Abstract
The older-adult population is constantly increasing, hence aging and mechanisms leading to aging are a topic raising increasing interest. Hypovitaminosis D is common amongst old patients and has been proposed as causative of several chronic diseases. Here we review the role of hypovitaminosis D and vitamin D supplementation in sarcopenia and dementia, from bench to bedside.
Keywords: vitamin D, brain, muscle, aging
1. Introduction
Due to an increased life expectancy, aging and the mechanisms leading to it have become a pivotal issue in scientific research. Together with innocuous and physiological changes due to aging, there are pathological changes that increase the risk of disease, disability, or death linked to the development of frailty and chronic diseases, hence older adults can be divided into the fit and the frail.
During aging, all systems and organs are physiologically reduced in their function. However, identifying the risk factors leading to “fit” or “frail” aging is fundamental in order to understand the physiopathological mechanisms leading to frailty and suggest preventive measures. Health maintenance in older age is one the most important challenges for future medicine. Hence, ameliorating the burden of chronic disease in the elderly is also one of the goals of future medicine. Among the chronic conditions that severely affect patient quality of life, diseases affecting mobility and cognition—namely, sarcopenia and dementia—are the most widely suffered. Both sarcopenia and dementia are a severe burden for older people and both diseases are hallmarks of frailty. Sarcopenia and its consequences (weakness, slowness, reduction of physical activity, and weight loss) are essential features of “physical frailty” [1]. Whereas cognitive impairment is a characteristic feature of “cognitive frailty”, this is a recently defined condition in which physical frailty coexists with cognitive impairment [2]. As both sarcopenia and dementia significantly contribute to frailty, herein we review the impact of hypovitaminosis D on these two diseases.
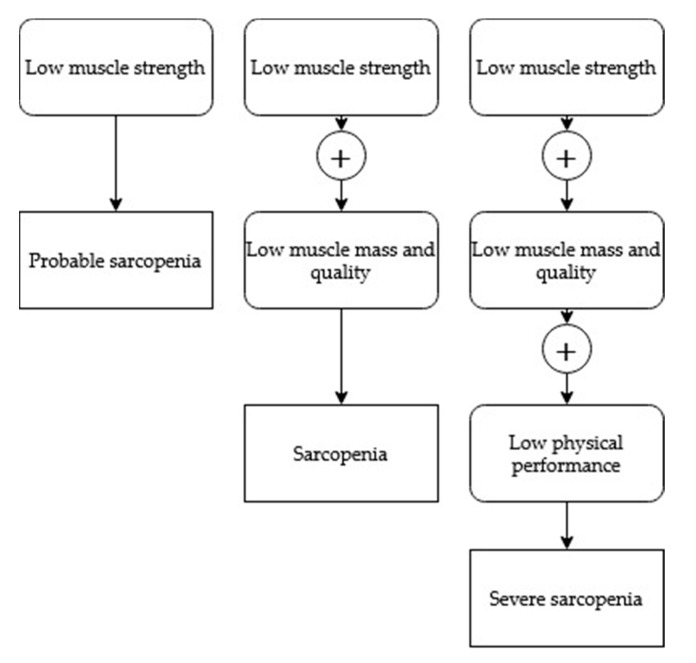
With the term “sarcopenia”, we indicate a condition characterized by both reduced muscle mass and muscle strength (“dynapenia”). These declines lead to an impairment in physical performance [3]. Due to the heterogeneity of the criteria used to diagnose sarcopenia, with different working groups providing their own definitions, it is difficult to compare the results of different studies. Table 1 summarizes the criteria used for the diagnosis of sarcopenia.
Table 1.
Different criteria for the diagnosis of sarcopenia.
| Criterium | Slowness | Weakness | Low Lean Mass | Summary Definition |
|---|---|---|---|---|
| International Working Group [7] | Gait speed < 1.0 m/s | Not included | ALM/ht2 ≤ 7.23 kg/m2 | Sarcopenia: slowness and low lean mass |
| EWGSOP [8] | Gait speed ≤ 0.8 m/s | Grip strength < 30 kg | ALM/ht2 ≤ 7.23 kg/m2 | (1) Sarcopenia: low lean mass plus slowness or weakness |
| (2) Severe sarcopenia: all three criteria | ||||
| FNIH Sarcopenia Project primary definition [9] | Gait speed ≤ 0.8 m/s | Grip strength < 26 kg | ALM/body mass index < 0.789 | (1) Weakness and low lean mass |
| (2) Slowness with weakness and low lean mass | ||||
| Baumgartner [10] | Not included | Not included | ALM/ht2 ≤ 7.23 kg/m2 | Low lean mass |
| Newman [11] | Not included | Not included | Residual of actual ALM*-predicted ALM from equation | Low lean mass |
* ALM: appendicular lean mass.
