Abstract
Background
Tuberculosis preventive therapy (TPT) is highly effective at preventing tuberculosis disease in household child contacts (<5 years), but is poorly implemented worldwide. In 2006, the World Health Organization recommended symptom-based screening as a replacement for tuberculin skin testing (TST) to simplify contact evaluation and improve implementation. We aimed to determine the effectiveness of this recommendation.
Methods
We conducted a pragmatic, cluster-randomized trial to determine whether contact evaluation using symptom screening improved the proportion of identified child contacts who initiated TPT, compared to TST-based screening, in Matlosana, South Africa. We randomized 16 clinics to either symptom-based or TST-based contact evaluations. Outcome data were abstracted from customized child contact management files.
Results
Contact tracing identified 550 and 467 child contacts in the symptom and TST arms, respectively (0.39 vs 0.32 per case, respectively; P = .27). There was no significant difference by arm in the adjusted proportion of identified child contacts who were screened (52% in symptom arm vs 60% in TST arm; P = .39). The adjusted proportion of identified child contacts who initiated TPT or tuberculosis treatment was 51.5% in the symptom clinics and 57.1% in the TST clinics (difference −5.6%, 95% confidence interval −23.7 to 12.6; P = .52). Based on the district’s historic average of 0.7 child contacts per index case, 14% and 15% of child contacts completed 6 months of TPT in the symptom and TST arms, respectively (P = .89).
Conclusions
Symptom-based screening did not improve the proportion of identified child contacts evaluated or initiated on TPT, compared to TST-based screening. Further research is needed to identify bottlenecks and evaluate interventions to ensure all child contacts receive TPT.
Clinical Trials Registration
Keywords: tuberculosis, pediatric, symptom-screening, tuberculin skin test, tuberculosis preventive therapy
This randomized controlled trial evaluated the effectiveness of the World Health Organization’s 2006 recommendation for a symptom-based screening on the uptake of tuberculosis preventive therapy (TPT) among child tuberculosis contacts. Despite this simplified evaluation process, TPT uptake remains poor.
Tuberculosis (TB) remains a leading cause of child mortality worldwide, with over 233 000 child deaths in 2017 [1, 2]. Children less than 5 years of age are particularly vulnerable, given their high risk of progression from infection to TB disease and the absence of a sensitive and specific diagnostic test for pediatric TB disease [3]. TB preventive therapy (TPT) reduces the risk of TB disease by more than 60% in children exposed to adults with TB, and has been a longstanding recommendation of the World Health Organization (WHO) for all household child contacts less than 5 years of age [4, 5]. This recommendation remains poorly implemented worldwide. In 2017, 23% of an estimated 1.3 million children less than 5 years of age with household exposure to Mycobacterium tuberculosis initiated TPT [1]. Modeling studies suggest improving contact investigation and implementation of TB preventive therapy in child contacts less than 5 years would prevent 83 000 (83%) child deaths and 65 000 (17%) TB cases among children less than 5 years annually [6].
In high-burden settings, screening is used to identify those children requiring a physician evaluation for TB disease. In these settings, all household child contacts less than 5 years of age should receive either TPT or TB treatment, without ascertaining their TB infection status. Historically, children were screened using tuberculin skin testing (TST), which required a follow-up visit for interpretation 2–3 days after placement. All TST-positive children, irrespective of symptoms, were then referred for chest X-rays and physician evaluations prior to TPT initiation. TST-negative children immediately initiated TPT. Frequent and global TST and chest X-ray stockouts and variable skills in clinical pediatric evaluations and X-ray interpretations are significant barriers to TPT initiation [7–9]. Caregivers view the time and monetary costs of evaluations as excessive when considering their otherwise well-appearing child [9, 10]. Prior studies have shown poor retention in care during the TST screening process [9, 11].
In 2006, the WHO recommended symptom-based screening of child contacts after several studies showed this strategy to be safe and effective in a research setting [7, 8, 12–14]. The symptoms screened for include poor weight gain, fever, cough, and fatigue or reduced playfulness [7, 8, 14]. When symptoms are absent, TPT can be safely initiated; when present, a physician evaluation is indicated. We hypothesized that implementing symptom-based screening would simplify child contact evaluations, allowing for fewer clinic visits and referrals to the district hospital and thereby increasing the proportion of child contacts initiating TPT.
