link between gut dysbiosis and hypertension (HTN) in animal models and humans.1–4 Altered gut microbiota in HTN is associated with increased neural trafficking between the gut and autonomic brain regions,5 total number and activation of microglia and neuroinflammation,6,7 sympathetic nerve activity to the gut5 and intestinal tyrosine hydroxylase and norepinephrine levels5 suggesting a dysfunctional gut-brain axis in HTN. Moreover, fecal microbiota transplant (FMT) from HTN donors into normotensive recipient animals results in an increase in blood pressure (BP),2,8 impaired immune responses, activation of the sympathetic nervous system and changes in vascular function.9,10
Given the importance of maternal factors and developmental programming on the long-term health of offspring11–15 and involvement of gut microbiota and dysfunctional gut-brain axis in HTN,5,16,17 our objective was to investigate the hypothesis that maternal treatment with an antihypertensive drug would have beneficial outcomes on gut-brain axis and regulation of BP in their offspring. We elected to use the antihypertensive drug, captopril (CAP), in the spontaneously hypertensive rat (SHR) model of HTN to evaluate this hypothesis. The rationale for this was: (i) CAP is an angiotensin-converting enzyme inhibitor (ACEi) with reported long-lasting antihypertensive effect even after its withdrawal in the SHR.18–20 (ii) CAP induces sustained neuronal activity in central cardioregulatory regions.21 (iii) Structural and functional evidence shows that CAP possesses antibacterial properties. For example, it is shown to inhibit bacterial enzymes metallo-β-lactamases (MBLs) and N-succinyl-L,L diaminopimelic acid desuccinylase (DapE), resulting in bacterial death.22,23 Our data demonstrates that maternal treatment with CAP produces long-lasting antihypertensive effects by reshaping of gut microbiota and improvement of gut pathology and permeability, thus rebalancing the dysfunctional gut-brain axis in the male offspring.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. The detailed description of Materials and Methods is available in online-only Data Supplement. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Histological Analysis
Paraffin-embedded 4μm sections were stained with hematoxylin-eosin and Masson-trichrome to analyze general morphology and collagen content of intestinal samples. Fibrosis, crypt depth, number of goblet cells per 100 epithelial cells and thickness of smooth muscle cell layer were quantified in 10 random fields of view per section with an Olympus Model BX41 microscope (Olympus, Tokyo, Japan) and Image J software.24
Real-time RT-PCR
Total RNA was isolated from proximal colon using RNeasy Plus Mini Kit (Qiagen, Germantown, MA) according to manufacturer’s protocol. The hypothalamic tissue including PVN was dissected from freshly frozen brains.25
A coronal section was cut from −0.90 to −2.15 mm posterior to bregma. These sections were mounted on slides and the PVN was isolated using a brain punch (Stoelting Co., Wood Dale, IL) for mRNA extraction.
Gut microbiota analysis
Differentially significant features at each taxonomic level were identified using linear discriminant analysis (LDA) with effect size measurements (LEfSe) to generate a taxonomic cladogram. α value for the factorial Kruskal-Wallis test among groups was 0.05, and the threshold on the logarithmic LDA score for discriminative features was 2.0.26
Results
CAP treatment reshapes gut microbiota in the SHR dams:
