Abstract
Background
Vancomycin is the most commonly administered antibiotic in hospitalized patients, but optimal exposure targets remain controversial. To clarify the therapeutic exposure range, this study evaluated the association between vancomycin exposure and outcomes in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia.
Methods
This was a prospective, multicenter (n = 14), observational study of 265 hospitalized adults with MRSA bacteremia treated with vancomycin. The primary outcome was treatment failure (TF), defined as 30-day mortality or persistent bacteremia ≥7 days. Secondary outcomes included acute kidney injury (AKI). The study was powered to compare TF between patients who achieved or did not achieve day 2 area under the curve to minimum inhibitory concentration (AUC/MIC) thresholds previously found to be associated with lower incidences of TF. The thresholds, analyzed separately as co-primary endpoints, were AUC/MIC by broth microdilution ≥650 and AUC/MIC by Etest ≥320.
Results
Treatment failure and AKI occurred in 18% and 26% of patients, respectively. Achievement of the prespecified day 2 AUC/MIC thresholds was not associated with less TF. Alternative day 2 AUC/MIC thresholds associated with lower TF risks were not identified. A relationship between the day 2 AUC and AKI was observed. Patients with day 2 AUC ≤515 experienced the best global outcomes (no TF and no AKI).
Conclusions
Higher vancomycin exposures did not confer a lower TF risk but were associated with more AKI. The findings suggest that vancomycin dosing should be guided by the AUC and day 2 AUCs should be ≤515. As few patients had day 2 AUCs <400, further study is needed to define the lower bound of the therapeutic range.
Keywords: vancomycin, MRSA, bacteremia, outcomes
A multicenter prospective study was performed to evaluate the relationship between day 2 vancomycin exposure profiles and outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia. The collective findings suggest that vancomycin dosing should be guided by the area under the curve (AUC) and day 2 AUCs should be maintained between 400 and 515.
(See the Editorial Commentary by Rodvold on pages 1546–9.)
Vancomycin is the most commonly administered antibiotic in US hospitals and a mainstay for treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections for decades [1], yet optimal dosing of vancomycin is unclear [2]. For serious MRSA infections, current guidelines recommend targeting an area under the concentration time curve to minimum inhibitory concentration ratio (AUC/MIC) ≥400 [3]. As AUCs are not routinely determined in clinical practice, trough concentrations of 15–20 mg/L are used as a surrogate. Despite widespread clinical adoption of these recommendations [4], supportive data are limited and largely derived from single-center retrospective studies [5–11]. Furthermore, these critical vancomycin exposure targets were derived mostly with a simple and error-prone formula that approximated AUC values based upon the prescribed daily vancomycin dose and the patient’s estimated renal function [6–9].
Bayesian software programs can generate accurate and reliable estimates of daily vancomycin AUCs with limited sampling [12, 13]. Applying this method to estimate vancomycin exposures in a retrospective cohort of hospitalized, adult patients with MRSA bacteremia, Lodise and colleagues identified 2 AUC/MIC thresholds (AUC/MIC by broth microdilution [BMD] ≥650 and AUC/MIC by Etest ≥320) associated with a lower probability of treatment failure [14]. These thresholds were consistent with other recent studies that employed a similar Bayesian approach to estimate the vancomycin exposure profile [7, 10]. However, prospective validation of previously these defined AUC/MIC targets among patients with MRSA bacteremia utilizing individualized estimates of exposure based on measured concentrations has not been done. This multicenter, observational study sought to evaluate prospectively the critical day 2 AUC/MIC exposure-outcome findings from the previous study by Lodise et al.
METHODS
Study Design and Population
This prospective, observational study was conducted in 14 centers between November 2014 and December 2015. The primary objective was to evaluate the impact of vancomycin AUC/MIC exposures on treatment failure rates among adult, hospitalized patients with MRSA bloodstream infections. The hypothesis was that patients who achieved day 2 AUC/MIC ratios above the thresholds (high exposure group) identified by Lodise and colleagues [14] will have 17.5% lower rates of failure relative to those with values below these thresholds (low exposure group). The thresholds, analyzed separately as co-primary endpoints, were AUC/MICBMD ≥650 and AUC/MICEtest ≥320. Day 2 vancomycin exposure was selected to best approximate near steady-state conditions of the initial vancomycin regimen. This also reflects contemporary clinical practice, in which vancomycin levels are frequently obtained on day 2.
Eligible participants were adult hospitalized patients with MRSA bacteremia who were treated with vancomycin within a window of 24 hours prior to and 48 hours after MRSA index blood culture collection, and whose vancomycin treatment continued for at least 72 hours after the index blood sample. Exclusion criteria were absolute neutrophil count <500 cells/mL, renal replacement therapy for chronic renal failure during the first 5 days of vancomycin treatment, documented MRSA bacteremia within 60 days prior to the index blood sample, Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥26 [15], and current participation in any antibiotic treatment intervention trial.
Evaluable patients were those who met inclusion and exclusion criteria, had a microbiology result from the central laboratory for the index MRSA blood culture, had at least 2 vancomycin blood concentrations during the first 5 days of vancomycin therapy (at least 1 sample had to be a nontrough vancomycin blood concentration collected on days 1–4 of vancomycin therapy), and had available outcome data 30 days after index blood culture collection. The study was conducted with a waiver of informed consent, consistent with Code of Federal Regulations title 45 part 46d, and the institutional review board at each site approved the study (see Supplementary Appendix: Patient Data for baseline patient data collected for the study).
Microbiologic Data and Phenotypic Characterization
Staphylococcus aureus was identified by standard methods. Oxacillin susceptibility was determined according to Clinical and Laboratory Standards Institute guidelines [16]. Isolates were stored in trypticase soy broth with 20% glycerol at –70°C. Isolates were shipped to JMI Laboratories (North Liberty, Iowa) for determination of MICBMD, MICEtest (according to the manufacturer’s instructions, bioMérieux, Marcy l’Etoile, France), and heterogeneous vancomycin-intermediate S. aureus [17] (Supplementary Appendix: Microbiologic Methods).
