Abstract
Tuberculosis (TB) elimination requires innovative approaches. The new Global Tuberculosis Network (GTN) aims to conduct research on key unmet therapeutic and diagnostic needs in the field of TB elimination using multidisciplinary, multisectorial approaches. The TB Pharmacology section within the new GTN aims to detect and study the current knowledge gaps, test potential solutions using human pharmacokinetics informed through preclinical infection systems, and return those findings to the bedside. Moreover, this approach would allow prospective identification and validation of optimal shorter therapeutic durations with new regimens. Optimized treatment using available and repurposed drugs may have an increased impact when prioritizing a person-centered approach and acknowledge the importance of age, gender, comorbidities, and both social and programmatic environments. In this viewpoint article, we present an in-depth discussion on how TB pharmacology and the related strategies will contribute to TB elimination.
Keywords: tuberculosis, drug resistance, pharmacokinetics, pharmacodynamics, therapeutic drug monitoring
An enhanced understanding of tuberculosis (TB) pharmacology aims to use pharmacokinetics and pharmacodynamics, operational research, and therapeutic drug monitoring to support TB elimination.
TUBERCULOSIS ELIMINATION REQUIRES A NEW APPROACH
In September 2018, the United Nations General Assembly convened the historical first high-level meeting of member states’ leaders on tuberculosis (TB), committing to end the TB epidemic by 2030. The 3 pillars of the World Health Organization’s (WHO’s) TB elimination strategy include integrated person-centered prevention and care, bold policies and supportive systems, and intensified research and innovations. Several interventions are central to enacting the elimination strategy, including prevention, diagnosis, and treatment, as well as rehabilitation of disease sequelae [1, 2]. Interventions may have overlapping benefit, for example, the rapid diagnosis and effective treatment of TB will reduce individual morbidity and mortality and will also reduce transmission of Mycobacterium tuberculosis within the community.
In recent years, investments in research and development have resulted in the approval of new medications for treatment of TB, such as bedaquiline and delamanid. The pipeline includes several new regimens that are expected to dramatically shorten the duration of treatment and increase the cure of all types of TB. Based on the latest evidence from research and country implementation, in 2019, the new WHO consolidated guidelines on drug-resistant TB treatment that promote new and repurposed medicines and shorter treatment regimens were issued [3]. Despite these promising developments, it is known that the uptake of new medicines and regimens at the country level is painfully slow. Barriers to more rapid uptake at the country level have included concerns about safety, regulatory bottlenecks, and especially insufficient evidence of efficacy and safety [4] in combination with other medicines in treatment regimens. Despite the slower-than-anticipated uptake, the ultimate goal remains a treatment regimen individualized in terms of components and duration using a combination of the safest and most efficacious medicines built upon rapid whole-genome sequencing drug-susceptibility testing (DST). To best do so, DST data must be coupled with actionable knowledge of antibiotic pharmacokinetics (PK) and pharmacodynamics (PD).
GLOBAL TUBERCULOSIS NETWORK
The Global Tuberculosis Network (GTN), hosted by the World Association for Infectious Diseases and Immunological Disorders (WAidid), was launched in October 2018 at the second Conference of WAidid in Milan, Italy [5, 6]. The GTN involves an international group of TB experts joining their collective experience toward reaching the goals of the End TB Strategy. The overarching aim of GTN is to collaborate with existing initiatives directed toward the elimination of TB in the areas of research, advocacy, and training.
The GTN is presently composed of 3 pillars. Pillar 1: the following technical committees: TB Pharmacology; Diagnosis; Treatment; Epidemiology, Statistics, and Methodology; Prevention/Latent TB Infection; Pediatric; Basic Science; Clinical Trials; TB and Surgery; Migrants/Vulnerable Populations; TB Infection Control; Impact Evaluation, Strategies, and Global Health; and Clinical Support to Patients–TB Consilium; pillar 2: representatives of organizations committed to TB control and interested in participating in the GTN; and pillar 3: private and pharmaceutical sectors, with observer status. Discussions are ongoing regarding the possibility to activate a pillar 4 with individual membership.