We have previously established an association between antihypertensive effects of CAP with altered gut microbiota in the SHR.21 Therefore, our first objective was to determine if CAP influences gut microbiota in pregnant SHR dams. The experimental scheme is presented in Figure 1A. Significant suppression of Shannon diversity was observed when comparing non-pregnant with pregnant SHR (Figure 1B). Treatment with CAP during pregnancy returned diversity to the non-pregnant state, though it was not significantly different from the pregnant SHR (Figure 1B). Trends towards reduction of the Firmicutes/Bacteroidetes (F/B) ratio, a parameter to evaluate imbalance of microbial composition, were found in pregnant SHR (Figure 1B). Principal coordinate analysis (PCoA) illustrated that pregnancy reshaped gut microbial composition (blue vs red) (Figure 1C). In addition, maternal treatment with CAP altered microbial composition (orange) (Figure 1C). As shown in the cladogram (Figure 1D), the genus, Allobaculum, family Erysipelotrichaceae, and the class Erysipelotrichia were enriched in SHR with CAP treatment during pregnancy (red arrow). In addition, the order Clostridiales and class Clostridia were more abundant in SHR with maternal CAP (blue arrow) (Figure 1D). It is also interesting to note that genera in the order Clostridiales considered to be beneficial (i.e. Coprococcus, Oscillospira, Roseburia, Dehalobacterium), showed higher average abundance in pregnant SHR+CAP, compared with pregnant SHR (Figure 1E). Finally, a decrease in the body weight of SHR dams and their male offspring (Figures S1A, S1B) and a trend towards a decrease in litter size (Figure S1C) were observed as reported previously27. These observations suggest that CAP treatment for 3 weeks, although it has a modest influence, initiates the reshaping of gut microbiota in pregnant SHR.
Figure 1.
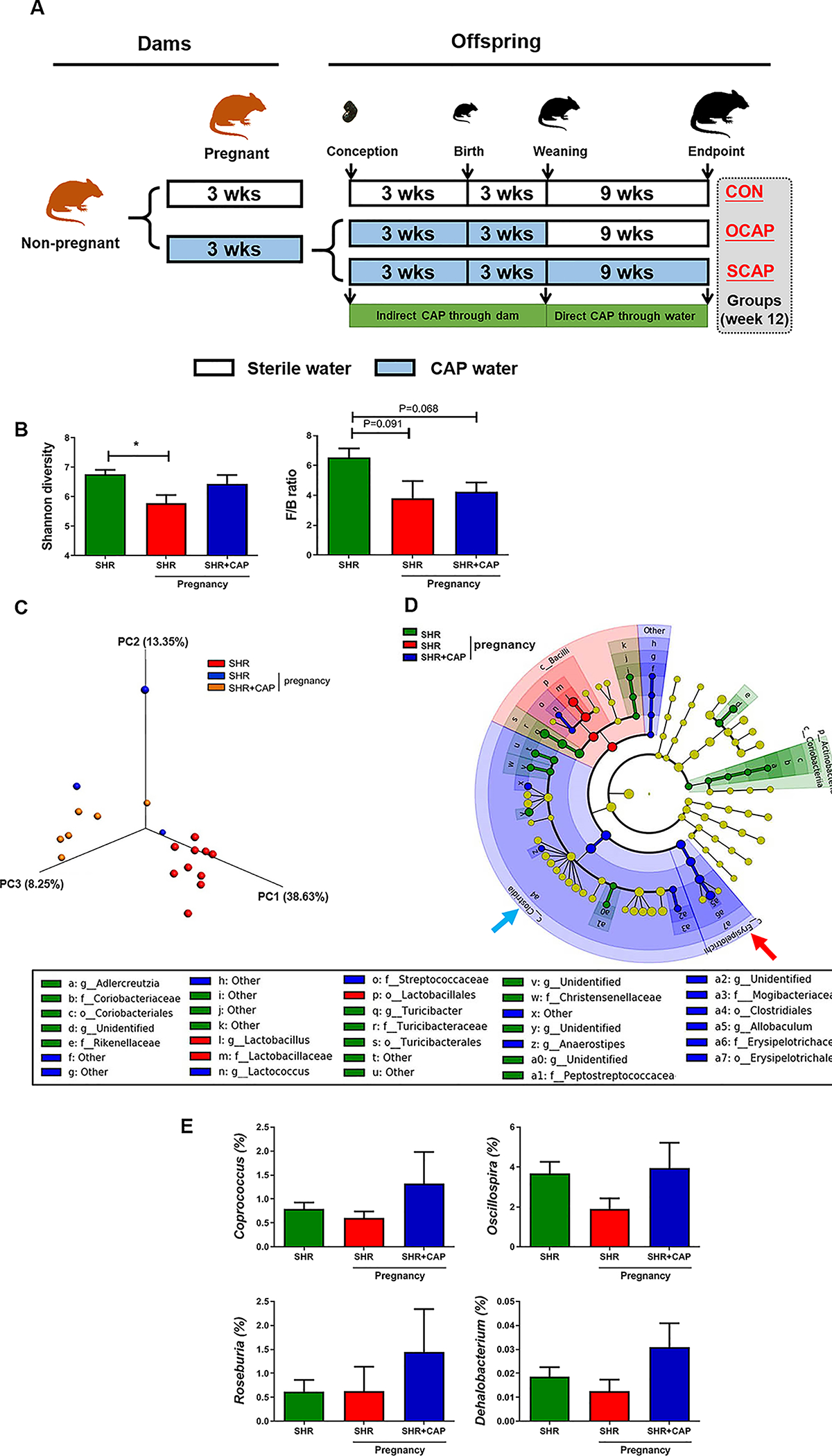
Maternal CAP treatment alters gut microbiota in SHR dams. (A) Schematic diagram of the experimental design. Pregnant rats were randomly assigned to two groups, fed with either sterile water or captopril containing water (CAP) until weaning. Rats from CAP group were further subdivided at weaning into two groups (i.e. maternal route of treatment with captopril (OCAP) and sustained CAP (SCAP) based on access to CAP water. CON, control; CAP, captopril; OCAP, maternal treatment with captopril; SCAP, continuous treatment with captopril. (B) Shannon diversity and Firmicutes/Bacteroidetes ratio (F/B ratio) were lower in pregnant dams, and not significantly affected by CAP. (C) Principal coordinate analysis (PCoA) with unweighted UniFrac metric was used to present the dissimilarity of the groups. (D) Taxonomic structure and abundance of indicated phylotypes were analyzed by LefSe and presented in cladogram. Significant dominance of phylotypes in each group was indicated by color. (E) Abundance of individual bacterial genera with significant differences between the groups. OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n=10 rats in SHR group; n=3 in SHR pregnancy; n=6 in SHR+CAP pregnancy. Data are presented as mean ± SEM. * P<0.05 in one-way ANOVA followed by Tukey’s multiple comparison test.
Maternal CAP treatment alters gut microbial composition of SHR male offspring:
Next, we investigated whether there were alterations in gut microbiota in SHR male offspring exposed to CAP via the dam. Gut microbial analyses of rats at 12 weeks of age demonstrated decreased diversity and increased F/B ratio in the SHR+CON when compared with WKY+CON (Figure 2A), which is consistent with a previous publication.1 However, these changes were normalized in both CAP-treated groups (i.e. SHR+SCAP and SHR+OCAP) of SHR male offspring (Figure 2A). In contrast, these alterations in diversity and F/B ratio by CAP were not observed in the WKY at 12 weeks of age (Figure S2A).
Figure 2.
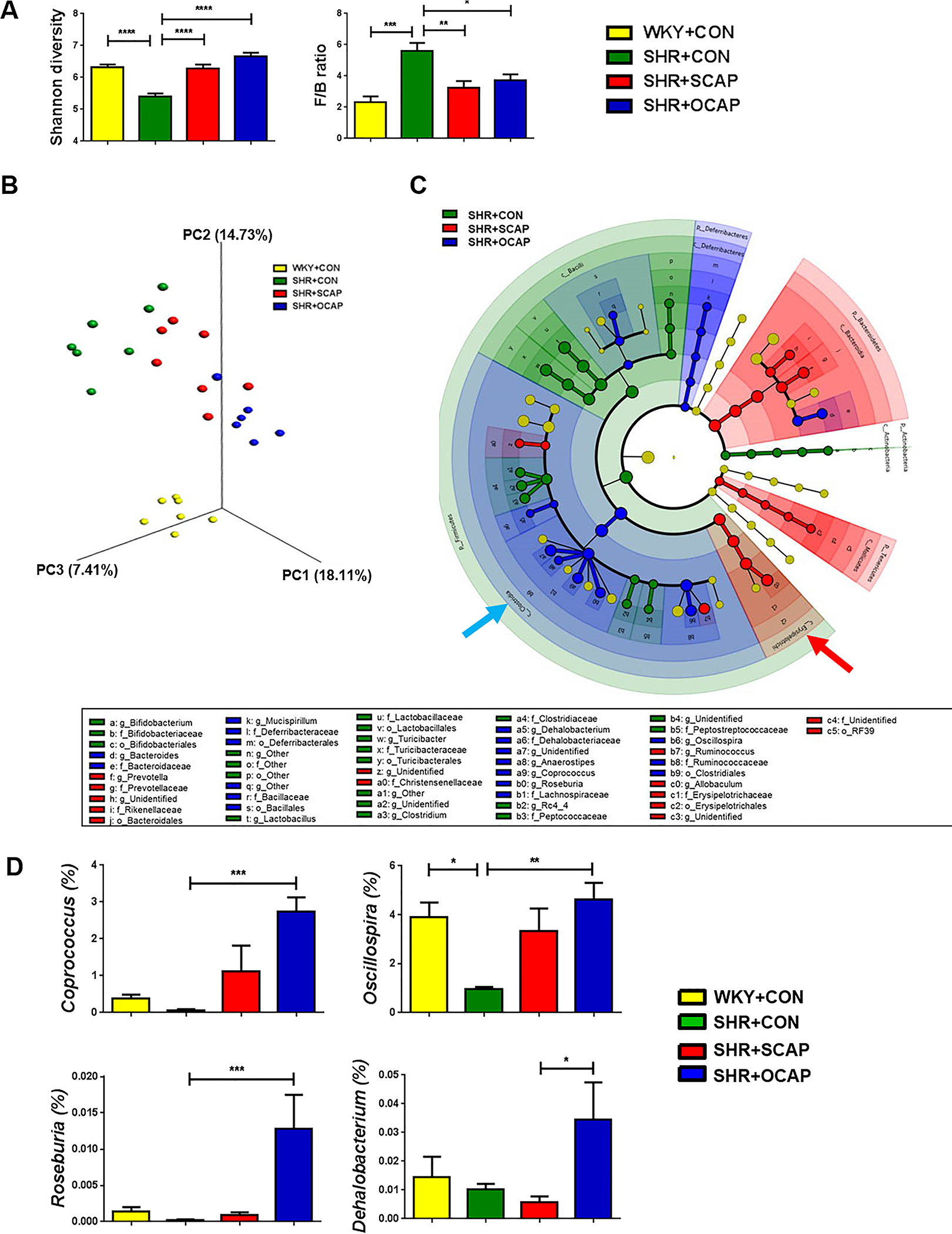
Maternal CAP treatment alters gut microbiota in SHR male offspring. (A) Shannon diversity and Firmicutes/Bacteroidetes ratio (F/B ratio) were affected by CAP in 12-week-old SHR male offspring. (B) Principal coordinate analysis (PCoA) with unweighted UniFrac metric was used to present the dissimilarity of the groups. (C) Taxonomic structure and abundance of indicated phylotypes were analyzed by LefSe and presented in cladogram. Significant dominance of phylotypes in each group was indicated by color. (D) Abundance of individual bacterial genera with significant differences between the groups. OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n= 6–7 rats per group. Data are presented as mean ± SEM. * P<0.05; **P<0.01; ***P<0.001 in one-way ANOVA followed by Tukey’s multiple comparison.
