Abstract
Background
Aspects of infant antibiotic exposure and its association with asthma development have been variably explored. We aimed to evaluate comprehensively and simultaneously the impact of dose, timing, and type of infant antibiotic use on the risk of childhood asthma.
Methods
Singleton, term-birth, non–low-birth-weight, and otherwise healthy children enrolled in the Tennessee Medicaid Program were included. Infant antibiotic use and childhood asthma diagnosis were ascertained from prescription fills and healthcare encounter claims. We examined the association using multivariable logistic regression models.
Results
Among 152 622 children, 79% had at least 1 antibiotic prescription fill during infancy. Infant antibiotic use was associated with increased odds of childhood asthma in a dose-dependent manner, with a 20% increase in odds (adjusted odds ratio [aOR], 1.20 [95% confidence interval {CI}, 1.19–1.20]) for each additional antibiotic prescription filled. This significant dose-dependent relationship persisted after additionally controlling for timing and type of the antibiotics. Infants who had broad-spectrum-only antibiotic fills had increased odds of developing asthma compared with infants who had narrow-spectrum-only fills (aOR, 1.10 [95% CI, 1.05–1.19]). There was no significant association between timing, formulation, anaerobic coverage, and class of antibiotics and childhood asthma.
Conclusions
We found a consistent dose-dependent association between antibiotic prescription fills during infancy and subsequent development of childhood asthma. Our study adds important insights into specific aspects of infant antibiotic exposure. Clinical decision making regarding antibiotic stewardship and prevention of adverse effects should be critically assessed prior to use during infancy.
Keywords: infant antibiotics, childhood asthma, drug utilization, dose-response relationship, clinical decision making
In a population-based cohort study, the association between infant antibiotics and risk of childhood asthma was found to be primarily driven by the number and spectrum of fills, irrespective of the timing, formulation, anaerobic coverage, and class of antibiotics.
Asthma is a chronic lung disease affecting >25 million people in the United States, of whom nearly 7 million are children [1]. The burden associated with asthma disproportionately affects minority and economically disadvantaged children, who are more likely to have uncontrolled disease and receive costly urgent care [2, 3]. While symptoms can be managed with medication [1], effective primary prevention strategies are lacking. A more thorough understanding of the relationship between modifiable environmental risk factors and asthma development is needed to improve asthma prevention.
Antibiotics are the most frequently prescribed medications for children and can be lifesaving for those suffering from severe bacterial infections [4]. However, antibiotics can negatively impact the diversity and function of the microbiome through collateral damage to commensal bacteria [5]. The human microbiome plays a role in shaping the developing immune system; therefore, dysbiosis may heighten the risk of chronic inflammatory diseases through immune dysregulation [6]. Studies have shown that the airway bacterial composition of subjects with asthma differs from that of healthy individuals [6, 7]. Additionally, divergent maturation patterns of the gut microbiota in early life have been associated with the development of asthma [8, 9].
Several studies have demonstrated an association between antibiotic exposure during infancy and an increased risk of asthma development [10–21]. We previously reported a dose-dependent relationship between increasing number of infant antibiotic courses and development of childhood asthma [22]. Although aspects of antibiotic use, such as dose, timing, and type, have been variably explored in previous studies, the comprehensive and simultaneous impact of these factors on asthma development has yet to be assessed. The objective of this study was to evaluate the association between the dose, timing, and type of antibiotic exposure during infancy and the development of childhood asthma by 6 years of age. Understanding and characterizing these relationships will aid in the development of clinical guidelines for safe use of antibiotics and enhance decision making for patients and clinicians.
METHODS
Study Population
We conducted a population-based cohort study of singleton, term-birth, non–low-birth-weight, and otherwise healthy children born during 1995–2003 and continuously enrolled in the Tennessee Medicaid program (TennCare). TennCare provides care to low-income pregnant women, children, and individuals who are elderly or have a disability. It covers approximately 20% of Tennessee’s population and 50% of the state’s children [23]. The study protocol was approved by the institutional review boards of Vanderbilt University and the Tennessee Department of Public Health.
Infant Antibiotic Use
Antibiotic use during the first 12 months of life was captured from medical claims prescription fill data of TennCare. Dates and names of antibiotics were recorded. Infant age at each antibiotic fill was calculated. Each fill was further classified by spectrum, formulation, anaerobic coverage, and class (Supplementary Table 1).
