Abstract
Background
Effective concentrations of antiretrovirals in the female genital tract (FGT) are critical for suppression of viral shedding or effective preexposure prophylaxis. The disposition of tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate (FTC-TP) in the FGT have been previously described. Despite widespread use, however, lamivudine triphosphate (3TC-TP) exposure in the FGT is unknown. Depot medroxyprogesterone acetate (DMPA) and vaginal dysbiosis have been implicated in increased risk of human immunodeficiency virus (HIV) acquisition, but whether they alter TFV-DP or 3TC-TP exposure, and therefore compromise prevention efficacy, is unknown.
Methods
Fifty premenopausal women living with HIV in Kampala, Uganda, and receiving daily tenofovir disoproxil fumarate/lamivudine were recruited. Ectocervical biopsies were obtained for quantification of TFV-DP and 3TC-TP using liquid chromatography–mass spectrometry. 16S ribosomal RNA gene sequencing was performed on DNA extracted from vaginal swabs. Wilcoxon rank-sum was used to test for differences between contraceptive groups.
Results
3TC-TP concentrations were on average 17-fold greater than TFV-DP concentrations in cervical tissues. TFV-DP concentrations in cervical biopsies were 76% greater in DMPA users compared with women using nonhormonal contraception (n = 23 per group). Abundance of Lactobacillus in vaginal swabs was correlated with 3TC-TP concentrations in cervical tissues.
Conclusions
We found that TFV-DP concentrations were significantly greater in DMPA users compared with women using nonhormonal contraception, suggesting that prevention efficacy is unlikely to be compromised by DMPA use. Similar to reports of FTC-TP, 3TC-TP exposure was significantly greater than TFV-DP in cervical tissue and was correlated with abundance of Lactobacillus. These data support lamivudine as an option for preexposure prophylaxis.
Clinical Trials Registration
Keywords: PrEP, tenofovir, lamivudine, microbiome, HIV, DMPA
Tenofovir diphosphate (TFV-DP) and lamivudine triphosphate (3TC-TP) concentrations were quantified in 50 Ugandan women living with human immunodeficiency virus. TFV-DP concentrations were higher in cervical tissue in women using depot medroxyprogesterone, and 3TC-TP concentrations were correlated with Lactobacillus relative abundance.
Antiretroviral exposure in the female genital tract (FGT) is critical for suppression of viral shedding or, in the case of preexposure prophylaxis (PrEP), human immunodeficiency virus (HIV) prevention. Tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate (FTC-TP) disposition in the FGT has been previously described, with comparatively low TFV-DP and high FTC-TP concentrations following oral dosing [1, 2]. However, despite widespread use, lamivudine triphosphate (3TC-TP) exposure in FGT is unknown. Several countries have endorsed lamivudine (3TC) (in combination with tenofovir disoproxil fumarate [TDF]) for PrEP; therefore, knowledge of exposure in FGT is warranted [3].
Depot medroxyprogesterone acetate (DMPA) is the most popular contraceptive method in sub-Saharan Africa [4]. Given the high HIV prevalence in this region, coadministration with PrEP is likely. Additionally, DMPA has been associated with increased risk of HIV infection [5]. In vitro, coincubation of CD4+ T cells with medroxyprogesterone acetate decreased TFV-DP concentrations and protection from HIV infection [6]. Therefore, we sought to determine whether DMPA decreased TFV-DP FGT exposure, potentially compromising PrEP efficacy.
Vaginal dysbiosis has also been associated with increased HIV risk [7]. A retrospective analysis from the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 study found different protection from HIV 1% TFV gel based on Lactobacillus status [8]. While this suggests the vaginal microbiome can affect the efficacy of topically applied medication, the effect when orally administered is less clear. A retrospective analysis of the Partners PrEP study did not find significant differences in efficacy based on Nugent score or Gardnerella, Bacteroides, or Lactobacillus abundance [9]. However, overall adherence to PrEP in the Partners PrEP study was high, and changes in drug exposure may not have been significant enough to put women at risk of drug failure. Donahue et al examined cervicovaginal fluid-to-plasma ratios of antiretroviral drugs and found a bimodal relationship between TFV penetration and α-diversity [10]. Few studies have measured active metabolite concentrations. We therefore sought to quantify the effect of the vaginal microbiome on intracellular active metabolite concentrations following oral dosing.
