Abstract
Purpose:
The majority of glaucoma patients do not take their medications as prescribed. Estimates of the cost-utility value of adherence to prescribed glaucoma medication are vital to implementing potentially effective interventions.
Design:
Cost-utility analysis using Monte Carlo microsimulations incorporating a series of Markov cycles (10,000 iterations per strategy).
Participants:
Glaucoma patients ≥ age 40 with a full life time horizon (up to 60 years).
Methods:
The analysis estimated glaucomatous progression based on data from the United Kingdom Glaucoma Treatment Study. Participants with glaucoma entered the model at age 40 with a mean deviation in the better seeing eye of −1.4dB ±−1.9d and −4.3dB ± −3.4dB in the worse seeing eye. Participants who worsened each year accumulate −0.8dB loss compared to −0.1dB loss for those who remained stable. Data from the Glaucoma Laser Trial and the Tube versus Trabeculectomy Studies were used to assign probabilities of worsening disease among treated patients. Claims data estimating rates of glaucoma medication adherence over four years was used to assign probability of adherence. Those with poor adherence were modeled as having outcomes similar to the placebo arm of the clinical trials. As patients’ mean deviation deteriorated, they transitioned between health states from mild (≥−6dB), to moderate (<−6dB to ≥−12dB), to severe glaucoma (<−12dB to ≥23dB) to unilateral (<−20dB) and bilateral blindness. At each health state, patients incurred the costs of treatment and established health utilities; ultimately, societal costs of low vision and blindness were included.
Main Outcome Measure:
Cost/QALY of glaucoma medication adherence.
Results:
Beginning at an initial glaucoma diagnosis at age 40, patients proceeded to single-eye blindness as early as 19 years among those who were nonadherent and 23 years for those remaining adherent. Total healthcare costs for adherent patients averaged $62,782 (standard deviation, SD: 34,107) while those for non-adherent patients averaged $52,722 (SD: 38,868). Non-adherent patients had a mean loss of 0.34 QALYs, resulting in a cost-effectiveness ratio of $29,600 per QALY gained.
Conclusion:
At a conservative willingness-to-pay of $50,000/QALY, there is much room to expand services to improve patient adherence.
Precis
At a standard willingness-to-pay of $50,000 per quality-adjusted life year (QALY), as the cost-utility of glaucoma medication adherence was estimated at $29,000/QALY, it would be cost-effective to expand services to improve adherence.
Non-communicable chronic diseases, such as diabetes and hypertension, are key drivers of morbidity and mortality in the United States (US).1 They often have few symptoms until the disease is rampant and require high levels of continuous self-management to optimize outcomes. Communication between the patient and a team of care providers, including specialty and primary care physicians, nurses, educators and pharmacists, is key to successful disease management.2,3 Arguably, this systems-based approach to improving chronic disease outcomes is the same type of approach that could improve outcomes in glaucoma care.
Despite many advances in treatment in the past two decades, glaucoma remains the third leading cause of blindness in the US.4 One significant contributor to this is that about half of glaucoma patients do not take their medications as prescribed,5,6 leading to many people with glaucoma being essentially un-treated. A recent Cochrane review found that interventions to improve glaucoma medication adherence had variable success,7 but those demonstrating success used personalized, one-on-one counseling strategies.8
As the US population ages, the prevalence of glaucoma is projected to increase by 140%, from 3.36 million to 4.72 million people affected, by 20409. In the current treatment paradigm, ophthalmologists are responsible for medical decision making, surgery, education, and counseling. With projected shortages in the ophthalmologist work force,10,11 and expected increases in the number of people with glaucoma,9 this work flow will need to shift to a paradigm of team-based care. For patients with diabetes, certified diabetes educators provide self-management counseling as part of the health care team caring for the patient. To support these services, Medicare reimburses 10 hours of self-management training after the first year of being diagnosed with diabetes and 2 hours of self-management training per year after that. To adequately address the needs of a growing patient population, certified glaucoma coaches could become an invaluable resource in the future if they could function similarly as part of our glaucoma care team.
The purpose of this study is to estimate the societal impact of optimal compared to poor glaucoma medication adherence in terms of costs and time-to blindness through a cost-utility analysis. Such an assessment will enable policy makers to understand the economics of implementing interventions such as payer policy change to cover glaucoma self-management counseling services to improve medication adherence.
Methods
Study Design
A cost-utility analysis was conducted that assessed the societal costs of optimal versus poor adherence to glaucoma medications among people 40 years of age and older with newly diagnosed glaucoma on a 60-year (full life) time horizon. The age of 40 was chosen as the entry age as it is the earliest age at which incident open-angle glaucoma commonly begins, and was the age of entry of participants into the United Kingdom Glaucoma Treatment Study (UKGTS), whose values we used to estimate glaucomatous progression over time.12,13 The UKGTS, published in 2014, was the first trial to compare the impact of a single medication (latanoprost) to placebo on visual function among glaucoma patients. The data generated facilitate a more precise estimation of the impact of glaucoma treatment on visual impairment than was possible in previous cost-effectiveness analyses. This cost-utility analysis took a societal perspective, incorporating direct medical costs, out of pocket expenses and costs to society from lost productivity, tax subsidies, and disability costs for those who are legally blind. The analysis focuses on a hypothetical cohort of patients who are adherent or non-adherent to medical treatment. The analysis assumes that all patients are adherent to scheduled visits and surgeries.
Model Design
The model was constructed as Monte Carlo microsimulations incorporating a series of Markov cycles using treatment (with medications and surgery), disease progression, resource utilization, and outcomes over one-year time horizons. The model used 10,000 iterations per strategy. All patients entered the model with mild glaucoma at age 40 and continued until death or age 100. The odds of disease progression determined the decibel reduction in each year of the model, the accumulation of which determined each subsequent state transition. Once disease progressed, a milder form of disease was not returned to; therefore, each year modeled represented either disease maintenance or progression (Figure 1). Trackers were used to accumulate decibels in each eye so that single-eye and full blindness could be modeled similar to real-world disease progression. TreeAge Pro 2018 R2.0 (Williamstown, MA) was used for all modeling analyses.
Figure 1.