While many countries have incorporated symptom screening into their guidelines [15], it remains poorly implemented [16]. There are limited data on the effectiveness of the symptom-based screening approach on TPT uptake. We conducted a pragmatic, cluster-randomized trial to measure the effectiveness of symptom-based screening on pediatric TPT uptake, when compared to TST-based screening, in a real-world setting.
METHODS
Study Population
We conducted a pragmatic, parallel, cluster-randomized trial in all 16 primary health clinics in the Matlosana subdistrict of North West Province, South Africa. Clinics were randomized to conduct child contact evaluations with either symptom-based or TST-based screening. All aspects of this evaluation were performed by clinic and/or hospital staff and recorded onto customized child contact management files. We included all children less than 5 years of age who were identified as a household contact (“child contact”) of a pulmonary and/or extrapulmonary TB index case that had been admitted to a participating clinic from October 2015 through February 2017. We excluded child contacts of index cases with documented rifampin resistance by Xpert MTB/RIF and/or isoniazid resistance by mycobacterial culture (Mycobacteria Growth Indicator Tube, MGIT, BD Diagnostics, Sparks, Maryland), as detected by line probe assays conducted by the South African National Health Laboratory Services.
Nurse Training and Program Implementation
Prior to the study initiation, a new child contact management file and register were designed with and tested among Matlosana nurses, to maximize their use. We trained all clinic TB staff on the use of the child contact management file and register; symptom assessment or TST placement and reading; contact tracing practices; and pediatric-specific counseling skills. Quarterly, a pediatrician provided ongoing mentorship, along with data and audit feedback on child contact identifications and linkage to care.
Child Contact Identification
Contact tracing was performed at the clinic, as currently recommended in South Africa [17]. TB staff were trained to ask about children living on the same plot of land [18].
Child Contact Evaluation
Children in the symptom arm were screened according to the 2013 South African Guidelines for Tuberculosis Management in Children [15]. The symptoms screened for included a cough or persistent fever for more than 2 weeks; documented weight loss or a failure to thrive; fatigue, manifested as tiredness or decreased playfulness; a neck mass; lethargy; and a wheeze nonresponsive to bronchodilators. The presence of any 1 symptom resulted in a positive screen (Supplementary Figure 1) [15]. Children in the TST arm were screened with both symptoms and TST. A positive TST was defined as ≥10 mm induration in a human immunodeficiency virus (HIV)-negative child or ≥5 mm in an HIV-positive child 48–72 hours after placement [15]. Asymptomatic children in the symptom arm and asymptomatic children with a negative TST in the TST arm were prescribed isoniazid (10-15 mg/kg/day) for 6 months. Symptomatic children in both arms and asymptomatic children with a positive TST in the TST arm were referred for evaluation by a public sector physician at the district hospital. This evaluation included a clinical history review and physical exam, followed by a chest X-ray and gastric aspirate if the clinical concern for TB disease persisted. Gastric aspirates were evaluated with MGIT culture and Xpert MTB/Rif by the South African National Health Laboratory Services, if the sample volume permitted. Children with microbiological evidence of Mycobacterium tuberculosis or a clinical TB diagnosis were initiated on treatment with rifampin, isoniazid, and pyrazinamide +/− ethambutol [15]. Children who developed TB symptoms while on TPT were evaluated by a physician.
Child Contact Follow-up
Children on TPT were followed monthly for the duration of TPT. Each follow-up visit included TB symptom screening, a side effect evaluation, and isoniazid distribution.
Outcomes and Ascertainment
The primary outcome was the difference in the proportion of identified child contacts initiating TPT or TB treatment in the symptom versus TST arms. Secondary outcomes included the cluster-adjusted proportion of estimated child contacts who progressed through each step of the care continuum (identification, screening, initiation, and completion of TPT) by arm. We calculated estimated child contacts using a historic ratio of 0.7 child contacts per index case who reported not living alone [19]. We identified child TB contacts using the index case’s contact tracing report and/or the child contact management file. We defined screening as a documented visit to the TB clinic within 6 months of the index case’s TB clinic admission. TB staff documented TPT initiation and outcomes on the child contact management file. Outcomes included the completion of therapy, loss to follow-up, discontinuation due to incident TB, side effects, a transfer out, other reasons, or death. Participants without marked treatment outcomes were considered to have completed TPT if a record review showed 5 monthly follow-up visits over a 6-month period. We defined a loss to follow-up as having no return visit within 4 months of the last recorded visit. All data were recorded by clinic staff and subsequently abstracted by study staff from index case and child contact management files.