METHODS
Study Population
Procedures for this study (NCT03377608) were reviewed and approved by the Research Ethics Committee of the Uganda Virus Research Institute, the Human Research Ethics Committee of the University of Witwatersrand, and the Uganda National Council of Science and Technology. Women enrolled in the BONE: Contraception and Anti-Retroviral Effects (BONE-CARE) study (a study examining cumulative effects of HIV infection, TFV use, and DMPA use on bone health [11]) were offered participation in this substudy if they were stable on their TDF-containing regimen and virally suppressed (plasma HIV RNA <50 copies/mL) for ≥6 months. Exclusion criteria included pregnancy, breastfeeding, symptomatic vaginal infection, abnormal vaginal bleeding, history of genital dysplasia or HPV, or use of oral/vaginal antibiotics or antifungals within 30 days (exception made for sulfamethoxazole-trimethoprim). Written informed consent was obtained prior to study participation.
All sampling took place at a single visit. A brief pelvic examination was performed to assess for gross abnormalities. Chlamydia trachomatis and Neisseria gonorrhoeae were detected in urine using the Becton Dickinson ProbeTec ET CT/GC Amplified DNA assay. Syphilis was tested on blood using rapid plasma reagin. Urine was collected for a point-of-care pregnancy human chorionic gonadotropin test. Vaginal swabs were collected on polyester swabs. Two cervical biopsies were obtained using Baby Tishcler Biopsy forceps (McKesson) from the outer third of the upper left and right quadrant of the cervix and snap-frozen in liquid nitrogen. Blood was collected into ethylenediaminetetraacetic acid tubes and centrifuged for plasma separation. Additional blood was collected into BD Vacutainer CPT tubes with sodium citrate, and peripheral blood mononuclear cells (PBMCs) were isolated according to protocol. Cells were lysed in 70:30 methanol:water.
Antiretroviral Quantification
Tenofovir and 3TC were quantified in plasma using a validated assay with a 1 ng/mL lower limit of quantification. In tissue biopsies and PBMCs, TFV-DP, 3TC-TP, deoxyadenosine triphosphate (dATP), and deoxycytidine triphosphate (dCTP) were quantified. Samples were extracted by protein precipitation. Isotopically labeled internal standards were used for all analytes except for 3TC-TP, which used isotopically labeled TFV-DP as its internal standard. In PBMCs, the dynamic range of assays was 0.02–100 ng/mL for dATP and TFV-DP, 0.2–600 ng/mL for 3TC-TP, and 0.06–100 ng/mL for dCTP. In tissue homogenate, the dynamic range was 0.02–20 ng/mL. Calibration standards and quality control samples met acceptance criteria of ±15% of nominal concentrations for plasma and PBMCs and ±20% for tissue.
Estradiol and Progesterone Quantification
Progesterone and estradiol were measured by a microplate enzyme immunoassay using reagents from Arbor Assays (Ann Arbor, Michigan). Prior to quantitation, hormones were extracted with hexane–ethyl acetate to remove water-soluble conjugated metabolites. Assay sensitivities for progesterone and estradiol are 0.07 ng/mL and 5 pg/mL, respectively, and interassay coefficients of variation for both assays are <10%.
Microbiome Sequencing
DNA was extracted from vaginal swabs and fluid, approximately 450 µL, using the DNeasy PowerSoil kit (Qiagen, Hilden, Germany) on the automated QIAcube platform. The V4 hypervariable region of the 16S ribosomal RNA (rRNA) gene was amplified and paired-end sequenced (300 nucleotides) on the Illumina MiSeq platform (Illumina, San Diego, California) using the 515F/806R primer set [12]. Sequence data were processed in “mothur” [13], as described previously [14]. In brief, sequences were pair-end joined and screened for quality. Operational taxonomic units (97% similarity) were classified against the Ribosomal Database Project version 16 release [15].
For statistical comparisons, samples were rarefied by random subsample to approximately 3000 sequence reads per sample. The α- and β-diversity indices were calculated as Shannon index [16] and Bray-Curtis distance [17].