Disease state transition diagram
Each bubble indicates a distinct health state included in the model. All straight lines indicate unidirectional state transitions while the looped arrows indicate the potential for remaining in a particular state in subsequent years of the model. The model structure was identical between both cohorts of hypothetically adherent and nonadherent individuals.
Health States
All participants entered the model with mild glaucoma and progressed to one of six different health states: mild glaucoma, moderate glaucoma, severe glaucoma, blindness in one eye, blindness in both eyes, and death. The classification is based on the mean deviation in the worse seeing eye. Glaucoma states were defined according to a modified Hodapp-Anderson-Parrish classification. Disease severity was determined using Humphrey visual field test values: mild glaucoma was defined as less than −6dB mean deviation; moderate glaucoma was defined as between −6dB to −12dB mean deviation; and severe glaucoma was defined as between - 12dB and −20dB. Blindness was defined as having greater than −20dB mean deviation.14 Annually, participants had a probability of death based on US census life tables.15
Progression from One Health State to Another
Participants with glaucoma entered the model at age 40 with a mean deviation in the better seeing eye of −1.4dB ±−1.9d and −4.3dB ± −3.4dB in the worse seeing eye, which correspond to the baseline values of participants in UKGTS. Of those UKGTS participants treated with latanoprost, 15.2% worsened compared to 25.6% of those treated with placebo over two years. Yearly progression among the proportion of patients whose glaucoma worsened was a median of −0.8dB/year.13 Participants who worsened each year accumulate −0.8Db loss compared to - 0.1dB loss for those who remain stable when they had mild and moderate glaucoma.16 When participants accrued enough decibel loss to have transitioned to severe glaucoma, a - 1.6dB/year loss rate was assumed given that advanced disease progresses more quickly than early disease and increasing age also predicts increasing annual decibel loss.17 We assumed that beta blockers 18 and alpha agonists19 have a similar effectiveness to prostaglandin analogues while carbonic anhydrase inhibitors are approximately 70% as effective.20 Therefore, when a participant worsened to requiring carbonic anhydrase inhibitors in addition to the first three classes of medications, the proportion of participants who worsened at that stage increased to 19.8% among those who were adherent and 33.3% among those who were not adherent. Those who progressed after receiving a prostaglandin analogue, whether they were adherent or non-adherent to the medications, would have treatment escalated in the following order: 1) laser trabeculoplasty, 2) beta blocker, 3) alpha agonist, 4) carbonic anhydrase inhibitor, 5) trabeculectomy with anti-metabolite, 6) glaucoma drainage implant. (Figure 1) Data from the following glaucoma surgery trials were used to assign probabilities of worsening disease among treated patients: the Glaucoma Laser Trial, 21 the Primary Tube Versus Trabeculectomy Study,22 and the Tube Versus Trabeculectomy Study.23 (Appendix 1, available at www.aaojournal.org).
Modeling the Impact of Adherence
To model the impact of adherence on glaucomatous progression, we used data from an analysis of beneficiaries ≥40 years old enrolled in a US managed care plan who were newly diagnosed and treated for open angle glaucoma. These data suggested that among 1,234 beneficiaries with incident open-angle glaucoma, only 15% had persistently good adherence (defined as a medication possession ratio of ≥75%) over four years of follow-up.21 Almost all (94%) of patients with good adherence during the first year of follow up also had good adherence in the subsequent three years of follow-up. Therefore, we assumed that once a participant was assigned to have good adherence in the model, they maintained good adherence over their lifetime. We then assumed that the group with good adherence would have the same outcomes as those in the treatment group in UKGTS while those with poor adherence would have outcomes similar to those in the placebo group in that trial. Not taking medications as prescribed was assumed to give the same outcome as taking a placebo medication as taking medications some of the time will lead to intraocular pressure fluctuation, which can lead to glaucomatous progression and poor outcomes.22-27
Model Validation
We compared our model output in terms of incidence of unilateral and bilateral blindness to rates seen in a population-based study in Olmsted County28 and in the Advanced Glaucoma Intervention Study. In the epidemiologic study in Olmstead County from 1981-2000, the probability of glaucoma-related blindness in at least one eye at 20 years was 13.5% (95% CI 8.8-17.9%). In the Advanced Glaucoma Intervention Study (AGIS),14 the ten-year cumulative incidence of unilateral visual field impairment comparable to legal blindness (>−20dB mean deviation) was between 7.3-18.5% depending on race and treatment.
Costs
Multiple estimates modeled the four main components of economic burden due to glaucoma: direct medical costs, indirect medical costs, and lost productivity. Direct medical costs were based on the cost of ophthalmologic examination for glaucoma using the American Academy of Ophthalmology’s Preferred Practice Guidelines. An initial visit included the cost of a New Patient Level IV Office visit, pachymetry, gonioscopy, Humphrey visual field testing, OCT and fundus photography. Follow-up visits for those with all stages of glaucoma included the cost for an annual dilated exam as a Level IV Office Visit, annual Humphrey visual field testing and annual OCT and a second examination for an intraocular pressure check billed as a Level III Office Visit. Those with moderate glaucoma incurred the cost of a second Level III Office visit and those with severe glaucoma incurred the cost of a third Level III office visit annually. Patients with severe glaucoma incurred the cost of a low vision office visit (one Level IV and one Level III visit annually). When glaucoma progressed to single eye blindness, the cost of an initial occupational therapy evaluation followed by four occupational therapy home visits were incurred during that year. (Table 1, available at www.aaojournal.org) We calculated costs for office visits based on the Center for Medicare and Medicaid Services (CMS) average reimbursement for Current Procedural Terminology (CPT) codes for offices that charge non-facility fees as recent research demonstrated that the majority of eye surgery and likely eye care is performed in offices and ambulatory surgical centers that do not charge the facility fee.29 A micro-costing approach was taken to allow for more robust varying of individual inputs. A micro-costing approach will allow us to use the model for future applications that would be better facilitated by having individual visit, procedure, and medication costs included in the base model.
Table 1.