Randomization
At baseline, all clinics performed TST-based screening, and none had systems to track child contacts through the screening and/or TPT processes. Clinics were assigned to the 2 arms with randomization constrained by TB case notifications rates and distance to the district hospital (measured in average transportation cost) to balance arms on cluster-level variables that were likely to be associated with successful screening and TPT initiation. Given the pragmatic nature of the trial, neither the clinic staff nor the child’s caregiver were masked to the study intervention.
Power and Sample Size
The study was powered based on an anticipated 1152 child contacts per arm (approximated using clinic TB notifications and a historical index-to-child contact ratio) [19], with 80% power and a 2-sided alpha of 5%, to detect a 40% increase (80% vs 40%) in TPT initiations in the symptom versus TST arms. This accounted for variability in clinic sizes and an estimated coefficient of variation of 0.3.
Statistical Analysis
All primary and secondary analyses were cluster-adjusted. The primary analysis was performed by intention-to-treat. For each cluster (clinic), we calculated the proportion of identified child contacts who initiated TPT or TB treatment, divided by the number of identified child contacts. To account for the correlation of data within a cluster, we calculated the unweighted mean of the clinic-level proportions (initiated/identified) for each arm and used an unpaired t test to assess the significance of the difference of these mean (cluster-adjusted) proportions. We used the Wilcoxon rank sum test to test the normality assumption and resistance to outliers. We conducted a post hoc sensitivity analysis, using the same methods and data, from when TST was in stock at all clinics (October 2015–August 2016).
For all secondary outcomes that constitute a step in the care continuum, we calculated the proportion of estimated child contacts who accessed each step of the TB prevention care continuum for each cluster (clinic), and then calculated the unweighted mean of these cluster-level proportions by arm. We used an unpaired t test to assess the significance of the difference of these mean (adjusted) proportions.
Ethical Approval
This study was approved by and granted a waiver of informed consent by the University of Witwatersrand Human Research Ethics Committee and the Johns Hopkins Medicine Institutional Review Board.
RESULTS
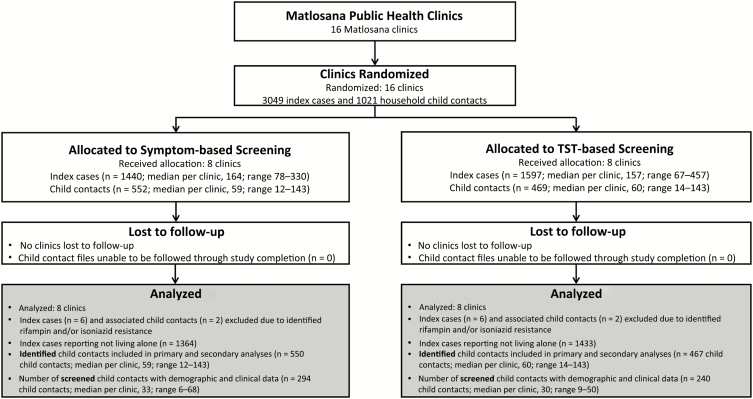
We randomly allocated 16 clinics to conduct symptom-based or TST-based screening (Figure 1). The index case recruitment was stopped 1 month early, due to a TST stockout in all 8 TST clinics. All clinics participated for the duration of the study and were included in all primary and secondary analyses. There were 3049 notified TB diagnoses (index cases). We excluded 6 index cases from each arm due to rifampicin- and/or isoniazid-resistant TB disease, resulting in 1440 and 1597 index cases in the symptom and TST arms, respectively. For both primary and secondary analyses, we included 1364 and 1433 index cases who reported not living alone and 550 and 467 identified child contacts in the symptom and TST arms, respectively. All child contacts had outcome data collected.
