Abstract
Early life stress (ELS) can be very harmful to an individual’s wellbeing and brain development. It is well established that childhood maltreatment is a significant risk factor for depression. ELS is positively correlated with depressive symptoms both in major depression disorder patients and healthy individuals, but the cognitive and neural mechanisms underlying this association are still unclear. In the present study, we calculate the within/between-network connectivity in 528 college students, and Pearson correlation was performed to investigate the relationship between network measures and ELS. Additionally, the same method was applied to verify these results in another sample. Finally, mediation analysis was performed to explore the cognitive and neural mechanisms regarding the association between ELS and depression. Correlation analysis indicated that ELS was positively correlated with the within-network connectivity of the ventral attention network (VAN), the dorsal attention network (DAN), the salience network (SN), the somatosensory network (SMN) and the between-network connectivity of ventral attention network-dorsal attention network (VAN-DAN), ventral attention network- somatosensory network (VAN-SMN), and ventral attention network-visual network (VAN-VN). Validation results indicated that ELS is associated with the within-network connectivity of VAN and DAN. Mediation analysis revealed that attention bias and the within-network connectivity of VAN could mediated the relationship between ELS and depression. Both behavioral and neural evidence emphasize ELS’s influence on individual’s emotion attention. Furthermore, the present study also provides two possible mediation models to explain the potential mechanisms behind the relationship between ELS and depression.
Subject terms: Social neuroscience, Neuroscience
Introduction
ELS refers to traumatic life event occurring childhood, including emotional, physical, and sexual abuse, emotional and physical neglect. Evidence suggests that ELS leads to prolonged phases of stress and even predict negative outcomes over the course of a lifetime1. In particular, maltreated children are more likely to develop posttraumatic stress disorder (PTSD)2,3, major depression disorder (MDD)4–6, anxiety disorder7,8, and substance abuse9,10 in the future. In addition, childhood maltreatment is also associated with lower level of well-being11,12, worse educational outcomes13,14 and shortened life expectancy15. With the development of MRI technology, Empirical researches have explored the influence of ELS on individual’s brain. The most prominent results oriented to the hippocampus and amygdala-brain regions which are sensitive to stress16–19. For example, adults who had experienced ELS demonstrated smaller volume of hippocampus, compared with controls20. ELS was associated with decreased amygdala volume21. Besides, studies have indicated that ELS also influence the volume of the prefrontal cortex, sensorimotor cortex and its related fiber-tract integrity21.These findings provide valuable evidences for comprehending how ELS modifies individual’s brain. However, recent studies confirmed that the human brain was consisted with complicated functional networks, and cognitive and affective processing are supported by the interaction of these networks, instead of the function of a single brain area. Thus, new pointcut is needed to explore the modifications and alternations that ELS makes to brain networks.
It has been suggested that identifying and processing emotional visual information is essential for emotion regulation22 and emotional attention bias is one of the most significant cognitive features in individuals who were exposed to ELS23,24. Empirical studies have supported that individuals with ELS demonstrated attention bias either toward or away from emotional stimuli9,25–27. Attention bias arises as early as 150 milliseconds after the presentation of the stimulus and modulate reactions occurring at this time scale usually depend on stimulus-driven (as opposed to voluntary) attention, a function rely on the participation of the VAN9,28. Considering the role of VAN in orienting and responding to salient stimuli29. It is reasonable to infer that attention bias might depend on the function of VAN.
ELS is believed to be a key risk factor for developing major depression disorder (MDD)30,31. Furthermore, previous studies have shown that there is a significant relationship between ELS and depression mood32,33. But the neural and cognitive mechanisms behind this association is still ambiguous. Thus, it is of great significance to discover the cognitive mechanism of how childhood maltreatment influence individual’s depressive emotion. In a sense, it may help to propose relevant interventions to decrease the negative influence of ELS. Evidence demonstrated that experimentally inducing attention bias to emotionally valanced stimulus causes measurable changes in mood in healthy individuals34,35, whereas attention bias modification can alleviate depressive symptoms36,37. Attention bias to traumatic experience might be an adaptive strategy to prepare for the forthcoming threat38, but from a long-term perspective, it will interfere with subsequent information processing, which might lead to a consistent negative influence. Presumably, we can infer that individual exposed to ELS might develop attention bias to emotionally valenced, which is detrimental to effective emotion regulation, and ultimately result in high level of depression when facing with stress. Both clinical and empirical studies emphasize the role of attention bias in developing and maintaining depression39,40. Thus we hypothesized that attention bias might play a key role in the relationship between ELS and depression,
Thus, the present study was designed to explore the influence of ELS on individual’s brain networks. Furthermore, we also attempted to decipher the neural and cognitive mechanism sustaining the association between ELS and depression. First, we investigate the relationship between ELS and within/between network connectivity with a big sample of 528 college students. To verify the results, machine learning method was applied to another small sample. Second, mediation analysis was performed to test whether there were particular neural basis mediate the relationship between ELS and depression. Finally, we explored whether attention bias could mediate the relationship between ELS and depression. Based on previous evidence, we hypothesized that attention bias and the functional connectivity (FC) of attention networks might be the behavioral and neural markers of ELS. In addition, there were two possible mediation mechanisms which might elucidate the potential neural and cognitive mechanisms sustaining the relationship between ELS and depression.