Model costs and utilities (available at available at www.aaojournal.org)
| Cost Parameter | Base Case Value |
|---|---|
| CPT Codes | |
| Level IV Office Visit, New Patient (CPT 99204)1 | $166.16 |
| Gonioscopy (CPT 92020)1 | $27.28 |
| Pachymetry (CPT 76514)1 | $15.43 |
| Visual Field Testing (CPT 92083)1 | $65.32 |
| Fundus Photography (CPT 92250)1 | $66.75 |
| Optical Coherence Tomography (CPT 92134)1 | $41.63 |
| Level IV Office Visit, Return Patient (CPT 99214)1 | $108.74 |
| Level III Office Visit, Return Patient (CPT 99213)1 | $73.93 |
| Low Vision Services, New Patient (CPT 99205)1 | $209.23 |
| Low Vision Services, Return Patient (CPT 99214)1 | $108.74 |
| Occupational Therapy, Initial Evaluation (CPT 97166)1 | $92.52 |
| Occupational Therapy Home Visits (CPT 97530 & CPT 97535)1 | $76.93 |
| Trabeculectomy with Anti-metabolite (CPT 66170)1 + $275 Anesthesia fee2 | $1387.91 |
| Trabeculectomy Revision (CPT 66185)1 + $275 Anesthesia2 | $1,133.46 |
| Tube Shunt Implant (CPT 66180) 1 + $275 Anesthesia2 | $1,431.69 |
| Tube Shunt Implant Complications (CPT 66185)1 + $275 Anesthesia2 | $1,133.46 |
| Laser Trabeculoplasty (CPT 65855)1 | $248.35 |
| Facility Fees for Glaucoma Surgery2 | $3,000-$7,000 |
| Medication Costs | |
| Latanoprost 2.5 mL3 | $15.55 (Akron, Inc) |
| Xalatan 2.5 mL3 | $203.78 (Pharmacia) |
| Timolol 0.5% 10 mL3 | $5.92 (Akron, Inc) |
| Timolol 0.5% GFS 5mL3 | $173.81 (Sandoz) |
| Brimonidine 0.2% 10 mL3 | $27.44 (Akron, Inc) |
| Alphagan P 0.1% 10 mL3 | $292.04 (Allergan) |
| Dorzolamide 2% 10 mL3 | $35.00 (Akron, Inc) |
| Azopt 2% 10 mL3 | $284.54 (Norvartis) |
| Moxifloxacin 3 mL3 | $39.00 (Akron, Inc) |
| Vigamox 3 mL3 | $164.26 (Novartis) |
| Prednisolone 10 mL × 2 bottles3 | $176.86 (Sandoz) |
| Pred Forte 10 mL × 2 bottles3 | $554.78 (Allergan) |
| Ketorolac tromethamine 0.5% 5mL × 2 bottles3 | $64.04 (Akorn) |
| Acular 0.5% 5mL × 2 bottles3 | $524.62 (Allergan) |
| Atropine 2 mL3 | $8.21 (Pharmedium Services) |
| Other Costs | |
| Direct Non-Medical Costs for the Blind4 | $15,047/year |
| Lost Productivity for the Blind for those <Age 654 | $10,866/year |
| Low Vision Aids for those with ≥ 1 Eye Blind2 | $200/year |
| Travel to appointments5 | $22.10/appointment |
| Utilities | |
| Mild Glaucoma6 | 0.92 |
| Moderate Glaucoma6 | 0.89 |
| Severe Glaucoma6 | 0.86 |
| Single eye blindness7 | 0.47 |
| Bilateral blindness 7 | 0.26 |
Centers for Medicare & Medicaid Services, Physician Fee Schedule. https://www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx Accessed 23 Feb. 2019.
Michigan Medicine Costs (internal communication, PANC).
Truven Health Analytics. Red Book Online [Database Online]. https://redbook.solutions.aap.org/.
Rein DB. The Economic Burden of Major Adult Visual Disorders in the United States. Arch Ophthalmol. 2006;124:1754-1760.
Schehlein EM, Im LT, Robin AL, Onukwugha E, Saeedi OJ. Nonmedical Out-of-Pocket Patient and Companion Expenditures Associated with Glaucoma Care. J Glaucoma. 2017;26:343-348.
Lee BS, Kymes SM, Nease RF, Sumner W, Siegfried CJ, Gordon MO. The impact of anchor point on utilities for 5 common ophthalmic diseases. Ophthalmology. 2008;115:898-903.e4.
Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327-331.
We assumed that patients would be initially treated with a prostaglandin analogue (Xalatan/Latanoprost). Those patients who worsened incurred costs for a laser trabeculoplasty including treatment with post-laser steroid (Pred Forte/Prednisolone Acetate). Those who continued to worsen would incur annual costs for a beta blocker (Timolol GFS/Timolol), alpha agonist (Alphagan P/Brimonidine), and a carbonic anhydrase inhibitor (Azopt/Dorzolamide), in that order. Glaucoma medication cost was estimated using Red Book prices.30 Brand name drugs were assumed to comprise 35% of medications prescribed.31 Those who continued to worsen incurred costs for a trabeculectomy followed by a glaucoma drainage implant with success rates and complication-related costs defined by outcomes in the Primary Tube Versus Trabeculectomy Study and the Tube Versus Trabeculectomy Study (Table 2, available at www.aaojournal.org). All patients undergoing trabeculectomy surgery incurred costs for moxifloxacin, atropine, and prednisolone post-operatively while those undergoing tube surgery incurred costs for moxifloxacin, prednisolone, and ketorolac.
Table 2.