Figure 1.
Study population. Abbreviation: TST, tuberculin skin test.
Index and Child Contact Characteristics at Baseline
Demographic and clinical data were available for 294/550 (53%) and 240/467 (51%) child contacts who presented to the clinic for screening in the symptom and TST arms, respectively. Key characteristics of screened child contacts were balanced between arms for child contacts, their associated index cases, and clinics (Tables 1 and 2; Supplementary Table 1). The median age of the TB index cases with associated child contacts was 35 years in both arms, and more than half were female. Most had pulmonary TB (87% in the symptom arm vs 84% in the TST arm), and nearly three-fourths were HIV positive. The median ages of child contacts were 24 months (interquartile range [IQR] 12–36) and 25 months (IQR 14–48) in the symptom and TST arms, respectively. Only 39% and 41% of child contacts were the children of index cases in the symptom and TST arms, respectively. HIV exposure was not recorded for 55% and 60% of children in the symptom and TST arms, respectively. Less than 1% of children in either arm were documented as HIV positive.
Table 1.
Baseline Characteristics of Index Cases with Associated Screened Child Contacts
| Index-level Characteristicsa | ||
|---|---|---|
| Characteristic | Symptom Clinics | TST Clinic |
| Number of index cases with identified and screened child contacts <5 years old | 214 | 183 |
| Age in years, median (IQR) | 35 (26–43) | 35 (27–43) |
| Age category | ||
| <5 years | 2 (1%) | 4 (2%) |
| 5–15 years | 18 (8%) | 6 (3%) |
| 16–35 years | 92 (43%) | 82 (45%) |
| 35–55 years | 85 (40%) | 72 (40%) |
| >55 years | 17 (8%) | 19 (10%) |
| Sex | ||
| Male | 90 (42%) | 62 (34%) |
| Female | 124 (58%) | 121 (66%) |
| Disease type | ||
| Pulmonary TB | 186 (87%) | 154 (84%) |
| Extrapulmonary TB | 20 (9%) | 22 (12%) |
| Pulmonary and extrapulmonary TB | 5 (2%) | 5 (3%) |
| Not recorded | 3 (1%) | 2 (1%) |
| Xpert MTB/RIF | ||
| Positive | 137 (64%) | 109 (59%) |
| Negative | 29 (14%) | 36 (20%) |
| Not recorded | 48 (22%) | 38 (21%) |
| Smear | ||
| Positive | 61 (29%) | 52(28%) |
| Negative | 107 (50%) | 102 (56%) |
| Not Recorded | 46 (21%) | 29 (16%) |
| Registration type | ||
| Newly registered by clinic | 126 (59%) | 84 (46%) |
| HIV status | ||
| Positive | 152 (71%) | 132 (72%) |
| Negative | 57 (27%) | 49 (27%) |
| Not recorded | 5 (2%) | 2 (1%) |
| HIV-positive and on cART at 2 months | 132 (87%) | 117 (89%) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin test.
aFor index cases with screened child contacts <5 years old.
Table 2.
Baseline Characteristics of Screened Child Contacts
| Child-level Characteristicsa | ||
|---|---|---|
| Symptom Clinics | TST Clinics | |
| Number of screened child contacts | 294 | 240 |
| Contact age in months, median (IQR) | 24 (12–36) | 24 (14–48) |
| Contact age group | ||
| <1 year | 62 (21%) | 46 (19%) |
| 1–2 years | 61 (21%) | 40 (17%) |
| ≥2 years | 154 (52%) | 147 (61%) |
| Not recorded | 17 (6%) | 7 (3%) |
| Contact sex | ||
| Male | 128 (44%) | 113 (47%) |
| Female | 153 (52%) | 110 (46%) |
| Not recorded | 13 (4%) | 17 (7%) |
| Relationship to index | ||
| Son/daughter | 115 (39%) | 98 (41%) |
| Grandchild | 50 (17%) | 58 (24%) |
| Niece/nephew | 63 (22%) | 37 (15%) |
| Other (sibling, etc) | 13 (4%) | 25 (11%) |
| Not recorded | 53 (18%) | 22 (9%) |
| Sleep location | ||
| Same bed | 76 (26%) | 63 (26%) |
| Same room | 21 (7%) | 19 (8%) |
| Same house | 150 (51%) | 117 (49%) |
| Different house | 6 (2%) | 4 (2%) |
| Other | 0 (0%) | 2 (1%) |
| Not recorded | 41 (14%) | 35 (14%) |
| HIV exposure | ||
| Exposed | 39 (13%) | 39 (16%) |
| Not exposed | 95 (32%) | 59 (25%) |
| Unknown/not recorded | 160 (55%) | 142 (59%) |
| HIV status | ||
| Positive | 1 (0.3%) | 2 (0.8%) |
| Negative | 159 (54%) | 169 (70%) |
| Unknown | 134 (46%) | 69 (29%) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin test.