Childhood Asthma Ascertainment
Asthma was ascertained between ages 4.5 years and 6 years using asthma-related healthcare encounters and asthma-specific medication use. Children with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 493.xx in any diagnostic field for inpatient, other hospital care, emergency department visit, and/or outpatient physician visit claims (2 outpatient visits separated by at least 30 days were required) were considered to have asthma. In addition, children with 2 fills for any short-acting β-agonist, 2 fills for montelukast in a 365-day period prior to US Food and Drug Administration approval of montelukast for allergic rhinitis, or a single fill of any other asthma-specific medication were considered as having asthma.
Statistical Analysis
The association between use, dose, timing, and type of antibiotics filled during infancy and the risk of childhood asthma was assessed using univariate and multivariable logistic regression models. Number of fills was treated as a continuous variable. The association between the number of antibiotic fills and childhood asthma was further evaluated by counting the number of fills during each month of infancy. We compared infants who did not have any antibiotic fills at each particular month in age to those who had 1 and ≥2 fills during that month. Timing of the first antibiotic fill and the risk of childhood asthma was assessed using infant age at the first fill. We classified infants into mutually exclusive categories based on the spectrum, formulation, anaerobic coverage, and class of antibiotics they ever had filled during infancy to determine the association between type of antibiotics and childhood asthma. The risk of childhood asthma by infant age at the first antibiotic fill and type of antibiotic fill was further assessed by comparing children who had only 1 fill with children who did not have any fills during infancy.
Children who had 2 antibiotic fills in a short period of time may have an increased risk of asthma. In a subset of children who had at least 2 antibiotic fills, we calculated the between-fill interval time (Supplementary Figure 1). The shortest interval time was considered the exposure of interest when multiple intervals (≥3 antibiotic fills during infancy) were estimated. The association of the shortest between-fill interval time and the risk of childhood asthma was assessed.
Because infant bronchiolitis is associated with childhood asthma in a severity-dependent manner [24] and is often an indication of receipt of antibiotics [25], we evaluated the interaction effect between the number of antibiotic fills and the most severe bronchiolitis healthcare encounter experienced during infancy on the risk of childhood asthma. Genetic predisposition for asthma increases the risk of asthma and has been shown to modify the association between infant antibiotic use and childhood asthma [10]. Thus, we assessed the potential interaction effect between the number of antibiotics and maternal asthma status, a marker of genetic predisposition for increased risk of asthma [10], on childhood asthma in the subset of children whose mother’s asthma status was available. A stratified analysis by maternal asthma status was conducted. All analyses were performed using R software, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and data management was performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina). Additional methodological details related to study population, antibiotic and asthma ascertainment, covariates, and statistical analysis can be found in the Supplementary Material.
RESULTS
Among 152 622 singleton, term-birth, non–low-birth-weight, otherwise healthy children enrolled in TennCare, 79% (n = 120 973) had at least 1 antibiotic fill during the first year of life (Table 1). Compared with children who did not have a fill during infancy, children who had at least 1 fill were more likely to be male, white, delivered by cesarean section, live in an urban area, have bronchiolitis healthcare encounters during infancy, and have mothers who smoked during pregnancy.
Table 1.
Characteristics of the Study Population of Children Born Between 1995 and 2003 With Continuous Enrollment in the Tennessee Medicaid Program (TennCare)