Model Inputs: Medical Services by Disease Stage (available at available at www.aaojournal.org)
| Disease Stage | Medical Service1 | Annual Frequency1 |
|---|---|---|
| New Patient | Office Visit Level IV, New Patient | 1 |
| Pachymetry | 1 | |
| Gonioscopy | 1 | |
| Visual Field Testing | 1 | |
| Fundus Photography | 1 | |
| Optical Coherence Tomography | 1 | |
| Mild Glaucoma (≥−6dB) | Office Visit Level IV | 1 |
| Office Visit Level III | 1 | |
| Visual Field Testing | 1 | |
| Optical Coherence Tomography | 1 | |
| Laser Trabeculoplasty | See Methods | |
| Moderate Glaucoma (<−6dB≥−12dB) | Office Visit Level IV | 1 |
| Office Visit Level III | 2 | |
| Visual Field Testing | 1 | |
| Optical Coherence Tomography | 1 | |
| Severe Glaucoma (<−12dB≥−20dB) | Office Visit Level IV | 1 |
| Office Visit Level III | 3 | |
| Visual Field Testing | 1 | |
| Optical Coherence Tomography | 1 | |
| Glaucoma Surgery | See Methods | |
| Single Eye Blindness (<−20dB) | Office Visit Level IV | 1 |
| Office Visit Level III | 3 | |
| Visual Field Testing | 1 | |
| Optical Coherence Tomography | 1 | |
| Low vision Services | 2 | |
| Occupational Therapy Evaluation* | 1 | |
| Occupational Therapy Home Visits* | 4 |
Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma PPP - American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-ppp-2015.
The cost of occupational therapy is not annual; it is a one-time cost over the first year of having one eye blind. It will recur if the second eye becomes blind.
Indirect medical costs included the cost of low vision aids, which we estimated at $200/year for those with visual impairment and blindness based on a discussion with our optometrists in the low-vision clinic. Indirect costs also included the out of pocket expense of traveling for appointments, which was estimated at $22.10/appointment (median cost) [range; mean (standard deviation), $11.1-$42.9; $44.1 (72.8)].32 Rein and colleagues calculated a number of other indirect costs for treating blindness in the US in 2004. The cost for guide dogs for the blind ($0.062 billion) and three government programs, the Department of Education’s Independent Living Services for Older, Blind Individuals ($0.029 billion), the American Printing House for the Blind ($0.016 billion), and the Library of Congress’ National Library Service for the Blind and Physically Handicapped ($0.05 billion). The cost for long-term care in nursing homes due to blindness is another significant indirect medical cost ($10.96 billion).33 The total estimate for direct non-medical costs was $11.12 billion for the 739,000 Americans blind in 2004, or $15,047 annually. Adapted to 2018 dollars, each person who is blind accrues indirect costs of $20,053 annually.
The cost of lost productivity due to visual impairment for those under age 65 was adapted from the work of Rein and colleagues. Compared to a labor force participation rate of 85% with average annual earnings of $33,195 among those age 40-64 with normal vision, the labor force participation rate 30% with average annual earnings of $21,074 among those who were blind. In total, Rein and colleagues estimated that blindness leads to $8.03 billion in annual productivity losses in 2004 dollars. The total estimate for lost productivity for those Americans who become blind before age 65 was $10,866 in 2004 dollars, and $14,481 in 2018 dollars.
Utilities
We used the utilities for glaucoma severity developed through a standard gamble method where participants were asked what risk of death they would be willing to trade – a value of 0 – for perfect health – a value of 1.34 We chose to use the utility values anchored in “perfect health” because these utilities may be more policy relevant than those anchored in “perfect vision” because the results are generalizable across medical conditions.34 Because there were no available utility values for unilateral and bilateral blindness anchored in “perfect health,” we used utility values anchored in “perfect vision.”35 We then conducted a sensitivity analysis to assess the impact of using utility values all derived from the standard gamble method anchored in “perfect vision,”36 as utilities anchored in “perfect vision” are lower than those anchored in “perfect health.”34 Utility values anchored in “perfect vision” were derived from work from Brown and Brown 36 in which they calculated that a 4dB loss (average loss for mild glaucoma) was associated with 20/25-2 vision and a utility of 0.902; a 9dB loss (average loss for moderate glaucoma) was associated with 20/40 vision and a utility of 0.800, a 16 dB loss (average loss for severe glaucoma) was associated with 20/70 vision and a 0.722 utility, and a ≥20dB loss (single eye blindness) was associated with 20/800 vision and a utility of 0.52.36 Bilateral blindness had a utility of 0.26.34
Sensitivity Analyses
Research completed on a smaller sample of patients prior to the UKGTS demonstrated a −1.0dB loss/year on average with a −2.0dB loss/year among those with worse disease and older age;37 therefore, we also used these assumptions in a sensitivity analysis as the UKGTS did not include participants with more severe disease. We modeled both the −2.0 db loss/year in two ways: 1) among participants who had progressed to moderate disease and 2) among participants who had progressed to severe disease. Additional sensitivity analyses included assessing the increased costs of medication above the wholesale acquisition price (120%-150%), varying the starting age at diagnosis (in 5-year increments), and assessing the impact of a higher success or failure rate from glaucoma surgery, varying the rates from 5%-20% for both trabeculectomy surgery and tube shunt surgery. Given recent utility analyses conducted in China 38 that found much lower utilities for glaucoma-related vision loss using the standard gamble method, we conducted a sensitivity analysis with a utility of 0.85±0.14 (varying from 0.71-0.99) for mild glaucoma and a utility of 0.75±0.2 (varying from 0.55-0.95) for moderate and severe glaucoma.
