Abstract
Background:
Characterization of breast cancer phenotypes has improved our ability to predict breast cancer behavior. Triple negative (TN) breast cancers have higher and earlier rates of distant events. It has been suggested that this behavior necessitates treating TNs faster than others, including use of neoadjuvant chemotherapy (NACT) if time to surgery is not rapid.
Methods:
A review of women diagnosed with noninflammatory, invasive breast cancer was conducted using the National Cancer Database for patients not having NACT, diagnosed between 2010 and 2014. Changes in overall survival due to delay were measured by phenotype.
Results:
351,087 patients met inclusion criteria, including 36,505 (10.4%) TNs, 77.9% hormone receptor-positive (HR+) and 11.7% HER2−enriched (HER2+). Phenotype, among other factors, was predictive of treatment delays. Adjusted median days from diagnosis to surgery and chemotherapy were 29.9, 31.6 and 31.5 (p<0.001) and 72.7, 78.0 and 74.4 (p<0.001) for TNs, HR+ and HER2+ cancers, respectively. After diagnosis, OS declined for all patients per month of preoperative delay (HR 1.104, p<0.001). In models separating or combining surgery and chemotherapy, this survival decline did not vary by breast cancer phenotype (p-values >0.3).
Conclusions:
Delays cause small but measurable effects overall, but the effect on survival does not differ among breast cancer phenotypes. Our data suggests that urgency between diagnosis and surgery or chemotherapy is similar for breast cancers of different subtypes. Although NACT is sometimes advocated solely to avoid treatment delays, this study does not suggest a greater surgical urgency for TNs compared with other breast cancer phenotypes.
INTRODUCTION
Breast cancer treatment in the United States requires a coordinated, multidisciplinary team effort to provide comprehensive care to patients. Although outcomes in breast cancer are improving, so is the complexity of its evaluation and management. Increasing numbers of physician appointments,1 imaging studies,2 second opinions sought,3 and even multidisciplinary care itself4 undoubtedly each have effects on the timeliness of this process and are the likely reason that the time to surgery has been increasing.5 The time to treatment from breast cancer diagnosis has also been proposed as a safety measure for facilities.6 Although there is no standard for time between diagnosis and surgery, minimization of delays between breast cancer diagnosis and treatment are perceived to be important because of the detrimental effects on outcomes that delays confer.7
Breast tumor phenotype has been found to correlate with outcomes and breast cancer behavior8 and is increasingly being used to guide treatment. Although phenotype, as assessed from histologic characteristics, does not completely correlate with tumor genotypes, the approximations made from the hormone receptor (HR), and HER2 status provide sufficient correlation to be used in clinical practice. Specifically, histologic differentiation between triple negative (TN), hormone receptor positive (HR+), and HER2−enriched (HER2+) breast cancers allows for tailoring of treatments for differing tumor behaviors.
TN tumors, in particular, are felt to be more aggressive because of their greater propensity to metastasize within the first three years. Although standards of care for surgical treatment between TN and other phenotypes do not differ,9 concerns may exist regarding the timing of treatment. In particular, there has been some suggestion that with delayed initiation of adjuvant chemotherapy, outcomes vary by tumor subtype.10 Concerns about delays in adjuvant chemotherapy may therefore translate into concerns about time to locoregional therapy, both because of the biological aggressiveness of the tumor, and because of some potential relationship between the timing of surgery and postoperative chemotherapy. Moreover, a need for neoadjuvant chemotherapy (NACT) could be proposed solely because of the perceived urgency of treatment. This study was thus performed to assess whether delays in time to surgery affect tumors of different phenotypes differently. If delays in the time to breast cancer surgery affect TN tumors more adversely than other subtypes, then NACT could be considered appropriate solely if it reduces the time to treatment.
METHODS
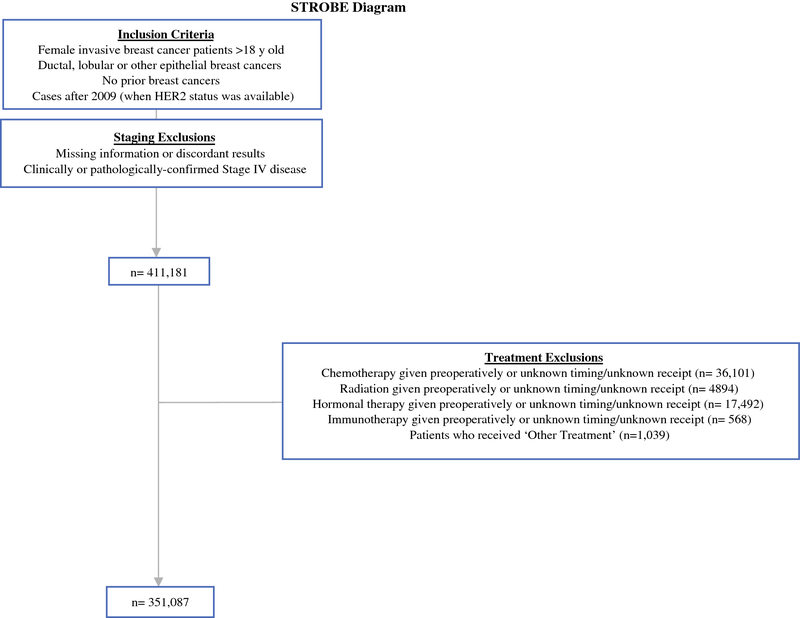
Data was retrieved from the National Cancer Database (NCDB) after receiving approval from our institutional review board (IRB) and the American College of Surgeons. The NCDB is a nationwide dataset that captures 66% of all breast cancer in the United States at Commission on Cancer (CoC) accredited hospitals.11 Women diagnosed with Stage I-III breast cancer were reviewed. Exclusion criteria included male patients, inflammatory breast cancer, in situ or metastatic disease, prior cancers, receipt of NACT, radiation therapy, hormone treatment or immunotherapy. As there is no definition of “delay”, here the term is used to refer to a longer time to treatment. NACT patients were excluded in order to provide a purer cohort, and to eliminate artificial magnification of the effect of delays on survival, as these patients have both, much longer times to surgery, and typically have a selection bias for far worse outcomes. Patients with missing diagnosis or treatment information were also excluded (Figure 1).
Figure 1.
Cohort inclusion and exclusion criteria
Breast cancer phenotypes were characterized as TN (ER−, PR−, HER2−); HR+ (ER+ and/or PR+, HER2−); and HER2+ (ER+/−, PR+/−, HER2+). Delays were analyzed continuously as days from diagnosis to surgery and surgery to chemotherapy, while all other variables were categorical. Follow up was calculated by the reverse Kaplan-Meier method.