| Characteristics | Total | No Antibiotic Use | Ever Used Antibiotics |
|---|---|---|---|
| Sample size | 152 622 (100.0) | 31 649 (20.7) | 120 973 (79.3) |
| Maternal age at delivery, ya | 22 (19–26) | 22 (19–26) | 22 (19–26) |
| Marital status (married) | 55 314 (36.2) | 11 168 (35.3) | 44 146 (36.5) |
| Maternal education | |||
| <12 y | 65 426 (42.9) | 13 370 (42.2) | 52 056 (43.0) |
| 12 y | 68 101 (44.6) | 13 695 (43.3) | 54 406 (45.0) |
| >12 y | 18 746 (12.3) | 4485 (14.2) | 14 261 (11.8) |
| Maternal smoking during pregnancy | 40 460 (26.5) | 7282 (23.0) | 33 178 (27.4) |
| Residence | |||
| Urban | 52 162 (34.2) | 7597 (24.0) | 44 565 (36.8) |
| Suburban | 35 616 (23.3) | 7197 (22.7) | 28 419 (23.5) |
| Rural | 64 713 (42.4) | 16 821 (53.1) | 47 892 (39.6) |
| Mode of delivery | |||
| Vaginal | 106 948 (70.1) | 22 996 (72.7) | 83 952 (69.4) |
| Assisted | 13 542 (8.8) | 2511 (7.9) | 11 031 (9.1) |
| Cesarean | 32 063 (21.0) | 6132 (19.4) | 25 931 (21.4) |
| Gestational age, wka | 39 (39–40) | 39 (39–40) | 39 (39–40) |
| Birth weight, ga | 3289 (3005–3600) | 3260 (2977–3572) | 3289 (3005–3600) |
| Male sex | 78 692 (51.6) | 15 033 (47.5) | 63 659 (52.6) |
| Infant race | |||
| White | 84 053 (55.1) | 14 597 (46.1) | 69 456 (57.4) |
| Black | 55 862 (36.6) | 13 711 (43.3) | 42 151 (34.8) |
| Other | 12 707 (8.3) | 3341 (10.6) | 9366 (7.7) |
| Most severe bronchiolitis healthcare encounter experienced during infancy | |||
| No visit | 122 010 (79.9) | 28 925 (91.4) | 93 085 (76.9) |
| Outpatient visit | 14 207 (9.3) | 1132 (3.6) | 13 075 (10.8) |
| Emergency department visit | 7835 (5.1) | 895 (2.8) | 6940 (5.7) |
| Hospitalization | 8570 (5.6) | 697 (2.2) | 7873 (6.5) |
| No. of living siblings at home | |||
| None | 62 373 (40.9) | 12 379 (39.1) | 49 994 (41.3) |
| 1 | 47 214 (30.9) | 9414 (29.7) | 37 800 (31.2) |
| ≥2 | 42 922 (28.1) | 9833 (31.1) | 33 089 (27.4) |
| Birth year | |||
| 1995 | 16 827 (11.0) | 2903 (9.2) | 13 924 (11.5) |
| 1996 | 16 570 (10.9) | 2816 (8.9) | 13 754 (11.4) |
| 1997 | 15 584 (10.2) | 2931 (9.3) | 12 653 (10.5) |
| 1998 | 16 330 (10.7) | 3085 (9.7) | 13 245 (10.9) |
| 1999 | 16 534 (10.8) | 3415 (10.8) | 13 119 (10.8) |
| 2000 | 17 678 (11.6) | 3817 (12.1) | 13 861 (11.5) |
| 2001 | 17 633 (11.6) | 3964 (12.5) | 13 669 (11.3) |
| 2002 | 17 357 (11.4) | 3992 (12.6) | 13 365 (11.0) |
| 2003 | 18 109 (11.9) | 4726 (14.9) | 13 383 (11.1) |
Data are presented as no. (%) unless otherwise indicated.
aData are expressed as median (interquartile range).
Among children who had at least 1 antibiotic fill during infancy, the median number of antibiotic fills was 4 (interquartile range [IQR], 2–5) (Table 2). The median infant age at the first fill was 229 days (IQR, 156–296 days). The majority of prescribed antibiotics were narrow spectrum (67.6%), oral formulation (96.8%), and had anaerobic activity (83.5%). Penicillin was the most frequently prescribed class of antibiotics (68.1%), with amoxicillin being the single most commonly prescribed antibiotic (52.8%).
Table 2.
Characteristics of Infant Antibiotic Use Among Infants Who Had At Least 1 Prescription Fill in the First Year of Life (n = 120 973)
| Characteristic | No. (%) |
|---|---|
| Total no. of antibiotic prescription fills | 349 673 (100.0) |
| No. of antibiotic fills per infanta | 4 (2–5) |
| Age at the first fill, da | 229 (156–296) |
| Spectrum | |
| Broad | 113 282 (32.4) |
| Narrow | 236 391 (67.6) |
| Formulation | |
| Oral | 338 565 (96.8) |
| Parenteral | 11 108 (3.2) |
| Anaerobic coverage | |
| Yes | 291 881 (83.5) |
| No | 57 792 (16.5) |
| Class | |
| Aminoglycoside | 78 (0.0) |
| Carbacephem | 8288 (2.4) |
| Cephalosporin | |
| First-generation | 10 734 (3.1) |
| Second-generation | 29 084 (8.3) |
| Third-generation | 14 976 (4.3) |
| Fourth-generation | 0 (0) |
| Glycopeptide | 18 (0.0) |
| Lincosamide | 470 (0.1) |
| Macrolide | 28 373 (8.1) |
| Penicillin | 238 195 (68.1) |
| Sulfonamide | 19 455 (5.6) |
| Carbapenem | 1 (0.0) |
| Monobactam | 0 (0) |
| Polypeptide | 0 (0) |
| Chloramphenicol | 0 (0) |
| Oxazolidinones | 1 (0.0) |
Data are presented as no. (%) unless otherwise indicated.
aData are expressed as median (interquartile range).