Statistical Analysis
The primary study question was the effect of DMPA on TFV-DP and 3TC-TP cervical concentrations. Secondary analyses explored associations between the vaginal microbiome and drug concentrations. Data are presented as median (interquartile range [IQR]) unless otherwise noted. Statistical analyses were performed using XLSTAT version 17.06 (Addinsoft, Belmont, Massachusetts) or SAS version 9.4 (SAS Institute, Cary, North Carolina) software. Bivariate analyses were performed using Wilcoxon rank-sum test or Spearman correlation test. Differences in community composition were evaluated using analysis of similarity (ANOSIM) of Bray-Curtis distances [18], and taxa that showed significant differences in relative abundance between groups were determined by linear discriminant analysis of effect size [19]. All statistics were evaluated at α = .05 with Bonferroni correction for multiple comparisons when appropriate.
To identify significant predictors of drug concentrations, multivariable linear regression was performed on log-transformed data using stepwise regression. Significance criteria for entry into the model and stay criteria were set at α = .15. Model criterion was evaluated on best adjusted r2 value.
RESULTS
Subject Characteristics
Fifty women were enrolled between November 2017 and March 2018. Twenty-five were receiving DMPA, 12 were using a copper intrauterine device (Cu-IUD), and 13 were using condoms as their primary contraceptive method. Characteristics of study participants at the time of sampling are reported in Table 1. Women <25 years old comprised 36% and 32% of DMPA and nonhormonal groups, respectively. All participants had been receiving TDF/3TC 300 mg/300 mg daily for at least 30 weeks. One participant on DMPA was receiving boosted 300 mg atazanavir, whereas the other 49 women were receiving 600 mg efavirenz. All participants were receiving daily sulfamethoxazole-trimethoprim 800 mg/160 mg.
Table 1.
Subject Characteristics
| Characteristic | Nonhormonala (n = 25) | DMPA (n = 25) |
|---|---|---|
| Age, y | 26.0 (23.9–30.7) | 26.4 (24.6–29.9) |
| Weight, kg | 59.1 (53.2–66.0) | 61.0 (55.8–66) |
| BMI, kg/m2 | 22.9 (21.3–25.0) | 24.8 (22.1–27.5) |
| Years since HIV diagnosis | 1.2 (1.1–1.7) | 1.6 (1.2–2.0) |
| Years on current ART regimen | 1.0 (0.9–1.2) | 1.0 (0.8–1.2) |
| Hours since last ART doseb | 17.2 (15.8–18.6) | 16.7 (15.0–17.5) |
| Months on contraceptive method | 20 (12–36) | 15 (12–48) |
| Days since last DMPA injection | NA | 34 (12–51) |
| Days since last menstrual periodc | 22 (13–29) | 19 (16–25) |
| Estradiol, pg/mL | 87.2 (63.8–125.9) | 27.3 (16.1 (39.3) |
| Progesterone, ng/mL | 5.5 (0.2–11.1) | 0.4 (0.2–0.5) |
| Sexually active, no. (%) | 23 (92) | 24 (96) |
| Gonorrhea, no. (%) | 2 (8)d | 2(8) |
| Chlamydia, no. (%) | 0 (0) | 0 (0) |
| Syphilise, no. (%) | 3 (12)d | 0 (0) |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; DMPA, depot medroxyprogesterone acetate; HIV, human immunodeficiency virus; NA, not applicable.
aWithin nonhormonal group, 12 of 25 (48%) of women were using copper intrauterine device.
bNinety-two percent of samples were collected between 12 and 20 hours postdose.
cReported for menstruating women only. Amenorrhea, a known and expected effect of DMPA use, was reported in 15 of 25 (60%) of DMPA users and 0 of 25 (0%) in the nonhormonal group.
dOne woman in the nonhormonal group tested positive for both gonorrhea and syphilis.
eTwo women had titers of 1:4; 1 woman had titer 1:16.
The majority (94%) of participants reported being sexually active. Thirty percent reported being married or in a monogamous relationship, while the remainder reported casual partner (60%), separated (6%), or single (4%).
Adherence
Three subjects, 1 on DMPA and 2 in the nonhormonal group, had plasma concentrations <1 ng/mL for both TFV and 3TC, suggesting no dose within the past 3–5 days. These subjects were excluded from further antiretroviral quantification. In the remaining women, TFV and 3TC plasma exposures were 66.4 (IQR, 51.3–83.5) ng/mL and 190.0 (IQR, 116–267) ng/mL, respectively, consistent with steady-state daily dosing [20, 21], and were not significantly different by contraceptive group.