In PCoA, we observed a clear separation of WKY+CON from all three SHR groups (Figure 2B). Within the three groups of SHR, both continuous and maternal CAP treatment altered gut microbial composition (Figure 2B). Although both groups showed distinct clusters from the SHR+CON, divergence was also observed between SHR+SCAP and SHR+OCAP (Figure 2B, Table S1). Cladogram analysis (Figure 2C) of the three SHR groups demonstrated the dominance of different bacterial phylotypes in each group. Similar to the changes in SHR dams+CAP, Allobaculum (genus), Erysipelotrichaceae (family), and Erysipelotrichia (class) were enriched in the SHR+SCAP (red arrow). The bacterial phylotypes enriched in the SHR+OCAP were mainly multiple bacterial genera in the Clostridiales Order (e.g. Anaerostipes, Coprococcus, Oscillospira, Roseburia, Dehalobacterium) (Figure 2C). Additionally, to illustrate quantified differences among groups, the bacterial genera enriched in the Clostridiales Order of the SHR+OCAP were graphed (Figure 2D). Interestingly, most of the bacteria enriched in the SHR+OCAP (e.g. Coprococcus, Oscillospira, Roseburia,) were also induced in the SHR+SCAP, compared with SHR+CON, though the abundance in SHR+SCAP was not high as that in SHR+OCAP (Figure 2D). The increases of these bacterial genera in both SHR+SCAP and SHR+OCAP suggest their responsiveness to CAP in SHR. The genera with a significant increase in SHR+OCAP, compared with SHR+CON, were butyrate-producing bacteria, Coprococcus, Roseburia and Oscillospira (Figure 2D).
Interestingly, PCoA of WKY+CON, WKY+SCAP, WKY+OCAP, along with SHR+CON, SHR+SCAP, SHR+OCAP from week 12 revealed alterations in microbial composition in WKY+OCAP, comparing to WKY+CON and WKY+SCAP (Figure S2B), which is similar to that observed in the SHR groups.
Maternal treatment of SHR with CAP decreases BP in male offspring:
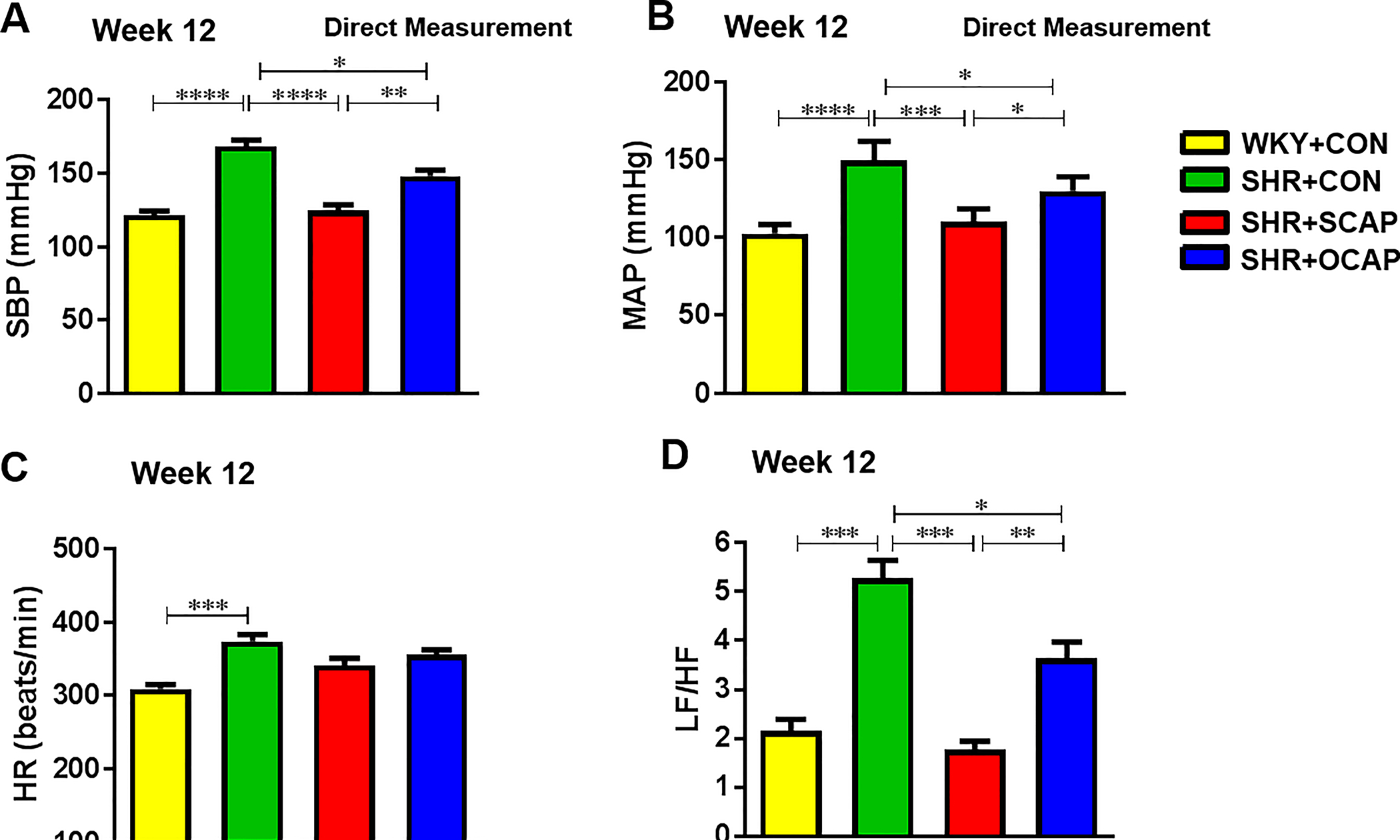
At 12 weeks of age, a significant elevation of SBP in the SHR was observed compared to age-matched WKY (169±8 mmHg versus121±5 mmHg; P<0.0001), consistent with previous reports.28 The SHR male offspring exposed to CAP throughout the experiment showed lower SBP (124±7 mmHg versus 169±8 mmHg; P<0.0001; Figure 3A). Importantly, the 12-week-old SHR with only maternal treatment with CAP demonstrated significantly lowered SBP and mean arterial BP (MAP) compared with SHR control (Figure 3A,3B). These BP data were obtained by direct measurement using radio telemetry, confirming data obtained by tail off (Figure S3A). Neither continuous CAP treatment nor maternal CAP treatment significantly lowered heart rate (HR) in 12-week-old SHR (Figure 3C). The ratios of heart weight to body weight (HW/BW) and left kidney weight to body weight (LKW/BW) that were increased in SHR+CON compared with WKY+CON rats were significantly ameliorated in SHR+SCAP and SHR+OCAP (Figure S3C, S3D). In contrast, we did not observe differences in the aforementioned cardiovascular parameters in the WKY at 12 weeks of age, although body weight was reduced by continuous CAP treatment (Figure S4).