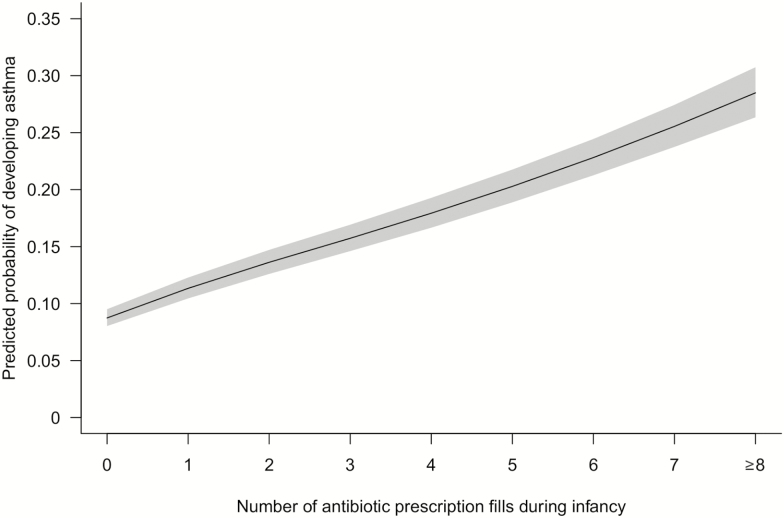
The prevalence of childhood asthma by 6 years of age was 14% (n = 20 984). Antibiotic fill during the first year of life significantly increased the odds of childhood asthma. Compared with children who did not have an antibiotic fill during infancy, children who had at least 1 fill had a nearly 2-fold increase in the odds of developing asthma by 6 years of age (odds ratio [OR], 1.86 [95% confidence interval {CI}, 1.79–1.94]; adjusted odds ratio [aOR], 1.88 [95% CI, 1.80–1.96]). A significant logit-linear (linear on the logit scale), dose-dependent relationship between antibiotic fills and childhood asthma was observed (Figure 1). With each additional fill during infancy, the odds of having asthma by 6 years of age increased by 18% (OR, 1.18 [95% CI, 1.17–1.19]) in the univariate analysis and 20% (aOR, 1.20 [95% CI, 1.19–1.20]) after adjusting for covariates.
Figure 1.
Infant antibiotic use is associated with an increased odds of childhood asthma in a dose-dependent manner (N = 152 622). The predicted probability was calculated using a multivariable logistic regression model adjusting for maternal smoking during pregnancy, maternal age at delivery, maternal education level, marital status, residence, gestational age at delivery, mode of delivery, number of living siblings at home, birth weight, infant race, infant sex, birth year, and birth month.
The number of antibiotic fills infants had (0, 1, ≥2) were counted at each month of age. The proportion of children who had at least 1 fill increased with advancing infant age (Supplementary Figure 2). At each particular month, having at least 1 antibiotic fill increased the odds of childhood asthma, adjusting for antibiotics filled at other months of age and covariates. The more antibiotic fills infants had at each particular month in age, the higher the odds of developing asthma.
We further examined the association between timing of first antibiotic fill and the odds of childhood asthma. The odds of developing asthma did not differ depending on the month in which the infant had their first antibiotic fill (Figure 2). This finding remained consistent after limiting to children who had at least 1 antibiotic fill during infancy and adjusting for the number of antibiotic fills (Supplementary Figure 3).
Figure 2.
Timing in month at the first antibiotic fill is associated with an increased odds of childhood asthma compared with infants who had no antibiotics filled during infancy (reference group) (N = 152 622). Adjusted odds ratios were calculated using a multivariable logistic regression model adjusting for maternal smoking during pregnancy, maternal age at delivery, maternal education level, marital status, residence, gestational age at delivery, mode of delivery, number of living siblings at home, birth weight, infant race, infant sex, birth year, birth month, and total number of prescription fills during infancy. Horizontal lines denote 95% confidence intervals. The number (%) of children in each group is given to the right of these lines.
Compared with children who did not have an antibiotic fill during infancy, antibiotic fills of any spectrum, formulation, anaerobic coverage, and class increased the odds of childhood asthma after covariate adjustment (Supplementary Figure 4). The odds of childhood asthma was increased among infants who had broad-spectrum-only antibiotic fills compared to those who had narrow-spectrum-only antibiotic fills (aOR, 1.10 [95% CI, 1.05–1.19]) but did not differ by timing, class, or anaerobic coverage of antibiotics during the first year of life (P > .05). The results were consistent comparing children who had only 1 antibiotic fill with children who never had antibiotics during infancy (data not shown).