Intracellular Metabolites and Endogenous Nucleotides in PBMCs
In PBMCs, TFV-DP, 3TC-TP, dATP, and dCTP did not differ by contraceptive group (Supplementary Table 1). Correlation coefficients and P values of correlation tests between analytes are reported in Supplementary Table 2. All 4 PBMC analytes were significantly correlated with each other, and TFV-DP and 3TC-TP were significantly correlated with respective plasma concentrations. There was no correlation between TFV-DP or 3TC-TP concentrations in PBMCs vs tissue. PBMC concentrations were not correlated with age (TFV-DP: P = .316; 3TC-TP: P = .611) or body mass index (BMI) (TFV-DP: P = .469; 3TC-TP: P = .544).
Endogenous hormones were not correlated with drug analytes or endogenous nucleotides; however, progesterone was positively correlated with the 3TC-TP to dCTP ratio in cells (r = 0.33, P = .027).
Intracellular Metabolites and Endogenous Nucleotides in Cervical Tissue
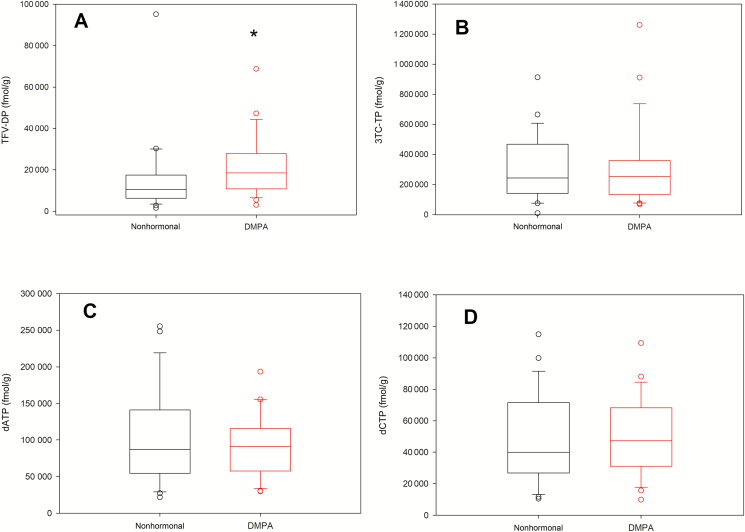
Measured concentrations of intracellular metabolites and their endogenous nucleotide analogues in cervical tissue are shown in Figure 1 and summarized in Supplementary Table 1. All tissue analytes were significantly correlated with each other (Supplementary Table 2). Median 3TC-TP concentrations were 17-fold higher than TFV-DP concentrations. Median TFV-DP was 76% higher in DMPA users compared with the nonhormonal group (P = .035). No differences were found in 3TC-TP, dATP, or dCTP between groups (Supplementary Table 1. Notably, TFV-DP and 3TC-TP in cervical tissue were more closely correlated with respective plasma concentrations of TFV or 3TC than with TFV-DP or 3TC-TP in PBMCs (Supplementary Table 2).
Figure 1.
Concentrations of intracellular metabolites and endogenous nucleotides in cervical tissue. A–D, Box plots showing distribution of analytes measured in cervical homogenates by contraceptive group: tenofovir diphosphate (A), lamivudine triphosphate (B), deoxyadenosine triphosphate (C), and deoxycytidine triphosphate (D). Abbreviations: 3TC-TP, lamivudine triphosphate; dATP, deoxyadenosine triphosphate; dCTP, deoxycytidine triphosphate; DMPA, depot medroxyprogesterone acetate; TFV-DP, tenofovir diphosphate. *P < .05, Wilcoxon rank-sum test.
Age was inversely correlated with 3TC-TP and dCTP in cervical tissues (3TC-TP: r = –0.41, P = .005; dCTP: r = –0.35, P = .014). Neither TFV-DP nor dATP in tissue was correlated with age (TFV-DP: r = –0.23, P = .13; dATP: r = –0.23, P = .11). However, there was a moderate inverse correlation between age and tissue TFV-DP within the nonhormonal group (r = –0.56, P = .006). BMI was not correlated with any analyte in tissue (TFV-DP: P = .608; 3TC-TP: P = .506; dATP: P = .105; dCTP: P = .142). Endogenous estradiol and progesterone at time of sample collection was not correlated with any tissue analyte (Supplementary Table 1); however, estradiol was negatively correlated with the TFV-DP to dATP ratio in cervical tissue (r = –0.33, P = .024). There was no correlation between the TFV-DP to dATP ratio and estradiol within contraceptive groups, suggesting that estradiol concentrations did not explain any greater variability than DMPA use.