Overall survival (OS) was used because the NCDB does not capture disease-specific survival, and because OS was felt to be the outcome of interest as it captures competing risks and mortality from treatment. The effect of surgical delay was assessed in the full cohort, while the effect of delay to chemotherapy was tested in the subset of patients who received chemotherapy. Because times to adjuvant chemotherapy have been suggested to affect TN tumors differently, two models were developed to assess OS in the chemotherapy subgroup. The first model included time from diagnosis to surgery, and separately included time from surgery to chemotherapy. The second model utilized one single time span from diagnosis (through surgery) to chemotherapy. Chemotherapy refers to chemotherapy and immunotherapy in order to incorporate HER2− directed therapy. Each model was run separately to individually assess the effect of time to surgery on OS.
Patients were grouped into 3 phenotypes: TN, HR+, and HER2+. Covariates of interest included patient, tumor, treatment, and treating facility characteristics. The association between covariates and phenotype were assessed via Chi-squared tests. Time until surgery was categorized as ≤30, 31–60, 61–90, and 91–180 days. We used ordinal logistic regression to assess the association between phenotype and delay group, adjusting for all pre-specified covariates of interest. Mixed effects models were used with a random intercept for facility ID, to account for clustering within facility. The effect of phenotype, delay, and the interaction between phenotype and delay on OS was assessed using Cox regression models, which adjusted for clustering using robust standard errors. Median adjusted delay by phenotype and patient characteristics was calculated based on quantile regression models. All statistical analyses were performed in SAS (version 9.4) or Stata (version 13).
RESULTS
Among 351,087 patients fulfilling inclusion criteria (Figure 1), 36,505 (10.5%) were TN, 273,521 (77.9%) were HR+, and 41,061 (11.7%) were HER2+. Mean and median times between diagnosis and surgery were 33.3 and 29 days, 35.5 and 31 days, and 35.3 and 30 days, respectively (p<0.001). Cohort characteristics by phenotype are shown in Table 1. TN and HER2+ tumors accounted for a greater proportion of tumors as age declined, from 9% of TN in those ≥70 to 12% in those <50, while HER2+ increased from 9% in those ≥70 to 16% in those <50. TN tumors accounted for 21% of cancers in Blacks, but only 9% in all other race categories, and comprised 11.1% of cancers in those having Hispanic ethnicity, and 10.4% of others. The proportion of tumors diagnosed that were TN declined from 11.7% in 2010 to 9.0% in 2014. Although 11.0% of ductal tumors were TN, only 1.1% of lobular tumors were TN. Tumor size was predominantly between 1 and 2 cm and either Stage I or II in TN, HR+, and HER2+ groups (p<0.001, Table 1), and breast conservation was elected in over 50% of patients across all phenotypes.
Table 1.
Cohort Characteristics by Phenotype
| Triple Negative | ER+ and/or PR +, HER2− | HER2 + | Total | P-value* | ||
|---|---|---|---|---|---|---|
| Age | <50 | 8,124 (22.3) | 48,458 (17.7) | 10,391 (25.3) | 66,973 (19.1) | <0.001 |
| 50–59 | 9,585 (26.3) | 65,305 (23.9) | 11,589 (28.2) | 86,479 (24.6) | ||
| 60–69 | 9,687 (26.5) | 80,324 (29.4) | 10,333 (25.2) | 100,344 (28.6) | ||
| ≥70 | 9,109 (25.0) | 79,434 (29.0) | 8,748 (21.3) | 97,291 (27.7) | ||
| Race | White | 27,808 (76.2) | 237,228 (86.7) | 33,863 (82.5) | 298,899 (85.1) | <0.001 |
| Black | 7,127 (19.5) | 22,920 (8.4) | 4,671 (11.4) | 34,718 (9.9) | ||
| Asian | 991 (2.7) | 8,581 (3.1) | 1,733 (4.2) | 11,305 (3.2) | ||
| Other/Unknown | 579 (1.6) | 4,792 (1.8) | 794 (1.9) | 6,165 (1.8) | ||
| Hispanic | No | 33,397 (91.5) | 251,757 (92.0) | 37,387 (91.1) | 322,541 (91.9) | <0.001 |
| Yes | 1,857 (5.1) | 12,538 (4.6) | 2,344 (5.7) | 16,739 (4.8) | ||
| Unknown | 1,251 (3.4) | 9,226 (3.4) | 1,330 (3.2) | 11,807 (3.4) | ||
| Insurance Status | Medicaid | 2,715 (7.4) | 14,345 (5.2) | 2,982 (7.3) | 20,042 (5.7) | <0.001 |
| Medicare | 12,999 (35.6) | 108,774 (39.8) | 12,761 (31.1) | 134,534 (38.3) | ||
| Not Insured | 804 (2.2) | 4,316 (1.6) | 934 (2.3) | 6,054 (1.7) | ||
| Other Government | 423 (1.2) | 2,756 (1.0) | 446 (1.1) | 3,625 (1.0) | ||
