(See the Major Article by Wake et al on pages 1683–90.)
In the era of human immunodeficiency virus (HIV) test and treat, priorities are concentrated on every person infected with HIV receiving antiretroviral therapy (ART) in order to improve survival and reduce HIV transmission. While this policy benefits the majority of those infected with HIV, those with advanced HIV disease (CD4 <200 cells/µL) have the potential for worse outcomes without further nuanced consideration. Persons with advanced HIV disease need to be screened and treated for opportunistic infections (tuberculosis and cryptococcal infection). Starting ART in persons with untreated asymptomatic cryptococcal antigenemia can be a fatal iatrogenic error [1, 2].
Cryptococcal disease causes ~15% of acquired immunodeficiency syndrome (AIDS)–related deaths, and overt clinical disease is preceded by asymptomatic cryptococcal antigenemia [3]. Screening for cryptococcal antigen (CrAg) is recommended among those entering into care with CD4 counts of less than 100 cells/µL and considered among those with CD4 counts of less than 200 cells/µL [4], because ~10% of cryptococcosis occurs among persons with CD4 counts of 100–200 cells/µL [5]. In the United States, 1 in 34 persons infected with HIV with CD4 counts of less than 100 cells/µL will be CrAg positive [6]. In the absence of CD4 testing (ie, test and treat), cryptococcal antigen screening of all patients initiating ART is cost-effective, if hospitalizations can be avoided [7]. Yet, even with preemptive fluconazole therapy, ~25% fail preemptive therapy, going on to develop meningitis or death [8–10]. Why people fail therapy is unknown, as many CrAg-positive people die after ART initiation without developing meningitis [1, 10].
Although “crypto meningitis” easily rolls off the tongue, Cryptococcus causes not just a meningitis but a meningoencephalitis. On postmortem examination of patients with cryptococcal meningitis, Cryptococcus has widespread dissemination throughout the body including the brain parenchyma. Such dissemination is often silent in persons infected with HIV living with AIDS until ART initiation. In the setting of partially treated or untreated cryptococcal infection, ART can cause immune reconstitution inflammatory syndrome (IRIS). Such unmasking or paradoxical IRIS manifestations can be diverse in anatomical location, including central nervous system, pulmonary, or systemic sepsis syndromes [11].
In this issue of Clinical Infectious Diseases, Wake and colleagues [12] report a detailed prospective cohort of 67 CrAg-positive patients with thorough baseline clinical screening and postmortem evaluation. In this study, 28% of the CrAg-positive patients later died despite negative lumbar punctures (LPs) at baseline. The majority of these deaths (8 of 13) were cryptococcal related, and the median time to death was 26 days. Three of the 4 persons who had autopsies had negative LPs at screening (with the fourth being asymptomatically positive). These patients became confused prior to death and had a positive cerebrospinal fluid (CSF) CrAg postmortem. This supports the idea that a negative LP in an asymptomatic patient with cryptococcal antigenemia is not reassuring.
This South African experience is similar to other reports. Meya et al [10] reported that among 152 asymptomatic Ugandan persons treated for cryptococcal antigenemia, 22% failed preemptive treatment, developing meningitis (10%) and/or death (20%). Overall, breakthrough meningitis unmasked on ART was fatal in 80%, of whom 20% had CSF pleocytosis with negative CSF CrAg (and death) [10]. In response to the Ugandan experience, multiple cohorts instituted LPs among CrAg-positive persons, as now recommended by 2018 World Health Organization guidelines [4]. In pooling the Ethiopian, South African, Tanzanian, and Ugandan cohort experiences [8, 9, 13, 14], plasma CrAg titers are positively associated with the probability of positive CSF CrAg (Figure 1). With plasma (or serum) CrAg titers of 1:80 or less, CSF involvement is uncommon, with less than 10% being CSF CrAg-positive in asymptomatic persons without headaches. As plasma titers increase, nearly ~60% of asymptomatic people are CSF CrAg positive with plasma titers of 1:1280 or greater.
Figure 1.
Plasma CrAg titer association with CSF CrAg status among 133 persons identified via CrAg screening programs in Ethiopia, South Africa, Tanzania, and Uganda. The prevalence of CSF CrAg positivity increases with both plasma CrAg titer as well as the presence of headache. Abbreviations: CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; neg, negative; pos, positive.