The association between the shortest between-fill interval time in days and odds of childhood asthma was assessed among children who had at least 2 antibiotic fills during infancy (n = 88 623). The median shortest between-fill interval time was 36 days (IQR, 23–69 days). After adjusting for total number of antibiotics fills infants had and other covariates, the shortest between-fill interval time was not associated with increased odds of childhood asthma (between-fill interval time: 69 vs 23 days; aOR, 1.05 [95% CI, .99–1.10]).
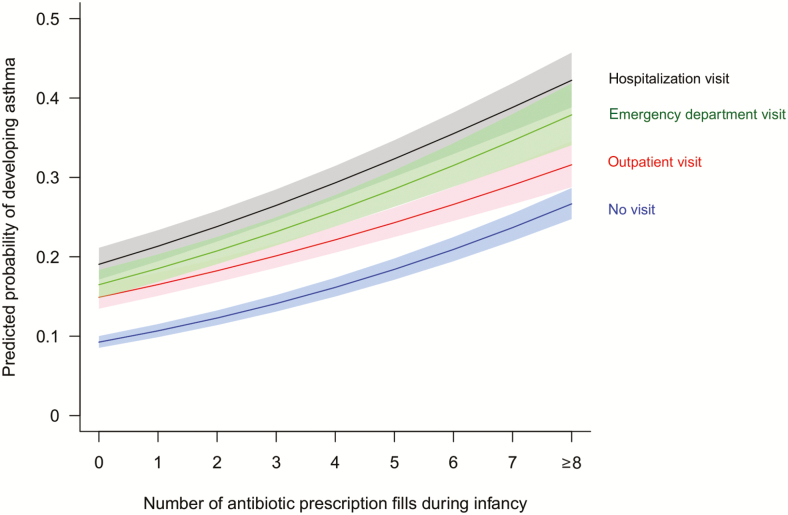
There was a statistically significant interaction effect between the severity of bronchiolitis healthcare encounters during infancy and the number of antibiotic fills on the odds of childhood asthma (P = .006; Figure 3). At each bronchiolitis healthcare encounter severity level, we observed a consistent dose-response relationship between infant antibiotic fills and childhood asthma. With each additional antibiotic fill, the odds of childhood asthma increased by 17% (aOR, 1.17 [95% CI, 1.16–1.18]), 13% (aOR, 1.13 [95% CI, 1.11–1.15]), 17% (aOR, 1.17 [95% CI, 1.13–1.20]), and 16% (aOR, 1.16 [95% CI, 1.13–1.19]) among children whose most severe visit for bronchiolitis during infancy was no visit, outpatient visit, emergency department visit, and hospitalization visit, respectively.
Figure 3.
Infant antibiotic use is associated with an increased odds of childhood asthma in a dose-dependent manner in every severity level of bronchiolitis healthcare encounters infants experienced during their first year of life (N = 152 622; P value for interaction effect = .006). The predicted probability was calculated using a multivariable logistic regression model adjusting for maternal smoking during pregnancy, maternal age at delivery, maternal education level, marital status, residence, gestational age at delivery, mode of delivery, number of living siblings at home, birth weight, infant race, infant sex, birth year, and birth month.
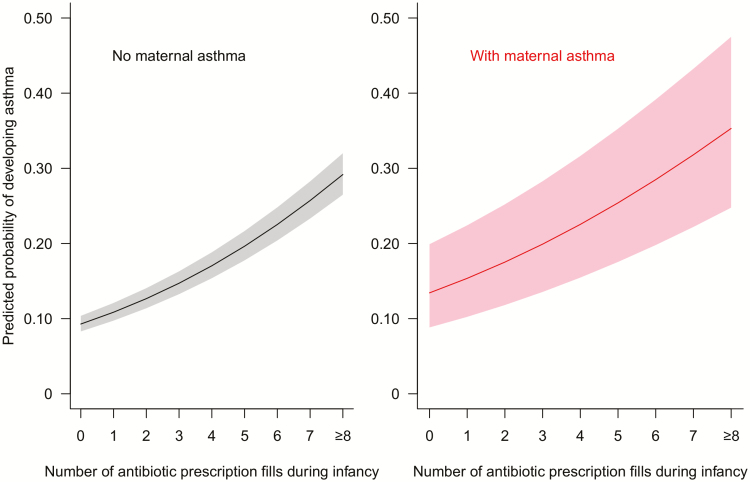
Maternal asthma represents a marker of genetic predisposition for childhood asthma. In a subset of children whose mothers were enrolled in TennCare (n = 87 148), we found a statistically significant interaction effect between infant antibiotic fill and maternal asthma on the odds of childhood asthma (P = .006). Children of mothers with asthma had higher odds of developing asthma by age 6 than children of mothers without asthma. However, regardless of maternal asthma status, the association between infant antibiotic fill and childhood asthma was consistent in a dose-dependent manner (Figure 4). With each additional antibiotic fill, the odds of developing asthma increased by 19% (without maternal asthma: aOR, 1.19 [95% CI, 1.18–1.20]) and 17% (with maternal asthma: aOR, 1.17 [95% CI, 1.13–1.22]), respectively.