| Private Insurance/Managed Care | 19,137 (52.4) | 140,428 (51.3) | 23,430 (57.1) | 182,995 (52.1) | ||
| Unknown | 427 (1.2) | 2,902 (1.1) | 508 (1.2) | 3,837 (1.1) | ||
| Education | ≥ 21% | 6,173 (16.9) | 35,322 (12.9) | 6,237 (15.2) | 47,732 (13.6) | <0.001 |
| 13% - 20.9% | 9,363 (25.6) | 62,778 (23.0) | 10,063 (24.5) | 82,204 (23.4) | ||
| 7%−12.9% | 11,903 (32.6) | 91,987 (33.6) | 13,500 (32.9) | 117,390 (33.4) | ||
| < 7% | 8,991 (24.6) | 82,939 (30.3) | 11,171 (27.2) | 103,101 (29.4) | ||
| Missing | 75 (0.2) | 495 (0.2) | 90 (0.2) | 660 (0.2) | ||
| Income | <$38,000 | 6,555 (18.0) | 37,127 (13.6) | 6,356 (15.5) | 50,038 (14.3) | <0.001 |
| $38,000 - $47,999 | 8,263 (22.6) | 57,696 (21.1) | 8,854 (21.6) | 74,813 (21.3) | ||
| $48,000 - $62,999 | 9,800 (26.8) | 73,943 (27.0) | 11,137 (27.1) | 94,880 (27.0) | ||
| $63,000 + | 11,800 (32.3) | 104,168 (38.1) | 14,608 (35.6) | 130,576 (37.2) | ||
| Missing | 87 (0.2) | 587 (0.2) | 106 (0.3) | 780 (0.2) | ||
| Setting | Large metropolitan | 18,958 (51.9) | 145,216 (53.1) | 21,666 (52.8) | 185,840 (52.9) | <0.001 |
| Small metropolitan | 11,318 (31.0) | 84,009 (30.7) | 12,484 (30.4) | 107,811 (30.7) | ||
| Suburban | 3,419 (9.4) | 23,586 (8.6) | 3,672 (8.9) | 30,677 (8.7) | ||
| Rural | 1,935 (5.3) | 13,694 (5.0) | 2,178 (5.3) | 17,807 (5.1) | ||
| Unknown | 875 (2.4) | 7,016 (2.6) | 1,061 (2.6) | 8,952 (2.5) | ||
| Miles to reporting facility | ≤10 | 19,948 (54.6) | 149,389 (54.6) | 21,938 (53.4) | 191,275 (54.5) | <0.001 |
| 11–20 | 8,600 (23.6) | 65,383 (23.9) | 9,968 (24.3) | 83,951 (23.9) | ||
| 21–40 | 4,640 (12.7) | 34,344 (12.6) | 5,364 (13.1) | 44,348 (12.6) | ||
| >40 | 3,180 (8.7) | 23,618 (8.6) | 3,637 (8.9) | 30,435 (8.7) | ||
| Unknown | 137 (0.4) | 787 (0.3) | 154 (0.4) | 1,078 (0.3) | ||
| Transition (new algorithm) | No | 18,073 (49.5) | 141,375 (51.7) | 20,316 (49.5) | 179,764 (51.2) | <0.001 |
| Yes | 11,090 (30.4) | 82,285 (30.1) | 12,514 (30.5) | 105,889 (30.2) | ||
| Unknown | 7,342 (20.1) | 49,861 (18.2) | 8,231 (20.0) | 65,434 (18.6) | ||
| Facility Volume | 1st quartile: 0–45 pts/year | 2,338 (6.4) | 15,951 (5.8) | 2,617 (6.4) | 20,906 (6.0) | <0.001 |
| 2nd quartile: 46–82 pts/year | 5,410 (14.8) | 37,598 (13.7) | 6,133 (14.9) | 49,141 (14.0) | ||
| 3rd quartile: 83–152 pts/year | 9,257 (25.4) | 67,027 (24.5) | 10,172 (24.8) | 86,456 (24.6) | ||
| 4th quartile: >152 pts/year | 19,500 (53.4) | 152,945 (55.9) | 22,139 (53.9) | 194,584 (55.4) | ||
| Year of diagnosis | 2010 | 6,899 (18.9) | 44,747 (16.4) | 7,233 (17.6) | 58,879 (16.8) | <0.001 |
| 2011 | 7,552 (20.7) | 51,301 (18.8) | 8,004 (19.5) | 66,857 (19.0) | ||
| 2012 | 7,532 (20.6) | 55,521 (20.3) | 8,297 (20.2) | 71,350 (20.3) | ||
| 2013 | 7,491 (20.5) | 59,954 (21.9) | 8,751 (21.3) | 76,196 (21.7) | ||
| 2014 | 7,031 (19.3) | 61,998 (22.7) | 8,776 (21.4) | 77,805 (22.2) | ||
| Charlson score | 0 | 29,608 (81.1) | 225,958 (82.6) | 34,416 (83.8) | 289,982 (82.6) | <0.001 |
| 1 | 5,490 (15.0) | 38,894 (14.2) | 5,436 (13.2) | 49,820 (14.2) | ||
| 2 | 1,086 (3.0) | 6,915 (2.5) | 946 (2.3) | 8,947 (2.5) | ||
| ≥3 | 321 (0.9) | 1,754 (0.6) | 263 (0.6) | 2,338 (0.7) | ||
| Histology | Ductal | 33,509 (91.8) | 239,127 (87.4) | 39,096 (95.2) | 311,732 (88.8) | <0.001 |
| Lobular | 382 (1.0) | 31,588 (11.5) | 1,339 (3.3) | 33,309 (9.5) | ||
| Other | 2,614 (7.2) | 2,806 (1.0) | 626 (1.5) | 6,046 (1.7) | ||
| Tumor Size | <10mm | 5,571 (15.3) | 68,993 (25.2) | 8,074 (19.7) | 82,638 (23.5) | <0.001 |
| 10–19mm | 12,906 (35.4) | 114,689 (41.9) | 14,587 (35.5) | 142,182 (40.5) | ||
| ≥20mm | 17,569 (48.1) | 86,789 (31.7) | 17,215 (41.9) | 121,573 (34.6) | ||
| Unknown | 459 (1.3) | 3,050 (1.1) | 1,185 (2.9) | 4,694 (1.3) | ||
| Grade/Differentiation | Well | 746 (2.0) | 84,099 (30.7) | 2,253 (5.5) | 87,098 (24.8) | <0.001 |
| Moderate | 6,035 (16.5) | 131,592 (48.1) | 14,290 (34.8) | 151,917 (43.3) | ||
| Poor | 27,769 (76.1) | 43,706 (16.0) | 21,991 (53.6) | 93,466 (26.6) | ||
| Undifferentiated/Anaplastic | 175 (0.5) | 264 (0.1) | 148 (0.4) | 587 (0.2) | ||
| Unknown | 1,780 (4.9) | 13,860 (5.1) | 2,379 (5.8) | 18,019 (5.1) | ||
| Analytic Stage | I | 18,100 (49.6) | 174,031 (63.6) | 21,344 (52.0) | 213,475 (60.8) | <0.001 |
| II | 15,472 (42.4) | 81,398 (29.8) | 15,117 (36.8) | 111,987 (31.9) | ||