Results
Model Validation
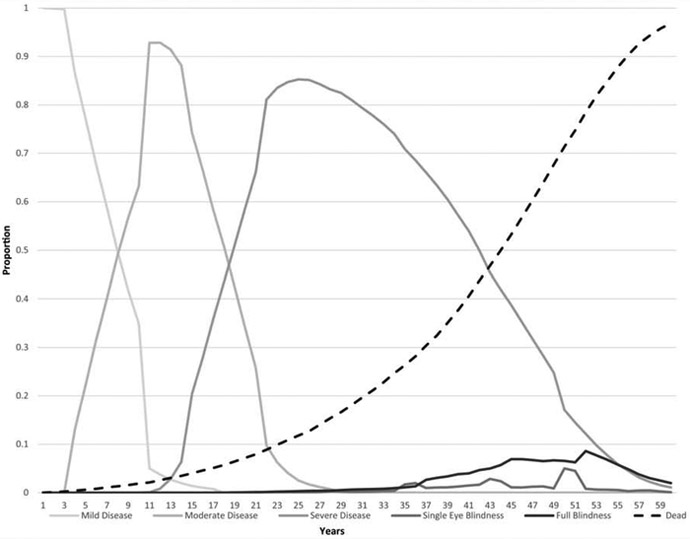
Figure 2 depicts the proportion of patients in each health state over the course of the model, separated by adherence status. Beginning at an initial glaucoma diagnosis at age 40, patients proceeded to single-eye blindness as early as 19 years among those who were consistently nonadherent and 23 years for those remaining adherent to therapy. The prevalence of blindness (either single eye or bilateral) by cohort among those alive were as follows: 0.2% (nonadherent) and 0% (adherent) at age 60; 1.1% (nonadherent) and 1.2% (adherent) at age 70; 9.2% (nonadherent) and 5.4% (adherent) at age 80; and 42.6% (nonadherent) and 19.0% (adherent) at age 90. Average annual costs (discounted) were $721 (SD: $212) for those with mild disease, $2,021 (SD: $380) for those with moderate disease, $897 (SD: $427) for those with severe disease, and $759 (SD: $1,669) for those who were single-eye blindness. When Lee and colleagues assessed direct medical costs for glaucoma by severity stage in a cross section of 150 patients, they found that those with mild disease incurred $1480/year in direct medical costs; those with moderate disease incurred $1765/year in direct medical costs, those with severe disease incurred $1915/year in direct medical costs and those with unilateral blindness (MD ≤−20 dB) incurred costs of $2464/year.11 The values in our study are lower overall because latanoprost, the first line agent for medical treatment of glaucoma, was not yet generically available during the Lee study. Additionally, the costs in this analysis are discounted in each year in which a participant runs through the model. Thus, health states farther in the future, such as single eye blindness, will have lower net present value than that observed from direct patient cost data. Discounting accounts for the fact that people place a lower value on their future state than their present state, and thus is an important component of modeling lifetime costs and utilities.
Figure 2.
Markov tracking of disease severity over time
Panel A. Adherent patients
Panel B. Nonadherent patients
The x-axis depicts time in the model while the y-axis is the proportion of each cohort in the included disease states of the model in each year.
Base Model
Over a period of up to 60 years following glaucoma diagnosis, total healthcare costs for adherent patients averaged $62,782 (SD: $34,107) while those for non-adherent patients averaged $52,722 (SD: $38,868). Non-adherent patients had a mean loss of 0.34 QALYs over a 60-year period compared to those who were adherent, and the resulting cost-effectiveness ratio was $29,600 per QALY gained.
Sensitivity Analyses
Several key model inputs were varied to examine their relative impact on the cost-utility values observed in the main analysis (Table 3). These sensitivity analyses included varying the rate of surgical success, the probability of surgical complications, and utility values of each disease severity. First, varying the costs of medications had only marginal impacts on the incremental cost-effectiveness ratios (ICERs) (not shown). However, employing different levels of utility across disease severity resulted in significant impact on resulting ICERs. At its lowest applied value (0.71), the utility of mild disease approaches a commonly accepted $100,000 per QALY willingness-to-pay threshold ($97,423/QALY) with only a marginal gain in effect (0.10 QALYs). In the case of moderate disease utilities, being adherent is actually dominated with fewer QALYs at a higher cost until utilities exceed 0.65. Adjusting the utility values of severe disease from their published high and low values resulted in incremental QALYs favoring being adherent ranging from gaining an additional 0.06 (high) to 1.31 (low) QALYs. This resulted in ICERs ranging from $7,685 (lower utility) to $172,071 (higher utility). However, it is important to note that some overlap in the utility values was possible given the distributions of estimates applied (based on published values). Interpretations of the sensitivity analyses tied to utility values should consider such overlap when interpreting the results on the high and low end of each distribution. Additional sensitivity analyses varying utility values based on “perfect vision” led to a mean loss of 0.54 QALYs over the 60-year observation period at an ICER of $18,591.
Table 3.
Sensitivity Analyses
| Parameter | Value | ICER Adherent |
|---|---|---|
| Mild disease utility | 0.71 | 97,432 |
| 0.92 | 29,600 | |
| 0.99 | 24,025 | |
| Moderate disease utility | 0.55 | Dominated* |
| 0.75 | 587,306 | |
| 0.95 | 21,038 | |
| Severe disease utility | 0.55 | 7,685 |
| 0.75 | 17,712 | |
| 0.95 | 172,071 | |
| Single-eye blindness utility | 0.33 | 29,009 |
| 0.47 | 29,600 | |
| 0.61 | 30,216 | |
| Bilateral blindness utility | 0.19 | 28,775 |
| 0.26 | 29,600 | |
| 0.33 | 30,475 | |
| Odds of laser failure | 0.0 | 58,202 |
| 0.204 | 29,678 | |
| 0.408 | 23,490 | |
| Probability of a successful trabeculectomy | 0.84 | Dominates** |
| 0.92 | 29,600 | |
| 1.0 | 58,651 | |
| Probability of a successful tube shunt implant | 0.86 | 79,016 |
| 0.93 | 29,600 | |
| 1.0 | 12,911 |
Dominated: results indicate that the strategy (adherence) led to lower effects at a higher cost compared to the other included strategy (nonadherence)
Dominates: results indicate that the strategy (adherence) led to higher effects at a lower cost compared to the other included strategy (nonadherence)
Comparatively less variability was seen when adjusting the utility values tied to both blind health states, which is likely a reflection of the limited time patients would have spent in either single or bilateral blindness health states. Varying the probability of successful surgical interventions—either trabeculectomy or tube—did significantly impact ICERs. Specific to trabeculectomy, being adherent dominates nonadherence if the odds of a success surgery fall below 90%. However, varying the rates of surgical complications had relatively little impact on resulting ICERs ($102-$137) even when the probabilities were double the base value (not shown). For laser trabeculoplasty among patients with mild disease, incremental changes in QALYs gained varied from 0.20 to 0.36 when varying the failure rate between 0% and 40.8% (double the base value). This resulted in the ICERs varying from $58,202 (0%) to $23,490 (40.8%) per QALY gained.