aFor screened children only.
Primary Outcome
The cluster-adjusted proportions of identified children who initiated TPT or TB treatment were 51.5% in the symptom clinics and 57.1% in the TST clinics (difference −5.6%, 95% CI −23.7% to 12.6%; P = .52; Table 3; Supplementary Figure 2). There was significant heterogeneity seen across clinics; the coefficient of variation for the primary outcome was 0.30.
Table 3.
Cluster-adjusted Proportion of Identified Child Contacts Initiating Tuberculosis Preventive Therapy by Arm
| Symptom | TST | Risk Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Cluster Number | Proportion of Identified Child Contacts Initiating TPT or TB Treatment | Proportion of Identified Child Contacts Initiating TPT or TB Treatment | ||
| 1 | 54.5% (36/66) | 25.7% (18/70) | … | … |
| 2 | 34.6% (18/52) | 55.9% (38/68) | … | … |
| 3 | 30.9% (13/42) | 78.2% (18/23) | … | … |
| 4 | 45.5% (65/143) | 77.3% (41/53) | … | … |
| 5 | 64.4% (58/90) | 58.2% (39/67) | … | … |
| 6 | 70.7% (29/41) | 31.5% (45/143) | … | … |
| 7 | 50.0% (6/12) | 65.5% (19/29) | … | … |
| 8 | 61.5% (64/104) | 64.3% (9/14) | … | … |
| Cluster summary | 51.5% | 57.1% | −5.6% (−23.7 to 12.6) | .52 |
Data are from an intent-to-treat analysis. Proportions of identified child contacts who initiated TPT or TB treatment are presented for each cluster (clinic). The unweighted mean of the clinic-level proportions is displayed by arm. An unpaired t test was used to assess the significance of the difference of the mean proportions between arms.
Abbreviations: CI, confidence interval; TB, tuberculosis; TPT, tuberculosis preventive therapy; TST, tuberculin skin test.
TST was fully in stock for 65% of the clinic-months. In a sensitivity analysis consisting of children evaluated during months when TST was available in all TST clinics, the adjusted proportion of children who initiated TPT was 58.1% in the symptom clinics and 56.5% in the TST clinics (risk difference 1.6%, 95% CI −16.7 to 19.8; P = .86; Supplementary Table 2).