Method
Participants
Dataset 1 contained 528 right-handed college students (157 males, age = 19.42 ± 1.40 years, age range: 16–26 years), 38 participants were excluded for its maximal motion between volumes in each direction > 3 mm, and rotation about each axis >3°. Dataset 2 contained 208 right-handed college students (86 males, age = 22.30 ± 1.47 years, age range: 18–27 years), 25 participants were excluded for its maximal motion between volumes in each direction >3 mm, and rotation about each axis >3° (see Table 1). Participants with a history of manic episodes, psychotic features or neurological illness were excluded. All participants were college students from Southwest University in China. They were informed of the procedure of the experiment, and finished written informed consent prior to participating in the study. For the participants under the age of 18 years, their parents were informed of the procedure and allowed them to participate in the experiment, and finished written informed consent prior to participating in the study. The study was approved by the Southwest University Brain Imaging Center Institutional Review Board. All research was performed in accordance with the relevant guidelines and regulations.
Table 1.
Demographic data.
| Variables | Dataset 1 (n = 528) | Dataset 2 (n = 208) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age | 19.42 | 1.40 | 16–26 | 22.30 | 1.47 | 18–27 |
| ELS | 37.35 | 8.34 | 25–72 | 35.39 | 9.16 | 25–77 |
| Depression | 7.05 | 6.45 | 0–30 | 7.24 | 5.76 | 0–27 |
| Present stress | 46.70 | 20.88 | 0–132 | 46.43 | 31.37 | 0-131 |
Note: SD = standard deviation;
Measure of the behavior variable
The childhood trauma questionnaire
The Childhood Trauma Questionnaire-Short Form (CTQ-SF) was used to evaluate individual’s traumatic experience during childhood41. The CTQ consisted of 25 clinical questions and 3 validity items. All the items were rated on a 5-point Likert scale (ranging from 1 never true to 5 very often true) enquiring about 5 types of childhood trauma including emotional, physical and sexual abuse and emotional and physical neglect. The CTQ-SF has demonstrated good reliability and validity42. The Cronbach’s alpha of DAQ in the dataset 1 was 0.74, indicating that the present questionnaire had good construct validity.
Depression
Individual’s difference in depression was evaluated by Beck Depression Inventory43. The BDI-II is a 21-item self-report questionnaire which measured the severity of depressive symptoms within the past week. Each of the 21 items was rated on 4-point Likert-type scale ranging from 0–3. Participants who get higher score in the BDI tend to experienced more depressive symptoms. The BDI- II is a reliable measure which is widely used to assesses the severity of depressive symptoms from non-clinical to clinical samples44.The Cronbach’s alpha of the scale was 0.85 in this sample,
Current stress
The Current stress was accessed by the Adolescent Self-Rating Life Events Checklist45. It consisted of 27 common negative life events which belonged to multiple social-stress domains: family (e.g., “Physical punishment by parents”), school (e.g., “Failure in a test”), interpersonal (e.g., “Break up with close friends”), and personal physical diseases (see Table 1), these life events were suggested to be the most frequently occurred to Chinese adolescents45. Each item begins with whether the participants experienced the particular event, and then there is a five-point Likert scale (ranging from 1 “not at all” to 5 “extremely severe”) to evaluate the perceived stress of each life event. The Cronbach’s alpha of the scale was 0.95 in this sample,
Dot probe task
102 participants completed the experiment outside of the MRI scanner. Eprime 2.0 software (https://pstnet.com/products/e-prime/) was used to control the presentation of stimuli and to record response accuracy and response time. The dot probe task was designed according to MacLeod46. At the beginning of the task, there was a fixation cross presented for 500 ms on the screen, followed by a pair of faces for 500 ms, after which the dots appeared, then the dots disappeared. The participants were then asked to identify whether the small dots were arranged horizontally or vertically by pressing the associated key. Before the real experiment started, participants were given five practice trials to familiarize themselves with the procedure. The task contained 8 conditions: 4 emotion face pairs (neutral and happy, NH, neutral and sad, NS, neutral and anger, NF, neutral and fear) x 2 probe locations (in the location of the emotional face or the neutral face) and was comprised of 2 blocks of 151 trails (NH 40 trails, NS 37 trails, NA 37 trails, NF 37 trails), with a short break between the blocks. Attention bias scores were calculated as follows:
Attentional bias score = 1/2[(RpLe - RpRe) + (LpRe - LpLe)]47
R = right position; L = left position; p = probe; e = emotional face. In this equation, RpLe represents the mean reaction time when the probe presents in the left and the emotional face presents in the right, and so on. This equation estimates the “attention capturing” quality of emotional faces by subtracting the mean reaction times for probes presenting in the same position of the emotional face from the mean reaction times for probes presenting in the opposite position of the emotional face47. Positive bias scores represent the allocation of attention toward the emotional face compare with the matched neutral faces, and negative bias scores represent the allocation of attention away from the emotional face relative to the matched neutral faces, that is attention avoidance.