Treatment Data
All antibiotic treatment and vancomycin concentration data during the first 5 days of vancomycin treatment were collected. Vancomycin dosing and monitoring were at the discretion of the treating clinician. As permitted by waiver of informed consent, vancomycin concentration could be assayed from leftover blood from standard-of-care blood draws. A trough sample was defined as one collected ≤2 hours prior to a vancomycin dose. A sample collected >2 hours prior to a vancomycin dose was considered a nontrough concentration. The vancomycin minimum concentration at hour 48 and AUC were estimated post hoc using the maximal a posteriori probability procedure in ADAPT 5 software, which has been demonstrated as a way to estimate AUCs with low bias and high precision with limited pharmacokinetic (PK) sampling [12, 13, 18] (full details on PK modeling can be found in Supplementary Appendix: Pharmacokinetic Modeling). The day 2 AUC values were calculated post hoc and were not used to guide the care of patients.
Outcomes
The primary outcome measure was treatment failure, defined as death within 30 days of index MRSA blood culture (30-day mortality) or growth of MRSA from a blood culture obtained ≥7 days after initiation of vancomycin therapy (ie, persistent bacteremia) [14, 19]. Secondary outcome measures included 30-day mortality, persistent bacteremia, and occurrence of acute kidney injury (AKI) among patients with a baseline creatinine <2.0 mg/dL. The occurrence of AKI was assessed from initiation of vancomycin to 48 hours postcompletion and was based on the definition of risk (postbaseline serum creatinine is ≥1.5 × baseline serum creatinine) in the modified RIFLE (risk, injury, failure, loss, and end-stage kidney disease) criteria utilizing serum creatinine values [20] and the vancomycin-induced nephrotoxicity (VINT) definition in the vancomycin consensus guideline statement (defined as either a 50% or 0.5 mg/dL increase in serum creatinine, whichever was greater) [3].
Desirability of outcome ranking (DOOR) analysis was conducted post hoc [21, 22]. Each patient was assigned an overall outcome based on the occurrences of 30-day mortality, persistent bacteremia, and AKI. The 5 outcome levels, from least to most desirable, were (1) death; (2) survival with treatment failure and AKI; (3) survival with treatment failure and no AKI; (4) survival with treatment success and AKI; and (5) survival with treatment success and no AKI. Partial credit weighting was used to explore whether varying the relative importance assigned to each outcome would affect the results [22] (Supplementary Appendix: DOOR and Partial Credit Scoring).
Statistical Methods
Based on the distribution of covariates among patients in a previous study [14], 250 evaluable patients were required for 80% power at a 2-sided α of .05 to detect a 17.5% difference in the primary treatment outcome variable between the prespecified dichotomous AUC/MIC exposure variables assuming a 1–1.5 split in the distribution of the 250 evaluable patients in each exposure group. Assuming 80% of patients would be evaluable, approximately 312 patients were needed for this study. Inference was based on 2-sided 95% confidence intervals (CIs) around treatment failure differences between high and low AUC/MIC exposure groups.
Secondary analyses consisted of estimating the differences between patients with high/low day 2 vancomycin exposures in proportions exhibiting the following outcomes: 30-day mortality, persistent bacteremia, and AKI. Kaplan-Meier plots were used to assess time to AKI. Log-binomial regression was used to quantify associations between each AUC/MIC exposure variable and dichotomous outcome variables after adjusting for covariates. For the log-binomial analyses, the AUC/MIC exposure covariate was forced into the model first. For covariates associated with the outcome of interest having a likelihood ratio statistic P ≤ .10, the covariate with the lowest Akaike information criterion (AIC) was added to the model. Additional covariates were added to the model until the AIC no longer decreased. Exploratory relative risk (RR) threshold and classification and regression tree (CART) analyses were also performed to identify alternative optimal AUC/MIC ratios associated with failure and day 2 AUC threshold values associated with AKI (Supplementary Appendix: Relative Risk Threshold and Classification and Regression Tree Analyses).
DOOR analysis was conducted to examine the associations between the day 2 AUC/MICBMD, AUC/MICEtest, and AUC and overall patient outcomes. Inverse probability of treatment weighting adjustments [23] were made for these prognostic factors: presence of endocarditis; baseline calculated creatinine clearance; APACHE II score; and presence of a prosthetic joint, cardiac prosthetic device, or intravascular prosthetic material. The ordinal outcomes included in the DOOR endpoint were also analyzed using a partial credit strategy (Supplementary Appendix: DOOR and Partial Credit Scoring). This approach is analogous to scoring an academic test, assigning 100% to the most desirable outcome, 0% to the least (eg, death), and “partial credit” to each intermediate DOOR rank.
RESULTS
Patients
Of 310 patients enrolled across the 14 centers, 265 were evaluable (Supplementary Figure 1). Five patients were enrolled in error, and 13 patients were found not to have met 1 or more entry criteria after enrollment (Supplementary Table 1). Of the 292 enrolled patients who met entry criteria, 27 were deemed not evaluable due to 1 or more of the following: (1) unavailability of ≥1 required vancomycin concentration (n = 26); (2) missing index MRSA isolate (n = 3); or (3) no 30-day outcome data (n = 6) (Supplementary Table 2).
The mean age of evaluable patients was 61 (standard deviation [SD], 17) years, and mean APACHE II score was 12 (SD, 6); 29% of patients had possible or definite endocarditis. Eighty-six percent of isolates had an MICBMD of 0.5 or 1 mg/L, and 97% of isolates had an MICEtest of 1 or 1.5 mg/L (Table 1). Estimation of patient-specific exposures was based on 800 available concentrations among the 265 evaluable patients. A plot of the final PK dataset predicted vs observed vancomycin concentrations is shown in Supplementary Figure 2. Altogether, 116 (44%) and 193 (73%) patients achieved an AUC/MICBMD ≥650 and AUC/MICEtest ≥320, respectively (Table 2). Twenty-six patients (9.8%) had a decrease in vancomycin dose after day 2, while 21 patients (7.9%) had a dose increase; data on dosing frequency changes were not available. Baseline characteristics and distribution of microbiologic phenotypes between AUC/MIC exposure groups and treatment failure status are shown in Table 2.