Continuum of Care
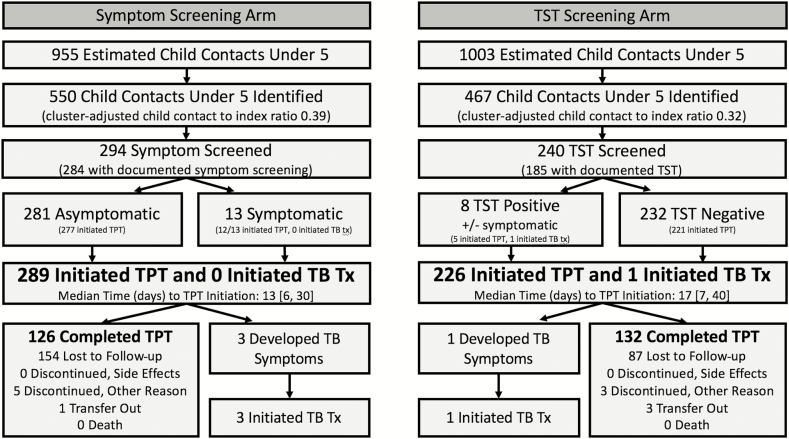
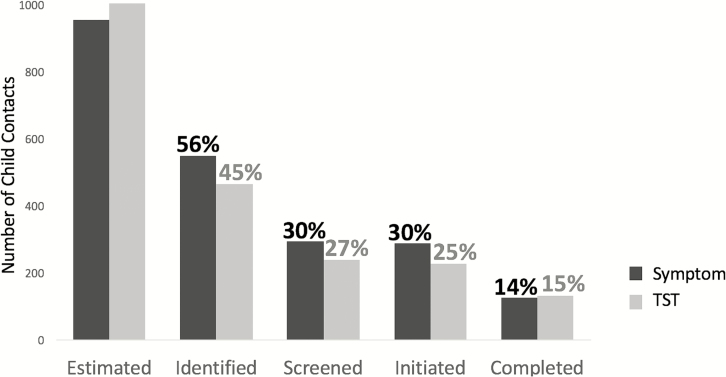
Contact tracing identified 550 and 467 child contacts from 1364 and 1433 TB index patients not reporting living alone (adjusted child-to-index ratios 0.39 and 0.32, respectively; P = .27) in the symptom and TST arms, respectively (Figure 2). The adjusted proportions of estimated child contacts who were identified were 55% (95% CI 38–73%) and 45% (95% CI 33–57%) in the symptom and TST arms, respectively (P = .27; Figure 3). The adjusted proportions of estimated contacts who were screened were 30% (95% CI 16–44%) and 27% (95% CI 21–36%) in the symptom and TST arms, respectively (P = .39). The adjusted proportions of estimated child contacts who initiated treatment (TPT or TB treatment) were 30% (95% CI 17–44%) and 25% (95% CI 15–36%) in the symptom and TST arms, respectively (P = .11). The adjusted proportions of estimated contacts who completed 6-month courses of TPT were 14% (95% CI 6–23%) and 15% (95% CI 6–24%) in the symptom and TST arms, respectively (P = .89).
Figure 2.
Number of child contacts progressing through each step of the cascade. All proportions are cluster adjusted and represent the proportion of estimated child contacts who progressed through that stage of the pediatric TB prevention care continuum. Child contacts were estimated by multiplying 0.7 by all adult index cases with pulmonary TB who did not report living alone. TPT in this study was 6 months of daily isoniazid. Numbers given in brackets indicate the interquartile ranges. Abbreviations: TB, tuberculosis; TPT, tuberculosis preventive therapy; TST, tuberculin skin test; Tx, treatment.
Figure 3.
Pediatric TB prevention continuum of care by arm from a cohort of child contacts associated with index cases admitted to TB clinics in Matlosana, South Africa from October 2015–February 2017. Adjusted proportions for each arm are displayed above each bar and represent the proportion of estimated child contacts progressing to that stage of TB preventive care. Child contacts were estimated by multiplying 0.7 by all adult TB index cases who did not report living alone. Abbreviations: TB, tuberculosis; TST, tuberculin skin test.
At baseline, only 13 and 8 child contacts had positive screens in the symptom and TST arms, respectively. There was 1 child in the TST arm who was clinically diagnosed with TB disease at their initial clinical evaluation. There were 4 children diagnosed with TB disease after TPT initiation: 3 in the symptom arm and 1 in the TST arm. These 4 children were asymptomatic at the time of TPT initiation and had initiated TPT 27–74 days prior to TPT discontinuation. All of these children clinically responded to TB treatment.
The majority of children who initiated but did not complete TPT were lost to follow-up. There were no reported discontinuations due to side effects, nor were any deaths reported. The adjusted proportions of estimated child contacts who completed at least 3 months of TPT were 20% (95% CI 9–31%) and 18% (95% CI 9–28%) in the symptom and TST arms, respectively, and the proportions who completed at least 1 month of TPT were 24% (95% CI 12–36%) and 21% (95% CI 11–31%) respectively.
DISCUSSION
The results of this pragmatic, cluster-randomized trial showed no significant difference in TPT uptake among child contacts following implementation of the WHO’s symptom-based screening guidelines, as compared to the previous standard of care: TST-based screening. Our results suggest that other interventions in the pediatric TB prevention care continuum are required to protect child contacts from developing TB disease. Child contact identification, linkage to care, and retention in care all require strengthening; over two-thirds of child contacts were either not identified or not linked to care, and only 1 in 2 who initiated treatment were retained in care for 6 months.