RsfMRI data acquisition and preprocessing
Image acquisition
Whole-brain images were quarried by a Siemens 3.0 T magnetic resonance scanner. Participant’s high-resolution anatomical image data was acquired through a T1- weighted magnetization-prepared rapid gradient-echo (3d MP-RAGE) sequence in sagittal plane (inversion time = 900 ms, repetition time = 1900 ms, echo time = 2.52 ms, flip angle = 9°, resolution matrix = 256 × 256, thickness = 1.0 mm, slices = 176, voxel size = 1 × 1 × 1 mm3). The functional image was coregistrated to T1 image.
The resting-state fMRI data scanning took 8 minutes, all participants were instructed to have a rest while keeping eyes closed, but stay awake during scanning. Participant’s whole brain functional slices were acquired in descending order by gradient-echoplanar imaging (EPI) sequences, and the detailed parameters were as follows: repetition time (TR) = 2000, echo time (TE) = 30 ms, slices = 32, flip angle = 90°, field of view (FOV) = 220 mm × 220 mm, andvoxel size 3.4 × 3.4 × 4 mm3, thickness = 3 mm¸ slice gap = 1 mm.
Image data preprocessing
Imaging data was applied with SPM8 toolbox (http://www.fil.ion.ucl.ac.uk/spm/ software/spm8/) and Data Processing Assistant for Resting- State fMRI (DPARSF) based on MATLAB 2012a platform (MathWorks Inc., Natick, MA) to preprocess. The first 10 EPI images were removed considering that the participants need time to get used to the scanning circumstance, and slice timing were conducted in the remaining images. Then the neuroimaging data underwent standard realignment and normalization (MNI space). Next the fMRI time-series were entered into spatial smoothing (8 mm FWHM). Additionally, band-pass temporal filtering (0.01–0.1 Hz) were conducted and then remove nuisance signal including white matter (WM) and cerebrospinal fluid (CSF), global mean signal regression, and 6 motion parameters. Finally, data quality control was performed and removed the participants whose maximal motion in each direction>3 mm, and rotation>3°.
Data analysis
Calculating of network measures
After preprocessing of resting-state MRI data, the power 264 template was adopted to extract the time course of each ROI48. Then 10 well-defined brain networks (contain 227 nodes) were chosen to enter into the follow-up analysis, including the default mode network (DMN), salience network (SN), fronto-parietal network (FPN), cingulo-opercular network (CON), sensorimotor network (SMN), visual network (VN), ventral attention network (VAN), dorsal attention network (DAN), auditory network (AN) and subcortical network (SUB). The within-network connectivity was calculated as follows:
For each brain network (a∈1,2, ⋯,10), the within-network connectivity was calculated as the average connectivity across all the links of the network normalized by the square of the number of nodes (ROIs). 𝐴𝑖𝑗 represent the 227 × 227 connectivity matrix; 𝑁𝑎 is the number of nodes within network 𝑎; 𝑖 and 𝑗 notes the Power ROIs. Higher level of within-network connectivity indicated stronger interaction between those regions within the particular network.
The pairwise connectivity between networks was computed as follows:
The pairwise connectivity between networks (𝑃𝐵𝑎−𝑏) was computed as the average connectivity across all the links between two networks, 𝑎 and 𝑏 (𝑎, 𝑏∈1,2, ⋯,10; 𝑎 ≠ 𝑏), normalized by the product of the number of nodes within the two networks49. Higher level of between-network connectivity indicated stronger interaction between those regions of the two networks.
Brain–behavior correlation analyses
We conducted pearson correlations analysis between the within/between-networks of connectivity and ELS, controlling for age and sex. The results were corrected by Benjamini and Hochberg FDR (BH_FDR) correction50.
Prediction analysis
To test the robustness of the brain-behavior relationship, we performed a machine-learning method named linear support vector regression (SVR) and cross-validation procedure with balanced ten-fold51. ELS was taken as the dependent variable and the network connectivity were taken as independent variables in the linear regression algorithm. The r(predicted, observed) was estimated by a four-fold cross-validation, and represent the prediction accuracy of the independent variable. We divided the data into four folds in order to keep the distributions of independent and dependent variables balanced. The prediction model was trained on the 75% sample and then was tested on the remaining participants. This procedure was conducted for 4 times to acquire a final r(predicted, observed) representing the correlation between the predict value and the actual value.
Generalization to a different sample
To examine to what extent the results could generalize to other sample, the same analysis was applied to Dataset 2. The analysis process was as follows: 1), preprocess the resting-state fMRI data, 2), construct brain networks based on the power 264 template, 3), calculate the within/between network connectivity, 4), Person correlation analysis was performed to investigate the relationship between ELS and network measures.
Mediation analysis
To examine whether the within-network connectivity of the VAN could explain the relationship between ELS and depression, a mediation analysis was conducted. The mediation model could partly explain the causal pathway by which the dependent variable (Y) was affected by the independent variable (X) affects a dependent variable (Y). Mediation analyses were performed by applying the indirect macro designed for SPSS52. In the current study, X is the ELS, Y is the depression, and M is the within-network connectivity of the VAN. Age and sex were entered as covariates in the mediation model. This macro uses bootstrapped sampling to estimate the indirect mediation effect. In this alogorithm, 2,000 bootstrapped samples were drawn, and bias corrected 95% bootstrap confidence intervals (CI) were reported. CI that do not include zero indicate a significant indirect effect of the independent variable on the dependent variable through the mediators. Besides, the same mediating method was performed to explore whether attention bias of a sad face could explain the association between ELS and depression.