Table 1.
Baseline Characteristics, Distribution of Microbiologic Phenotypes, Exposure Variables, and Outcomes
| Demographics and Baseline Characteristics | Value |
|---|---|
| Male sex, No. (%) | 168 (63) |
| Combined racial classes, No. (%) | |
| Asian | 4 (2) |
| Black | 70 (26) |
| Other | 6 (2) |
| White | 173 (65) |
| Unknown | 12 (5) |
| Ethnicity, No. (%) | |
| Not Hispanic or Latino | 190 (72) |
| Hispanic or Latino | 12 (5) |
| Unknown | 63 (24) |
| Age, y, mean (SD) | 60.7 (17.3) |
| Weight, kg, mean (SD) | 81.7 (24.9) |
| APACHE II score, mean (SD) | 12 (6) |
| Bacterial complications, No. (%) | |
| Presence of infective endocarditis: definite/possible | 78 (29) |
| Presence of internal prosthetic material | 63 (24) |
| Microbiologic phenotypes | |
| MICBMDa | |
| Range | 0.25–2.0 mg/La |
| MIC50/90 | 1.0/1 mg/L |
| MICEtestb | |
| Range | 0.5–2 mg/Lb |
| MIC50/90 | 1.5/1.5 mg/L |
| hVISA phenotype, No. (%) | 0 (0) |
| Day 2 vancomycin exposure variables, mean (SD) | |
| Cmin at 48 hours | 14.0 (6.2) |
| AUC | 586.9 (235.5) |
| AUC/MICBMD | 865.9 (425.2) |
| AUC/ MICEtest | 475.7 (259.4) |
| Outcomes, No. (%) | |
| Failure | 49 (18) |
| 30-d mortality | 30 (11) |
| Persistent bacteremia | 26 (10) |
| 60-d mortality | 42 (16) |
| Recurrence | 9 (3) |
| AKIc | 55 (26) |
| VINTc | 60 (28) |
Abbreviations: AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; AUC, area under the curve; Cmin, minimum blood plasma concentration; hVISA, heterogeneous vancomycin-intermediate Staphylococcus aureus; MIC50/90, minimum inhibitory concentration that inhibits 50% and 90% of the isolates; MICBMD, minimum inhibitory concentration by broth microdilution; MICEtest, minimum inhibitory concentration by Etest; SD, standard deviation; VINT; vancomycin-induced nephrotoxicity.
aPercentage of isolates with MICBMD of 0.5 and 1 mg/L: 25% and 72%, respectively.
bPercentage of isolates with MICEtest of 1 and 1.5 mg/L: 29% and 57%, respectively.
cAmong patients with baseline serum creatinine <2.0 mg/dL.
Table 2.
Comparison of Baseline Characteristics Between Patients With Drug Exposures Above Versus Below the Area Under the Curve/Minimum Inhibitory Concentration by Broth Microdilution of 650 and Failure Versus Nonfailure
| Secondary Covariates | Failure Status | AUC/MICBMD | AUC/MICEtest | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure | Nonfailure | P Value | <650 | ≥650 | P Value | <320 | ≥320 | P Value | |
| (n = 49) | (n = 216) | (n = 149) | (n = 116) | (n = 72) | (n = 193) | ||||
| Male sex | 30 (61) | 138 (64) | .73 | 91 (61) | 77 (66) | .37 | 41 (57) | 127 (66) | .18 |
| Race | |||||||||
| Asian | 0 (0) | 4 (2) | .55 | 3 (2) | 1 (1) | .08 | 1 (1) | 3 (2) | .95 |
| Black | 15 (31) | 55 (25) | 37 (25) | 33 (28) | 18 (25) | 52 (27) | |||
| Other | 2 (4) | 4 (2) | 1 (1) | 5 (4) | 1 (1) | 5 (3) | |||
| White | 31 (63) | 142 (66) | 98 (66) | 75 (65) | 48 (67) | 125 (65) | |||
| Unknown | 1 (2) | 11 (5) | 10 (7) | 2 (2) | 4 (6) | 8 (4) | |||
| Weight, kg, mean (SD) | 79.5 (21.5) | 82.2 (25.6) | .85 | 78.9 (23.9) | 85.2 (25.7) | .04 | 77.21 (22.69) | 83.31 (25.47) | .06 |
| Body mass index, mean (SD) | 28.1 (7.9) | 27.6 (7.8) | .57 | 26.9 (7.3) | 28.8 (8.6) | .11 | 26.86 (6.96) | 28.04 (8.26) | .38 |
| Age, y, mean (SD) | 68.8 (13.9) | 58.8 (17.5) | <.01 | 61.8 (17.5) | 59.3 (17.0) | .32 | 58.69 (17.94) | 61.41 (17.02) | .22 |
| Residence in ICU at time of index blood culture collection | 14 (29) | 45 (21) | .24 | 29 (19) | 30 (26) | .21 | 18 (25) | 41 (21) | .51 |
| Type of MRSA | |||||||||
| Hospital/healthcare acquired | 40 (82) | 147 (68) | .06 | 102 (68) | 85 (73) | .39 | 46 (64) | 141 (73) | .15 |
| Community acquired | 9 (18) | 69 (32) | 47 (32) | 31 (27) | 26 (36) | 52 (27) | |||
| Residence in healthcare institution for >72 h in past 180 d | 32 (65) | 115 (53) | .13 | 74 (50) | 73 (63) | .03 | 33 (46) | 114 (59) | .05 |
| Length of hospital stay prior to index culture, d, median (IQR) | 0 (0–0) | 0 (0–0) | .76 | 0 (0–0) | 0 (0–0) | .45 | 0 (0–0) | 0 (0–0) | .35 |
| APACHE II score, mean (SD) | 15.1 (5.5) | 11.7 (5.4) | <.01 | 11.9 (5.5) | 12.8 (5.7) | .25 | 11.36 (5.52) | 12.67 (5.56) | .10 |
| Estimated creatinine clearance at baseline, mL/min, mean (SD) | 60.4 (42.5) | 91.1 (57.7) | <.01 | 83.0 (49.9) | 88.3 (63.8) | .77 | 91.54 (52.64) | 83.13 (57.69) | .13 |