The GTN has already organized working groups to identify priorities and conduct research, initially focused on TB diagnosis and therapeutics.
TB PHARMACOLOGY SECTION
In accordance with GTN’s perspectives, the TB Pharmacology Committee will promote the identification and study of critical knowledge gaps in our current understanding of TB pharmacology.
Preclinical models will be used to explore and determine the relationship between drug exposure and bacterial response, that is, microbial kill or acquired resistance. Information on drug dosing from these models will guide dosing strategies in real life. Acknowledging the funding gap for TB, operational research will be used in addition to clinical trials to evaluate PK/PD-guided dosing of currently available drugs. In addition to research, implementation strategies and accompanying tools will be developed to facilitate bedside PK/PD-guided dosing.
Our aim in this viewpoint article is to provide a framework of PK/PD strategies to bridge bench to bedside. The committee identified the following strategies to bridge bench to bedside: minimum inhibitory concentration (MIC) and hollow fiber systems, translation of in vitro PK/PD to the bedside, research in an operational setting, and PK/PD at the bedside.
MIC and Hollow Fiber Infection Systems to Redefine Dosing Strategies
Anti-TB drug sensitivity tests are routinely performed on the first isolate of M. tuberculosis grown in culture to provide data for tailored therapy or to confirm the susceptibility to a therapy started on the basis of epidemiological and patient-related data or, more recently, on molecular results from direct specimens. Routine DST is performed in liquid or solid media at a critical concentration (CC); based on the results, strains are classified as “sensitive” or “resistant.” The MIC is the lowest static antibiotic concentration able to inhibit bacterial growth. MIC testing has been proposed as a better strategy for aligning a phenotypic test with mutations that confer drug resistance. The advantage of MIC identification is that it provides a range of values as a presumptive indication of sensitivity to the drugs [7].
In addition to well-known factors such as cavitary disease, comorbidities (eg, human immunodeficiency virus), treatment length and completion, and medication adherence, clinical studies have revealed that variability in long-term patient outcomes can be explained by drug concentrations and M. tuberculosis isolate MICs and, hence, the ratios of drug concentrations to MIC; in other words, PK/PD [8–16]. These same aspects also drive acquired drug resistance at the level of the TB cavity in patients, based on poor penetration to the site of infection [17]. Some of these studies, such as the clinical study of ethionamide analyzed using machine learning (ML), show that as the MIC increases, patient outcomes get worse until an MIC is reached above which outcomes are uniformly poor (ie, at the susceptibility breakpoint) [14].Conversely, ML-based analysis of clinical data in children and adults with TB shows that outcomes improve as concentrations of drugs in combination therapy increase until a threshold is reached above which outcomes are uniformly good [8–11, 18, 19]. The MIC and drug concentration-threshold ceilings therefore give us targets that doses administered to patients should achieve.
The relationships between PK/PD exposures and M. tuberculosis outcomes have most commonly been derived in the hollow fiber system model of TB (HFS-TB), which uses human-like antibiotic concentration-time profiles encountered in the lungs. Although the HFS does not necessarily reflect the complete clinical condition of lung pathology during TB [20], as long as the PK/PD output of the model is predictive of what happens in the clinical setting, it can be used to inform dose optimization [21]. The HFS-TB, together with Monte Carlo simulations, has a forecasting accuracy of within 94% of the value of MIC susceptibility breakpoints, optimal dose, and PK/PD exposures associated with effect in clinical studies [12, 14, 21]. Table 1 shows the HFS-TB PK/PD parameters and susceptibility breakpoints for WHO-approved anti-TB drugs. By taking into account the PK variability of the anti-TB drugs, the MIC variability, and drug penetration indices, the ability of different doses to achieve the optimal PK/PD exposures and the susceptibility breakpoint can be derived [22, 23].