| III | 2,933 (8.0) | 18,092 (6.6) | 4,600 (11.2) | 25,625 (7.3) | ||
| Surgery | Breast Conservation | 21,491 (58.9) | 177,485 (64.9) | 20,696 (50.4) | 219,672 (62.6) | <0.001 |
| Mastectomy | 10,236 (28.0) | 57,339 (21.0) | 12,494 (30.4) | 80,069 (22.8) | ||
| Mastectomy with Reconstruction | 4,778 (13.1) | 38,697 (14.1) | 7,871 (19.2) | 51,346 (14.6) | ||
| Chemotherapy | Yes | 27,145 (74.4) | 72,160 (26.4) | 30,380 (74.0) | 129,685 (36.9) | <0.001 |
| No | 9,360 (25.6) | 201,361 (73.6) | 10,681 (26.0) | 221,402 (63.1) | ||
| Radiation | Yes | 22,660 (62.1) | 177,546 (64.9) | 23,845 (58.1) | 224,051 (63.8) | <0.001 |
| No | 13,845 (37.9) | 95,975 (35.1) | 17,216 (41.9) | 127,036 (36.2) | ||
| Endocrine therapy | Yes | 1,005 (2.8) | 236,233 (86.4) | 25,494 (62.1) | 262,732 (74.8) | <0.001 |
| No | 35,500 (97.2) | 37,288 (13.6) | 15,567 (37.9) | 88,355 (25.2) | ||
| Immunotherapy | Yes | 204 (0.6) | 992 (0.4) | 11,431 (27.8) | 12,627 (3.6) | <0.001 |
| No | 36,301 (99.4) | 272,529 (99.6) | 29,630 (72.2) | 338,460 (96.4) | ||
| Nodes examined | 0–1 | 7,531 (20.6) | 67,945 (24.8) | 8,026 (19.5) | 83,502 (23.8) | <0.001 |
| 2 | 7,065 (19.4) | 58,560 (21.4) | 7,755 (18.9) | 73,380 (20.9) | ||
| 3–4 | 8,763 (24.0) | 65,677 (24.0) | 9,183 (22.4) | 83,623 (23.8) | ||
| ≥5 | 12,904 (35.3) | 79,839 (29.2) | 15,814 (38.5) | 108,557 (30.9) | ||
| Unknown | 242 (0.7) | 1,500 (0.5) | 283 (0.7) | 2,025 (0.6) | ||
| Nodes positive | 0 | 26,902 (73.7) | 197,054 (72.0) | 27,115 (66.0) | 251,071 (71.5) | <0.001 |
| ≥1 | 8,572 (23.5) | 66,301 (24.2) | 12,926 (31.5) | 87,799 (25.0) | ||
| No nodes examined | 944 (2.6) | 9,707 (3.5) | 928 (2.3) | 11,579 (3.3) | ||
| Unknown | 87 (0.2) | 459 (0.2) | 92 (0.2) | 638 (0.2) | ||
| *Chi-square test |
Cohort characteristics divided by delay group are presented in e Table 1. TN tumors had a median delay of 29.95 days (CI95% 29.68, 30.22), compared with 31.56 days for HR+, (CI95% 31.48, 31.65; p<0.001) and 31.50 days for HER2+ (CI95% 31.29, 31.71; p<0.001), with other adjusted times to surgery enumerated in e Table 2. Predictors of time to surgery are found in Table 2. TN tumors had a shorter time to surgery than HR+ and HER2+ tumors (OR 0.82, CI95% 0.79, 0.84; p<0.001). The greatest predictors of longer time to surgery included performance of mastectomy with reconstruction (OR 2.99, CI95% 2.92, 3.07; p<0.001), Medicaid insurance (OR 1.76, CI95% 1.71,1.82; p<0.001), Black race (OR 1.71, CI95% 1.67, 1.76; p<0.001), other government insurance (OR 1.34, CI95% 1.25, 1.43; p<0.001), and mastectomy without reconstruction (OR 1.36, CI95% 1.32, 1.39; p<0.001). The greatest predictor of shorter time to surgery was for patients having no comorbidities (OR 0.7, CI95% 0.64,0.76; p<0.001).
Table 2.
Predictors of Delay to Surgery
| Odds Ratio Estimates | |||||
|---|---|---|---|---|---|
| Estimate | 95% Confidence Limits | P-value | |||
| Phenotype | HER2+ | REF | |||
| TN | 0.82 | 0.79 | 0.84 | <0.001 | |
| HR+ | 1.01 | 0.98 | 1.03 | 0.625 | |
| Age | <50 | REF | |||
| 50–59 | 0.98 | 0.96 | 1.00 | 0.047 | |
| 60–69 | 0.96 | 0.94 | 0.98 | 0.001 | |
| ≥ 70 | 0.84 | 0.81 | 0.86 | <0.001 | |
| Race | White | REF | |||
| Black | 1.71 | 1.67 | 1.76 | <0.001 | |
| Asian | 1.03 | 0.99 | 1.07 | 0.183 | |
| Other/Unknown | 1.11 | 1.05 | 1.17 | 0.002 | |
| Hispanic | No | REF | |||
| Yes | 1.3 | 1.26 | 1.35 | <0.001 | |
| Unknown | 0.97 | 0.93 | 1.02 | 0.192 | |
| Insurance | Private Insurance | REF | |||
| Medicaid | 1.76 | 1.71 | 1.82 | <0.001 | |
| Medicare | 1.12 | 1.09 | 1.14 | <0.001 | |
| Other Government | 1.34 | 1.25 | 1.43 | <0.001 | |
| Not Insured | 1.44 | 1.36 | 1.52 | <0.001 | |
| Unknown | 1.13 | 1.05 | 1.21 | 0.001 | |
| Education | ≥ 21% | REF | |||
| 13% - 20.9% | 0.96 | 0.93 | 0.98 | 0.002 | |
| 7%−12.9% | 0.92 | 0.89 | 0.95 | <0.001 | |
| < 7% | 0.86 | 0.83 | 0.89 | <0.001 | |
| Income | < $38,000 | REF | |||
| $38,000 - $47,999 | 0.97 | 0.95 | 1.00 | 0.023 | |
| $48,000 - $62,999 | 0.97 | 0.94 | 0.99 | 0.015 | |
| $63,000 + | 0.90 | 0.87 | 0.94 | <0.001 | |
| Setting | Large metropolitan | REF | |||
| Small metropolitan | 0.95 | 0.92 | 0.98 | 0.001 | |
| Suburban | 0.91 | 0.87 | 0.94 | <0.001 | |
| Rural | 0.86 | 0.81 | 0.90 | <0.001 | |
| Unknown | 0.99 | 0.94 | 1.04 | 0.670 | |