Figure 4.
Infant antibiotic use is associated with an increased odds of childhood asthma in a dose-dependent manner in children of mothers with (n = 3884) and without (n = 83 264) maternal asthma. The predicted probability was calculated using a multivariable logistic regression model adjusting for number of prenatal antibiotic prescription fills, maternal smoking during pregnancy, maternal age at delivery, maternal education level, marital status, residence, gestational age at delivery, mode of delivery, number of living siblings at home, birth weight, infant race, infant sex, birth year, and birth month.
DISCUSSION
To our knowledge, the present study is the first to comprehensively and simultaneously assess the relationship between use, dose, timing, and type of antibiotics during infancy and childhood asthma. The use of antibiotics during infancy significantly increased the risk of childhood asthma. Moreover, the association between infant antibiotic use and childhood asthma was dose-dependent, with an increase in odds of 20% observed for each additional antibiotic filled. This dose-dependent association persisted across severity levels of infant bronchiolitis healthcare encounters and among infants born to mothers with asthma. The risk of childhood asthma was increased by the use of broad-spectrum antibiotics but did not differ by the timing, class, or anaerobic coverage of antibiotics filled during infancy.
Our findings of a dose-response relationship between increasing number of infant antibiotic fills and the risk of developing childhood asthma are consistent with our previous findings [22] and the findings of several other studies [10, 11, 14, 18–20, 25, 26], including in 2 large, population-based cohorts [20, 26]. What is novel and important about the present study is that timing, class, and anaerobic coverage of antibiotics had little impact on the significant dose-response relationship. Fill of antibiotics at any time during infancy increased the risk of childhood asthma, regardless of the number of antibiotics filled at other times during infancy. Our result that timing of antibiotic fill was not associated with increased odds of childhood asthma is supported by previous studies that categorized antibiotic use in time blocks of ≥6 months [11, 27]. In contrast, Wjst and colleagues observed an increased odds of childhood asthma with earlier administration of first antibiotics (never vs 0–6 months: aOR, 9.9 [95% CI, 3.1–31.4]; never vs 7–12 months: aOR, 5.2 [95% CI, 1.7–15.6]; and never vs 1–2 years: aOR, 3.8 [95% CI, 1.2–11.7]) [28]. As we observed in our study, earlier administration of antibiotics is often a marker of frequent administration of antibiotics. The association of early administration disappeared after adjusting for the total number of antibiotics filled during infancy. In addition to not adjusting for the total number of antibiotics used during infancy, the study performed by Wjst and colleagues also suffered from inherent limitations due to its cross-sectional study design, self-reported outcome, and self-reported recall of exposure (reported at 5 years of age).
We classified antibiotics by spectrum, formulation, anaerobic coverage, and class. Use of antibiotics, regardless of their type, increased the risk of childhood asthma. The more fills infants had, the higher the risk of childhood asthma. Fill of broad-spectrum antibiotics was associated with a higher risk of childhood asthma compared with narrow-spectrum antibiotics, a finding that has been reported in previous studies [19, 26]. However, the risk of childhood asthma did not differ by class, formulation, and anaerobic coverage of antibiotics. The mixed findings may be the result of challenges with classifying antibiotics into broad- and narrow-spectrum categories, including the lack of a validated algorithm and changing antibiotic resistance patterns over time [29, 30]. Our findings are in line with the microflora hypothesis, which suggests that antibiotic-driven dysbiosis of microbiota composition may alter the maturation of the immune system and increase the risk of developing allergic diseases [31].