Table 1.
Pharmacokinetics and Pharmacodynamics Exposure and Minimum Inhibitory Concentration Breakpoints as Targets for Optimized Doses
| World Health Organization Classification | Drug | Hollow Fiber System Model of Tuberculosis and Monte Carlo Experiments Derived | Clinical Study Derived | Reference | ||
|---|---|---|---|---|---|---|
| PK/PD Exposure Target (Free Drug) in Lung | PK/PD MIC or Susceptibility Breakpoint, mg/L | PK or PK/PD Derived in Blood | MIC or Susceptibility Breakpoint, mg/L | |||
| First-line | ||||||
| Rifampin | AUC0-24/MIC >1360; peak/MIC >75 | 0.0625 | AUC0-24 >35.4 mg*h/L; peak >8.2 mg/L | 0.125; 0.0695 | [24] | |
| Isoniazid | AUC0-24/MIC >567 | 0.0312 | AUC0-24 >52 mg*h/L | 0.0312; 0.0334 | [24] | |
| Ethambutol | Peak/MIC >0.51; AUC0-24/MIC >119 | 4 | Peak/MIC >0.46 | 4 | [24] | |
| Pyrazinamide | AUC0-24/MIC >209 | 50 | AUC0-24 >363 mg*h/L; AUC0-24/MIC >11.3 | 50 | [25] | |
| Multidrug-Resistant Tuberculosis | ||||||
| Group A | ||||||
| Moxifloxacin | AUC0-24/MIC = 56a | 1 | … | … | [26] | |
| Levofloxacin | AUC0-24/MIC = 146; AUC0-24/MIC = 360a | 0.5 | AUC0-24/MIC = 160 | … | [13] | |
| Gatifloxacin | AUC0-24/MIC = 184 | 0.5/2 | AUC0-24 >50.29 | 0.5/2 | [12] | |
| Linezolid | AUC0-24/MIC = 119 | 2 | ... | ... | [27] | |
| Bedaquiline | ... | ... | ... | ... | … | |
| Group B | ||||||
| Clofazimine | ... | ... | … | … | … | |
| Cycloserine | Time above MIC = 30% | 64 | ... | ... | [28] | |
| Group C | ||||||
| Delamanid | ... | ... | ... | ... | … | |
| Imipenem/cilastatin | ... | ... | ... | ... | … | |
| Meropenem | ... | ... | ... | ... | … | |
| Amikacin | Peak/MIC = 10.13 | ... | Peak >67 mg/L | ... | [19, 29] | |
| Ethionamide | AUC0-24/MIC >56.2 | 2.5 | ... | 2.5 | [14] | |
| P-aminosalicylic acid | ... | ... | ... | ... | … |
Abbreviations: AUC, area under the concentration time curve; MIC, minimum inhibitory concentration; PD, pharmacodynamics; PK, pharmacokinetics.
aResistance suppression target.