| Diabetes mellitus | 15 (31) | 75 (35) | .58 | 42 (28) | 48 (41) | .02 | 19 (26) | 71 (37) | .11 |
| Heart failure (class II–IV) | 13 (27) | 18 (8) | <.01 | 17 (11) | 14 (12) | .87 | 8 (11) | 23 (12) | .86 |
| COPD | 8 (16) | 36 (17) | .95 | 28 (19) | 16 (14) | .28 | 8 (11) | 36 (19) | .14 |
| Transplanted organ | 0 (0) | 14 (6) | .07 | 9 (6) | 5 (4) | .53 | 4 (6) | 10 (5) | .90 |
| Active malignancy | 7 (14) | 35 (16) | .74 | 26 (17) | 16 (14) | .42 | 10 (14) | 32 (17) | .59 |
| Receipt of immunosuppressive drugs in last 30 d | 5 (10) | 35 (16) | .29 | 29 (19) | 11 (9) | .02 | 11 (15) | 29 (15) | .96 |
| Decubitus ulcers (stage II–IV) | 8 (16) | 22 (10) | .22 | 16 (11) | 14 (12) | .73 | 6 (8) | 24 (12) | .35 |
| Cerebrovascular accident | 6 (12) | 21 (10) | .60 | 17 (11) | 10 (9) | .46 | 6 (8) | 21 (11) | .54 |
| Surgery requiring >48 h hospitalization in 30 d prior to date of index culture | 4 (8) | 27 (13) | .39 | 14 (9) | 17 (15) | .19 | 6 (8) | 25 (13) | .30 |
| Presence of infective endocarditis | 22 (45) | 56 (26) | <.01 | 38 (26) | 40 (34) | .11 | 19 (26) | 59 (31) | .51 |
| Preexisting valvular heart disease | 11 (22) | 18 (8) | <.01 | 17 (11) | 12 (10) | .78 | 6 (8) | 23 (12) | .41 |
| Previous infective endocarditis | 2 (4) | 5 (2) | .49 | 3 (2) | 4 (3) | .47 | 3 (4) | 4 (2) | .34 |
| Cardiac prosthetic device (eg, pacemaker, cardioverter- defibrillator, prosthetic valve) | 9 (18) | 14 (6) | <.01 | 9 (6) | 14 (12) | .08 | 7 (10) | 16 (8) | .71 |
| Prosthetic joints | 4 (8) | 16 (7) | .86 | 10 (7) | 10 (9) | .56 | 4 (6) | 16 (8) | .45 |
| Intravascular prosthetic material (eg, grafts, stents) | 8 (16) | 24 (11) | .31 | 15 (10) | 17 (15) | .26 | 7 (10) | 25 (13) | .47 |
| Receipt of antibiotic for at least 48 h in the 30 d prior to index culture | 15 (31) | 77 (36) | .50 | 56 (38) | 36 (31) | .27 | 23 (32) | 69 (36) | .56 |
| Receipt of vancomycin ≥48 h prior to index culture in 30 d prior to index culture | 7 (14) | 18 (8) | .20 | 9 (6) | 16 (14) | .03 | 4 (6) | 21 (11) | .19 |
| Polymicrobial BSI | 1 (2) | 19 (9) | .11 | 11 (7) | 9 (8) | .91 | 4 (6) | 16 (8) | .45 |
| Source of bacteremia infection: possibly to definitely | |||||||||
| Intravenous catheter | 13 (27) | 58 (27) | .96 | 39 (26) | 32 (28) | .80 | 20 (28) | 51 (26) | .83 |
| Urinary tract | 7 (14) | 36 (17) | .68 | 23 (15) | 20 (17) | .69 | 11 (15) | 32 (17) | .80 |
| Osteoarticular (bone and joint) | 7 (14) | 39 (18) | .53 | 30 (20) | 16 (14) | .18 | 12 (17) | 34 (18) | .86 |
| Skin and soft tissue | 23 (47) | 95 (44) | .71 | 75 (50) | 43 (37) | .03 | 33 (46) | 85 (44) | .79 |
| Abdominal source | 3 (6) | 17 (8) | .68 | 12 (8) | 8 (7) | .72 | 7 (10) | 13 (7) | .41 |
| Central nervous system | 2 (4) | 6 (3) | .63 | 4 (3) | 4 (3) | .72 | 1 (1) | 7 (4) | .34 |
| Respiratory tract | 7 (14) | 37 (17) | .63 | 24 (16) | 20 (17) | .81 | 8 (11) | 36 (19) | .14 |
| Other | 13 (27) | 30 (14) | .03 | 16 (11) | 27 (23) | <.01 | 11 (15) | 32 (17) | .80 |
| Receipt of β-lactam during first 7 d of vancomycin or >24 h | 25 (51) | 124 (57) | .42 | 79 (53) | 70 (60) | .23 | 39 (54) | 110 (57) | .68 |
| Receipt of aminoglycoside during first 7 d of vancomycin or >24 ha | 4 (8) | 4 (2) | .02 | 4 (3) | 4 (3) | .72 | 2 (3) | 6 (3) | .89 |
| Receipt of clindamycin during first 7 d of vancomycin or >24 h | 3 (6) | 6 (3) | .24 | 6 (4) | 3 (3) | .52 | 2 (3) | 7 (4) | .73 |
| Receipt of fluoroquinolone during first 7 d of vancomycin or >24 h | 2 (4) | 13 (6) | .60 | 8 (5) | 7 (6) | .82 | 3 (4) | 12 (6) | .52 |
| Receipt of rifampin during first 7 d of vancomycin or >24 ha | 4 (8) | 6 (3) | .07 | 5 (3) | 5 (4) | .69 | 0 (0) | 10 (5) | .05 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; AUC, area under the curve; BSI, bloodstream infection; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; MICBMD, minimum inhibitory concentration by broth microdilution; MICEtest, minimum inhibitory concentration by Etest; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation.
aFifteen patients received an aminoglycoside and/or rifampin. Among these patients, 5 were treatment failures; 3 of the 5 failures had an intravascular prosthetic device present.