Results
The neural correlates of ELS
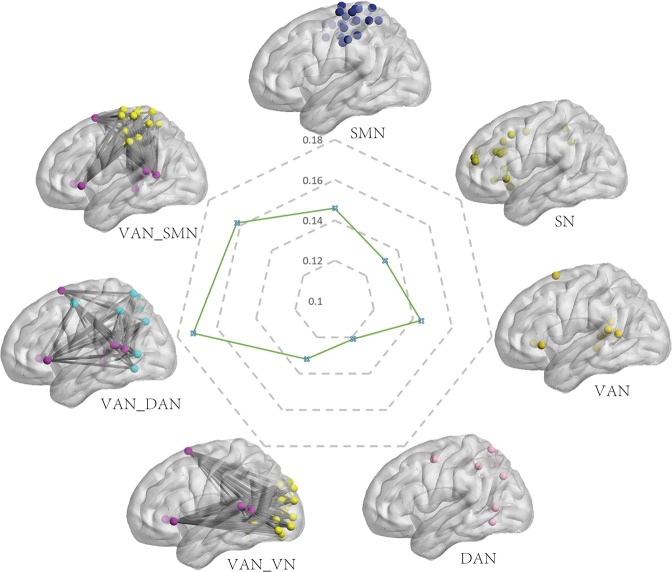
The demographic data are presented in Table 1. There was no gender difference in ELS (t = 2.682, p = 0.377), and ELS was not related to age (r = 0.033, p = 0.439). Within-network results indicated that ELS is positively correlated with within-network connectivity of the VAN, DAN, SMN-H, SN, and between-network of the VAN-DAN, VAN-VN, VAN-SMN (see Table 2 and Figure 1). Then prediction analysis was performed to test the robustness of the relationship between ELS and the within-network connectivity of these networks. Prediction results demonstrated that within/between-network connectivity of these networks could predict ELS (r = 0.139, p < 0.005, see Figure 2), after controlling for age, gender and current stress. Due to the relatively small ample size of the Dataset 2 and its resultant low statistical power, the Dataset 2 only partly verified the present results. The results of Dataset 2 indicated that ELS was positively correlated with BDI score (r = 0.230, p < 0.005), the within-network connectivity of the VAN (r = 0.224, p < 0.005) and the DAN (r = 0.165, p < 0.05).
Table 2.
Within/between-network connectivity associated with ELS.
| Network | r | p | pB H_FDR | |
|---|---|---|---|---|
| Within-network | VAN | 0.144 | 0.001 | <0.05 |
| DAN | 0.121 | 0.007 | <0.05 | |
| SN | 0.132 | 0.003 | <0.05 | |
| SMN | 0.146 | 0.001 | <0.05 | |
| Between-network | VAN-DAN | 0.172 | 0.001 | <0.05 |
| VAN-SMN | 0.162 | 0.001 | <0.05 | |
| VAN-VN | 0.132 | 0.001 | <0.05 |
Abbreviations: VAN, ventral attention network, DAN, dorsal attention network, SN, salience network, SMN, sensorimotor network, VN, visual network.
Figure 1.
Within/between-network connectivity associated with ELS. The radar map shows the correlation coefficients of the within/between-network connectivity with ELS. Abbreviations: VAN, ventral attention network, DAN, dorsal attention network, SN, salience network, SMN, sensorimotor network, VN, visual network.
Figure 2.
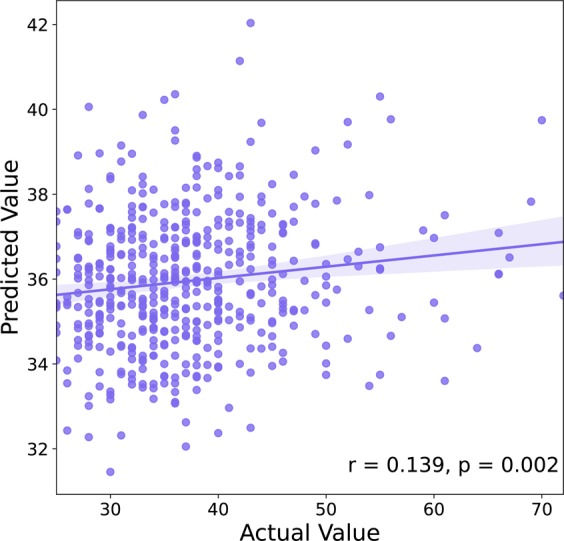
The prediction results. The within-network connectivity of VAN, DAN, SN, SMN and the between-network connectivity of VAN_DAN, VAN_SN, VAN_SMN could predict individual difference of ELS.