| Facility Volume | 1st quartile: 0–45 pts/year | 0.73 | 0.64 | 0.82 | <0.001 |
| 2nd quartile: 46–82 pts/year | 0.78 | 0.70 | 0.88 | <0.001 | |
| 3rd quartile: 83–152 pts/year | 0.83 | 0.74 | 0.93 | 0.002 | |
| 4th quartile: >152 pts/year | REF | ||||
| Distance (miles) | ≤ 10 | REF | |||
| 11–20 | 0.99 | 0.97 | 1.01 | 0.148 | |
| 21–40 | 0.98 | 0.95 | 01.0 | 0.035 | |
| >40 | 1.04 | 1.00 | 1.07 | 0.044 | |
| Unknown | 1.31 | 0.67 | 2.54 | 0.428 | |
| Transition | No | REF | |||
| Yes | 1.58 | 1.55 | 1.61 | <0.001 | |
| Unknown | 0.97 | 0.95 | 0.99 | 0.003 | |
| Year of Diagnosis | 2010 | 0.72 | 0.70 | 0.74 | <0.001 |
| 2011 | 0.76 | 0.75 | 0.78 | <0.001 | |
| 2012 | 0.83 | 0.81 | 0.85 | <0.001 | |
| 2013 | 0.92 | 0.90 | 0.93 | <0.001 | |
| 2014 | REF | ||||
| Comorbidities | 0 | 0.70 | 0.64 | 0.76 | <.0001 |
| 1 | 0.79 | 0.72 | 0.85 | <0.001 | |
| 2 | 0.90 | 0.83 | 0.99 | 0.033 | |
| ≥3 | REF | ||||
| Histology | Ductal | REF | |||
| Lobular | 1.10 | 1.07 | 1.12 | <0.001 | |
| Other | 1.00 | 0.95 | 1.05 | 0.914 | |
| Grade | Well | REF | |||
| Moderate | 0.98 | 0.96 | 1.00 | 0.014 | |
| Poor | 0.86 | 0.84 | 0.88 | <0.001 | |
| Undifferentiated/Anaplastic | 0.91 | 0.77 | 1.08 | 0.273 | |
| Unknown | 0.99 | 0.95 | 1.02 | 0.522 | |
| Tumor Size | <10mm | REF | |||
| 10–19mm | 0.98 | 0.96 | 0.99 | 0.008 | |
| ≥ 20mm | 0.93 | 0.91 | 0.96 | <0.001 | |
| Unknown | 1.05 | 0.98 | 1.11 | 0.157 | |
| Stage | I | 0.91 | 0.87 | 0.94 | <0.001 |
| II | 0.94 | 0.91 | 0.97 | <0.001 | |
| III | REF | ||||
| Surgery Type | Breast Conservation | REF | |||
| Mastectomy | 1.36 | 1.32 | 1.39 | <0.001 | |
| Mastectomy with Reconstruction | 2.99 | 2.92 | 3.07 | <0.001 | |
| Chemotherapy | No | REF | |||
| Yes | 0.79 | 0.78 | 0.81 | <0.001 | |
| Radiation | No | REF | |||
| Yes | 0.83 | 0.82 | 0.85 | <0.001 | |
| Endocrine therapy | No | REF | |||
| Yes | 0.87 | 0.86 | 0.89 | <0.001 | |
| Nodes examined | 0–1 | REF | |||
| 2 | 1.02 | 1.00 | 1.04 | 0.143 | |
| 3–4 | 1.00 | 0.98 | 1.03 | 0.672 | |
| ≥5 | 0.96 | 0.94 | 0.98 | 0.002 | |
| Positive Nodes | 0 | REF | |||
| ≥1 | 1.13 | 1.1 | 1.16 | <0.001 | |
| No nodes examined | 1.31 | 1.26 | 1.37 | <0.001 | |
TN = Triple negative, HR+ = Hormone Receptor-Positive, HER2+ = HER2−enriched, REF=Referent
Median follow up was 3.5 years. Predictors of overall survival (OS) are illustrated in Table 3. Per month of delay to surgery there was a HR of 1.104 (CI95% 1.08, 1.13; p<0.001). Phenotype was a predictor of OS with hazard ratios for HR+ and HER2+ tumors relative to TN of 0.78 (CI95% 0.75, 0.82; p<0.001) and 0.74 (CI95% 0.704, 0.78; p<0.001), while Black race was not a predictor with phenotype adjustment. A test of interaction between time to surgery and phenotype found that delays do not affect survival differently by phenotype (p=0.334).
Table 3.
Multivariable model for predictors of Overall Survival. (See Table 4 for tests of interaction between phenotypes and effects of delay.)
| Hazard Ratio | 95% Confidence Limits | p-value | |||
|---|---|---|---|---|---|
| Delay (per month) | 1.10 | 1.08 | 1.13 | <0.001 | |
| Phenotype | TN | REF | . | . | . |
| HR+ | 0.78 | 0.75 | 0.82 | <0.001 | |
| HER2+ | 0.74 | 0.70 | 0.78 | <0.001 | |
| Age | <50 | REF | . | . | . |
| 50–59 | 1.13 | 1.07 | 1.19 | <0.001 | |
| 60–69 | 1.36 | 1.29 | 1.44 | <0.001 | |
| ≥70 | 2.59 | 2.43 | 2.76 | <0.001 | |
| Race | White | REF | . | . | . |
| Black | 1.01 | 0.97 | 1.06 | 0.578 | |
| Asian | 0.57 | 0.52 | 0.64 | <0.001 | |
| Other/Unknown | 0.78 | 0.68 | 0.89 | 0.002 | |
| Hispanic | No | REF | . | . | . |
| Yes | 0.63 | 0.57 | 0.70 | <0.001 | |
| Unknown | 1.00 | 0.93 | 1.09 | 0.914 | |
| Insurance | Private Insurance/ Managed Care | REF | . | . | . |
| Medicaid | 1.53 | 1.43 | 1.63 | <0.001 | |
| Medicare | 1.44 | 1.37 | 1.50 | <0.001 | |
| Other Government | 1.16 | 0.99 | 1.37 | 0.072 | |
| Not Insured | 1.37 | 1.21 | 1.55 | <0.001 | |
| Unknown | 1.21 | 1.06 | 1.39 | 0.006 | |
| Education | ≥ 21% | REF | . | . | . |
| 13% - 20.9% | 1.03 | 0.98 | 1.08 | 0.218 | |
| 7%−12.9% | 1.02 | 0.97 | 1.08 | 0.446 | |
| < 7% | 0.95 | 0.89 | 1.01 | 0.145 | |
| Missing | 2.31 | 1.12 | 4.76 | 0.023 | |
| Income | < $38,000 | REF | . | . | . |
| $38,000 - $47,999 | 0.97 | 0.93 | 1.01 | 0.173 | |
| $48,000 - $62,999 | 0.92 | 0.88 | 0.97 | 0.002 | |
| $63,000 + | 0.88 | 0.82 | 0.93 | <0.001 | |