Outcomes
Treatment failure did not differ by high vs low vancomycin exposure (AUC/MICBMD ≥650: 22% vs 15%, P = .15; AUC/MICEtest ≥320: 21% vs 11%, P = .07) (Table 3). No significant differences in proportions of patients exhibiting 30-day mortality or persistent bacteremia were noted between patients with drug exposures above vs below an AUC/MICBMD of 650 or AUC/MICEtest of 320 (Table 3). Results of the log-binomial analyses that adjusted for covariates associated with each outcome of interest at P ≤ .1 were consistent with the bivariate comparisons (Table 3). The exploratory RR threshold and CART analyses did not locate alternative optimal critical AUC/MIC and AUC exposure thresholds associated with a lower risk of failure after multivariable adjustment for covariates associated with failure at P ≤ .1 (Supplementary Figure 3 and Table 4). As part of the exploratory analyses, an AUC/MICBMD of 400 was tested and was not found to be associated with treatment failure.
Table 3.
Bivariate Comparison of Primary and Secondary Outcomes Between Patients With Drug Exposures Above Versus Below the Area Under the Curve/Minimum Inhibitory Concentration by Broth Microdilution of 650
| Outcome | AUC/MICBMD | Proportion, % | Risk Difference (95% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Treatment failure | <650 | 15.44 | 0.07 (–.03 to .17) | 0.03 (–.06 to .12) |
| ≥650 | 22.41 | |||
| Persistent bacteremia | <650 | 8.05 | 0.04 (–.03 to .11) | 0.01 (–.07 to .09) |
| ≥650 | 12.07 | |||
| 30-d mortality | <650 | 9.46 | 0.04 (–.03 to .12) | 0.04 (–.02 to .10) |
| ≥650 | 14.16 | |||
| AKIa | <650 | 21.0 | 0.12 (.00–.24) | 0.09 (–.04 to .22) |
| ≥650 | 33.0 | |||
| VINTa | <650 | 22.6 | 0.14 (.01–.26) | 0.12 (–.00 to .25) |
| ≥650 | 36.4 | |||
| AUC/MICEtest | Proportion, % | |||
| Treatment failure | <320 | 11.1 | 0.10 (.01–.19) | 0.07 (–.07 to .22) |
| ≥320 | 21.2 | |||
| Persistent bacteremia | <320 | 5.56 | 0.06 (–.01 to .13) | 0.07 (–.05 to .19) |
| ≥320 | 11.4 | |||
| 30-d mortality | <320 | 7.04 | 0.06 (–.02 to .14) | 0.07 (–.06 to .20) |
| ≥320 | 13.16 | |||
| AKIa | <320 | 14.3 | 0.17 (.05–.28) | 0.15 (.02–.29) |
| ≥320 | 30.9 | |||
| VINTa | <320 | 15.9 | 0.18 (.06–.29) | 0.16 (.02–.30) |
| ≥320 | 33.6 |
Abbreviations: AKI, acute kidney injury; AUC, area under the curve; CI, confidence interval; MICBMD, minimum inhibitory concentration by broth microdilution; MICEtest, minimum inhibitory concentration by Etest; VINT; vancomycin-induced nephrotoxicity.
aPatients with baseline serum creatinine (<2.0 mg/dL).
Table 4.
Unadjusted and Final Model Risk Differences for Failure by Exploratory Day 2 Area Under the Curve (AUC) and AUC/Minimum Inhibitory Concentration Predictors
| Integrated Exposure (PK/PD) Measure | Exposure Category | No. of Patients | Proportion, % | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|---|
| Point Estimate | (95% CI) | Point Estimate | (95% CI) | ||||
| CART AUC/MICBMD cutpoint | ≤345.88 | 30 | 3.33 | 0.17 | (.09–.25) | 0.17 | (–.13 to .48) |
| >345.88 | 235 | 20.43 | |||||
| RR AUC/MICBMD cutpoint | <500 | 85 | 11.76 | 0.10 | (.01–.19) | 0.03 | (–.06 to .11) |
| ≥500 | 180 | 21.67 | |||||
| CART AUC/MICEtest cutpoint | <344.9 | 81 | 9.88 | 0.12 | (.04–.21) | 0.10 | (–.04 to .24) |
| ≥344.9 | 184 | 22.28 | |||||
| RR AUC/MICEtest cutpoint | <350 | 83 | 10.84 | 0.11 | (.02–.20) | 0.08 | (–.06 to .22) |
| ≥350 | 182 | 21.98 |
Abbreviations: AUC, area under the curve; CART, classification and regression tree; CI, confidence interval; MICBMD, minimum inhibitory concentration by broth microdilution; MICEtest, minimum inhibitory concentration by Etest; PK/PD, pharmacokinetic/pharmacodynamic; RR, relative risk.
Acute Kidney Injury
In total, 212 patients had a baseline creatinine <2.0 mg/dL. The rates of AKI and VINT were higher in patients in the high AUC/MIC groups (Table 3), consistent with results of the Kaplan-Meier time-to-AKI analyses (Supplementary Figures 4 and 5), which demonstrated differences in AKI and VINT between the binary AUC/MIC exposure groups. In the log-binomial regression, several CIs around adjusted risk differences excluded the value zero for binary AUC/MIC exposure variables, indicating an increased risk of AKI and VINT with higher vancomycin exposure (Table 3). Exploratory RR threshold and CART analyses indicated that patients with an AUC ≥793 relative to those with an AUC ≤343 were at greater risk for AKI and VINT (Table 5).
Table 5.