The study was not powered to detect small differences in TPT uptake between the 2 arms; we hypothesized a large increase in TPT uptake. Our sample size assumptions were largely correct, except for a higher than expected baseline proportion of TPT uptake in the TST arm. Post hoc power calculations demonstrated 80% power to detect a 40% increase in TPT initiations between arms. TST stockouts may have affected the TPT uptake by either limiting progression through the continuum of care or resulting in symptom-based screening being performed in both arms. A sensitivity analysis, excluding the time with TST stockouts, showed no difference between the 2 arms.
TPT initiation was a well-functioning step in the care continuum, where a surprising 97% of screened child contacts were initiated on TPT, irrespective of arm. This was unexpected based on our pretrial assessment and prior published care continuums in South Africa and other high-burden settings [9, 11]. Better than expected proportions of child contacts may have been initiated on TPT in the control arm due to the implementation of the TPT register and medical file, the institution of audits and feedback, participation in the clinical trial, or the combination of any or all of these factors.
The introduction of a child contact management file allowed us to define a pediatric TB care continuum for an entire South African subdistrict. Health system strengthening activities, including healthcare worker training and mentorship, documentation tools, audits and feedback, evidence-based screening strategies, and prioritization from the local TB program, could not together improve the care continuum to more than 15% TPT coverage. Had a 1-month TPT regimen been available, coverage would have increased to 23%, given heavy losses early in the care continuum, suggesting that short-course TB prevention regimens alone will not be sufficient to improve pediatric TB prevention. Nearly all clinics used facility-based services during this study: few utilized community-based TB services providing directly observed therapy to the index case. The integration of pediatric TB prevention into other clinic-based and community-based services may improve pediatric TPT uptake.
We anticipated a larger number of child contacts screening positive in both arms and, therefore, more TB diagnoses. Frankly symptomatic children may have presented directly to the hospital, where they were recorded only as index cases, or may have died without a TB diagnosis. Modeling studies suggest that more than 95% of pediatric TB mortality is among children who fail to be diagnosed with TB disease antemortem [2]. While symptom-based screening resulted in fewer clinic visits, it did not result in fewer referrals to the district hospital, due to few children screening positive in either arm. Symptom screening was accepted by both nurses and caregivers, as evidenced by the high proportion (99%) of screened child contacts initiating TPT.
There were 4 child contacts who developed TB symptoms within several weeks of TPT initiation and were diagnosed with TB disease. These cases may reflect mildly symptomatic child contacts who initially screened negative or children nonadherent to TPT who progressed to TB disease. These children all responded to first-line TB treatment without any concern for drug resistance. Symptom screening can be conducted safely in a real-world setting, but requires ongoing symptom screening after TPT initiation.
This trial was conducted in a semiurban South African subdistrict where nurse training and support may be of better quality than in many rural sub-Saharan African communities, thereby possibly limiting its generalizability. Even still, the subdistrict suffered many financial constraints, resulting in significant nursing shortages; overall, the clinics were functioning at 57% of their nursing capacity at the time of the study completion.
In conclusion, simplifying pediatric TB screening did not result in improved TPT uptake, as compared with TST-based screening. Clinic nurses appropriately initiated TPT using symptom-based screening in a real-world setting. Symptom screening can be conducted safely and without a greater burden to the healthcare system, as compared to TST-based screening, and should remain the standard of care. Further research is needed to improve child contact identification, linkage to care, and retention in TB preventive care to reduce TB-associated child morbidity and mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. S.-A., C. S., E. V., N. M., and R. E. C. conceptualized this study. N. S.-A., E. V., N. M., and R. E. C. acquired funding. N. S.-A., G. L. and B., M. T. conducted the investigation. N. S.-A., S. C., N. M., and R. E. C. developed the methodology. N. S.-A., G. L. B., M. T., C. S., Z. M., K. M., E. V., and N. M. managed the project administration. S. C. curated the data. S. C., N. S.-A., and R. E. C. conducted the formal analysis. N. S.-A. wrote the original draft. N. S.-A., S. C., G. L. B., M. T., K. M., C. S., Z. M., E. V., N. M., and R. E. C. reviewed and edited drafts. Deidentified individual-level participant data and an accompanying data dictionary will be made publicly available at United States Agency for International Development’s (USAID) Data Development Library (https://www.usaid.gov/development-data-library).