There are some limitations to using MICs. First, although commercial plates exist, MIC testing has not been fully standardized; however, the European Committee on Antibiotic Susceptibility Testing mycobacterial subgroup is working on a standardized protocol to be used for defining MIC distribution of TB drugs. For several drugs, the lower bound of the MICs is truncated, which prevents adequate quality control [30]. Second, specific mutations in rpoB, the gene that contains the primary resistance-determining region for the rifamycins, are associated with poorer clinical outcome despite a sensitive result by phenotypic tests. Furthermore, MICs of those specific rpoB mutants show that MICs are higher than the wild-type distribution and that their distribution crosses the CC for the drug [31–33]. Interestingly, the MICs of these isolates and the rpoB mutation are correctly classified based on HFS-TB–derived PK/PD exposures and Monte Carlo simulations and ML of clinical data [23, 34]. In the case of isoniazid, resistance is associated with mutations in different genes both in coding and noncoding regions (mainly, katG, inhA, fabG1), and the different mutants show different MICs for the drug [35]. Mutations in inhA promoter are usually associated with a favorable outcome when treated with higher doses of the drug despite the fact that some strains will be reported as resistant based on phenotypic DST at the CC [35]. The same phenomenon is observed with moxifloxacin: DST at the CC cannot accurately predict if strains could be treatable with higher doses of the drug. The WHO recently established the clinical breakpoint (CB) [36] to identify strains that are potentially treatable with higher doses of moxifloxacin. The MIC distribution of strains genotypically mutated in gyrA/B shows a bimodal distribution. Even the use of the CB can result in a misclassification of some mutant-bearing mutations as sensitive to high doses of fluoroquinolones while resistance is highly likely. For gatifloxacin, such a dose-dependent zone has been defined, with higher doses being effective with specific gyrA/B mutations based on HFS-TB data and clinical data [12]. Large multicenter studies such as CRyPTIC that correlate MICs to genomic mutations will shed light on how to use molecular markers to properly guide therapy decisions [7, 37].
Translation of In Vitro PK/PD to the Bedside
Antimicrobial drugs act in predictable ways. They have specific targets within the mycobacteria, and specific concentrations are needed to inhibit the growth of the organisms in vitro (MIC). Once the drugs are thoroughly studied in vitro, the findings can be confirmed in models of animals with active immune systems. Specific sites of infection can be studied in these models (eg, cerebrospinal fluid, brain, bone, pulmonary cavities).
Armed with the knowledge of the pharmacodynamically linked index, for example, maximum concentration (Cmax)/MIC, area under the concentration time curve (AUC)/MIC, or time > MIC, dosing regimens that maximize the probability of target attainment can be constructed. In general, one targets “large,” infrequent doses to sequentially drive down the number of viable organisms using drugs that are “concentration dependent” or one maintains concentrations above some measure of inhibition for as long as possible within each dosing interval (“time-dependent” drugs). Most antimycobacterial drugs can be dosed to maximize the free (unbound) drug AUC/MIC.
Because 3 mycobacteria populations appear to coexist in vivo, that is, those that actively divide, grow slowly in acidic environments, and are nonreplicating persisters, the pharmacodynamically linked index should be determined for all 3 populations using appropriate models. At the initiation of therapy, the largest population is in log-phase growth. Small numbers appear to be present within the acid phase and among nonreplicative persister phenotypes. However, these latter populations will emerge as the cause of failures or relapses if the chosen drugs fail to eliminate them.
Both mycobacterial populations and human populations show considerable interindividual variability. With the former, variability involves lineage-specific features, locations within the infected host, interactions with host-defense cells, the growth state, and susceptibility to the drugs used. Hosts vary widely based on age, immune function, renal and hepatic function, extent and duration of disease, and extent of cavity formation, which in turn reflect host genetic variability. Given this large number of variables, the probability that 1 regimen with 1 set of fixed doses is the right regimen for all patients is approximately zero. Therefore, individualized therapy is required if failures, relapses, and the selection of additional drug resistance are to be avoided.
Research in an Operational Setting
It is imperative that clinical data that reflect the heterogeneity of patients in operational settings are used to validate models that predict PK/ PD relationships. Given a target drug exposure, observational PK studies can optimize dosing according to age, weight, comorbidity, drug–drug interactions, or genotype. Population PK models accurately account for PK variability, and use of sparse-sampling, large population studies is feasible. As various sampling designs can be combined, data can be pooled across studies. These models can then be used to simulate optimal drug doses for different clinical scenarios. The importance of evaluating dosing guidelines in the relevant patient populations and optimizing doses accordingly has recently been demonstrated in observational pediatric studies evaluating drug exposure by age and weight [38]. Not-withstanding, there is often insufficient evidence to support dosing guidelines for infants, children, pregnant women, patients prescribed concomitant medicines that potentially interact with anti-TB drugs, and other special populations.