A recognized limitation of many of the studies that have assessed this relationship to date was the failure to reduce confounding by indication and reverse causality [25, 32]. It is plausible that infection itself or other genetic and environmental risk factors that predispose infants to infection is the causal factor increasing the risk of childhood asthma and that the association between antibiotics and childhood asthma is not causal. We attempted to reduce and distinguish the effects of infection from antibiotic use. Although it is likely that both infection and antibiotic use contribute to the risk of childhood asthma, the strongest evidence against confounding by indication in our study is the finding of an association between antibiotic use and asthma among children with no bronchiolitis healthcare encounters. We observed a similar increase in the odds of childhood asthma with increasing antibiotics among infants with mothers who did and did not have asthma, after adjusting for maternal prenatal antibiotic fill and other covariates. As these variables are markers of genetic and environmental predisposition for childhood asthma, these findings reduce the plausibility of reverse causation. An additional strength of our study was our use of a previously validated algorithm to define childhood asthma, an algorithm that is similar to that used by other US Medicaid systems [33–37]. Use of a large, administrative-based cohort allowed us to assess more specific aspects of antibiotic use in infancy, eliminate potential recall bias through self-report of exposure and outcome, and include many covariates to diminish potential confounding effects. However, although many interesting and important associations could be observed within our large sample population, some of the associations, while significant, were small in magnitude and thus may not be clinically meaningful.
Although our study had many strengths, we acknowledge the limitations. While we observed a significant dose-response relationship, we can neither confirm nor refute the causative role of antibiotics in the development of childhood asthma. We could not include several factors that are known to be associated with asthma risk, including breastfeeding, exposure to mold or pets, daycare attendance, and other environmental exposures. Fill data were used as a surrogate for antibiotic exposure; however, we do not know whether patients administered the filled medication. It is likely that most fills were administered, as prescription of antibiotics is an indication of infection and filling the prescription is the first step of treatment. The use of fill data for this purpose has been previously validated [38]. Our study population includes mainly subjects with a low socioeconomic status; thus, results from our study may not be generalizable to other populations. However, about 50% of children born in Tennessee each year are enrolled in TennCare [23], and although the magnitude of the effect size may differ, there is no reason to believe that the results are substantially different from other US populations. Last, our use of ICD-9-CM codes to ascertain disease conditions and covariates may lend itself to some misclassification.
CONCLUSIONS
In this large, population-based cohort study, the risk of childhood asthma increased in a dose-response manner in relationship to the number of antibiotic prescription fills infants had during the first year of life. The risk of childhood asthma was increased by the use of broad-spectrum antibiotics but did not differ by the timing, class, or anaerobic coverage of antibiotics filled during infancy. In light of the mounting evidence supporting the detrimental effect of infant antibiotic use on childhood asthma, patients and healthcare providers should critically weigh the risks and benefits of antibiotic use in infants before administration and consider possible alternative treatment strategies when available, and the research community should focus on how the adverse effects of antibiotics could be prevented when their use is required.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are indebted to the Tennessee Bureau of TennCare and the Tennessee Department of Health, Office of Policy, Planning, and Assessment for providing the data.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Financial support. This work was supported by the Agency for Healthcare Research and Quality (grant number R01 HS018454 to T. V. H.) and the National Institutes of Health (grant numbers K24 AI77930 to T. V. H.; R21 HL133742 and R21 HL123829 to P. W.; T32 HL87738 and 5T32 GM007569-41 to C. S.; R21 HL129020 to C. Y.; T32 5T32HL087738-13 to B. M. D.; and UL1 RR024975 [now 2 UL1 TR000445]).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. National Heart, Lung, and Blood Institute. Asthma Available at: https://www.nhlbi.nih.gov/health-topics/asthma. Accessed 20 August 2018.