Figure 3.
Maternal CAP treatment persistently lowers systolic blood pressure (SBP), mean arterial pressure (MAP), heart rate (HR) and autonomic balance in SHR male offspring. (A) Continuous treatment of CAP and maternal treatment with CAP lowered SBP in the 12-week-old SHR. (B) SCAP and OCAP treatment with CAP lowered MAP in the 12-week-old SHR. (C) Changes in HR in the control, continuous CAP and maternal CAP of SHR. (D) Continuous treatment of CAP and maternal treatment with CAP attenuated increased low frequency/high frequency (LF/HF) ratio in the SHR of 12-week old. OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n= 6–7 rats per group. Data are represented as mean ± SEM. * P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 in one-way ANOVA followed by Tukey’s multiple comparison.
Maternal CAP treatment persistently attenuates sympathetic activity of SHR male offspring:
The increase of BP in the SHR has been closely associated with increased sympathetic activity.29 Therefore, we performed variability analysis of SBP obtained by radio telemetry at week 12. The ratio of low frequency to high-frequency variables (LF/HF) was increased in the SHR+CON, compared with WKY+CON (Figure 3D). Importantly, this effect was significantly attenuated in male offspring by continuous CAP treatment and by maternal treatment of CAP (Figure 3D).
The paraventricular nucleus of the hypothalamus (PVN) integrates multiple input signals and controls the sympathetic outflow. Activation of microglia within the PVN region has been described in SHR with established HTN.6 Thus, we investigated the effects of maternal CAP on activation of microglia and neuroinflammation in the PVN. Activated microglia were defined as an ameboid morphology with a larger cell body and thickened and shortened processes.30 A 75% increase in microglia per 200×200 μm2 and a 107% increase in activated microglia were observed in the PVN of SHR+CON rats compared with WKY+CON (Figure 4A, 4B). However, these increases were significantly attenuated in both the continuous CAP treatment group and the maternal treatment with CAP group in the PVN (Figure 4A, 4B). Next, we measured proinflammatory cytokines in the PVN. We observed 410% increases in mRNA for tumor necrosis factor (TNF)-α, 357% for interleukin (IL)-1β and 330% for IL-6 in the PVN of SHR+CON rats, compared with WKY+CON rats (Figure 4C). Importantly, these effects were attenuated in both SHR+SCAP and SHR+OCAP groups (Figure 4C), supporting a persistent beneficial effect of maternal CAP exposure on autonomic neuroinflammation in male offspring. In contrast, neither microglia number, their activation, nor the proinflammatory cytokines changed in the PVN of WKY+SCAP and WKY+OCAP (Figure S5).
Figure 4.
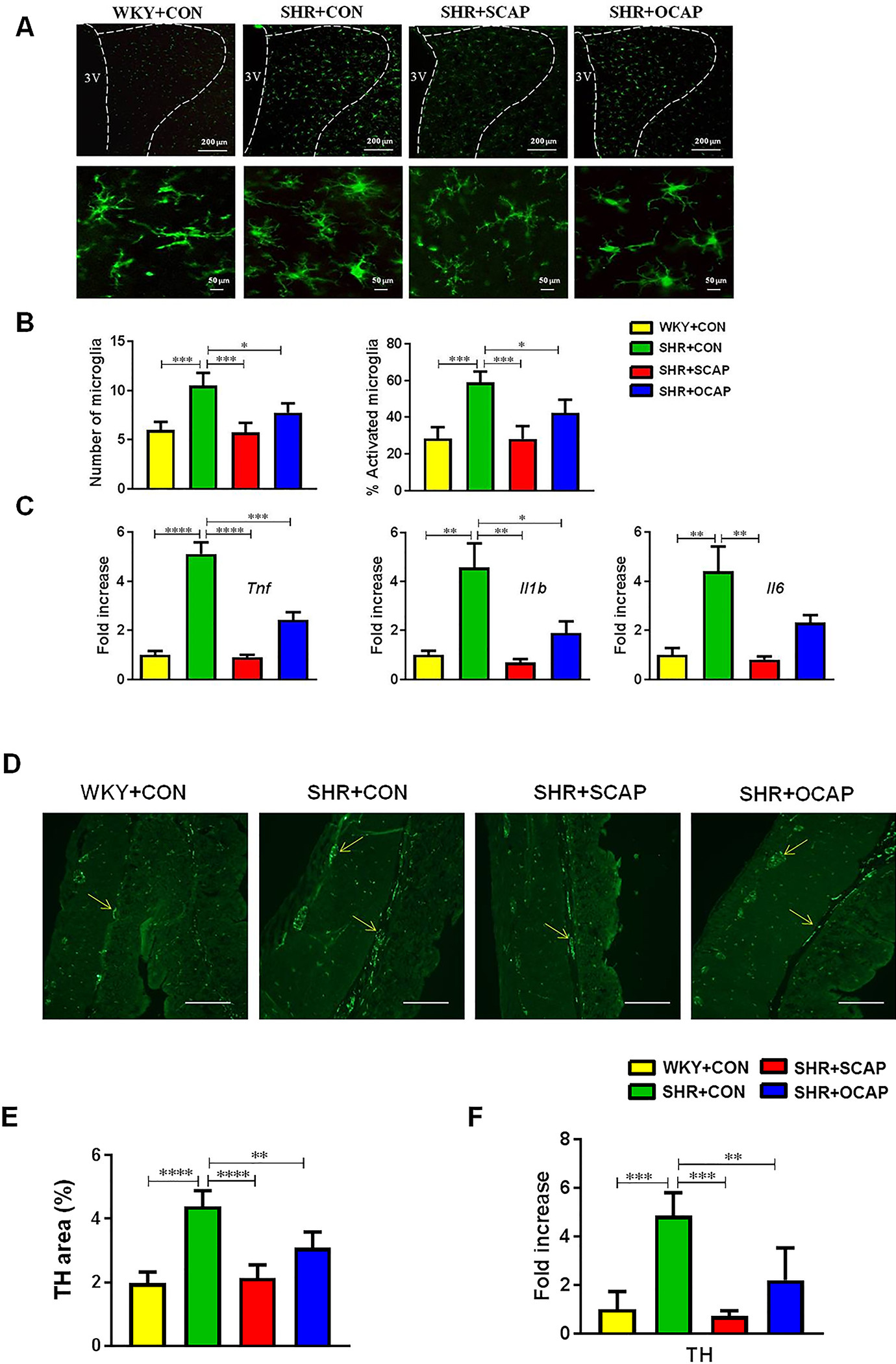
Maternal CAP treatment attenuates microglia activation and neuroinflammation and decreases colonic tyrosine hydroxylase (TH) in SHR male offspring. (A) Representative immunofluorescence images of Iba1 (ionized-calcium binding adaptor molecule 1) staining of microglia in the paraventricular nucleus (PVN). (B) Quantification of microglia within the 200×200 μm2 cell body area of PVN (n=5 rats per group). Activated microglia manifest a more “ameboid” morphology, characterized by larger cell bodies with thickened and shortened processes (n=5 rats per group). (C) The mRNA levels of TNF-α, IL-1β and IL-6 were determined by qPCR and normalized to GAPDH. Data were presented by fold changes over the WKY+CON group. (D) Representative images of TH immunoreactivity. (E) Quantification of TH staining by percentage of TH positive area (n=6–7 rats per group). (F) The mRNA for TH was measured by qPCR and normalized to GAPDH (n=6–7 rats per group). OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n=5 rats per group. Data are represented as mean ± SEM. * P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 in one-way ANOVA followed by Tukey’s multiple comparison. 3V, third ventricle.