Challenges in the analysis of PK/PD relationships within clinical data include a lack of sensitive markers of treatment response, inaccurate extrapolation of PK over time, high-dimensional data with multiple time-varying covariates that sometimes display multicolinearity and complex interactions, and heterogeneity within and between study populations. ML techniques have aided in our understanding of the complex and multidimensional relationships between anti-TB drug exposures and treatment outcomes. These data-driven and hypothesis-generating analyses have provided useful insights into complex real-world data from patient cohorts [10].
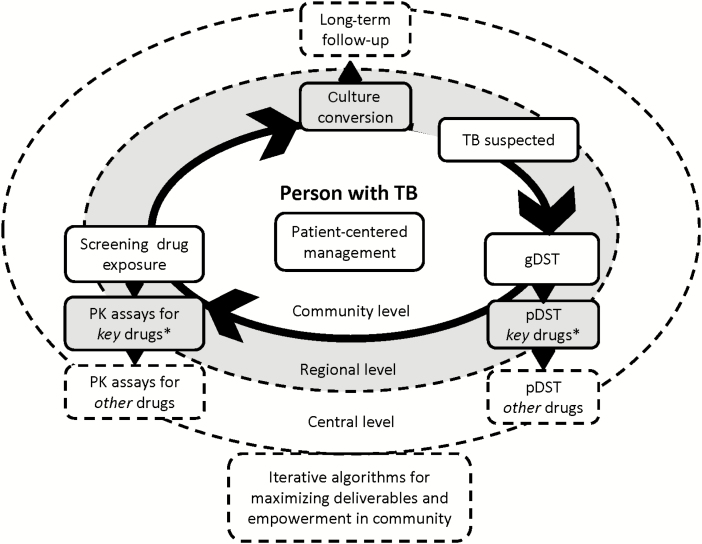
Such PK and PK/PD studies should ideally be integrated in phase 3 or 4 clinical trials (Figure 1); however, they add complexity and costs because of sample collection, processing, storage and shipment, and good laboratory practices including proficiency testing. Observational studies nested in operational settings are important to represent the diversity of patient populations, including special populations and patients with comorbid conditions that exclude them from clinical trials. These studies would ideally leverage data from the wider patient population that are captured in routine data systems.
Figure 1.
Individualized management of tuberculosis using operational PK/PD research. In general, PK/PD research is patient-centered and uses information on the susceptibility of the pathogen being either phenotypic or genotypic, subsequently using a measure of drug exposure and correlate both in relation to treatment outcome or any other measure of treatment response in a real-life setting. Abbreviations: gDST, genotypic drug susceptibility testing; pDST, phenotypic drug susceptibility testing; PK, pharmacokinetic; TB, tuberculosis; TDM, therapeutic drug monitoring. * key drugs for TDM include rifampicin, pyrazinamide, isoniazid, levofloxacin, moxifloxacin, and linezolid.
Data-sharing platforms allow maximal use of such data by providing a resource for researchers to examine large datasets, validate the data, and extrapolate test findings. While the clinical measures currently used to characterize PK, M. tuberculosis susceptibility, and disease response are limited, they provide an important reference framework for preclinical models. The role of integrated-systems pharmacology models that use multiple levels of preclinical data together with clinical evidence to translate PK/PD relationships into clinical guidance has yet to be fully realized [39].
PK/PD at the Bedside
A fundamental tenet agreed upon by world leaders at the 2018 UN High Level Meeting on TB was that solutions should be rooted in human rights and therefore be person-centered. Therein, solutions must be adaptable and capable of response to the individual, acknowledging microbial, host, and environmental/social circumstances [40]. A pan TB regimen has been proposed to provide easy-to-use and effective TB treatment. The advantages for fixed-dose combinations for compliance has been long recognized. Alternatively, knowing that PK/PD data demonstrate the importance of individualized dosing and regimen construction, operational research could assist in prioritization; patients will benefit from this strategy. In the few instances where individualized management strategies have thus far been routinely offered to target specific patients with TB, such as early therapeutic drug monitoring for diabetes-related TB, improved microbiologic and programmatic outcomes have been realized [41]. Although a target approach seems more realistic than TDM for every patient, the major bottleneck to person-centered clinical benefit to date has been a lack of later-phase clinical trials and a path for scale-up in settings most burdened by TB [42].