Correlations between ELS, network measures and depression
A correlation analysis was conducted to explore the relationship between ELS, network measures and depression. After controlling for age, gender and current stress, ELS was significantly correlated with depression (r = 0.204, p < 0.001), and the within-network connectivity of the VAN was also associated with depression (r = 0.137, p < 0.005). These results suggested that a intensely association exists between the within-network connectivity of the VAN, ELS and depression. To test the hypothesis that attention bias sustains the relationship between ELS and depression, we also investigate the relationship between ELS, attention bias and depression.
Mediation results
The first mediation analysis indicated that the within-network connectivity of the VAN significantly mediate the relationship between ELS and depression (indirect effect = 0.01, 95% CI = [0.004, 0.04], p < 0.05, see Figure 3), Standardized regression coefficients are present in the path diagram, which represent the covariant relationship between two variables. The second mediation analysis indicated that the attention bias to a sad face had a significant mediating effect on the relationship between ELS and depression (indirect effect = 0.07, 95% CI = [0.007, 0.125], p < 0.05, see Figure 4)
Figure 3.
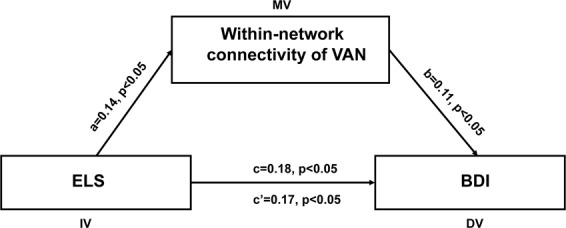
The within-network connectivity of VAN (Ventral attention network) mediate the relationship between ELS (Early life stress) and depression. The depicted diagram shows that ELS predicts displaced depression through the within-network connectivity of VAN after controlling for age, gender. Standardized regression coefficients are present in the path diagram.
Figure 4.
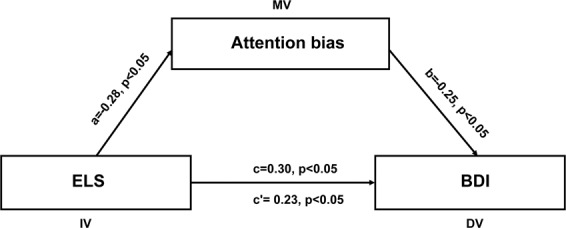
Attention bias mediate the relationship between ELS (Early life stress) and depression. The depicted diagram shows that ELS predicts displaced depression through attention bias after controlling for age, gender. Standardized regression coefficients are present in the path diagram.
Discussion
This study extends existing knowledge about the neural basis of ELS by revealing that FC within/between large-scale brain networks were related to the individual differences of ELS. Fist, positive associations were observed between ELS and the within-network connectivity of the VAN, DAN, SN, SMN. Second, we found a positive relationship between ELS and the between-network connectivity of the VAN- DAN, VAN-VN, VAN-SMN. Finally, we found that attention bias and the within-network connectivity of the VAN mediate the relationship between ELS and depression. These results therefore provide a convincible neurobiological association for the changes in attention associated with childhood trauma and depression.
Positive associations were observed between ELS and the within-network connectivity of the VAN, DAN and the between-network connectivity of the VAN-DAN, which fitted well with previous studies that aberrant function of brain regions in attention network were associated with ELS and depression. For example, exposure to ELS was associated with increased activation of inferior parietal lobule during working memory task53. Previous studies detected increased functional connectivity between dmpfc, dlpfc (key node of DAN), vmpfc and ACC54 in MDD patients. The VAN was confirmed to be involved in stimulus-driven attention29. For instance, detecting a salient target might require the involvement of the VAN (i.e., “invalid” targets in the Posner spatial cueing paradigm)55–58. Whereas the DAN embodies top-down attention control59,60. DAN activity might increase when individuals orientate their attention to the current task56,61,62
Generally, these two networks tend to cooperate during normal cognitive processes. During an ongoing cognitive process, that task-relevant signals from the dorsal attention network might “filter” stimulus-driven signals in the ventral attention network, while stimulus-driven “circuit-breaking” signals from the ventral attention network offer an interruption to the dorsal attention network, reorienting the attention toward salient stimuli63. Several studies have demonstrated the association between childhood trauma and aberrant threat detection and response. Specifically, clinical study suggested that vigilance mediates the association between childhood neglect and negative symptoms in male psychotic patients64, exposure to ELS is associated with attention bias to a threatening stimulus, such as a fearful face65, or the emotional valence word66–68. Overall, this increased within/between-network connectivity of attention systems might indicate a higher state of vigilance and awareness in individuals exposed to ELS.
In addition, the results also demonstrated that ELS was positively correlated with the within-network connectivity of the SN, SMN, and the between-network connectivity of VAN-VN, VAN-SMN. The SN is suggested to be involved in responding to behaviorally salient stimuli69 and implement the coordination of behavioral responses70. Increased SN activity has been observed during situations in which changing behavior is more likely to be needed71. Previous study has indicated that the SN is involved in supporting stable attention for a current goal72. In this study, this association between ELS and the within-network connectivity of the SN might suggest constant attention toward the external environment. The abnormal anatomical structure of the SMN is widely reported in ELS related studies73,74. The SMN is related to the processing of incoming sensory stimuli and generating motor outputs75–79. Previous studies have suggested that depressive temperament was associated with alternated neuronal variability in the SMN80, Increased within-network connectivity of the SMN might support being more reactive to internal and external signals for initiating response. The VN was involved in processing primary visual information and projecting to higher sensory areas81. The stronger interaction between the SMN, VN and VAN might indicate a more sensitive perception of internal and external stimulus.