One of the most used algorithms in clinical practice is the algorithm recently developed by the European Working Group on Sarcopenia in Older People (EWGSOP) [4] (Figure 1).
Figure 1.
EWGSOP clinical practice algorithm for the diagnosis of sarcopenia.
According to the EWGSOP definition, the estimated prevalence of sarcopenia is 10–40% amongst elderly individuals in the community [5,6], however, this prevalence rises in settings of care for older patients. Due to increasing life expectancy, the number of patients living with sarcopenia is expected to grow to more than 200 million in the next 40 years [3].
Being sarcopenic increases sanitary costs and decreases quality of life in older patients [12,13], hence the correct identification of risk factors linked to sarcopenia is important to precociously identify these patients and to set up an early intervention. In our “aging world”, cognitive impairment is also constantly increasing. The World Alzheimer Report 2018 [14] states that about 50 million people worldwide suffer from dementia and the epidemiological projection envisions that these numbers are expected to rise to 152 million by 2050.
The most diffuse form of dementia is Alzheimer’s disease (AD). Other types of dementia are vascular dementia, mixed dementia, and Lewy body dementia. Dementia and sarcopenia are responsible for an enormous increase in sanitary costs; the United States spent about $818 billion for patients with dementia in 2015, and these costs have increased by more than 30% since 2010. The cost distribution is not homogeneous and the majority of costs burden high-income countries [15].
Both sarcopenia and dementia have been associated with hypovitaminosis D, which has been suggested as one of the causative mechanisms. Although different values have been used as references and the agreed threshold for the diagnosis of hypovitaminosis D is still debated, hypovitaminosis D is widely diffuse among older people. Guidelines from different scientific societies and different countries establish the threshold for hypovitaminosis D as being under 50 nmol/L or 75 nmol/L of blood 25(OH) vitamin D (25(OH)D3) [Table 2].
Table 2.
Different vitamin D cut-offs used in clinical practice.
Despite these different thresholds, the majority of studies indicate that with 25(OH)D3 lower than 50 nmol/L, bone metabolism is impaired and there is an increased risk of fractures, falls, and myopathy [20,21]. Priemel and colleagues demonstrated that pathological mineralization defects of bone occur in patients with a serum 25(OH)D3 below 75 nmol/L [22]. In contrast with the above mentioned studies, two recent randomized controlled clinical trials [23,24] indicated that only individuals with baseline 25(OH)D3 levels lower than 30 nmol/L will benefit from vitamin D supplement use.
Clinicians working on vitamin D generally agree to maintain 25(OH)D3 levels between 20 and 125 nmol/L. These prudential levels are associated with a healthy skeleton and avoid possible toxic effects. Recent papers have raised caveats concerning toxic effects of high doses of vitamin D: the administration of a bolus of vitamin D3 higher than 50,000 UI may result in an increased risk of falls and fractures [25,26]. Moreover, levels of vitamin D higher than 150 nmol/L have been associated with increased mortality in a wide population study [27].
Hypovitaminosis D has been described as common to different chronic diseases linked to senescence, and the incidence of hypovitaminosis D increases as age increases.
Even adopting the most conservative cut-off value for hypovitaminosis D (lower than 50 nmol/L), it is a frequent condition amongst people aged 65 years or more. In the U.S., the prevalence of vitamin D deficiency and vitamin D insufficiency were found to be 28.9% and 41.4%, respectively [28].
Hypovitaminosis D in older age groups is mainly due to the reduced ability of the skin to synthetize cholecalciferol from its precursor: 7-dehydrocholesterol. Together with a reduced synthesis of vitamin D, older subjects showed a reduced expression of vitamin D receptors (VDRs). These two phenomena cooperate in the amplification of the effect of hypovitaminosis D during aging [29].
Hypovitaminosis D has been identified as a common feature between diseases widely diffused in senescence such as osteoporosis, sarcopenia, and cognitive impairment.
Although the most well-known clinical effect of hypovitaminosis D is osteoporo-malacia, here we will focus on the role of vitamin D in the pathogenesis of sarcopenia and cognitive impairment, analyzing studies from experimental models and their clinical relevance.