Disclaimer. The funders had no role in the study design, data collection, data analysis or interpretation, or writing of and decision to publish this manuscript.
Financial support. This work was supported by Project SOAR (Supporting Operational AIDS Research) funded by USAID, cooperative agreement AID-OAA-A-14-00060 to R. E. C. and N. M.; http://www.projsoar.org, the Harvard Medical School Richardson Fellowship, the Johns Hopkins’s Children’s Center’s Baurenschmidt Award, and the National Institutes of Health (grant numbers T32 AI052071, 4KL2TR001077-04, and P30AI094189). The North West Department of Health provided all public-sector care in Matlosana.
Potential conflict of interest. R. E. C. has received personal fees from Merck, Otsuka, and Janssen, outside the submitted work. N. M.’s institution has received grants from Pfizer and Roche, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 2. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5:e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 4. Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev 2000; 2 : CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis 2014; 14:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodd PJ, Yuen CM, Becerra MC, Revill P, Jenkins HE, Seddon JA. Potential effect of household contact management on childhood tuberculosis: a mathematical modelling study. Lancet Glob Health 2018; 6:e1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kruk A, Gie RP, Schaaf HS, Marais BJ. Symptom-based screening of child tuberculosis contacts: improved feasibility in resource-limited settings. Pediatrics 2008; 121:e1646–52. [DOI] [PubMed] [Google Scholar]
- 8. Triasih R, Robertson CF, Duke T, Graham SM. A prospective evaluation of the symptom-based screening approach to the management of children who are contacts of tuberculosis cases. Clin Infect Dis 2015; 60:12–8. [DOI] [PubMed] [Google Scholar]
- 9. Szkwarko D, Hirsch-Moverman Y, Du Plessis L, Du Preez K, Carr C, Mandalakas AM. Child contact management in high tuberculosis burden countries: a mixed-methods systematic review. PLOS One 2017; 12:e0182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutherford ME, Hill PC, Triasih R, Sinfield R, van Crevel R, Graham SM. Preventive therapy in children exposed to Mycobacterium tuberculosis: problems and solutions. Trop Med Int Health 2012; 17:1264–73. [DOI] [PubMed] [Google Scholar]
- 11. Osman M, Hesseling AC, Beyers N, et al. Routine programmatic delivery of isoniazid preventive therapy to children in Cape Town, South Africa. Public Health Action 2013; 3:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva, Switzerland: World Health Organization, 2006. [PubMed] [Google Scholar]
- 13. Martinez L, Shen Y, Handel A, et al. Effectiveness of WHO’s pragmatic screening algorithm for child contacts of tuberculosis cases in resource-constrained settings: a prospective cohort study in Uganda. Lancet Respir Med 2018; 6:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnet M, Kyakwera C, Kyomugasho N, et al. Prospective cohort study of the feasibility and yield of household child tuberculosis contact screening in Uganda. Int J Tuberc Lung Dis 2017; 21:862–8. [DOI] [PubMed] [Google Scholar]
- 15. National Department of Health Republic of South Africa. Guidelines for the management of tuberculosis in children Available at: https://www.health-e.org.za/wp-content/uploads/2014/06/National-Childhood-TB-Guidelines-2013.pdf. Accessed 17 May 2018.
- 16. Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PLOS Med 2011; 8:e1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Department of Health RoSA. National tuberculosis management guidelines. Available at: http://www.tbonline.info/media/uploads/documents/south_african_national_tuberculosis_management_guidelines_%282009%29.pdf. Accessed 7 August 2017.
- 18. Van Wyk SS, Mandalakas AM, Enarson DA, Gie RP, Beyers N, Hesseling AC. Tuberculosis contact investigation in a high-burden setting: house or household? Int J Tuberc Lung Dis 2012; 16:157–62. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro AE, Variava E, Rakgokong MH, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med 2012; 185:1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.