Further analysis revealed that there were positive association between the within-network connectivity of the VAN, attentional bias and ELS, which might suggest that adults who have experienced ELS have an altered within-network connectivity of the VAN associated with attention bias. In order to explore the neural and cognitive mechanisms for the influence of ELS on depression, we constructed two mediation models to decipher the potential relationship between ELS, depression, within-network connectivity of the VAN and attention bias. There is no doubt that attentional bias is a key cognitive feature in people with ELS, but the orientation of attentional bias is still controversial. Some studies indicated that ELS is related to attentional bias toward threatening or sad face82,83, while others suggested attentional bias away from threatening stimuli84,85. The present results supported the latter point. According to Posner’s study, longer stimulus onset asynchronies (more than 300 ms) might result in a reliable disadvantage for valid trails, during which the attention was first engaged by the cue and then disengaged from it (that is attention avoidance). Presumably, attention avoidance might be a defensive response to reducing exposure to negative stimuli, but from a long-term perspective it attentional avoidance might hinder effective emotion regulation86,87. Specifically, exposure to ELS might lead to attentional avoidance, which might associate with ineffective emotion regulation. Eventually, individual who has experienced ELS will develop a higher depressive state after stress events. Previous studies demonstrated that major depressive disorder was associated with abnormal connectivity of regions involved in cognitive control of attention88,89, indicating an aberrant attention process to salient cues. As for the role of the VAN in the relationship between ELS and depression, one possible explanation is that increased within-network connectivity of VAN might relate to a stronger stimulus-driven attention toward threaten stimuli, which conform to the sensibility to threatening stimuli in individual with higher depression.
Altogether, the present study provided behavioral and neural evidence for the alternations of ELS on individual’s attention. Based on these results, we can infer that attention is a key factor to explain how individual who have experienced ELS develop depression. Besides, attention bias modification training could serve as a potential intervention method to reduce the negative impact of ELS.
Acknowledgements
This research was supported by the Chongqing doctoral research and innovation project (CYB19102). National Natural Science Foundation of China (31470981; 31571137; 31500885; 31600878; 31771231), Project of the National Defense Science and Technology Innovation Special Zone, Chang Jiang Scholars Program, National Outstanding Young People Plan, the Program for the Top Young Talents by Chongqing, the Fundamental Research Funds for the Central Universities (SWU1609177), Natural Science Foundation of Chongqing (cstc2015jcyjA10106), Fok Ying Tung Education Foundation (151023), the Research Program Funds of the Collaborative Innovation Center of Assessment toward Basic Education Quality at Beijing Normal University.
Author contributions
J.Q. and C.D. initiated and supervised this project. J.Q. and Y.M. conceived and designed the experiment. Y.M. and H.X. interpreted the results and write the manuscript. All authors contributed to the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yu Mao and Hong Xiao.
Contributor Information
Cody Ding, Email: dingc@umsl.edu.
Jiang Qiu, Email: qiuj318@swu.edu.cn.
References
- 1.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American journal of psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson MP, Kaslow NJ, Lane DB, Kingree J. Childhood maltreatment, PTSD, and suicidal behavior among African American females. Journal of Interpersonal Violence. 2000;15:3–15. doi: 10.1177/088626000015001001. [DOI] [Google Scholar]
- 3.Grassi-Oliveira, R. & Stein, L. M. Childhood maltreatment associated with PTSD and emotional distress in low-income adults: the burden of neglect. Child Abuse & Neglect (2008). [DOI] [PubMed]
- 4.Nemeroff CB, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depression and anxiety. 1999;9:169–174. doi: 10.1002/(SICI)1520-6394(1999)9:4<169::AID-DA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Bifulco A, Moran PM, Baines R, Bunn A, Stanford K. Exploring psychological abuse in childhood: II. Association with other abuse and adult clinical depression. Bulletin of the Menninger Clinic. 2002;66:241–258. doi: 10.1521/bumc.66.3.241.23366. [DOI] [PubMed] [Google Scholar]
- 7.Simon NM, et al. Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depression and anxiety. 2009;26:1027–1032. doi: 10.1002/da.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce LC, Heimberg RG, Blanco C, Schneier FR, Liebowitz MR. Childhood maltreatment and social anxiety disorder: Implications for symptom severity and response to pharmacotherapy. Depression and Anxiety. 2012;29:132–139. doi: 10.1002/da.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological bulletin. 2007;133:1. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Stein JA, Leslie MB, Nyamathi A. Relative contributions of parent substance use and childhood maltreatment to chronic homelessness, depression, and substance abuse problems among homeless women: Mediating roles of self-esteem and abuse in adulthood. Child abuse & neglect. 2002;26:1011–1027. doi: 10.1016/S0145-2134(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 11.Bellis MA, Hughes K, Jones A, Perkins C, McHale P. Childhood happiness and violence: a retrospective study of their impacts on adult well-being. BMJ open. 2013;3:e003427. doi: 10.1136/bmjopen-2013-003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurius PS, Green S, Logan-Greene P, Borja S. Life course pathways of adverse childhood experiences toward adult psychological well-being: A stress process analysis. Child abuse & neglect. 2015;45:143–153. doi: 10.1016/j.chiabu.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romano E, Babchishin L, Marquis R, Fréchette S. Childhood maltreatment and educational outcomes. Trauma, Violence, & Abuse. 2015;16:418–437. doi: 10.1177/1524838014537908. [DOI] [PubMed] [Google Scholar]