- 2. Forno E, Celedón JC. Health disparities in asthma. Am J Respir Crit Care Med 2012; 185:1033–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baltrus P, Xu J, Immergluck L, Gaglioti A, Adesokan A, Rust G. Individual and county level predictors of asthma related emergency department visits among children on Medicaid: a multilevel approach. J Asthma 2017; 54:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang YJ, Boushey HA. The microbiome and asthma. Ann Am Thorac Soc 2014; 11(Suppl 1):S48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One 2010; 5:e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol 2015; 135:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stokholm J, Blaser MJ, Thorsen J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 2018; 9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1401 US children. Am J Epidemiol 2011; 173:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol 2013; 24:762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wickens K, Pearce N, Crane J, Beasley R. Antibiotic use in early childhood and the development of asthma. Clin Exp Allergy 1999; 29:766–71. [DOI] [PubMed] [Google Scholar]
- 13. Cohet C, Cheng S, MacDonald C, et al. Infections, medication use, and the prevalence of symptoms of asthma, rhinitis, and eczema in childhood. J Epidemiol Community Health 2004; 58:852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahn KM, Lee MS, Hong SJ, et al. Fever, use of antibiotics, and acute gastroenteritis during infancy as risk factors for the development of asthma in Korean school-age children. J Asthma 2005; 42:745–50. [DOI] [PubMed] [Google Scholar]
- 15. Flöistrup H, Swartz J, Bergström A, et al. Parsifal Study Group Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol 2006; 117:59–66. [DOI] [PubMed] [Google Scholar]
- 16. Foliaki S, Nielsen SK, Björkstén B, Von Mutius E, Cheng S, Pearce N; ISAAC Phase I Study Group Antibiotic sales and the prevalence of symptoms of asthma, rhinitis, and eczema: the International Study of Asthma and Allergies in Childhood (ISAAC). Int J Epidemiol 2004; 33:558–63. [DOI] [PubMed] [Google Scholar]
- 17. Karimi M, Mirzaei M. Antibiotic use and symptoms of asthma, allergic rhinitis and eczema in children. Iran J Pediatr 2009; 19:141–6. [PubMed] [Google Scholar]
- 18. McKeever TM, Lewis SA, Smith C, et al. Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands general practice research database. J Allergy Clin Immunol 2002; 109:43–50. [DOI] [PubMed] [Google Scholar]
- 19. Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest 2007; 131:1753–9. [DOI] [PubMed] [Google Scholar]
- 20. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009; 123:1003–10. [DOI] [PubMed] [Google Scholar]
- 21. Martel MJ, Rey E, Malo JL, et al. Determinants of the incidence of childhood asthma: a two-stage case-control study. Am J Epidemiol 2009; 169:195–205. [DOI] [PubMed] [Google Scholar]
- 22. Wu P, Feldman AS, Rosas-Salazar C, et al. Relative importance and additive effects of maternal and infant risk factors on childhood asthma. PLoS One 2016; 11:e0151705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tennessee State Government Division of TennCare. TennCare overview. Available at: https://www.tn.gov/tenncare/information-statistics/tenncare-overview.html. Accessed 20 March 2019.
- 24. Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009; 123:1055–61.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su Y, Rothers J, Stern DA, Halonen M, Wright AL. Relation of early antibiotic use to childhood asthma: confounding by indication? Clin Exp Allergy 2010; 40:1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almqvist C, Wettermark B, Hedlin G, Ye W, Lundholm C. Antibiotics and asthma medication in a large register-based cohort study—confounding, cause and effect. Clin Exp Allergy 2012; 42:104–11. [DOI] [PubMed] [Google Scholar]
- 27. Kusel MM, de Klerk N, Holt PG, Sly PD. Antibiotic use in the first year of life and risk of atopic disease in early childhood. Clin Exp Allergy 2008; 38:1921–8. [DOI] [PubMed] [Google Scholar]
- 28. Wjst M, Hoelscher B, Frye C, Wichmann HE, Dold S, Heinrich J. Early antibiotic treatment and later asthma. Eur J Med Res 2001; 6:263–71. [PubMed] [Google Scholar]
- 29. Sanchez GV, Roberts RM, Albert AP, Johnson DD, Hicks LA. Effects of knowledge, attitudes, and practices of primary care providers on antibiotic selection, United States. Emerg Infect Dis 2014; 20:2041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Acar J. Broad- and narrow-spectrum antibiotics: an unhelpful categorization. Clin Microbiol Infect 1997; 3:395–6. [DOI] [PubMed] [Google Scholar]
- 31. Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy 2005; 35:1511–20. [DOI] [PubMed] [Google Scholar]
- 32. Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics 2011; 127:1125–38. [DOI] [PubMed] [Google Scholar]
- 33. Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008; 178:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wakefield DB, Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr Pulmonol 2006; 41:962–71. [DOI] [PubMed] [Google Scholar]
- 35. Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc 2000; 48:651–7. [DOI] [PubMed] [Google Scholar]
- 36. Hartert TV, Speroff T, Togias A, et al. Risk factors for recurrent asthma hospital visits and death among a population of indigent older adults with asthma. Ann Allergy Asthma Immunol 2002; 89:467–73. [DOI] [PubMed] [Google Scholar]
- 37. Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005; 352:2082–90. [DOI] [PubMed] [Google Scholar]
- 38. Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Québec. J Clin Epidemiol 1995; 48:999–1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.