No women reported history of any sexually transmitted infection; however, 6 women tested positive for gonorrhea and/or syphilis on the day of sampling (Table 1). Sensitivity analyses performed excluding these 6 subjects with sexually transmitted infections did not affect overall results or significance.
Microbiome and Effect on Drug Concentrations
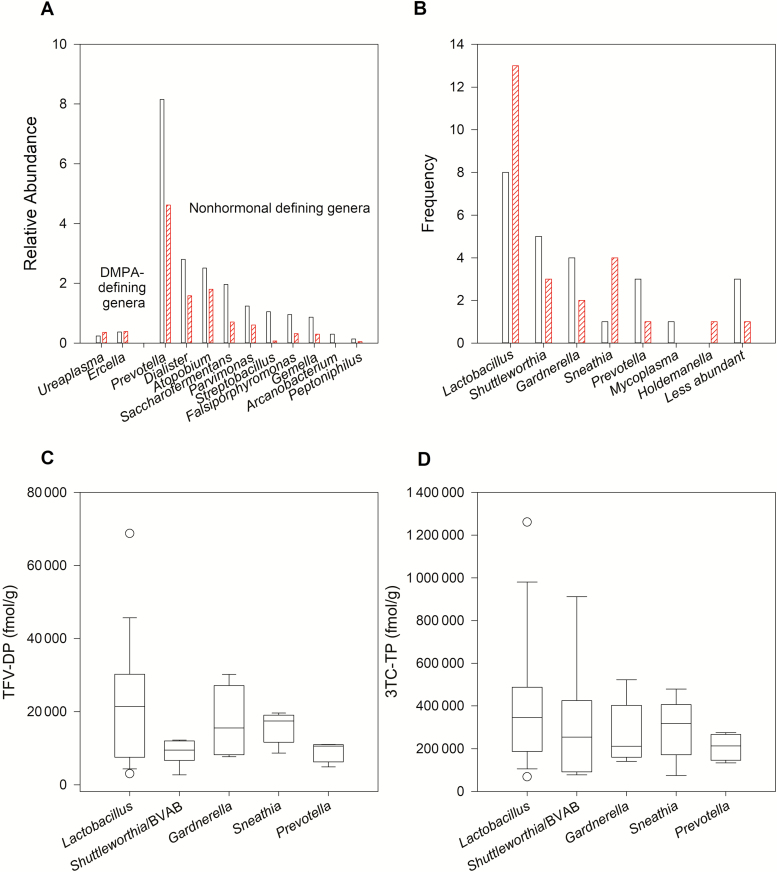
Women receiving DMPA had lower bacterial α-diversity compared to the nonhormonal group (mean Shannon diversity: 1.36 vs 2.00, P = .035). The relative abundance of Lactobacillus was inversely correlated with age (r = –0.31, P = .027) but did not differ between groups. The α-diversity in the vaginal microbiome was negatively correlated with the TFV-DP to dATP ratio in cervical tissue (r = –0.40, P = .006) but not with TFV-DP concentrations (r = –0.22, P = .13). 3TC-TP and dCTP concentrations in cervical tissue were correlated with relative abundance of Lactobacillus (r = 0.32, P = .033 and r = 0.30, P = .044, respectively). No vaginal genera were significantly correlated with cervical TFV-DP or dATP. TFV-DP was inversely correlated with Shuttleworthia (or bacterial vaginosis–associated bacteria) abundances within DMPA users (r = –0.43, P = .040) but not within nonhormonal contraceptive users (r = –0.14, P = .5). Endogenous hormones were not correlated with α-diversity or abundance of any genera.
Linear discriminant analysis of effect size identified 12 genera differentially abundant between groups (Figure 2A). Of those, only Prevotella, Atopobium, Dialister, Parvimonas, and Saccharofermentans comprised >1% of the relative abundance, and all were >1000-fold greater in the nonhormonal group compared with the DMPA group. Among the top 5 most prevalent genera (Figure 2B), TFV-DP and 3TC-TP concentrations were consistently highest when Lactobacillus was the most dominant genera (Figure 2C and 2D).
Figure 2.