- 14.Pieterse D. Childhood maltreatment and educational outcomes: Evidence from South Africa. Health economics. 2015;24:876–894. doi: 10.1002/hec.3065. [DOI] [PubMed] [Google Scholar]
- 15.Corso PS, Edwards VJ, Fang X, Mercy JA. Health-related quality of life among adults who experienced maltreatment during childhood. American journal of public health. 2008;98:1094–1100. doi: 10.2105/AJPH.2007.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neuroscience research. 1996;26:235–269. doi: 10.1016/S0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 17.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 18.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MM, et al. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. Journal of child psychology and psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin L, Chia F-S. Characterizing temperate rocky shores from the perspective of an early juvenile snail: the main threats to survival of newly hatched Nucella emarginata. Marine Biology. 1995;122:625–635. doi: 10.1007/BF00350684. [DOI] [Google Scholar]
- 23.Davis JS, et al. Attachment anxiety moderates the relationship between childhood maltreatment and attention bias for emotion in adults. Psychiatry research. 2014;217:79–85. doi: 10.1016/j.psychres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romens SE, Pollak SD. Emotion regulation predicts attention bias in maltreated children at‐risk for depression. Journal of Child Psychology and Psychiatry. 2012;53:120–127. doi: 10.1111/j.1469-7610.2011.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child maltreatment. 2009;14:148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-Haim Y, et al. Life-threatening danger and suppression of attention bias to threat. American Journal of Psychiatry. 2010;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- 27.Wald I, et al. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychological medicine. 2011;41:699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]
- 28.Sylvester CM, et al. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:1326–1336. e1325. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown J, Cohen P, Johnson JG, Smailes EM. Childhood abuse and neglect: specificity of effects on adolescent and young adult depression and suicidality. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1490–1496. doi: 10.1097/00004583-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. Journal of Clinical Psychiatry. 2004;65:249–254. doi: 10.4088/JCP.v65n0217. [DOI] [PubMed] [Google Scholar]
- 32.Raes F, Hermans D. On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: Brooding versus reflection. Depression and Anxiety. 2008;25:1067–1070. doi: 10.1002/da.20447. [DOI] [PubMed] [Google Scholar]
- 33.Gibb B, Alloy L, Abramson L. Global reports of childhood maltreatment versus recall of specific maltreatment experiences: Relationships with dysfunctional attitudes and depressive symptoms. Cognition and Emotion. 2003;17:903–915. doi: 10.1080/02699930244000408. [DOI] [Google Scholar]
- 34.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of abnormal psychology. 2002;111:107. doi: 10.1037/0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, H. et al. Early Life Stress and Trauma and Enhanced Limbic Activation to Emotionally Valenced Faces in Depressed and Healthy Children. Journal of the American Academy of Child & Adolescent Psychiatry53, 800-813.e810. [DOI] [PMC free article] [PubMed]
- 36.Hakamata Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peckham AD, McHugh RK, Otto MW. A meta‐analysis of the magnitude of biased attention in depression. Depression and anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 38.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological bulletin. 1986;99:20. doi: 10.1037/0033-2909.99.1.20. [DOI] [PubMed] [Google Scholar]
- 39.Clark DM, Wells A. A cognitive model of social phobia. Social phobia: Diagnosis, assessment, and treatment. 1995;41:00022–00023. [Google Scholar]
- 40.Yang W, Ding Z, Dai T, Peng F, Zhang JX. Attention bias modification training in individuals with depressive symptoms: a randomized controlled trial. Journal of behavior therapy and experimental psychiatry. 2015;49:101–111. doi: 10.1016/j.jbtep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein DP, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 42.Fink, L. A., Bernstein, D., Handelsman, L., Foote, J. & Lovejoy, M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. The American journal of psychiatry (1995). [DOI] [PubMed]
- 43.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78:490–498. [Google Scholar]
- 44.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 45.Liu X, Liu L, Yang J, Zhao G. Reliability and validity of the adolescents self-rating life events checklist. Chinese Journal of Clinical Psychology. 1997;5:34–36. [Google Scholar]
- 46.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of abnormal psychology. 1986;95:15. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- 47.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of abnormal psychology. 2004;113:127. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 48.Power, J. D. et al. Functional network organization of the human brain. 72, 665–678 (2011). [DOI] [PMC free article] [PubMed]
- 49.Yu M, et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proceedings of the National Academy of Sciences. 2019;116:8582–8590. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995;57:289–300. [Google Scholar]