Yet, this dichotomy between positive versus negative CSF CrAg to guide therapy misses that cryptococcosis is a disseminated disease with brain, pulmonary, and systemic involvement in addition to CSF. As Ssebambulidde et al [15] reported, among 1201 Ugandans presenting with headache and meningitis symptoms, 4% had symptomatic cryptococcal antigenemia with negative CSF studies but probable brain parenchymal involvement and early meningitis. In-hospital mortality was an unacceptable 32% with fluconazole preemptive therapy, with a median time to death of 7 days, which was similar to the 31% in-hospital mortality among those with documented CSF CrAg-positive “meningitis” treated with amphotericin-combination therapy [15].
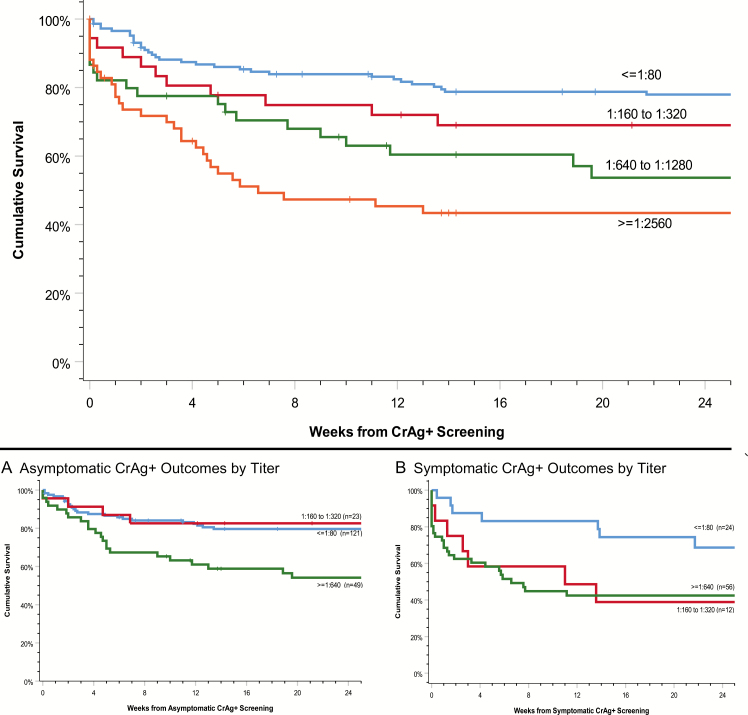
As we have seen in prior studies, Wake et al reported that CrAg titers were significantly higher in those with cryptococcal disease compared with asymptomatic CrAg antigenemia alone, and CrAg titer predicted death [12]. Among the 4 prospective cohorts describing cryptococcal antigenemia, the same findings were demonstrated—namely, as plasma titer increases, mortality increases (Figure 2) [16]. Among persons with low titers of 1:80 or less, symptoms of headache were not associated with survival. Symptoms influenced survival among persons with CrAg titers of 1:160 to 1:320, whereby those presenting with headache had 40% worse survival than asymptomatic persons with similar titers (P = .009). At or above 1:640 titers, survival was relatively poor, regardless of symptoms (P = .077).
Figure 2.
Survival in CrAg-positive persons by plasma CrAg titer. Among asymptomatic persons in whom CSF CrAg positivity was excluded, similar outcomes occurred among those with a CrAg titer ≤1:320 regardless of symptoms (P = .68). CNS symptoms most dramatically decreased survival among those with plasma CrAg titers of 1:160 to 1:320, frequently associated with a positive CSF CrAg. Poor survival occurred among persons with plasma CrAG titers >1:640, regardless of symptoms. Neither symptoms nor CSF CrAg status predicted CNS dissemination in this group with high plasma titers. Abbreviation: CrAg, cryptococcal antigen.
To decrease the mortality among CrAg-positive persons, 3 strategies are possible. First is to initiate antifungal therapy as soon as possible. A new positive blood CrAg test is a critical result, and patients need to return for therapy expeditiously. Additional medication adherence measures may be necessary for this specific population [17].
Second, experts and guidelines advocate for diagnostic LPs on all CrAg-positive persons, regardless of symptoms. Yet, among asymptomatic CrAg-positive persons, 60% of deaths occurred in CSF CrAg-negative persons [8, 9, 13, 14]. A negative CSF CrAg may be falsely reassuring. In these 4 cohorts, survival among those with plasma CrAg titer of 1:640 or higher and negative CSF CrAg was less than 30% when treated with fluconazole preemptive therapy. Among persons with high titers, LP should likely be used to assess and control increased intracranial pressure and less about delineating if CSF involvement is present, as CNS infection is highly probable in the brain, regardless of CSF results.