2. Vitamin D and Aging
2.1. Vitamin D and Muscle Health: From Bench to Bedside
VDRs are expressed in human muscle fibers, especially during their early developmental phases, and decrease upon their maturation [30]. It has been demonstrated in vitro that vitamin D plays an active role in muscle maturation as myoblasts can differentiate into myocytes thanks to a signal mediated by VDRs [31]. Besides its genomic effects, vitamin D has non-genomic rapid-onset effects that can play a role in muscle contraction as it is involved in the regulation of membrane calcium channels [32,33]. Vitamin D increases calcium influx in muscle cell cytoplasm within minutes in a dose-dependent manner [34,35] through activation of two kinases, namely, c-Src and PI3K [36]. PI3K activation leads to an increasing level of inositol triphosphate (IP3) and diacylglycerol (DAG); IP3 induces calcium displacement from the sarcoplasm, while DAG, along with calcium in the cytosol, is a key component in the activation of protein kinase C (PKC). PKC interacts with calcium channels on the cell membrane, leading to more calcium influx in the cytosol [37,38]. Calcium binds to the troponin–tropomyosin complex, resulting in the exposure of active binding sites, enabling muscle contraction [39].
Experimental models of knock-out mice for VDR confirmed this role, as in VDR null mice, the muscle mass is reduced and the fibers have a lower diameter in respect to wild-type littermates [40].
Moreover, muscle development and maturation is impaired in VDR null mice. These mice express transcriptional factors typical of early muscle development, such as myf5, E2A, and myogenin, for a longer period, suggesting that the VDR is a key player in correct muscle growth and maturation [40].
The action of vitamin D in muscle development is independent of its effect on blood calcium levels, as has been demonstrated in VDR null mice with normal blood calcium [40].
Besides the role on muscle development and maturation, a role for vitamin D has also been postulated in the control of muscle atrophy. Vitamin D has been implicated in muscular protein degradation through the control of the ATP-ubiquitin-dependent system [41]. In rats, a significant increase in catalytic activity and in protein ubiquitination during vitamin D deficiency have been demonstrated [41]. The increased muscle atrophy associated with vitamin D deficiency is associated with reduced anaerobic capacity and tolerance to exercise, together with a disruption of muscle morphology demonstrated by low cross-sectional areas of muscle fibers and the reduction of fast fibers [42].
As VDR expression increases after muscular injury, it has also been suggested that vitamin D may have a role in muscle regeneration [32,43]. This could be very important in patients affected by sarcopenia who have reduced muscular regeneration.
Taken together, these experimental data strongly suggest a role for vitamin D in muscle health. As regards humans, despite studies’ heterogeneity, a physiological role for vitamin D has been envisioned.
In humans, the role of vitamin D in muscle health is supported by several data: in patients with VDR mutations or severe vitamin D deficiency, there is a generalized muscle atrophy and muscular sufferance which appears even before the appearance of the altered bone turnover [43]. Associated studies have demonstrated that in older age, vitamin D deficiency is strongly linked to muscle weakness and loss, suggesting that hypovitaminosis D in the elderly may be an important factor in the development of sarcopenia [39,44,45]. In a large study on more than 4000 older community-dwelling adults, Aspell et al. showed that patients with vitamin D lower than <30 nmol/L were more likely to have impaired muscle function with a reduction in physical performance and muscle strength, but not an increased risk of falls [46].
2.2. Vitamin D Supplements and Muscle Health
Despite the accumulating evidence on the link between vitamin D deficiency and muscle health (especially in older adults), the role of vitamin D supplementation in recovering muscle mass and function is yet to be proven. Meta-analysis and systematic literature reviews were able to find only a minor, often non-statistically significant, improvement in muscle strength with vitamin D supplementation, even when associated with calcium supplementation and exercise [47,48].
A closer look at recent randomized controlled clinical trials, even ones included in the aforementioned papers, demonstrates a great deal of heterogeneity in patients, levels of vitamin D at baseline, doses of vitamin D supplementation, and even in tests used to measure muscle strength and sarcopenia. While it is true that the older population is itself a very heterogeneous group, the selection of a more precise section of the population might help in finding results that are more suitable to clarify whether vitamin D supplementation has a role in the prevention and treatment of age-related muscle loss.
Moreover, some trials have raised some caveats suggesting that a high bolus of cholecalciferol does not prevents falls, but, on the contrary, seems to increase the risk of falling and may be ineffective in improving bone mineral density and bone turnover [26].
In order to understand these conflicting results, it is important to highlight that the subjects included in those papers were not affected by hypovitaminosis D and were treated with doses much higher than the ones recommended in clinic [19]. Hence, the conclusions from those papers can only be that too much vitamin D, if it is not needed, may be detrimental for unknown reasons.