Vaginal microbiota and cervical drug concentrations. A, Relative abundance of the 12 genera that separated the depot medroxyprogesterone acetate (DMPA) users (hashed bars) from the nonhormonal (clear bars) group in linear discriminant analysis of effect size. The nonhormonal group was defined by greater abundance of potentially pathogenic bacteria associated with bacterial vaginosis. B, Number of women with each of the most prevalent genera identified, shown for nonhormonal contraception users (clear bars) and DMPA users (hashed bars). C and D, Cervical concentrations of tenofovir diphosphate (C) and lamivudine triphosphate (D) by the most prevalent genera. Abbreviations: 3TC-TP, lamivudine triphosphate; BVAB, bacterial vaginosis–associated bacteria; DMPA, depot medroxyprogesterone acetate; TFV-DP, tenofovir diphosphate.
Drug Exposure in Copper IUD Users
When comparing tissue analytes between condom users, Cu-IUD users, and DMPA users, the 3 groups had different TFV-DP concentrations (P = .029, Kruskal-Wallis test). In pairwise comparisons, TFV-DP was not significantly different between condom users and DMPA users (median, 14 570 vs 18 477 fmol/g, respectively; P = .418) but was significantly lower in Cu-IUD users (median, 9375; vs DMPA, P = .007; vs condom, P = .094). 3TC-TP, dATP, and dCTP were not significantly different in 3-way or pairwise comparisons based on contraceptive use.
Vaginal microbiomes did not significantly differ between condom and Cu-IUD users based on α-diversity (1.92 vs 2.08, P = .424) or ANOSIM (P = .737). However, Fusobacterium and Prevotella were significantly more abundant in Cu-IUD users in linear discriminant analysis, whereas condom users were defined by Arcanobacterium, Peptoniphilus, and Saccharofermentans.
Multivariable Regression to Identify Predictors of Drug Exposure in Cervical Tissue
In multivariable analysis, DMPA use, age, and TFV plasma concentrations were identified as significant in the best model to predict cervical TFV-DP concentrations. Age, 3TC plasma concentrations, and progesterone concentrations were significant predictors of cervical 3TC-TP. Other parameters tested in models included BMI, estradiol concentrations, α-diversity, and Lactobacillus abundance.
DISCUSSION
DMPA use has been associated with increased risk of HIV acquisition [5]; therefore, understanding PrEP efficacy in DMPA users is warranted. This study compared intracellular metabolites of TFV and 3TC following oral dosing, and their endogenous nucleotide analogues, in PBMCs and cervical tissue in women receiving DMPA compared to women using nonhormonal contraceptive methods. TFV-DP concentrations in cervical tissues were 76% greater in DMPA users, whereas 3TC-TP, dATP, and dCTP were comparable between groups. Our findings suggest that DMPA is unlikely to compromise efficacy of oral TFV-based PrEP. This is consistent with a retrospective analysis of the Partners PrEP study, which found similar HIV protection in DMPA users compared with users of nonhormonal contraception [22]. Our findings are also consistent with a recent report of increased vaginal TFV-DP with DMPA use in women using 1% TFV gel [23]. In our study, TFV-DP exposure in PBMCs was lower in DMPA users, suggesting a mechanism unique to the FGT. In one study, estradiol and progesterone increased TFV-DP formation in CD4+ cells isolated from the FGT, but had no effect on CD4+ cells isolated from blood [24]. As DMPA use dramatically reduces endogenous levels of estrogen and progesterone, these in vitro findings are consistent with our observations reported here. Our findings on DMPA were specific to TFV-DP and not 3TC-TP, which may be related to different transport and phosphorylation pathways of TFV vs 3TC [25, 26].
Overall, TFV-DP cervical concentrations in our study were about 2-fold higher than concentrations after a single dose of TDF, a difference consistent with accumulation from first dose to steady state [27]. Compared to concentrations previously reported in HIV-negative US women, cervical dATP in our study were slightly higher but within range of previous reports, whereas dCTP concentrations in our study were consistently approximately 10-fold lower [27], perhaps due to population differences.