To further investigate whether persistent changes in PVN inflammation led to alteration of sympathetic input to the gut of SHR with established HTN, we measured tyrosine hydroxylase (TH), the enzyme that synthesizes norepinephrine in synapses of sympathetic nerves, in the proximal colon. We found an increase in the mean intensity of TH fluorescence (Figure 4D, 4E), and an increase in Th mRNA in the proximal colon of SHR+CON (Figure 4F) compared with those in WKY+CON. Continuous CAP treatment significantly attenuated the increased immunoreactivity of TH (Figure 4D, 4E) and its mRNA (Figure 4F) in the 12-week-old SHR, but not in age- and treatment-matched WKY (Figure S6). Consistently, maternal treatment with CAP attenuated the increase in TH immunoreactivity (Figure 4D, 4E) and mRNA (Figure 4F) in the SHR at 12 weeks of age. However, no changes in gut TH were found in the treatment matched WKY at 12 weeks of age (Figure S6).
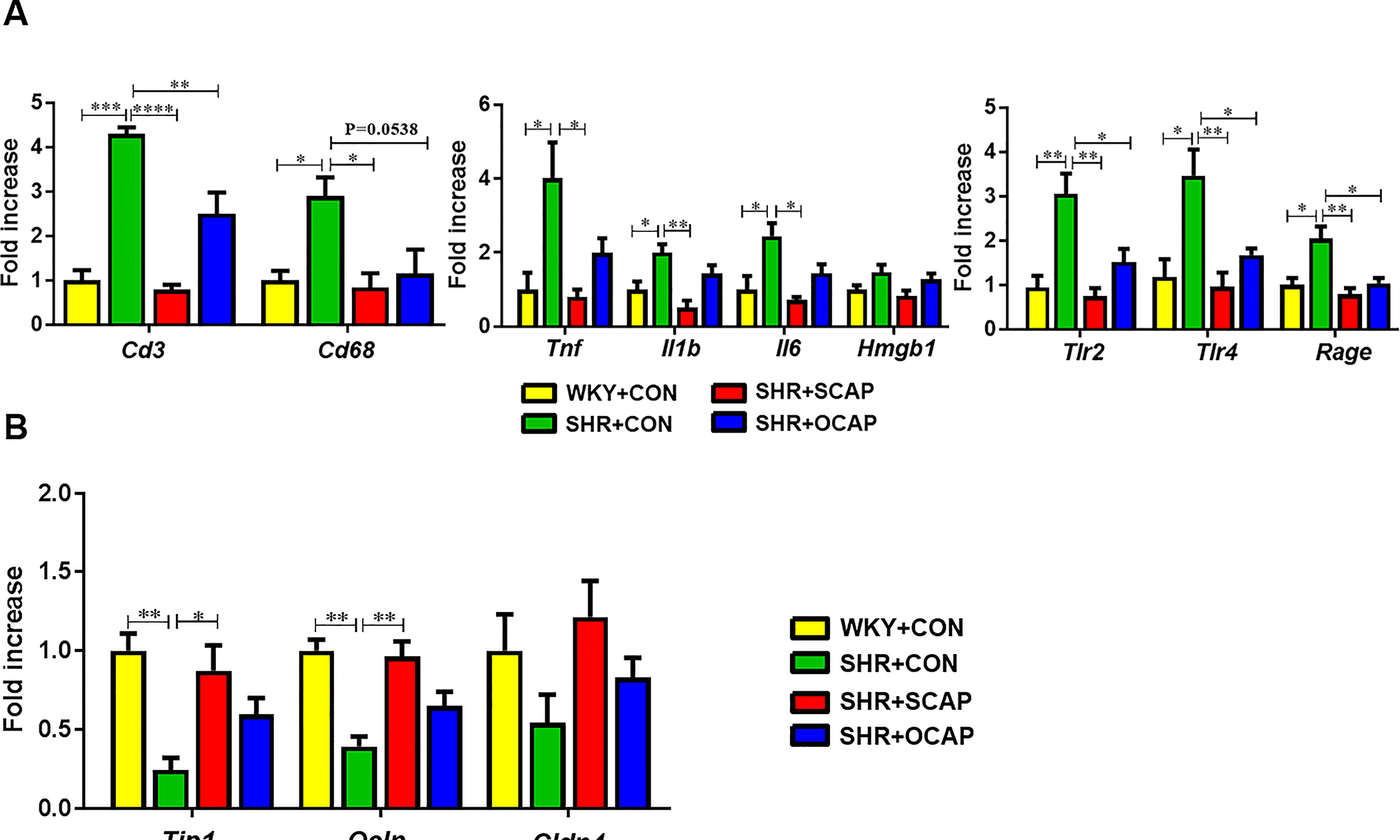
Maternal CAP treatment persistently ameliorates gut inflammation, permeability, and pathology in the SHR male offspring:
Guided by the changes in the sympathetic drive to the gut and gut microbiota, we investigated whether there was any alteration in gut pathology, inflammatory status and/or permeability. At 12 weeks of age, Cd3, Cd68, Tnf, Il1b, Il6, Tlr2 (toll-like receptor 2), Tlr4 (toll-like receptor 4), and Rage (receptor for advanced glycationend-products) were all increased in the proximal colon of SHR+CON, compared with WKY+CON (Figure 5A). Continuous CAP treatment in SHR significantly lowered the expression of these genes (Figure 5A). Importantly, the SHR with only maternal treatment with CAP also exhibited reduced expression of these genes (Figure 5A). Next, we observed decreased mRNA for the tight junction proteins Tjp1 (tight junction protein 1), Ocln (occludin) and Cldn4 (claudin 4) in the proximal colon of SHR (Figure 5B) and mRNA for these tight junction proteins were restored in SHR male offspring exposed to maternal CAP (Figure 5B). SHR with established HTN exhibited reduced expression of Tjp1 and Olcn. Maternal treatment with CAP significantly increased gene expression of these tight junction proteins in the SHR at 12 weeks of age (Figure 5B). Therefore, these data suggest that maternal treatment with CAP improves intestinal immunity and permeability prior to the onset of HTN, and the protective effect was maintained until at least 12 weeks of age. In contrast, neither continuous nor maternal treatment with CAP significantly changed the expression of the aforementioned immunity-related genes in the WKY (Figure S7A). In addition, mRNA of tight junction proteins was not affected by CAP (Figure S7B).