Third, enhanced antifungal therapy for CrAg-positive persons could improve survival by more aggressively treating presumed Cryptococcus in the brain, even if yeasts are not yet present in CSF. The authors suggest adjunctive flucytosine with fluconazole. This combination, while highly effective, remains unavailable in much of sub-Saharan Africa and expensive in the United States ($425/day). Another likely efficacious option is single-dose liposomal amphotericin at 10 mg/kg, which has been used in cryptococcal meningitis [18]. A randomized clinical trial will start in June 2019 using this strategy for asymptomatic cryptococcal antigenemia. Either (or both) of these options would be appealing compared with a mortality rate of more than 50% among persons with titers of 1:640 or higher. Although these therapies may have limited availability in low- and middle-income countries currently, one simple, immediate step would be to increase the fluconazole dose to 800 mg/day for 10 weeks in asymptomatic CrAg-positive persons. Earlier ART initiation in persons with high titers and disseminated infection is unlikely to ameliorate mortality, based on experience with earlier ART in cryptococcal meningitis [19]; however, data are needed to inform practice. The current recommendation is to initiate ART 2 weeks after antifungal therapy.
In summary, Wake and colleagues identified that cryptococcal disease is a significant cause of mortality in persons with asymptomatic cryptococcal infection, despite negative LPs. Plasma CrAg titers are strongly associated with mortality and can identify those at highest risk of death, even in the absence of symptoms. Cryptococcal disease is a spectrum ranging from pulmonary infection, to subclinical disseminated infection, to meningoencephalitis. CSF studies alone are not sufficient to rule out disseminated infection. Treatment of cryptococcal antigenemia is a readily implementable intervention to reduce AIDS-related deaths, but further improvements in preemptive antifungal therapy are needed.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers U01AI125003, R01AI118511, K23AI138851) to improve cryptococcal antigenemia outcomes.
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or =100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Letang E, Muller MC, Ntamatungiro AJ, et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children Available at: http://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/. Accessed 1 April 2018. [PubMed]
- 5. Tugume L, Rhein J, Hullsiek KH, et al. ; COAT and ASTRO-CM Teams HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J Infect Dis 2019; 219:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKenney J, Bauman S, Neary B, et al. Prevalence, correlates, and outcomes of cryptococcal antigen positivity among patients with AIDS, United States, 1986–2012. Clin Infect Dis 2015; 60:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajasingham R, Meya DB, Greene GS, et al. Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: a cost-effectiveness modeling analysis. PLoS One 2019; 14:e0210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV cin Uganda. J Acquir Immune Defic Syndr 2018; 78:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wake RM, Britz E, Sriruttan C, et al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis 2018; 66:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meya DB, Kiragga AN, Nalintya E, et al. Reflexive laboratory-based cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4 <100 cells/µL: a stepped-wedge, cluster-randomized trial. J Acquir Immune Defic Syndr 2019; 80:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 2010; 7:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wake RM, Govender NP, Omar T, et al. Cryptococcal-related mortality despite fluconazole pre-emptive treatment in a cryptococcal antigen (CrAg) screen-and-treat programme. Clin Infect Dis 2019; 69: In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beyene T, Zewde AG, Balcha A, et al. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis 2017; 65:2126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faini D, Kalinjuma AV, Katende A, et al. Laboratory-reflex cryptococcal antigen screening is associated with a survival benefit in Tanzania. J Acquir Immune Defic Syndr 2019; 80:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ssebambulidde K, Bangdiwala AS, Kwizera R, et al. Symptomatic cryptococcal antigenemia presenting as early cryptococcal meningitis with negative CSF analysis. Clin Infect Dis 2019; 68:2094–8. doi: 10.1093/cid/ciy817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol 2019; 57:e01238–18. doi: 10.1128/JCM.01238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mfinanga S, Chanda D, Kivuyo SL, et al. ; REMSTART Trial Team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 18. Jarvis JN, Leeme TB, Molefi M, et al. Short-course high-dose liposomal amphotericin B for human immunodeficiency virus-associated cryptococcal meningitis: a phase 2 randomized controlled trial. Clin Infect Dis 2019; 68:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]