These data are the first to describe 3TC-TP exposure in the FGT. Although efficacy of 3TC for PrEP has never been formally tested, several countries have recommended TDF/3TC for PrEP due to its wider availability and cost-benefit compared to TDF/emtricitabine [3]. Similar to previous reports for FTC-TP [1, 2], 3TC-TP exposure in cervical tissues was more than a log higher than TFV-DP when comparing molar concentrations. Although 3TC-TP concentrations did not differ by contraceptive method, our multivariable regression did identify an inverse relationship between 3TC-TP in cervical tissues and progesterone concentrations in plasma. Although we did not observe an effect on PBMCs, Anderson et al previously reported that in vitro, progesterone decreases 3TC-TP formation in PBMC by 22% [28]. Overall, the data generated here provide a pharmacologic rationale for 3TC as a PrEP agent.
Increasing age was associated with decreased exposure of drug metabolites and endogenous nucleotides, an effect specific to FGT as there was no correlation between age and PBMC drug concentrations. Our group previously found significantly lower ex vivo conversion of TFV and emtricitabine to their phosphorylated metabolites in cervical tissue from postmenopausal women [29]. The mechanism is unknown but is unlikely to be hormone related as the women in our current study were all premenopausal, and age remained a significant predictor of drug concentrations even after adjusting for hormone use as a covariate. Future studies with an expanded age range that account for other physiologic variables associated with aging, such as inflammation response and cervical ectopy, will be required to elucidate this mechanism.
We observed decreased microbial diversity in DMPA users. This is consistent with reports that DMPA either has a neutral or protective effect against bacterial vaginosis [30, 31]. Whether this is due to progestin regulation or to decreased menses associated with DMPA use is not clear. Regardless, as diversity in the vaginal microbiome increases, so does the abundance of potentially proinflammatory taxa. In vitro, Klatt et al found that the anaerobic genera Gardnerella, Prevotella, and Mobiluncus metabolize TFV to an inactive metabolite, adenine, whereas Lactobacillus has minimal effect [8]. Although our observational study can only identify correlations and not causation, decreased diversity in the vaginal microbiome and reduced abundance of proinflammatory taxa could be a potential mechanism of greater FGT drug exposure with DMPA use.
Cu-IUD users had the lowest TFV-DP concentrations compared with DMPA or condom users. Overall, vaginal microbial diversity in Cu-IUD users was not significantly different than condom users; however, there was increased Prevotella and Fusobacterium abundance in the Cu-IUD users. This is consistent with a recent study that followed 48 women initiating Cu-IUD contraception and found increased bacterial vaginosis prevalence as well as increased Gardnerella vaginalis and Atopobium vaginae concentrations [30]. These data further support the rationale of vaginal dysbiosis regulating drug exposure in the FGT.
There are limitations to the interpretation of our study. First, we could not control for menstrual cycle in menstruating women. However, previous studies found that menstrual cycle did not affect drug exposure in cervicovaginal fluid [32]. Furthermore, Thurman et al found with topical dosing that while vaginal TFV was increased in the luteal phase, TFV-DP was similar across the menstrual cycle [23]. Menses can affect the vaginal microbiome, but changes are most predominant during active bleeding, which none of the women in our study were experiencing at time of sample collection. In addition, we did not find any differences in the vaginal microbiome between women reporting amenorrhea and those with normal cycles. Second, use of 16S rRNA gene sequencing limited our interpretation of the vaginal microbiome. Using this approach, we could only determine relative and not absolute abundance, which may have limited our ability to identify correlations between drug concentrations and specific bacterial taxa. Due to short sequence reads, we were only able to reliably identify bacteria at the genus and not species level [33], and we could not account for fungal species, which may also have an impact on drug metabolism and may also have been associated with Cu-IUD use. Last, to avoid confounding results, we excluded women with factors that may also influence the vaginal microbiome (eg, antibiotic use or symptomatic vaginal infection). This may have led to vaginal microbiota profiles that are not generalizable to the general population of female PrEP users.
CONCLUSIONS
These data provide pharmacokinetic support that DMPA is unlikely to compromise TFV-based PrEP efficacy. These data fill a critical gap by describing exposure of the active metabolite of 3TC in the FGT, providing reassurance in 3TC use for PrEP. We also present a plausible mechanism by which DMPA increases concentrations of genital metabolites in mucosal tissues via decreased diversity in the vaginal microbiome.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the following members of the Bone: Contraception and Anti-retroviral Effects protocol team for review of the study proposal: Noah Kiwanuka, MBChB, MPH, PhD; Philippa Musoke, MBChB, FRCP, PhD; and John Pettifor, MD, PhD. Sequence processing and analysis was done using the resources of the Minnesota Supercomputing Institute. Drug concentrations were measured at the University of North Carolina Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Laboratory. Progesterone and estradiol concentrations were measured at the University of Southern California Reproductive Endocrine Research Laboratory.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant numbers K08 AI134262 to M. R. N. and R01 AI118332 to F. M. K.); the University of Minnesota Deborah Powell Women’s Center; the University of Minnesota Academic Health Center; and the University of Minnesota College of Pharmacy.