- 51.Supekar K, et al. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proceedings of the National Academy of Sciences. 2013;110:8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40:879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- 53.Quidé Y, et al. Effects of childhood trauma on working memory in affective and non-affective psychotic disorders. Brain imaging and behavior. 2017;11:722–735. doi: 10.1007/s11682-016-9548-z. [DOI] [PubMed] [Google Scholar]
- 54.Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological psychiatry. 2001;50:651–658. doi: 10.1016/S0006-3223(01)01263-X. [DOI] [PubMed] [Google Scholar]
- 55.Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. Journal of cognitive neuroscience. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- 56.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature neuroscience. 2000;3:292. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 57.Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macaluso E, George N, Dolan R, Spence C, Driver J. Spatial and temporal factors during processing of audiovisual speech: a PET study. Neuroimage. 2004;21:725–732. doi: 10.1016/j.neuroimage.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 59.Bundesen C. Visual selection of features and objects: Is location special? A reinterpretation of Nissen’s (1985) findings. Perception & Psychophysics. 1991;50:87–89. doi: 10.3758/BF03212208. [DOI] [PubMed] [Google Scholar]
- 60.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual review of neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 61.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature neuroscience. 2000;3:284. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 62.Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. Journal of Neuroscience. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience. 2002;3:201. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 64.Mansueto G, et al. Childhood adversities and psychotic symptoms: the potential mediating or moderating role of neurocognition and social cognition. Schizophrenia research. 2019;206:183–193. doi: 10.1016/j.schres.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 65.Choi K, Jeon G-S, Cho S-I. Prospective study on the impact of fear of falling on functional decline among community dwelling elderly women. International journal of environmental research and public health. 2017;14:469. doi: 10.3390/ijerph14050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wingenfeld K, et al. Associations of childhood trauma, trauma in adulthood and previous-year stress with psychopathology in patients with major depression and borderline personality disorder. Child Abuse & Neglect: The International Journal. 2011;35:647–654. doi: 10.1016/j.chiabu.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 67.McNally RJ, Clancy SA, Schacter DL. Directed forgetting of trauma cues in adults reporting repressed or recovered memories of childhood sexual abuse. Journal of abnormal psychology. 2001;110:151. doi: 10.1037/0021-843X.110.1.151. [DOI] [PubMed] [Google Scholar]
- 68.Puetz VB, et al. Altered neural response to rejection‐related words in children exposed to maltreatment. Journal of child psychology and psychiatry. 2016;57:1165–1173. doi: 10.1111/jcpp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaty RE, Benedek M, Kaufman SB, Silvia PJ. Default and executive network coupling supports creative idea production. Scientific reports. 2015;5:10964. doi: 10.1038/srep10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry. 2013;170:616–623. doi: 10.1176/appi.ajp.2013.12070950. [DOI] [PubMed] [Google Scholar]
- 74.Khan A, et al. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Frontiers in psychiatry. 2015;6:42. doi: 10.3389/fpsyt.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basalyga G, Salinas E. When response variability increases neural network robustness to synaptic noise. Neural Computation. 2006;18:1349–1379. doi: 10.1162/neco.2006.18.6.1349. [DOI] [PubMed] [Google Scholar]
- 76.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nature reviews neuroscience. 2008;9:292. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garrett DD, McIntosh AR, Grady CL. Moment-to-moment signal variability in the human brain can inform models of stochastic facilitation now. Nature Reviews Neuroscience. 2011;12:612. doi: 10.1038/nrn3061-c1. [DOI] [PubMed] [Google Scholar]
- 78.Zou Q-H, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zuo X-N, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conio B, et al. Opposing patterns of neuronal variability in the sensorimotor network mediate cyclothymic and depressive temperaments. Human brain mapping. 2019;40:1344–1352. doi: 10.1002/hbm.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas Yeo B, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fani N, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological medicine. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gibb, B. E., Benas, J. S., Grassia, M. & McGeary, J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child & Adolescent Psychology38, 415–426. [DOI] [PMC free article] [PubMed]
- 84.Mansell W, Clark DM. How do I appear to others? Social anxiety and processing of the observable self. Behaviour research and therapy. 1999;37:419–434. doi: 10.1016/S0005-7967(98)00148-X. [DOI] [PubMed] [Google Scholar]
- 85.Pishyar R, Harris LM, Menzies RG. Attentional bias for words and faces in social anxiety. Anxiety, Stress & Coping. 2004;17:23–36. doi: 10.1080/10615800310001601458. [DOI] [Google Scholar]
- 86.Koole SL. The psychology of emotion regulation: An integrative review. Cognition and emotion. 2009;23:4–41. doi: 10.1080/02699930802619031. [DOI] [Google Scholar]
- 87.Werner KH, Goldin PR, Ball TM, Heimberg RG, Gross JJ. Assessing emotion regulation in social anxiety disorder: The emotion regulation interview. Journal of Psychopathology and Behavioral Assessment. 2011;33:346–354. doi: 10.1007/s10862-011-9225-x. [DOI] [Google Scholar]
- 88.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaiser RH, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Social cognitive and affective neuroscience. 2014;10:654–663. doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]