| Missing | 0.75 | 0.39 | 1.44 | 0.392 | |
| Setting | Large metropolitan | REF | . | . | . |
| Small metropolitan | 1.11 | 1.06 | 1.16 | <0.001 | |
| Suburban | 1.10 | 1.04 | 1.18 | 0.002 | |
| Rural | 1.10 | 1.02 | 1.20 | 0.019 | |
| unknown | 1.01 | 0.92 | 1.10 | 0.845 | |
| Facility Volume | 1st quartile: 0–45 pts/year | REF | . | . | . |
| 2nd quartile: 46–82 pts/year | 0.89 | 0.83 | 0.95 | 0.005 | |
| 3rd quartile: 83–152 pts/year | 0.82 | 0.77 | 0.87 | <0.001 | |
| 4th quartile: >152 pts/year | 0.76 | 0.71 | 0.81 | <0.001 | |
| Distance (miles) | ≤10 | REF | . | . | . |
| 11–20 | 0.92 | 0.89 | 0.95 | <0.001 | |
| 21–40 | 0.88 | 0.84 | 0.93 | <0.001 | |
| >40 | 0.82 | 0.77 | 0.88 | <0.001 | |
| Unknown | 0.96 | 0.71 | 1.29 | 0.777 | |
| Transition | No | REF | . | . | . |
| Yes | 0.93 | 0.90 | 0.96 | <0.001 | |
| Unknown | 0.96 | 0.92 | 1.01 | 0.088 | |
| Year of Diagnosis | 2010 | REF | . | . | . |
| 2011 | 1.06 | 1.02 | 1.11 | 0.001 | |
| 2012 | 1.08 | 1.03 | 1.12 | 0.001 | |
| 2013 | 1.13 | 1.07 | 1.18 | <0.001 | |
| 2014 | 1.13 | 1.06 | 1.20 | 0.002 | |
| Charlson comorbidity score | 0 | REF | . | . | . |
| 1 | 1.47 | 1.42 | 1.52 | <0.001 | |
| 2 | 2.09 | 1.97 | 2.21 | <0.001 | |
| ≥3 | 3.03 | 2.77 | 3.32 | <0.001 | |
| Histology | Ductal | REF | . | . | . |
| Lobular | 0.98 | 0.93 | 1.02 | 0.311 | |
| Other | 1.05 | 0.96 | 1.15 | 0.266 | |
| Grade | Well | REF | . | . | . |
| Moderate | 1.14 | 1.10 | 1.18 | 0.001 | |
| Poor | 1.70 | 1.62 | 1.78 | <0.001 | |
| Undifferentiated/Anaplastic | 2.17 | 1.76 | 2.69 | <0.001 | |
| Unknown | 1.29 | 1.19 | 1.40 | <0.001 | |
| Tumor Size | <10mm | REF | . | . | . |
| 10–19mm | 1.37 | 1.31 | 1.43 | <0.001 | |
| ≥20mm | 1.95 | 1.85 | 2.06 | <0.001 | |
| Unknown | 1.10 | 0.95 | 1.26 | 0.203 | |
| Stage | I | REF | . | . | . |
| II | 1.28 | 1.22 | 1.34 | <0.001 | |
| III | 2.86 | 2.67 | 3.05 | <0.001 | |
| Surgery Type | Breast Conservation | REF | . | . | . |
| Mastectomy | 0.98 | 0.94 | 1.02 | 0.232 | |
| Mastectomy with Reconstruction | 0.58 | 0.54 | 0.61 | <0.001 | |
| Chemotherapy | No | REF | . | . | . |
| Yes | 0.60 | 0.57 | 0.62 | <0.001 | |
| Radiation | No | REF | . | . | . |
| Yes | 0.59 | 0.57 | 0.61 | <0.001 | |
| Endocrine therapy | No | REF | . | . | . |
| Yes | 0.53 | 0.51 | 0.55 | <0.001 | |
| Nodes Examined | 0–1 | REF | . | . | . |
| 2 | 0.92 | 0.88 | 0.97 | 0.007 | |
| 3–4 | 0.87 | 0.83 | 0.91 | <0.001 | |
| ≥5 | 0.90 | 0.86 | 0.94 | <0.001 | |
| Unknown | 1.11 | 0.91 | 1.34 | 0.304 | |
| Positive Nodes | 0 | REF | . | . | . |
| ≥1 | 1.56 | 1.50 | 1.63 | <0.001 | |
| No nodes examined | 2.16 | 2.04 | 2.28 | <0.001 | |
| Unknown | 1.65 | 1.28 | 2.15 | 0.002 | |
Chemotherapy was administered to 129,685 patients. Adjusted times from diagnosis to chemotherapy are shown in e Table 3, noting 72.71 (CI95% 72.33, 73.08) days for TN, compared with 77.96 days in HR+ (CI95% 77.76, 78.15; p<0.001), and 74.41 days in HER2+ (CI95% 74.16, 74.66; p<0.001). Mean and median times from surgery to chemotherapy were 45.7 and 41.0 days overall with times as short as 44.0 and 39.0 days in those having surgery 1–30 days from diagnosis, to 61.0 and 53.0 days postoperative in those having surgery 120–180 days from diagnosis (e Table 4). Chemotherapy in TN patients was administered less frequently in older women, declining progressively from 92.0% in those <50 to 41.4% in those ≥70 for patients having no comorbidities, and from 92.3% to 38.0%, 86.8% to 36.3% and 77.3% to 25.7% in those having comorbidity scores of 1, 2, and ≥3, respectively for these age groups.
Two models were created for OS in the chemotherapy subgroup. The first included time from diagnosis to surgery and separately included time from surgery to chemotherapy. In this model, longer times from diagnosis to surgery per month (1.09, CI95% 1.05,1.13; p<0.001) and from surgery to chemotherapy per month (HR 1.10, CI95% 1.08,1.12; p<0.001) each impaired survival, with phenotype also significant (e Table 5). The test for interaction between time from diagnosis to surgery and phenotype, and time from surgery to chemotherapy and phenotype found no significant effect of phenotype on the decline in survival with increasing delay (p=0.334 and p=0.305, respectively; Table 4).
Table 4. Adjusted hazard ratios for survival, per month of increasing delay.