Figure 5.
Maternal CAP treatment reduces mRNA for gut inflammatory and tight junction proteins in SHR male offspring. (A) Immunity-related genes (i.e. Cd3, Cd68, Tnf, Il1b, Il6, Hmgb1, Tlr4 and Tlr2) and (B) tight junction proteins mRNA (i.e. Tjp1, Ocln and Cldn4) in proximal colon of 12-week-old SHR were measured by qPCR and normalized to GAPDH. Data are shown as fold change over the WKY+CON group. OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n= 6 rats per group. Data are mean ± SEM. *P<0.05; **P<0.01; ***P<0.001 in one-way ANOVA followed by Tukey’s multiple comparison.
Finally, we evaluated the effects of maternal CAP on gut pathology. Increases in fibrotic area, thickness of muscle layer, and decrease in numbers of goblet cells per 100 epithelial cells and crypt depth were observed in proximal colon of 12-week-old SHR (Figure 6). Continuous CAP treatment led to a significant reversal in fibrotic area, thickness of muscle layer and increased numbers of goblet cells in SHR+SCAP (Figure 6). Furthermore, maternal treatment with CAP persistently ameliorated gut pathology in SHR+OCAP by decreasing fibrotic area and thickness of muscular layer (Figure 6). However, no differences in the number of goblet cells nor crypt depth were found between SHR+OCAP and SHR+CON (Figure 6). Neither continuous nor maternal treatment with CAP altered gut pathology in the WKY (Figure S8).
Figure 6.
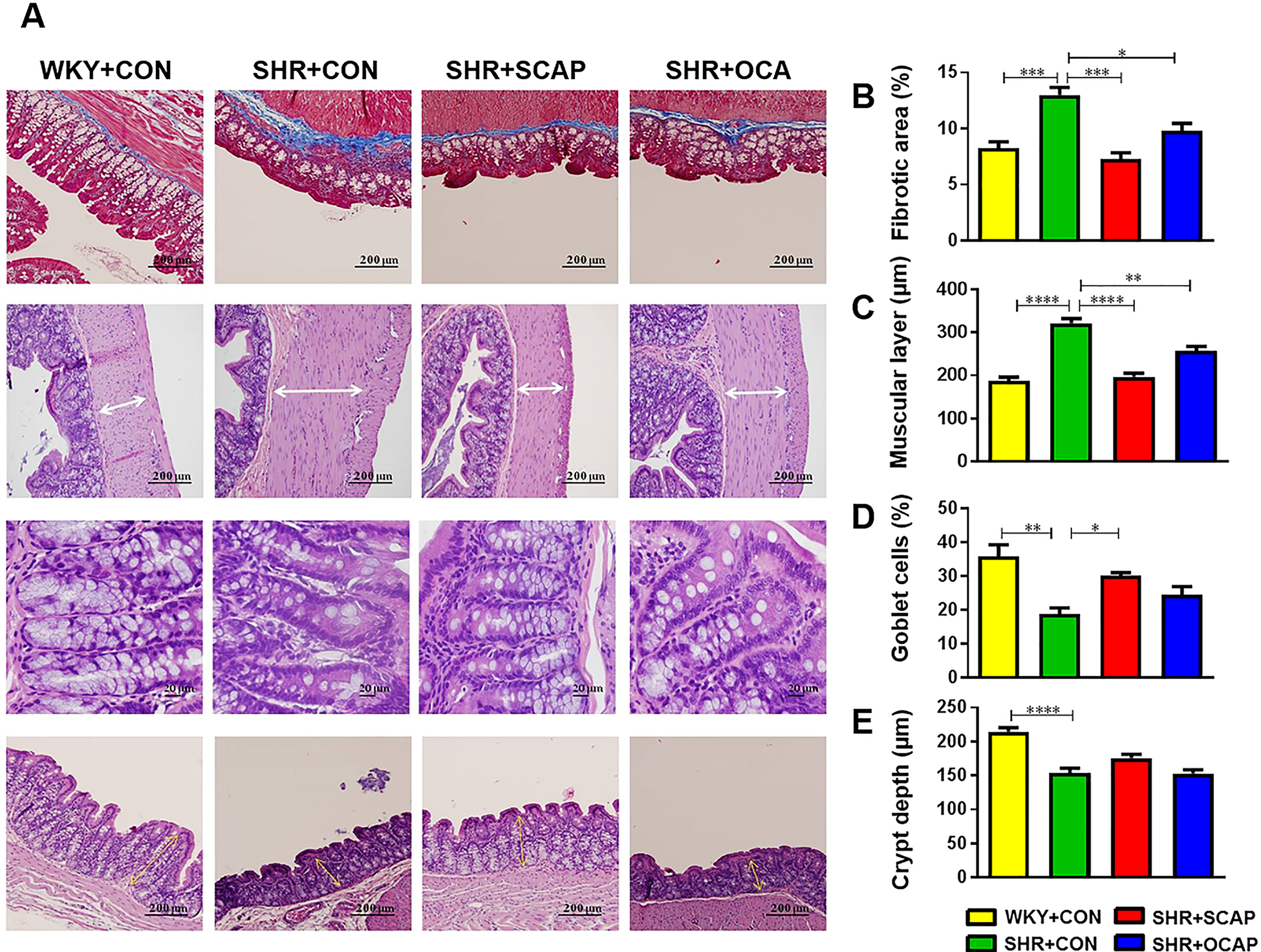
Maternal CAP treatment improves gut pathology in SHR male offspring. (A) Representative hematoxylin-eosin (H&E) and Masson-trichrome staining images of proximal colon of indicated rat groups. (B-E) Quantitative analysis of Masson-trichrome staining to demonstrate the fibrotic area in the proximal colon. Quantitative analysis of H&E staining to demonstrate the thickness of muscle layer, numbers of goblet cells per 100 epithelial cells and crypt depth in the proximal colon. OCAP, maternal treatment with captopril; SCAP, sustained treatment with captopril. n= 5 rats per group. Data are mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 in one-way ANOVA followed by Tukey’s multiple comparison.
In summary, these findings suggest that treatment with CAP via the dam persistently improves intestinal immunity, permeability, and pathology in SHR+OCAP at 12 weeks of age, associated with a bloom of beneficial bacteria, higher microbial diversity and lower BP.
Discussion
The most important observation of this study is that maternal treatment of SHR dams with CAP has beneficial effects on the hypertensive status of their male offspring. This includes lower BP, reshaped gut microbiota, improved gut pathology and decreased neuroinflammation. As a result, male offspring exposed to only maternal CAP exhibit long-term attenuation of HTN. In addition, several features of gut microbial alterations in SHR dams+CAP (i.e. diversity, Clostridiales Order, Erysipelotrichales Order) are comparable to those observed in the SHR+SCAP and SHR+OCAP male offspring. Therefore, we suggest that CAP induces changes in the gut microbial communities that are transmitted and are likely to be accentuated in male offspring. This indicates that amelioration of a dysfunctional gut-brain axis is transmitted from mother to male offspring despite of discontinuation of antihypertensive treatment. These findings shed light on the maternal influence on the gut-brain axis in HTN.