In addition to examining the impact of these variables on the ICERs, we also examined the relative impact of varying the progression rate of glaucoma by disease severity. Adjusting the decibel loss to −2.0 per year once severe disease was reached among those worsening led to reducing the time to single eye blindness to as early as 22 years for those who were adherent and as early as 19 years for those who were nonadherent. In this case, an additional 0.45 QALYs were gained by being consistently adherent at an average added lifetime cost of $3,901. The resulting ICER for this analysis was $8,689 per QALY. When adjusting decibel loss to −2.0 per year among patients worsening to moderate and severe disease, the time to first blindness reduced to 14 years among those adherent and13 years among those nonadherent to topical therapy. An additional 0.43 QALYs were gained by being adherent and were done so at lower costs of care: $79,905 (adherent) versus $83,928 (nonadherent). Consequently, adherence dominated being nonadherent (ICER -$9,339 per QALY).
A final sensitivity analysis was conducted on the age at initial glaucoma diagnosis (i.e., the age at which they would enter the model) by varying the model entry age from 40 up to 75 years of age. A general upward trend in ICERs was observed using 5-year increments in diagnosis age, which peaked at an initial diagnosis age of 50 years ($69,147/QALY gained) before generally declining. (Figure 3)
Figure 3.
Sensitivity Analysis: The Impact of Varying Age at Diagnosis on ICER
The x-axis depicts initial age at diagnosis of mild glaucoma (in five-year increments) and the y-axis depicts the incremental cost-effectiveness ratio (ICER)
Discussion
In this cost-utility analysis comparing optimal to poor glaucoma medication adherence, we demonstrated that being adherent to glaucoma medications resulted in improved quality of life for a relatively low increase in lifetime healthcare costs. Being adherent to glaucoma medications cost $29,600 per quality-adjusted life year (QALY) over a person’s lifetime in our base model. If we use utility values anchored in “perfect vision” instead of a combination of “perfect health” for glaucoma severity and “perfect vision” for blindness, we find that being adherent to glaucoma medications costs only $18,591/QALY. If someone is a “fast progressor,”39 with an average of −2.0 dB loss/year once they have moderate or severe disease, being adherent to medications dominates being non-adherent (−$9,339/QALY). At an average willingness-to-pay of $50,000/QALY, there is much room to expand services to improve patient adherence and still be under this standard willingness-to-pay threshold. Yet, there are not currently systematic ways to provide self-management support to patients with glaucoma. In one study of 279 video-recorded patient visits where a new medication was prescribed, less than one-third of patients received any education or counseling about their glaucoma or their treatment.40 The current economic analysis showed that it would be beneficial to society to invest in ensuring that glaucoma patients have the support they need to optimize their medication adherence.
To ensure that the results of this model were valid, we compared our average time to blindness with the average time to blindness identified in the most recent epidemiologic study from Olmstead County, MN between 1981-2000. The rates of blindness among the non-adherent cohort in this model are very similar to that seen in the epidemiological survey in Olmstead County, MN where the 20-year incidence of blindness in at least one eye was estimated at 13.5% [95% CI 8.8% −17.9%] at an average age of 81.2 ± 10.3.28 Our estimate for unilateral or bilateral blindness among the 80-year olds in our cohort was 9.2% for the nonadherent cohort, within the 95% confidence interval of the Olmstead County data and within the estimates of the Advanced Glaucoma Intervention Study ten-year cumulative incidence of blindness, 7.3-18.5%.14 The estimate of blindness for the 80 year olds in our cohort who were adherent was only 5.4%, demonstrating the incremental value of being adherent to glaucoma medications as people age. Because there are many fewer participants who remain alive at 90 years of age, the rates of blindness in our cohort are quite high in this group, with 19.0% blind in at least one eye among the adherent cohort compared to 42.6% blind in among the nonadherent cohort. This exponential relationship between prevalence of blindness and age is also reflected in population level estimates of blindness in the National Health and Nutrition Examination Survey (NHANES) data where the prevalence of blindness was <1% among those age 40-49 and 12% among those ≥age 80.41 These high levels of visual impairment and blindness in the elderly contribute to decreased independence and increased nursing facility utilization in this age group.42,43
To ensure that the model was internally valid and the findings were robust, we conducted a number of sensitivity analyses. Varying the costs of medication resulted in only modest changes to the model’s results, which is likely a result of probability-weighted values used, the set values of adherence, and the large differences in costs applied between generic and branded medications. Estimates employing real-world whole lifetime adherence data would likely to lead to more robust findings tied to medication cost and ICERs as the level of adherence in each cohort would have significant impacts on overall medication spend. Similarly, the rate of glaucomatous visual loss—represented by set values of decibels each year—is also likely to vary if real-world data were used for moderate and severe glaucoma in addition to mild glaucoma. However, we believe that our model is a likely representation of time to blindness given available epidemiological data. Varying the rates of complications after trabeculectomy or tube shunt surgery did not substantially change the outcome, which was not surprising given that we did not apply different utility values to those experiencing complications and it was assumed these complications were managed rather than allowing them to lead to long-term consequences.
In the US, the threshold most commonly used in cost effective analysis is $50,000 USD per QALY gained. This cutoff was determined using the approach of benchmark interventions. In this approach the cost-effectiveness of a previous ‘benchmark’ intervention that has already been adopted is used as the value threshold for acceptable cost-effectiveness. The threshold in the US was based off an estimate of the cost-effectiveness of dialysis for chronic renal disease.44 Newer studies place the estimate of the threshold in the US using this approach at $150,000 USD per QALY gained.45 The World Health Organization promotes an approach that deems an intervention cost-effective if it costs less than three times the national gross domestic product (GDP) per disability-adjusted life year (DALY) avoided.46 Using the threshold based on per capita GDP approach, an intervention would be deemed cost effective if the it cost less than $160,500 USD per DALY averted. Given these alternative cutoffs, population based adherence interventions may be more cost-effective than would be assumed given the traditional $50,000 USD per QALY gained threshold.