Acute Kidney Injury by Exploratory Day 2 Area Under the Curve Exposures
| Categorical Outcome | Integrated Exposure (PK/PD) Measure | Exposure Category | No. of Patients | Proportion, % | Cut-point Level Comparison | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|---|---|---|
| Point Estimate | (95% CI) | Point Estimate | (95% CI) | ||||||
| AKI | CART AUC cutpoint | ≤343 | 30 | 10 | High vs low | 0.29 | (.08–.49) | 0.26 | (.00–.51) |
| >343 to 793 | 151 | 26.49 | High vs medium | 0.12 | (–.06 to .31) | 0.12 | (–.07 to .32) | ||
| ≥793 | 31 | 38.71 | Medium vs low | 0.16 | (.04–.29) | 0.14 | (–.06 to .33) | ||
| RR AUC cutpoint | <550 | 104 | 21.15 | … | 0.09 | (–.02 to .21) | 0.12 | (–.01 to .24) | |
| ≥550 | 108 | 30.56 | … | ||||||
| VINT | CART AUC cutpoint | ≤343 | 30 | 10 | High vs low | 0.32 | (.12–.52) | 0.27 | (.01–.54) |
| >343 to 793 | 151 | 29.14 | High vs medium | 0.13 | (–.06 to .32) | 0.13 | (–.07 to .32) | ||
| ≥793 | 31 | 41.94 | Medium vs low | 0.19 | (.06–.32) | 0.15 | (–.06 to .35) | ||
| RR AUC cutpoint | <550 | 104 | 23.08 | … | 0.10 | (–.02 to .22) | 0.12 | (.00–.25) | |
| ≥550 | 108 | 33.33 | … |
Abbreviations: AKI, acute kidney injury; AUC, area under the curve; CART, classification and regression tree; CI, confidence interval; PK/PD, pharmacokinetic/pharmacodynamic; RR, relative risk; VINT; vancomycin-induced nephrotoxicity.
DOOR Risk-benefit Analysis
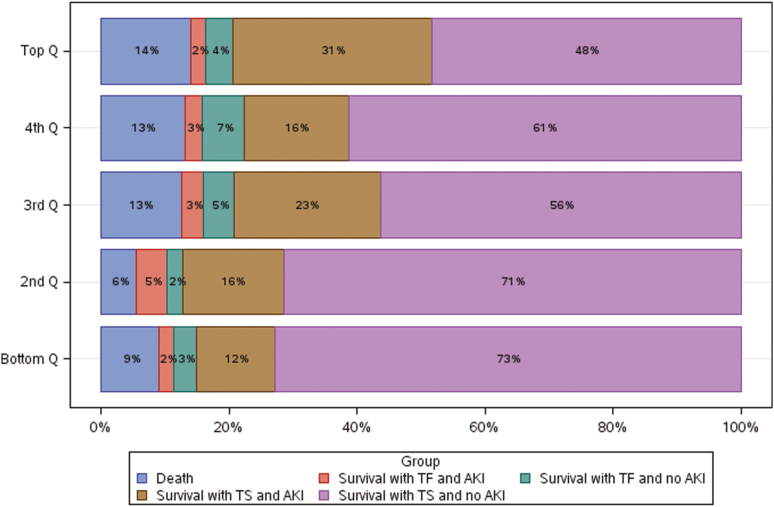
Of the 106 patients in the 2 lowest AUC exposure quintiles (AUC ≤515), 72% (95% CI, 68%–76%) experienced the best global outcome, compared with the 3 higher exposure quintiles pooled (55% [95% CI, 52%–59%] of 159 patients) (Figure 1). Results of the day 2 AUC/MICBMD and AUC/MICEtest DOOR analyses were consistent with the day 2 AUC DOOR analysis (Supplementary Figures 6 and 7). Varying the partial credit weighting did not identify alternative exposure thresholds associated with more favorable global outcomes (Supplementary Figures 8 and 9). Instead, under a range of partial credit scoring systems, outcomes still appeared better at lower AUC exposure thresholds.
Figure 1.
Desirability of outcome ranking analysis by area under the curve quintiles: bottom quintile, 94.3–≤389.8; second quintile, >389.8–≤515.7; third quintile, >515.7–≤620.8; fourth quintile, >620.8–≤757.4; top quintile, >757.4–≤1755.0. Abbreviations: AKI, acute kidney injury; Q, quintile; TF, treatment failure; TS, treatment success
DISCUSSION
In this prospective, multicenter study of adult patients with MRSA bacteremia, higher day 2 AUC/MIC exposures were not associated with a lower rate of failure but were associated with nephrotoxicity. The lack of benefit with higher day 2 AUC/MIC exposures is unlikely to be attributable to selecting the wrong thresholds, as exploratory analyses did not identify alternative optimal targets. Absence of benefit with higher vancomycin exposure additionally held true for analyses restricted to patients with an APACHE II score >10 and patients with infective endocarditis. Secondary efficacy outcome measures also did not differ between the prespecified AUC/MIC exposure groups.
While efficacy was not associated with vancomycin exposure, the incidence of AKI was higher in patients with an AUC/MICBMD ≥650 and AUC/MICEtest ≥320. As is typical for nonimmunologic drug-related AKIs, most events occurred after 5 days of therapy (Supplementary Figures 4 and 5). The observed association between vancomycin exposure and nephrotoxicity is most plausibly driven by the AUC, as an antibacterial MIC has no pathophysiologic relationship to a patient’s kidney function, and thus no causal association with AKI. Although this study lacked power to discriminate between dichotomous, ordinal, and continuous expressions of AUCs, an AUC-nephrotoxicity relationship clearly was present and existed in a stepwise fashion that persisted in the multivariable analyses, with incidence of AKI greatest among patients with an AUC ≥793 relative to those with an AUC ≤343 (Table 5). The AUC thresholds associated with increased risk of AKI in this study are notably consistent with previous reports [24–26].