The nonsignificant interaction terms indicate that there is no difference between phenotypes regarding the effect of delay on survival.
| Point Estimate | 95% Confidence Interval | P-Value | ||
|---|---|---|---|---|
| Diagnosis to Surgery | ||||
| Overall | 1.09 | 1.05 | 1.13 | <0.001 |
| By phenotype | 0.334 (interaction)* | |||
| TN | 1.10 | 1.04 | 1.17 | |
| HR+ | 1.08 | 1.03 | 1.13 | |
| HER2+ | 1.07 | 0.99 | 1.16 | |
| Surgery to Chemotherapy | ||||
| Overall | 1.10 | 1.08 | 1.12 | <0.001 |
| By phenotype | 0.305 (interaction) | |||
| TN | 1.11 | 1.08 | 1.15 | |
| HR+ | 1.09 | 1.07 | 1.12 | |
| HER2+ | 1.13 | 1.09 | 1.16 | |
| Diagnosis to Chemotherapy | ||||
| Overall | 1.10 | 1.08 | 1.12 | <0.001 |
| By phenotype | 0.340 (interaction) | |||
| TN | 1.11 | 1.08 | 1.14 | |
| HR+ | 1.09 | 1.07 | 1.11 | |
| Her2+ | 1.12 | 1.09 | 1.15 | |
This interaction is for the cohort having chemotherapy (n= 129,685). The p (interaction) for the entire cohort (n = 351,807) is 0.825
TN = Triple negative, HR+ = Hormone Receptor-Positive, HER2+ = HER2−enriched
The second OS model included time from diagnosis through surgery to chemotherapy as a single time period. Similar results were seen in the effect of OS by phenotype. In this model, each month of delay to chemotherapy had a HR of 1.10 (CI95% 1.08,1.12; p<0.001); e Table 6). The test for interaction between time from diagnosis through surgery to chemotherapy and phenotype found no significant effect of phenotype on the decline in survival with increasing delay (p=0.340) (Table 4). Hazard ratios per month of delay for OS for each model (diagnosis until surgery and surgery until chemotherapy, and diagnosis through surgery until chemotherapy) are elaborated in Table 4.
DISCUSSION
Times to treatment of breast cancer have been evaluated in several studies, and increasing delay has been found to have detrimental effects for surgery, chemotherapy, and radiotherapy.12 Despite this negative effect on survival, waiting times for breast cancer operations have been found to be increasing.13–15 The time between diagnosis and surgery can lengthen for a variety of reasons, including greater use of imaging or biopsies15, preoperative multidisciplinary evaluation4, and second opinions requiring transfers of care16, while socioeconomic factors may also contribute.4,13,15,16 Such delays to surgery have survival implications in their own right, but also have a downstream effect by pushing back times to adjuvant treatment.
We also know that overall and disease-free survival are, in part, dependent upon breast cancer phenotype.17 The phenotype of a breast cancer is both prognostic and predictive, with clinical response to chemotherapy differing among subtypes.18 Paradoxically, even though TN or basal-like tumors have a higher response rate to chemotherapy than their HR + or HER2− enriched counterparts, their prognosis remains worse.19 Systemic therapy, given either in the neoadjuvant setting or as adjuvant therapy, remains the standard in patients with TN tumors, however, because it significantly decreases the risk of recurrence and improves survival rates.20 Our study reflects this paradigm in that 74.4% of the TN cancers here received chemotherapy.
Neoadjuvant chemotherapy has become one standard option in TN breast cancers, in part because of concern about the aggressiveness of the disease, but also because of the clinical response rate seen in the neoadjuvant setting. Concerns about treatment delays for TN tumors exist because it has an earlier propensity to recur distantly, compared with other phenotypes. Although one study evaluating delays in TN breast cancers has found that times under 90 days did not adversely affect survival21, a more recently presented abstract noted that maximal benefit for patients with TN breast cancer was achieved when chemotherapy was administered within one month of surgery22, leaving the exact timing controversial, but confirming that time is of the essence. The patterns of care seen here confirm this attitude, with phenotype being a predictor of surgical delay (p<0.0001) and TN breast cancers undergoing surgery approximately 2 days earlier than their counterparts. Postoperatively, median times to chemotherapy in TN patients were also 2 and 4 days shorter than HER2+ and HR+ tumors, respectively. While these small differences would not translate into a clinically significant benefit, they likely illustrate that concern exists about delays and that efforts are made to expedite care in this group on the part of clinicians.
Delays in chemotherapy have long been known to affect survival.12 In fact, the quality measure endorsed by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network recommends that administration of adjuvant chemotherapy occur within 120 days to Stage I-III women with breast cancer.12,23 What is not yet clear, however, is how delays may affect breast cancer phenotypes differently. To our surprise, we found no differences in the detrimental effect of preoperative delays on the outcomes of the three breast cancer phenotypes (p=0.334) with an overall 10.4% increase in hazards of death per month delay.
These results are subject to several limitations. The NCDB does not provide a representative sample of US cancer cases, but instead provides data from Commission on Cancer centers, and phenotype information was not available for more than half of the cases and was therefore excluded from our analysis. Furthermore, as in any observational study, the relationship between delay and survival may be subject to bias from unmeasured confounders. The NCDB does not collect disease-specific survival and so we cannot distinguish, for instance, between younger women dying of TNBC and older women dying of other causes. Finally, it must be noted however, that because our study focused on times to surgery as first modality, it cannot be generalized to the population receiving NACT.
The question of phenotypes and delays has been explored in a study evaluating times to chemotherapy by Chavez-MacGregor and colleagues, reviewing patients from the California Cancer Registry from 2005 to 2010.10 Their study of 24,843 patients noted an increase in adverse outcomes when chemotherapy is administered > 90 days from surgery, consistent with our findings. Their study found, however, that the effect of delays was greater on the TNs subset of patients than others. In contrast, an earlier single-center study performed by the same group from their institutional database, found that longer times to chemotherapy, specifically those >60 days, were detrimental over all breast cancers, and that Stage III, trastuzumab-treated HER2+ tumors and TN breast cancers were those that did worse.24 We utilized two models in this analysis because unfortunately neither of these studies evaluated delays to surgery and delays to chemotherapy either separately or in combination to determine whether delays from these intervals had differing impacts on outcomes. In fact, the effect of delays did not impact outcome differently by phenotype when considering the time to surgery (p=0.825) and time to chemotherapy (p=0.305) within the same model.
When comparing factors associated with treatment delays in Chavez-MacGregor’s study with our own, both studies found that black race, Hispanic ethnicity, insurance status and lower socioeconomic status were associated with delays in treatment. Interestingly, we found that, while black breast cancer patients have worse outcomes, when adjusting for phenotype, those differences resolved. These findings are consistent with Silber et al25, who noted that presentation characteristics account for a substantial proportion of such outcome differences.