An interesting question is whether high BP causes these changes or alterations in the gut-brain axis is responsible for high BP. FMT from SHR to normotensive rats results in an increase in BP, enhanced autonomic activity, neuroinflammation and impaired vascular function.9,10 In contrast, FMT from WKY rats attenuates these pathophysiologies in SHR.9,10 Similar effects of FMT on BP from hypertensive patients and animals into normal animals have been observed by others.2,8 Therefore, the FMT studies argue in favor of the concept that gut microbiota-initiated changes of the gut-brain axis are responsible for high BP.
Previous studies have demonstrated that altered gut microbiota, gut permeability and immune status coupled with neuroinflammation in the autonomic regions of the brain have significant influence on the development and establishment of HTN.1,5,7,31,32 Treatment with CAP attenuates those changes and lowers BP.5,33 Early studies have also shown that the antihypertensive effect of CAP is maintained in SHR male offspring long after withdrawal of CAP.18 However, knowledge gaps remained regarding the pathways involved, and mechanisms of this prolonged antihypertensive influence. Our present study addresses this gap in knowledge and suggests that maternal imprinting of amelioration of the dysfunctional gut-brain axis could be one such mechanism. Evidence for this conclusion includes: (1) CAP treatment of SHR alters gut microbiota, gut permeability, tight junction proteins and attenuates gut pathology.5,21 In addition, decreases in activated microglia and neuroinflammatory markers by CAP have been documented.33 These alterations are maintained in SHR male offspring even after discontinuation of CAP treatment. (2) Comparable patterns of certain beneficial bacterial phylotypes between CAP-treated dams and their male offspring. (3) Changes in the gut and the brain are associated with attenuation of high BP and cardiac pathology.21 In the gut, we tested the expression of markers for adaptive immune cells (T cells, Cd3), innate immune cells (macrophages, Cd68), pro-inflammatory mediators (Tnf, Il1b, Il6, Hmgb1), and microbial sensors (Tlr4 for gram-negative bacterial lipopolysaccharide, LPS; Tlr2 for gram-positive bacterial lipoteichoic acid, LTA).34 These mediators were significantly suppressed in the SHR male offspring with treatment of CAP. In the brain, significant decreases in microglia activation (morphology) and neuroinflammation (Tnf, Il1b, Il6) in the PVN were demonstrated in the SHR male offspring with CAP treatment.
The precise mechanisms of maternal imprinting of gut-brain axis are unknown but may involve inflammation, nutritional status during pregnancy, genetic inheritance and acquirement of epigenetic modification as have been suggested in metabolic diseases, such as diabetes, obesity, and HTN.12,35–38 Recent evidence has demonstrated that postnatal colonization of the gut by microorganisms plays an important role in priming the health of offspring.39,40 In our study, the SHR dams treated with CAP exhibited certain changes comparable to those in their male offspring, indicating the microbes colonizing the gut of the offspring in the postnatal period are significantly influenced by the dam. We observed that bacterial genera enriched in both SHR+OCAP and SHR+SCAP showed significantly higher abundance in SHR+OCAP group. Coprococcus and Roseburia have protective effects against atherosclerosis and are decreased in both animals and patients with high BP.1,2,41 They are short-chain fatty acid producers, which are also decreased in high salt diet-induced HTN.42 Consistent with this, we observed decreased plasma butyrate in HTN.31,43 Oscillospira has been associated with lean animals and humans.44,45 Oscillospira are beneficial microbes with the capability to metabolize complex carbohydrates that are depleted in rats with AngII-induced hypertension.46 Taken together, the sustained lowering of BP in the SHR+OCAP rats at 12 weeks appears to be, at least partially, attributed to the positive alteration in gut microbiota. However, we do not want to imply that postnatal colonization of the pups with the dam’s gut microbiota is the sole factor responsible for maternal imprinting of HTN. Other mechanisms involving kidney47, blood vessels and heart48, and metabolism49 must also be at play in the antihypertensive effects of CAP, which may explain why SCAP rats show approximately 20mmHg lower BP than OCAP rats.
The observation of significant influence of CAP on gut microbial composition suggests the possibility to repurpose CAP to use as an antibacterial agent. Recent metagenomic analysis of stool samples from 1135 participants from a cohort in the Netherlands showed that use of ACE inhibitors (ACEi), among other drugs, had significant impacts on gut microbiota.50 Some of these associations were replicated in 2700 individuals in the British TwinUK study.51 These alterations in gut microbiota may be acquired because of (1) CAP’s inhibitory effects on MBLs, an enzyme to hydrolyze most β-lactam antibiotics22 and DapE, an exclusively bacterial enzyme for cell wall protein synthesis.23 (2) CAP’s inhibitory effects on P-glycoprotein 1 and its bacterial homolog ATP Binding Cassette (ABC) transporters.52 In addition, other classes of antihypertensives such as ARBs, beta-blockers, prazosin and verapamil also influence ABC transporters53 and subsequently increase CAP bioavailability. Therefore, further studies are needed to test whether the repurposing of CAP as an antibacterial agent contributes to its transgenerational antihypertensive effects in offspring.
In conclusion, maternal CAP treatment persistently alters the gut-brain axis and attenuates hypertension of male offspring. This prolonged effect may be partially mediated by modulation of the gut microbiota by CAP. Thus, validated repurposing/redesigning of a safer derivative of captopril for gut microbiota modulation could be a valuable strategy to consider for HTN treatment.
Perspectives
Our current study demonstrates the impacts of captopril, an antihypertensive angiotensin-converting enzyme inhibitor (ACEi) with potential antibacterial activity, on the gut microbiota of pregnant hypertensive dams, and its maternal beneficial impacts on the gut-brain axis and blood pressure of the male offspring. Our study suggests that the dual actions (i.e. antibacterial, blood pressure-lowering) of a safe and repurposed captopril or its derivatives could be a major advancement in providing transgenerational protection against hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
The repurposing of the FDA approved drugs has become an emerging field of study. Our current study demonstrates the impact of captopril, an antihypertensive angiotensin-converting enzyme inhibitor (ACEi) with potential antibacterial activity, on the gut microbiota of pregnant hypertensive dams, and its maternal beneficial impact on the gut-brain axis and blood pressure of the male offspring.
What Is Relevant?
Our study provides the foundation for repurposing of a safe and more effective captopril. This is based on current knowledge of the role of the gut microbiota in hypertension including transmission of the gut microbiota from mother to offspring. Our data support the concept that targeting the microbiota during pregnancy is beneficial for the offspring cardiovascular health.
Summary
The repurposing of FDA-approved drugs has become a significant field of study. Our data show that featured changes in gut microbiota in dams treated with captopril are inherited in male offspring, which are associated with beneficial alterations in the gut-brain axis. Thus, our study suggests that the dual actions (i.e. antibacterial for gut microbiota, blood pressure-lowering for cardiovascular health) of safe repurposed antihypertensives to lower blood pressure could be a major advancement in providing transgenerational protection against hypertension.