Potential conflicts of interest. T. T. B. has served as a consultant to ViiV Healthcare, Gilead Sciences, Merck, Theratechnologies, and EMD-Serono. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seifert SM, Chen X, Meditz AL, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses 2016; 32:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodges-Mameletzis I, Dalal S, Msimanga-Radebe B, Rodolph M, Baggaley R. Going global: the adoption of the World Health Organization’s enabling recommendation on oral pre-exposure prophylaxis for HIV. Sex Health 2018; 15:489–500. [DOI] [PubMed] [Google Scholar]
- 4. Tsui AO, Brown W, Li Q. Contraceptive practice in sub-Saharan Africa. Popul Dev Rev 2017; 43:166–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS 2016; 30:2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen Z, Rodriguez-Garcia M, Patel MV, Bodwell J, Kashuba ADM, Wira CR. Hormonal contraceptives differentially suppress TFV and TAF inhibition of HIV infection and TFV-DP in blood and genital tract CD4+ T cells. Sci Rep 2017; 7:17697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018; 18:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356:938–45. [DOI] [PubMed] [Google Scholar]
- 9. Heffron R, McClelland RS, Balkus JE, et al. Partners PrEP Study Team Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study. Lancet HIV 2017; 4:e449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donahue Carlson R, Sheth AN, Read TD, et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J Infect Dis 2017; 216:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiweewa FM, Kiwanuka N, Scholes D, et al. Bone mineral density in a cohort of young adult women using Depo-Provera and tenofovir, Kampala, Uganda. In: 19th International Workshop on Adverse Drug Reactions and Co-morbidities in HIV, Milan, Italy, 23–25 October 2017. Available at: http://www.natap.org/2017/AdverseReactComor/AdverseReactComor_12.htm. Accessed 9 May 2019.
- 12. Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009; 75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staley C, Kaiser T, Vaughn BP, et al. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome 2018; 6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 2009; 37:D141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shannon CE, Weaver W. The mathematical theory of communication. Urbana: University of Illinois Press, 1949. [Google Scholar]
- 17. Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 1957; 27:325–49. [Google Scholar]
- 18. Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 1993; 18:117–43. [Google Scholar]
- 19. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43:595–612. [DOI] [PubMed] [Google Scholar]
- 21. Yuen GJ, Lou Y, Bumgarner NF, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother 2004; 48:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heffron R, Mugo N, Were E, et al. Partners PrEP Study Team Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS 2014; 28:2771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thurman AR, Schwartz JL, Brache V, et al. Effect of hormonal contraception on pharmacokinetics of vaginal tenofovir in healthy women: increased tenofovir diphosphate in injectable depot medroxyprogesterone acetate users. J Acquir Immune Defic Syndr 2019; 80:79–88. [DOI] [PubMed] [Google Scholar]
- 24. Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Kashuba AD, Wira CR. Sex hormones regulate tenofovir-diphosphate in female reproductive tract cells in culture. PLoS One 2014; 9:e100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pastor-Anglada M, Cano-Soldado P, Molina-Arcas M, et al. Cell entry and export of nucleoside analogues. Virus Res 2005; 107:151–64. [DOI] [PubMed] [Google Scholar]
- 26. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson PL, King T, Zheng JH, MaWhinney S. Cytokine and sex hormone effects on zidovudine- and lamivudine-triphosphate concentrations in vitro. J Antimicrob Chemother 2008; 62:738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicol MR, Brewers LM, Kashuba ADM, Sykes C. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS 2018; 32:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218:622.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013; 27(Suppl 1):S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheth AN, Evans-Strickfaden T, Haaland R, et al. HIV-1 genital shedding is suppressed in the setting of high genital antiretroviral drug concentrations throughout the menstrual cycle. J Infect Dis 2014; 210:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edgar RC. Accuracy of taxonomy prediction for 16S rRNA and fungal ITS sequences. PeerJ 2018; 6:e4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.