Although our study differs from the Chavez-MacGregor study by noting that phenotypes were not affected differently by delays, neither study’s results apply to those receiving neoadjuvant therapy. The cohorts were markedly different in size (24,843 patients in the California Cancer Registry versus 351,087 patients in our NCDB study), and characteristics, with their patient population representing much higher risk patients, even though both excluded neoadjuvant patients who may have worse outcomes. Stage III breast cancers comprised 20% of their cohort versus 7.3% in our study and they did not exclude secondary cancers (753 or 3% of their study population) as was done here.10
The poorer survival of patients with TN breast cancers has impelled many oncologists to recommend neoadjuvant chemotherapy (NACT) for newly diagnosed patients, and studies like those above support the idea that chemotherapy should be expedited. Although NACT is the ultimate way to expedite systemic therapy and leads some to suggest that NACT should be pursued for TN tumors because of timing alone, National Surgical Adjuvant Breast and Bowel Protocols (NSABP) B-18 and B-27 found that preoperative chemotherapy and adjuvant chemotherapy are equivalent,26 contradicting that concept, and suggesting that outcomes from chemotherapy delays before surgery are comparable to chemotherapy delays after surgery.
Most TN tumors do respond well to chemotherapy, and both adjuvant and neoadjuvant paradigms have been developed for use in this disease. It must be noted, however, that our findings do not have any implications for standard NACT indications, and instead, only inform us that timing of treatment should not be added to this list of indications for NACT when given for TN tumors specifically.
Ultimately it is well known that the aggressive behavior of TN tumors stems from their capacity to develop distant recurrences in the short term more frequently than other subtypes, while receptor positive tumors have a longer time to relapse, and longer follow up could conceivably show differences in outcomes. Although with local failure not being the primary issue, surgical indications and procedures do not differ between TN tumors and other phenotypes. It therefore makes sense that the median time between diagnosis and surgery for TN tumors compared with other subtypes does not statistically change outcomes.
The greater concern should be the time to chemotherapy, and specifically whether delays to surgery affect the timing of chemotherapy sufficiently to change those outcomes, but our results may be explained when considering the tumor’s entire lifespan. It must be remembered that tumors do not begin their existence at diagnosis; they begin at inception and continue to grow during their “silent interval,” until diagnosis is ultimately possible. It is thought that this period, before diagnosis, constitutes the majority of a tumor’s lifespan. The time to chemotherapy is much longer from inception than from diagnosis or surgery, and therefore differences in delay, regardless of phenotype, represent only a small fraction of the tumor’s overall lifespan. While it has been possible to distinguish outcome changes for delays in large cohorts despite this, we have found that the influence of delay on the three phenotypes differs, if at all, by only a small fraction of those small drops in outcome. This results in no demonstrable clinically or statistically significant differences between the phenotypes.
It should be noted, that while delays did not differ between subtypes, our study still reinforces the fact that delays over all breast cancer phenotypes do affect survival and should be minimized, although we believe that the urgency of breast cancer treatment is universal and similar between phenotypes. Our data have found that TN tumors are thus not unique candidates for expedited surgery as versus other breast cancer subtypes.
ACKNOWLEDMENTS
This work was supported by a United States Public Health Services grant for analysis of the data via support of our biostatistics facility, and by generous private donor support from the Marlyn Fein Chapter of the Fox Chase Cancer Center Board of Associates, for analysis and interpretation of the data.
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Supplementary Material
Synopsis:
This cohort study using the National Cancer Database reports on delays from diagnosis to surgery and diagnosis to chemotherapy in the non-neoadjuvant setting by breast cancer phenotype. We discovered a similar decrease in overall survival with delays, across all three breast cancer phenotypes.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures: The authors declare no conflicts of interest.
REFERENCES
- 1.Bleicher RJ, Ruth K, Sigurdson ER, et al. : Trends in the patient’s preoperative time burden during the evaluation of breast cancer. Oncology 27 S1:22–23, 2013 [Google Scholar]
- 2.Dinan MA, Curtis LH, Hammill BG, et al. : Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. Jama 303:1625–31, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Hillen MA, Medendorp NM, Daams JG, et al. : Patient-Driven Second Opinions in Oncology: A Systematic Review. Oncologist 22:1197–1211, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churilla TM, Egleston BL, Murphy CT, et al. : Patterns of multidisciplinary care in the management of non-metastatic invasive breast cancer in the United States Medicare patient. Breast Cancer Res Treat 160:153–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleicher RJ, Ruth K, Sigurdson ER, et al. : Preoperative Delays in the US Medicare Population With Breast Cancer. Journal of Clinical Oncology 30:4485–92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polverini AC, Nelson RA, Marcinkowski E, et al. : Time to Treatment: Measuring Quality Breast Cancer Care. Ann Surg Oncol 23:3392–402, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Bleicher RJ, Ruth K, Sigurdson ER, et al. : Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol 2:330–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker JS, Mullins M, Cheang MCU, et al. : Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. Journal of Clinical Oncology 27:1160–1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar P, Aggarwal R: An overview of triple-negative breast cancer. Arch Gynecol Obstet 293:247–69, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. : Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol 2:322–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Stewart AK, Winchester DP, et al. : The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15:683–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleicher RJ: Timing and Delays in Breast Cancer Evaluation and Treatment. Ann Surg Oncol 25:2829–2838, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liederbach E, Sisco M, Wang C, et al. : Wait times for breast surgical operations, 2003–2011: a report from the National Cancer Data Base. Ann Surg Oncol 22:899–907, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Smith EC, Ziogas A, Anton-Culver H: Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg 148:516–23, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Bleicher RJ, Ruth K, Sigurdson ER, et al. : Preoperative delays in the US Medicare population with breast cancer. J Clin Oncol 30:4485–92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleicher RJ, Chang C, Wang CE, et al. : Treatment delays from transfers of care and their impact on breast cancer quality measures. Breast Cancer Res Treat 173:603–617, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Chia SK, Bramwell VH, Tu D, et al. : A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 18:4465–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey LA, Dees EC, Sawyer L, et al. : The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–34, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Prat A, Pineda E, Adamo B, et al. : Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24 Suppl 2:S26–35, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Yagata H, Kajiura Y, Yamauchi H: Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer 18:165–73, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Eastman A, Tammaro Y, Moldrem A, et al. : Outcomes of delays in time to treatment in triple negative breast cancer. Ann Surg Oncol 20:1880–5, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Morante Z DlC-KG, Pinto J et al. : Localized triple negative breast cancer in extremes of life [abstract]. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium Cancer Res 78:Abstract nr P1-15-06, 2018 [Google Scholar]
- 23.Desch CE, McNiff KK, Schneider EC, et al. : American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol 26:3631–7, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. : Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32:735–44, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silber JH, Rosenbaum PR, Clark AS, et al. : Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389–97, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Rastogi P, Anderson SJ, Bear HD, et al. : Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–85, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.