While the cost of quantitative analytic assays such as mass spectrometry and high-performance liquid chromatography (HPLC) has been frequently cited as a major barrier to implementation of therapeutic drug monitoring, cost-effectiveness fails to adequately weigh the personal gain for the individual. When such arguments were previously applied as “economistic rationalization” for restricting access to second-line drugs to treat multidrug-resistant TB, they were subsequently shown to instead amplify the epidemic [43]. We contend that implementation should not focus on questions of whether or not individualized management should be available but rather, how best to prioritize its use, and how best to maximize its deliverables. As technologies such as medications are appropriately designated as essential, affordability and global solidarity toward negotiating access naturally follows.
We acknowledge the relative logistical hurdles of collecting multiple venous blood samples within a dosing interval, centrifuging and transporting those specimens frozen to a referral laboratory for processing using single analyte assays, each with separate reagents and quality-control procedures, and then delivering those drug concentration results to a clinician for interpretation and action. High-quality TDM including the analysis, interpretation, and integration of those results in direct patient care requires training in pharmacology that is often lacking and therefore considered another hurdle hindering implementation of TDM. Multianalyte assays that combine the analysis of several key drugs in a single test will reduce the number of laboratory procedures and reduce specimen volume. However, the most likely innovation to remove the implementation bottleneck will be an alternative to sample collection that either bypasses the cold chain and the necessity for phlebotomy entirely or that serves as an initial screening test prior to conventional serum HPLC or mass spectrometry [44]. Dried blood spots have been used in a variety of environmental conditions, can be shipped at room temperature, and, for many critical drugs, can replicate serum results [45]. Alternatively, as a screening tool, promising work continues in both saliva and urine colorimetric detection assays that could be performed at the point-of-care and may prove semiquantitative, delivering a readout of low or normal/high that for the majority of patients would obviate the need for serum testing [46]. Thus, laboratory capacity development notwithstanding, we envision an approach to individualized management that can be adapted for the priorities of each patient, as depicted in Figure 1.
CONCLUSIONS
Optimization of TB treatment in daily practice is challenging for many medical and socioeconomic reasons. Yet, shifting focus to person-centered strategies that individualize drug dosing and drug regimen composition, as outlined in this viewpoint, will offer the person with TB the best opportunity for rapid sputum culture conversion that can reduce the transmission of TB, prevent acquired drug resistance, and ultimately shorten the total treatment duration. Providing the earliest and most optimal individualized treatment to people with TB, wherever they access care, will be critical for global TB elimination along with multidisciplinary approaches that address poverty and the catastrophic individual costs to people with TB and their families, novel approaches to reduce the latent TB reservoir and the incipient TB transmitted from people with subclinical disease, and improved vaccines for high-burden settings [1]. We recognize that a massive influx in public funding and widespread scale-up of public–private initiatives will be necessary to facilitate this approach. With regard to optimizing TB treatment, we argue that appropriate support is needed from regulatory authorities that can provide guidance on individualized dosing using TDM, as has been done for other antimicrobial drugs such as vancomycin. Regulatory support of off-label use of repurposed drugs based on publically available data in addition to fast-track approval of new drugs will help to expand the therapeutic armamentarium [47]. Providing people with TB, their care providers, the public health community, and researchers flexibility, as in the case of optimized TB treatment, will bring long-awaited precision to the goal of global TB elimination.