The findings also suggest that vancomycin dosing should be guided by the AUC instead of the AUC/MIC ratio, and Bayesian software programs and simple analytic equations makes possible real-time, accurate measurement of the AUC with limited PK sampling [12, 13]. The MIC value is of less importance for several reasons. First, there is a narrow range of vancomycin MICBMD values (the gold standard) among contemporary MRSA isolates (observed here and in other studies), with values of 0.5 or 1 mg/L in most institutions [27, 28]. Second, there is inherent imprecision of MIC measurement, with a range of accuracy of ±1 log2 dilutions [16, 29], and a high degree of variability between MIC testing methods typically used in healthcare institutions [29, 30]. Third, MIC values are typically not available within the first 72 hours of index culture collection, and thus cannot easily be incorporated into the initial dosing regimen. Fourth, MIC has no causal relationship with AKI; vancomycin exposure is the physiologic driver of AKI.
For the day 2 AUC target range, we believe the collective study findings suggest that day 2 AUCs should be maintained below approximately 515 to maximize efficacy and minimize the likelihood of nephrotoxicity. This recommendation is supported by the results of the post hoc DOOR analyses that demonstrated that patients in the lower 2 AUC quintiles (day 2 AUCs ≤515) had the best global outcomes (Figure 1). Additionally, the exploratory RR threshold and CART AUC-AKI analyses suggest that the risks of AKI and VINT were lowest among those with day 2 AUCs within this range (Table 5). Although global outcomes were similar between the 2 lower DOOR quintiles, we believe it is prudent to target an AUC of at least 400 since <20% of patients in this study had AUC values <400 and it is unclear whether efficacy outcomes are maintained at day 2 AUC values less than this threshold.
This study has several limitations. First are those inherent to an observational study design, including study selection bias due to the evaluability of patients, confounding, and nonstandardized clinical management (including vancomycin dosing, monitoring, blood culture collection, and duration of therapy). However, baseline characteristics, comorbid conditions, measures of disease severity, and source control efforts were comparable between the high and low vancomycin exposure groups. This was a study of adult, nonneutropenic, nondialysis patients, and the observed findings may not be applicable to other populations. Nearly all MRSA isolates had vancomycin MICs <2 mg/L. Although isolates with higher vancomycin MIC values are infrequently encountered in clinical practice, this is an important subset of MRSA bloodstream infections for which further study is needed. Our definition of treatment failure was limited to objective measures to minimize any biases that may result from assessing and interpreting observational clinical data [19], but may not include all outcomes that are relevant to patients. Pharmacokinetic sampling was not completely standardized and included all PK samples collected over the first 5 days of therapy in an effort to gain the best individualized estimate of each patient’s PK profile. As renal function varies over the initial course of therapy, we selected a population PK model as a Bayesian prior that made vancomycin clearance proportional to creatinine clearance. This permitted PK parameters to be estimated in the presence of changing renal function. Finally, the impact of dosing, dosing frequency, duration of therapy, and therapy switches were not considered, as the focus was to evaluate the association between the day 2 vancomycin exposure profile and outcomes. These covariates should be considered in future studies.
In conclusion, this study found no difference in treatment failure between the a priori specified vancomycin exposure groups among adults with MRSA bacteremia. While not associated with treatment failure, higher day 2 vancomycin exposures were associated with more AKI. These results have important implications for clinical practice. Clinicians and guideline authors should reassess the balance of benefits and risks of targeting higher AUC/MIC for patients with MRSA bacteremia. In addition, the findings suggest that vancomycin dosing should be guided by the AUC instead of the AUC/MIC ratio and day 2 AUCs should be maintained below 515 to maximize efficacy and minimize the likelihood of AKI. It is unclear whether efficacy outcomes are maintained at day 2 AUC values <400 as few patients in this study had AUCs below this threshold. Further study is needed to define the lower bound of the day 2 AUC therapeutic range.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. P. L. led the development of the research question, study design, implementation of the study protocol, analysis and interpretation of data, and drafting the report, along with T. P. L., T. H., H. C., and V. G. F. Jr.; M. Si., M. J. Z., C. B. C., P. C. P., M. K., P. R., F. P. S., M. Sc., R. G. W., M. R., J. S., S. C. B., S. S., M. B., A. S., and D. W. oversaw the operational delivery of the study protocol and recruitment. S. L. R., M. F., and S. E. were study statisticians. All authors provided critical review and final approval of the manuscript.
Acknowledgments. The authors acknowledge Dr Kurt Stevenson for his participation in the study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported herein was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number UM1AI104681). V. G. F. Jr. was supported by a midcareer mentoring award from the NIH (grant number K24-AI093969).