Applying the conservative estimate of willingness-to-pay in the US of $50,000/QALY, if a person was diagnosed with glaucoma at age 40 and had a 38-year average life span,47 a service that was $550/year that improved medication adherence would likely be highly cost-effective from a societal standpoint. If a program that helped improve medication adherence helped improve any other aspect of a person’s health-related quality-of-life, the program costs could potentially exceed $550/year while still being highly cost-effective. Currently, the average Centers for Medicare and Medicaid Services (CMS) reimbursement for diabetes self-management training (CPT G0108) is $54.19 per 30-minute session. A person with newly diagnosed diabetes or poorly controlled diabetes can be referred for 10 hours of self-management training over one year with 2 hours yearly after that for follow-up support, which costs $541.90 for the first year followed by $216.76/year in subsequent years. At least this level of self-management support would be deemed highly cost-effective in decreasing the time to glaucoma-related visual impairment and blindness. Future models can confirm the rates by which such interventions would be cost-effective should they demonstrate an impact on adherence.
A Cochrane Review of medication adherence interventions identified four successful components: theory-based interventions, personally tailored education, motivational interviewing-based counseling, and reminder systems.48 Programs that have incorporated reminders,49 personally tailored education,50 and theory-based counseling interventions using a motivational interviewing style of counseling51 have shown great initial promise in improving glaucoma medication adherence, improving average medication adherence by 15-19 percentage points, though programs that did not incorporate these techniques were less successful.8 A program that combined all of these successful elements along with longitudinal support, and was able to be delivered by a trained para-professional staff member integrated into the eye care provider care team has the potential to greatly improve medication adherence, and thus health related quality of life.
Both the strengths and limitations of this study lie in the data sources used to inform the Markov model. Because the UKGTS was the first study to assess the impact of treating mild glaucoma with medications versus placebo on glaucomatous visual field loss, we were able to get a very precise estimate of progression among patients with mild glaucoma. However, the study did not include people with moderate or severe disease, so we conducted sensitivity analyses to examine the impact of our assumptions of glaucomatous progression in these disease stages. As more trials examining the effect of various treatments on glaucomatous visual field loss are undertaken, data for such economic analyses will become more and more precise. Another limitation is the need to use the assumption that those patients with poor adherence have outcomes similar to the placebo arm in randomized clinical trials of glaucoma medication efficacy. Better data assessing the precise impact of adherence on visual field progression is needed. Our direct medical costs were lower than those estimated by Lee and colleagues33 after accounting for inflation as their study assessed actual expenditures whereas ours used predicted expenditures following the Preferred Practice Guidelines where future costs were discounted. If costs are actually higher than our estimates for later stages of disease, our cost/QALY for adherence may actually be an overestimate (i.e., services to improve adherence would have a lower ICER and be even more cost-effective). A strength of this study lies in its societal perspective as it models both the direct costs of medical care and the indirect costs to individuals and society from blindness. Future work is needed to model societal costs of poor glaucoma medication adherence using national estimates of glaucoma incidence.
In conclusion, this is the first study that has modeled the cost-effectiveness of improving glaucoma medication adherence from a societal perspective. We found that being adherent to glaucoma medications improves individual’s health-related quality of life for a relatively low increase in lifetime healthcare costs. From even a conservative cost-effectiveness standpoint, there is opportunity to allocate more resources to improving adherence while remaining highly cost-effective. Therefore, it is imperative to focus on developing cost-effective programs to better support people in taking their glaucoma medications on time, every day.
Supplementary Material
Acknowledgments
Funding Agency: National Eye Institute (K23EY025320, PANC) and Research to Prevent Blindness Career Development Award (PANC). The funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No conflicting relationships exist for any author.
This work was presented in part at: The Association for Research in Vision and Ophthalmology Vancouver, BC, April 29, 2019.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.CDC. FastStats - Deaths and Mortality. https://www.cdc.gov/nchs/fastats/deaths.htm.
- 2.Best Practices. Disease management: the new tool for cost containment and quality care. Natl Governors Assoc. 2003. [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. Chronic Conditions Among Medicare Beneficiaries. www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions/downloads/2012chartbook.pdf.
- 4.Eye Disease Statistics. https://www.aao.org/eye-disease-statistics.
- 5.Olthoff CMG, Schouten JSAG, van de Borne BW, Webers CAB. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. [DOI] [PubMed] [Google Scholar]
- 6.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: A systematic review. Patient Prefer Adherence. 2011;5:441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterman H, Evans JR, Gray TA, Henson D, Harper R. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev. 2013;2013:CD006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman-Casey PA, Dayno M, Robin AL. Systematic review of educational interventions to improve glaucoma medication adherence: An update in 2015. Expert Rev Ophthalmol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology. 2014;121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 10.Higginbotham EJ. The Physician Workforce Discussion Revisited. Arch Ophthalmol. 2012;130:648–649. [DOI] [PubMed] [Google Scholar]
- 11.Lee PP. Access to Care. Arch Ophthalmol. 2007;125:406. [DOI] [PubMed] [Google Scholar]
- 12.Congdon N, O’Colmain B, Klaver CCW, et al. Causes and Prevalence of Visual Impairment Among Adults in the UnitedStates. Arch Ophthalmol. 2004;122:477–485. [DOI] [PubMed] [Google Scholar]
- 13.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. [DOI] [PubMed] [Google Scholar]