Notes
Acknowledgments. This article has been developed by the Pharmacology Committee of the Global Tuberculosis Network and is part of the scientific activities of the World Health Organization Collaborating Centre for TB and Lung Diseases, Fondazione S. Maugeri, Tradate, Italy.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Visca D, Zampogna E, Sotgiu G, et al. Pulmonary rehabilitation is effective in patients with tuberculosis pulmonary sequelae. Eur Respir J 2019;53 pii: 1802184. [DOI] [PubMed] [Google Scholar]
- 2. Tiberi S, Torrico MM, Rahman A, et al. Managing severe tuberculosis and its sequelae: from intensive care to surgery and rehabilitation. J Bras Pneumol 2019; 45:e20180324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva, Switzerland: WHO; 2019:1–104. Available at: https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1. [PubMed] [Google Scholar]
- 4. Akkerman O, Aleksa A, Alffenaar JW, et al. ; Members of the International Study Group on New Anti-Tuberculosis Drugs and Adverse Events Monitoring Surveillance of adverse events in the treatment of drug-resistant tuberculosis: a global feasibility study. Int J Infect Dis 2019; 83:72–6. [DOI] [PubMed] [Google Scholar]
- 5. Silva DR, Rendon A, Alffenaar JW, et al. Global TB Network: working together to eliminate tuberculosis. J Bras Pneumol 2018; 44:347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rendon A, Silva DR, Esposito S, Sotgiu G, Migliori GB, Chakaya JM. Launching the Global TB Network. Int J Tuberc Lung Dis 2019; 23:123–4. [DOI] [PubMed] [Google Scholar]
- 7. Rancoita PM V, Cugnata F, Gibertoni Cruz AL, et al. Validating a 14-drug microtiter plate containing bedaquiline and delamanid for large-scale research susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2018;62 pii: e00344–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood N, Pasipanodya JG, Denti P, et al. Concentration-dependent antagonism and culture conversion in pulmonary tuberculosis. Clin Infect Dis 2017; 64:1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasipanodya JG, Smythe W, Merle CS, et al. Artificial intelligence-derived 3-way concentration-dependent antagonism of gatifloxacin, pyrazinamide, and rifampicin during treatment of pulmonary tuberculosis. Clin Infect Dis 2018; 67:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chigutsa E, Pasipanodya JG, Visser ME, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deshpande D, Pasipanodya JG, Srivastava S, et al. Gatifloxacin pharmacokinetics/pharmacodynamics-based optimal dosing for pulmonary and meningeal multidrug-resistant tuberculosis. Clin Infect Dis 2018;67(suppl_3):S274–S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deshpande D, Pasipanodya JG, Mpagama SG, et al. Levofloxacin pharmacokinetics/pharmacodynamics, dosing, susceptibility breakpoints, and artificial intelligence in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande D, Pasipanodya JG, Mpagama SG, et al. Ethionamide pharmacokinetics/pharmacodynamics-derived dose, the role of MICs in clinical outcome, and the resistance arrow of time in multidrug-resistant tuberculosis. Clin Infect Dis 2018;67(suppl_3):S317–S326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colangeli R, Jedrey H, Kim S, et al. ; DMID 01-009/Tuberculosis Trials Consortium Study 22 Teams Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med 2018; 379:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swaminathan S, Pasipanodya JG, Ramachandran G, et al. drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dheda K, Lenders L, Magombedze G, et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am J Respir Crit Care Med 2018; 198:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srivastava S, Pasipanodya JG, Ramachandran G, et al. A long-term co-perfused disseminated tuberculosis-3D liver hollow fiber model for both drug efficacy and hepatotoxicity in babies. EBioMedicine 2016; 6:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Modongo C, Pasipanodya JG, Magazi BT, et al. Artificial intelligence and amikacin exposures predictive of outcomes in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother 2016; 60:5928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 2015; 61(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]