Sources of Funding
This study was supported by NIH grants R01 HL132448 and HL033610 to MKR, National Natural Science Foundation of China Nos. 81700373 to HL, and Biocodex Foundation USA grant to TY.
Footnotes
Conflict of interest
All authors confirm that there are no conflicts of interest.
Reference
- 1.Yang T, Santisteban MM, Rodriguez V, et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015;65(6):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Q, Gu Y, Li X, et al. Alterations of the Gut Microbiome in Hypertension. Front Cell Infect Microbiol. 2017;7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mell B, Jala VR, Mathew AV, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santisteban MM, Qi Y, Zubcevic J, et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 2017;120(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi P, Grobe JL, Desland FA, et al. Direct pro-inflammatory effects of prorenin on microglia. PLoS One. 2014;9(10):e92937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santisteban MM, Ahmari N, Carvajal JM, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117(2):178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toral M, Robles-Vera I, de la Visitación N, et al. Role of the immune system in vascular function and blood pressure control induced by fecal microbiota transplantation in rats. Acta Physiol (Oxf). 2019:e13285. [DOI] [PubMed] [Google Scholar]
- 10.Toral M, Robles-Vera I, de la Visitación N, et al. Critical Role of the Interaction Gut Microbiota - Sympathetic Nervous System in the Regulation of Blood Pressure. Front Physiol. 2019;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng T, Zhang J, Sommer K, et al. Effects of Environmental Exposures on Fetal and Childhood Growth Trajectories. Ann Glob Health. 2016;82(1):41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaeger K, Saben JL, Moley KH. Transmission of Metabolic Dysfunction Across Generations. Physiology (Bethesda). 2017;32(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu ST, Niu X, Raetz M, Savani RC, Hooper LV, Mirpuri J. Maternal high-fat diet results in microbiota-dependent expansion of ILC3s in mice offspring. JCI Insight. 2018;3(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasinger JH, Davis GK, Newsome AD, Alexander BT. Developmental Programming of Hypertension: Physiological Mechanisms. Hypertension. 2016;68(4):826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T, Zubcevic J. Gut-Brain Axis in Regulation of Blood Pressure. Front Physiol. 2017;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berecek KH, Reaves P, Raizada M. Effects of early perturbation of the renin-angiotensin system on cardiovascular remodeling in spontaneously hypertensive rats. Vascul Pharmacol. 2005;42(3):93–98. [DOI] [PubMed] [Google Scholar]
- 19.Wu JN, Berecek KH. Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension. 1993;22(2):139–146. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Edwards DG, Berecek KH. Effects of early captopril treatment and its removal on plasma angiotensin converting enzyme (ACE) activity and arginine vasopressin in hypertensive rats (SHR) and normotensive rats (WKY). Clin Exp Hypertens. 1996;18(2):201–226. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Aquino V, Lobaton GO, et al. Sustained Captopril-Induced Reduction in Blood Pressure Is Associated With Alterations in Gut-Brain Axis in the Spontaneously Hypertensive Rat. J Am Heart Assoc. 2019;8(4):e010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusof Y, Tan DTC, Arjomandi OK, Schenk G, McGeary RP. Captopril analogues as metallo-β-lactamase inhibitors. Bioorg Med Chem Lett. 2016;26(6):1589–1593. [DOI] [PubMed] [Google Scholar]
- 23.Dutta D, Mishra S. L-Captopril and its derivatives as potential inhibitors of microbial enzyme DapE: A combined approach of drug repurposing and similarity screening. J Mol Graph Model. 2018;84:82–89. [DOI] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma RK, Oliveira AC, Kim S, et al. Involvement of Neuroinflammation in the Pathogenesis of Monocrotaline-Induced Pulmonary Hypertension. Hypertension. 2018;71(6):1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.al-Shabanah OA, al-Harbi MM, alGharably NM, Islam MW. The effect of maternal administration of captopril on fetal development in rat. Res Commun Chem Pathol Pharmacol. 1991;73(2):221–230. [PubMed] [Google Scholar]
- 28.Park S, Shin J, Hong Y, et al. Forced exercise enhances functional recovery after focal cerebral ischemia in spontaneously hypertensive rats. Brain Sci. 2012;2(4):483–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62(12):1111–1118. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Arjona MDM, Grondona JM, Granados-Durán P, Fernández-Llebrez P, López-Ávalos MD. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front Cell Neurosci. 2017;11:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond). 2018;132(6):701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zubcevic J, Santisteban MM, Perez PD, et al. A Single Angiotensin II Hypertensive Stimulus Is Associated with Prolonged Neuronal and Immune System Activation in Wistar-Kyoto Rats. Front Physiol. 2017;8:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asraf K, Torika N, Apte RN, Fleisher-Berkovich S. Microglial Activation Is Modulated by Captopril:. Front Cell Neurosci. 2018;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. [DOI] [PubMed] [Google Scholar]
- 35.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, EpiSCOPE Mo. Epigenetics and human obesity. Int J Obes (Lond). 2015;39(1):85–97. [DOI] [PubMed] [Google Scholar]
- 36.Bosse JD, Lin HY, Sloan C, et al. A low-carbohydrate/high-fat diet reduces blood pressure in spontaneously hypertensive rats without deleterious changes in insulin resistance. Am J Physiol Heart Circ Physiol. 2013;304(12):H1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doris PA. Genetics of hypertension: an assessment of progress in the spontaneously hypertensive rat. Physiol Genomics. 2017;49(11):601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sales VM, Ferguson-Smith AC, Patti ME. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017;25(3):559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167(4):915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. [DOI] [PubMed] [Google Scholar]
- 41.Mehta N, Jiv Singh Nagpal S, Confer B, Vargo J, Bhatt A. Migration and Erosion of Cervical Spine Hardware into the Esophageal Lumen Causing Odynophagia and Dysphagia. ACG Case Rep J. 2016;3(4):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bier A, Braun T, Khasbab R, et al. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, Magee KL, Colon-Perez LM, et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol (Oxf). 2019;226(2):e13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konikoff T, Gophna U. Oscillospira: a Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016;24(7):523–524. [DOI] [PubMed] [Google Scholar]
- 46.Sharma RK, Yang T, Oliveira AC, et al. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circ Res. 2019;124(5):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumbers ER, Burrell JH, Menzies RI, Stevens AD. The effects of a converting enzyme inhibitor (captopril) and angiotensin II on fetal renal function. Br J Pharmacol. 1993;110(2):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeffer JM, Pfeffer MA, Mirsky I, Braunwald E. Prevention of the development of heart failure and the regression of cardiac hypertrophy by captopril in the spontaneously hypertensive rat. Eur Heart J. 1983;4 Suppl A:143–148. [DOI] [PubMed] [Google Scholar]
- 49.de Kloet AD, Krause EG, Kim DH, Sakai RR, Seeley RJ, Woods SC. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009;150(9):4114–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson MA, Verdi S, Maxan ME, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9(1):2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61(25):2495–2502. [DOI] [PubMed] [Google Scholar]
- 53.Choi MS, Yu JS, Yoo HH, Kim DH. The role of gut microbiota in the pharmacokinetics of antihypertensive drugs. Pharmacol Res. 2018;130:164–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