- 14.Ederer F, Gaasterland DA, Dally LG, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004;111:651–664. [DOI] [PubMed] [Google Scholar]
- 15.Arias E, Heron M, Xu J. United States Life Tables 2014. Natl Vital Stat Reports. 2017;66:1–64. [PubMed] [Google Scholar]
- 16.Nouri-Mahdavi K, Brigatti L, Weitzman M, Caprioli J. Comparison of methods to detect visual field progression in glaucoma . Ophthalmology. 1997;104:1228–1236. [DOI] [PubMed] [Google Scholar]
- 17.Katz J, Gilbert D, Quigley HA, Sommer A. Estimating progression of visual field loss in glaucoma. Ophthalmology. 1997;104:1017–1025. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Valk R, Webers CAB, Hendrikse F, De Vogel SC, Prins MH, Schouten JSAG. Predicting intraocular pressure change before initiating therapy: timolol versus latanoprost. Acta Ophthalmol. 2008;86:415–418. [DOI] [PubMed] [Google Scholar]
- 19.DuBiner HB, Mroz M, Shapiro AM, Dirks MS. A comparison of the efficacy and tolerability of brimonidine and latanoprost in adults with open-angle glaucoma or ocular hypertension: A three-month, multicenter, randomized, double-masked, parallel-group trial. Clin Ther. 2001;23:1969–1983. [DOI] [PubMed] [Google Scholar]
- 20.O’Donoghue EP. A comparison of latanoprost and dorzolamide in patients with glaucoma and ocular hypertension: a 3 month, randomised study. Br J Ophthalmol. 2000;84:579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular Pressure Control and Long-term Visual Field Loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caprioli J, Coleman AL. Intraocular Pressure Fluctuation. Ophthalmology. 2008;115:1123–1129.e3. [DOI] [PubMed] [Google Scholar]
- 24.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635. [DOI] [PubMed] [Google Scholar]
- 25.Hong S Long-term Intraocular Pressure Fluctuation and Progressive Visual Field Deterioration in Patients With Glaucoma and Low Intraocular Pressures After a Triple Procedure. Arch Ophthalmol. 2007;125:1010–1013. [DOI] [PubMed] [Google Scholar]
- 26.Lee PP, Walt JW, Rosenblatt LC, Siegartel LR, Stern LS. Association Between Intraocular Pressure Variation and Glaucoma Progression: Data from a United States Chart Review. Am J Ophthalmol. 2007;144:901–907.e1. [DOI] [PubMed] [Google Scholar]
- 27.Fujino Y, Asaoka R, Murata H, et al. Evaluation of Glaucoma Progression in Large-Scale Clinical Data: The Japanese Archive of Multicentral Databases in Glaucoma (JAMDIG). Investig Opthalmology Vis Sci. 2016;57:2012–2020. [DOI] [PubMed] [Google Scholar]
- 28.Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-Term Trends in Glaucoma-Related Blindness in Olmsted County, Minnesota. Ophthalmology. 2014;121:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagg BC, Talwar N, Mattox C, Lee PP, Stein JD. Trends in Use of Ambulatory Surgery Centers for Cataract Surgery in the United States, 2001-2014. JAMA Ophthalmol. 2018;136:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truven Health Analytics. Red Book Online [Database Online]. https://redbook.solutions.aap.org/.
- 31.Newman-Casey PA, Woodward MA, Niziol LM, Lee PP, De Lott LB. Brand Medications and Medicare Part D: How Eye Care Providers’ Prescribing Patterns Influence Costs. Ophthalmology. 2018;125:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schehlein EM, Im LT, Robin AL, Onukwugha E, Saeedi OJ. Nonmedical Out-of-Pocket Patient and Companion Expenditures Associated with Glaucoma Care. J Glaucoma. 2017;26:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rein DB. The Economic Burden of Major Adult Visual Disorders in the United States. Arch Ophthalmol. 2006;124:1754–1760. [DOI] [PubMed] [Google Scholar]
- 34.Lee BS, Kymes SM, Nease RF, Sumner W, Siegfried CJ, Gordon MO. The impact of anchor point on utilities for 5 common ophthalmic diseases. Ophthalmology. 2008;115:898–903.e4. [DOI] [PubMed] [Google Scholar]
- 35.Brown MM. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown GC, Brown MM. Patient Preference-Based Comparative Effectiveness and Cost-Utility Analysis of the Prostamides for Open-Angle Glaucoma. J Ocul Pharmacol Ther. 2019;35:145–160. [DOI] [PubMed] [Google Scholar]
- 37.Katz J, Gilbert D, Quigley HA, Sommer A. Estimating progression of visual field loss in glaucoma. Ophthalmology. 1997;104:1017–1025. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Liang Y, Chen Y, Musch DC, Zhang C, Wang N. Utility Analysis of Vision-related Quality of Life in Patients With Glaucoma and Different Perceptions from Ophthalmologists. J Glaucoma. 2015;24:508–514. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan BC, Malik R, Shuba LM, Rafuse PE, Nicolela MT, Artes PH. Rates of Glaucomatous Visual Field Change in a Large Clinical Population. Investig Opthalmology Vis Sci. 2014;55:4135–4143. [DOI] [PubMed] [Google Scholar]
- 40.Sleath B, Blalock SJ, Carpenter DM, et al. Ophthalmologist-patient communication, self-efficacy, and glaucoma medication adherence. Ophthalmology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou C-F, Frances Cotch M, Vitale S, et al. Age-Related Eye Diseases and Visual Impairment Among U.S. Adults. Am J Prev Med. 2013;45:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Y-P, Wong DT. Self-reported visual impairment in elderly Canadians and its impact on healthy living. Can J Ophthalmol. 2008;43:407–413. [DOI] [PubMed] [Google Scholar]
- 43.Crews J, Campbell V. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Heal. 2004;95:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to Pay for a Quality-adjusted Life Year. Med Decis Mak. 2000;20:332–342. [DOI] [PubMed] [Google Scholar]
- 45.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What Is the Price of Life and Why Doesn’t It Increase at the Rate of Inflation? Arch Intern Med. 2003;163:1637. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Choosing Interventions That Are Cost-Effective. http://www.who.int/choice/en/.
- 47.CDC. FastStats - Life Expectancy. https://www.cdc.gov/nchs/fastats/life-expectancy.htm.
- 48.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014:CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boland MV, Chang DS, Frazier T, Plyler R, Jefferys JL, Friedman DS. Automated Telecommunication-Based Reminders and Adherence With Once-Daily Glaucoma Medication Dosing. JAMA Ophthalmol. 2014;132:845–850. [DOI] [PubMed] [Google Scholar]
- 50.Okeke CO, Quigley HA, Jampel HD, et al. Interventions Improve Poor Adherence with Once Daily Glaucoma Medications in Electronically Monitored Patients. Ophthalmology. 2009;116:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreer LE, Owsley C, Campbell L, Gao L, Wood A, Girkin CA. Feasibility, Patient Acceptability, and Preliminary Efficacy of a Culturally Informed, Health Promotion Program to Improve Glaucoma Medication Adherence Among African Americans: “ G laucoma Management O ptimism for A frican Americans L iving with Glau. Curr Eye Res. 2016;41:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.