- 21. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 22. Gumbo T, Alffenaar JC. Pharmacokinetic/pharmacodynamic background and methods and scientific evidence base for dosing of second-line tuberculosis drugs. Clin Infect Dis 2018; 67:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 2010; 54:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis 2010; 201:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 2009; 53:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190:1642–51. Available at: http://jid.oxfordjournals.org/content/190/9/1642.abstract. [DOI] [PubMed] [Google Scholar]
- 27. Srivastava S, Magombedze G, Koeuth T, et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother 2017;61 pii: e00751–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deshpande D, Alffenaar JWC, Köser CU, et al. D-cycloserine pharmacokinetics/pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis 2018;67(suppl_3):S308–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srivastava S, Modongo C, Siyambalapitiyage Dona CW, Pasipanodya JG, Deshpande D, Gumbo T. Amikacin optimal exposure targets in the hollow-fiber system model of tuberculosis. Antimicrob Agents Chemother 2016; 60:5922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schön T, Matuschek E, Mohamed S, et al. Standards for MIC testing that apply to the majority of bacterial pathogens should also be enforced for Mycobacterium tuberculosis complex. Clin Microbiol Infect 2019; 25:403–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williamson DA, Roberts SA, Bower JE, et al. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2012;16:216–20. [DOI] [PubMed] [Google Scholar]
- 32. Miotto P, Cabibbe AM, Borroni E, Degano M, Cirilloa DM. Role of disputed mutations in the rpoB gene in interpretation of automated liquid MGIT culture results for rifampin susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miotto P, Tessema B, Tagliani E, et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J 2017; 50 pii: 1701354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 2014; 58:6111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghodousi A, Tagliani E, Karunaratne E, et al. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype comprised of overlapping MIC distributions with different underlying resistance mechanisms. Antimicrob Agents Chemother 2019; 63 pii: e00092–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Technical Expert Group. Technical Expert Group Meeting Report: critical concentrations for drug susceptibility testing for TB medicines. Geneva: World Health Organization (WHO), 2017. [Google Scholar]
- 37. The CRyPTIC Consortium and the 100000 Genomes Project. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N Engl J Med 2018; 379:1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McIlleron H, Chirehwa MT. Current research toward optimizing dosing of first-line antituberculosis treatment. Expert Rev Anti Infect Ther 2019; 17:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pienaar E, Sarathy J, Prideaux B, et al. Comparing efficacies of moxifloxacin, levofloxacin and gatifloxacin in tuberculosis granulomas using a multi-scale systems pharmacology approach. PLoS Comput Biol 2017; 13:e1005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Burgt EP, Sturkenboom MG, Bolhuis MS, et al. End TB with precision treatment! Eur Respir J 2016; 47:680–2. [DOI] [PubMed] [Google Scholar]
- 41. Alkabab Y, Keller S, Dodge D, Houpt E, Staley D, Heysell S. Early interventions for diabetes related tuberculosis associate with hastened sputum microbiological clearance in Virginia, USA. BMC Infect Dis 2017; 17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alffenaar J-WC, Heysell SK, Mpagama SG. Therapeutic drug monitoring: the need for practical guidance. Clin Infect Dis 2018; 68:1065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nicholson T, Admay C, Shakow A, Keshavjee S. Double standards in global health: medicine, human rights law and multidrug-resistant TB treatment policy. Health Hum Rights 2016; 18:85–102. [PMC free article] [PubMed] [Google Scholar]
- 44. Alffenaar JWC. Therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Infect Dis 2017; 64:104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghimire S, Bolhuis MS, Sturkenboom MGG, et al. Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur Respir J 2016; 47:1867–9. [DOI] [PubMed] [Google Scholar]
- 46. van den Elsen SHJ, Oostenbrink LM, Heysell SK, et al. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther Drug Monit 2018; 40:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alffenaar J-WC, Sintchenko V, Marais BJ. Acquired drug resistance: recognizing potential of repurposed drugs. Clin Infect Dis 2019. pii: ciz334. [DOI] [PubMed] [Google Scholar]