Potential conflicts of interest. T. P. L. has served as a consultant for Allergan, Melinta, Motif, Paratek, Nabriva, and Merck & Co; has served as a scientific advisor for Melinta, Motif, and Paratek; has served on the speaker’s bureau for Melinta, Sunovion; and has received grants from Merck & Co. H. C. has received grants and personal fees from Allergan. V. G. F. Jr. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals, and Genentech; has NIH Small Business Technology Transfer/Small Business Innovation Research grants pending with Affinergy, Locus, and Medical Surface; has served as a consultant for Achaogen, Affinium, AmpliPhi Biosciences, Astellas Pharma, Arsanis, Affinergy, Basilea Pharmaceutica, Bayer, Cerexa, ContraFect, Cubist, Debiopharm, Durata Therapeutics, Galderma, Grifols, Genentech, Green Cross, Janssen, MedImmune, Merck, The Medicines Co, Pfizer, Novartis, NovaDigm Therapeutics, Tetraphase, Theravance Biopharma, Trius, and XBiotech; has received honoraria from Theravance Biopharma and Green Cross; has a patent pending in sepsis diagnostics; has served as a grant investigator for research grants for Theravance, Novartis, and Cubist/Merck; and has received royalties from UpToDate. M. Si. has received grant funding from Cubist Pharmaceuticals (now Merck & Co) through his institution; has received consulting fees through his institution from Curetis GmBH, Paratek Pharmaceuticals, and Cutis Pharma; and was a primary investigator on clinical trials for AstraZeneca, Cempra, Aradigm, Cubist Pharmaceuticals (Now Merck & Co), Synthetic Biologics, Debiopharm, Bayer Healthcare, Theravance, Seres Therapeutics, Rempex, Vela Diagnostics, AM-Pharma, Abbott Molecular, Gilead Sciences, NeuMoDx Molecular, Nabriva Therapeutics, Sanofi Pasteur, Diasorin Molecular, Curetis GmbH, Pfizer, Cidara Therapeutics, Shire, ContraFect, Aridis Pharmaceuticals, Epigenomics, Genentech, Finch Therapeutics, MedImmune, Research and Development LLC, The Medicines Co, Summit Therapeutics, and Iterum Therapeutics International; and also has a patent for methods of diagnosing increased risk of developing methicillin-resistant Staphylococcus aureus (MRSA) or community-associated MRSA issued, and a patent pending for the detection and treatment of MRSA and surgical site infection (SSI). S. E. received grants from NIAID/NIH during the conduct of the study; has received personal fees from Takeda/Millennium, Pfizer, Roche, Novartis, Achaogen, Huntington’s Study Group, Auspex, Alcon, Merck, Chelsea, Mannkind, QRx Pharma, ACTTION, Genentech, Affymax, FzioMed, Amgen, GSK, Boehringer-Ingelheim, American Statistical Association, US Food and Drug Administration, Osaka University, City of Hope, National Cerebral and Cardiovascular Center of Japan, NIH, Muscle Study Group, Society for Clinical Trials, Dug Information Association, University of Rhode Island, New Jersey Medical School/Rutgers, PPRECISE, Statistical Communications in Infectious Diseases, Cubist, AstraZeneca, Teva, Repros, Austrian Breast and Colorectal Cancer Study Group/Breast International Group and the Alliance Foundation Trials, Zeiss, Dexcom, American Society for Microbiology, Claret Medical, Vir, Arrevus, Five Prime, Shire, Alexion, Gilead, Spark, Clinical Trials Transformation Initiative, Nuvelution, Tracon, Deming Conference, Antimicrobial Resistance and Stewardship Conference, World Antimicrobial Congress, WAVE, Advantagene, Braeburn, Cardinal Health, Lipocine, Microbiotix, and Stryker. M. J. Z. has received research grants from Merck, Pfizer, Genetech, and Medimmune, as well as honoraria from Contrafect for consulting. M. Sc. has received personal fees from Achaogen, SIGA Technologies, Paratek, Premier, and Bayer; has received grants from the CARE Foundation, Nevakar, Allergan, Allecra, and Merck & Co; and has received travel support from Astellas. M. Sc. also has a patent WO2017161296A1 pending. M. R. received grants from the NIAID during the conduct of the study; and has received grants from Crestovo, Finch, and Contrafect. S. C. B. has served on the advisory board for The Medicines Co; has received grants from Rempex Pharmaceuticals; and has received grants to her institution from The Medicines Co and Shionogi. A. S. has served as a consultant for Paratek. D. W. received research funding from Theravance Biopharma during the conduct of the study. T. L. H. has been a consultant for Basilea Pharmaceutica, Genentech, Motif Bio, The Medicines Co, and Theravance. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rybak MJ, Rotschafer JC, Rodvold KA. Vancomycin: over 50 years later and still a work in progress. Pharmacotherapy 2013; 33:1253–5. [DOI] [PubMed] [Google Scholar]
- 3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009; 29:1275–9. [DOI] [PubMed] [Google Scholar]
- 4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy 2013; 33:1256–63. [DOI] [PubMed] [Google Scholar]
- 5. Brown J, Brown K, Forrest A. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother 2012; 56:634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2013; 57:1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung Y, Song KH, Cho Je, et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 2014; 43:179–83. [DOI] [PubMed] [Google Scholar]
- 8. Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 2011; 52:975–81. [DOI] [PubMed] [Google Scholar]
- 9. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–42. [DOI] [PubMed] [Google Scholar]
- 10. Casapao AM, Lodise TP, Davis SL, et al. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 2015; 59:2978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin Ther 2013; 35:772–9. [DOI] [PubMed] [Google Scholar]
- 12. Neely MN, Youn G, Jones B, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 2014; 58:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev 2014; 77:50–7. [DOI] [PubMed] [Google Scholar]
- 14. Lodise TP, Drusano GL, Zasowski E, et al. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 2014; 59:666–75. [DOI] [PubMed] [Google Scholar]
- 15. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests; approved standards. 9th ed. M2–M9. Wayne, PA: CLSI, 2006. [Google Scholar]
- 17. Wootton M, MacGowan AP, Walsh TR, Howe RA. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J Clin Microbiol 2007; 45:329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource, 2009. [Google Scholar]
- 19. Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:1000–8. [DOI] [PubMed] [Google Scholar]
- 20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit:risk evaluation. Stat Biopharm Res 2016; 8:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model 1986; 7:1393–512. [Google Scholar]
- 24. Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 2012; 58:308–12. [DOI] [PubMed] [Google Scholar]
- 25. Zasowski EJ, Murray KP, Trinh TD, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 2018; 62. doi:10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 2018; 52:805–10. [DOI] [PubMed] [Google Scholar]
- 27. Jones RN. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin Infect Dis 2006; 42(Suppl 1):S13–24. [DOI] [PubMed] [Google Scholar]
- 28. Farrell DJ, Castanheira M, Mendes RE, Sader HS, Jones RN. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008–2010). Clin Infect Dis 2012; 55(Suppl 3):S206–14. [DOI] [PubMed] [Google Scholar]
- 29. Rybak MJ, Vidaillac C, Sader HS, et al. Evaluation of vancomycin susceptibility testing for methicillin-resistant Staphylococcus aureus: comparison of Etest and three automated testing methods. J Clin Microbiol 2013; 51:2077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruzel MC, Lewis CT, Welsh KJ, et al. Determination of vancomycin and daptomycin MICs by different testing methods for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2011; 49:2272–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.