Although calcium is important for muscle contraction, randomized clinical trials show no additional effect of calcium on muscle strength in young athletes both female [49] and male [50]. Furthermore, in older community-dwelling women, the administration of yogurts fortified with vitamin D (200 IU) and calcium (400 mg) given twice a day was not able to increase gait performance [51].
2.3. Vitamin D and Cognitive Health: From Bench to Bedside
The observation that the VDR is also expressed in the central nervous system (CNS) and that CNS is per se able to synthetize calcitriol thanks to the expression of the 25-hydroxylase and 1α-hydroxylase [52] has raised the hypothesis that vitamin D may have a role in brain health and in cognitive performance.
In a rat model of Alzheimer’s disease (AD), rats fed with low levels of vitamin D lost their cognition abilities more rapidly in respect to those fed a control diet [53]. Moreover, in mice with low levels of vitamin D, the production of amyloid-β (AB) is increased and there is an increased formation of amyloid plaques, as is typically observed in patients affected by AD [54]. In transgenic mice who spontaneously accumulate AB and develop AD, a diet supplemented with cholecalciferol is able to reduce the amyloid plaque formation by enhancing the amyloid clearance. Consistent with histological changes, vitamin D supplements ameliorate animal cognitive performance [55,56].
The mechanisms through which vitamin D reduces the accumulation of AB and the formation of amyloid plaque are not completely clear. It has been suggested that vitamin D increases the AB clearance by the blood–brain barrier, increasing its brain-to-blood efflux though both genomic and non-genomic action [57,58]. Moreover, in vitro primary cultures of cortical neurons show that vitamin D is directly implicated in the production of Aβ and can downregulate its expression [59]. Several genes involved in the pathogenesis of AD have a vitamin D responding element within their sequences [60]. These genes are deregulated if vitamin D deficiency occurs during growth [61,62]. However, it has never been demonstrated that hypovitaminosis D during growth may influence cognitive performance in adult life.
Despite all the data obtained from in vitro and from animal models, the role of vitamin D in cognition is far from being elucidated. Its role is probably complex and mediated through the cross talk with other factors such as estrogen and insulin [56]. Transcriptomic analysis of the neocortex of healthy mice and those affected by AD showed that after vitamin D treatment there is a deregulation of pathways related to inflammation and immune response, neurotransmission, vascular processes, and hormonal alterations, suggesting a complex and multiple role for vitamin D rather than a single one in the development of dementia [56].
In humans, some studies have suggested that lower levels of vitamin D are associated with poorer cognition in patients affected by cognitive problems, however a faster loss of cognitive performance has not been demonstrated [63,64]. Furthermore, in older subjects complaining of memory deficits without a diagnosis of dementia, hypovitaminosis D has been associated with lower cognitive performance, namely, poorer mental flexibility [65]. On the other hand, a recent study performed with the Mendelian randomization method found no evidence that hypovitaminosis D may be a cause of cognitive deficits in mid- or later life [66].
Due to the high heterogeneity of the methods used and of the population analyzed, it is difficult to find homogeneous results. Nevertheless, a recent meta-analysis including both patients with impaired cognition and healthy subjects suggests that poorer vitamin D status is associated with poorer cognitive performance with respect to high vitamin D levels, however, the author acknowledges possible publication biases [67].
Data obtained in animals and associated studies in humans have brought substantial attention to the possible role of vitamin D supplements in preventing cognitive decline in humans. However, the data obtained in different studies are difficult to interpret and do not clearly highlight the role of vitamin D in the pathogenesis and treatment of dementia. Interventional studies are less common than observational ones, and results are more inconclusive because the protocols used for supplementation and the population included are largely different. The durations of treatment vary from a single dose to 18 weeks, with different dosages and different molecules. Dhesi and colleagues [68] used ergocalciferol as a single intramuscular injection of 600,000 IU, Przybelski and colleagues used oral ergocalciferol of 50,000 IU three times a week for four weeks [69], Dean and colleagues used oral cholecalciferol capsules of 5000 IU daily [70], whereas Pettersen used oral cholecalciferol in two different doses of 4000 IU versus 400 IU daily for 18 weeks [71]. Moreover, the population enrolled was different with regards to age: old subjects [72] versus young [47]. The settings are also different: community-dwelling residents versus nursing home residents [73,74]. The vitamin D status at inclusion in the study is also different: in the study by Dhesi and colleagues [68], the subjects included were vitamin D deficient, while in the others they could be deficient or not [69,70]. In addition, the study designs are different, as two studies used a placebo-controlled group [68,70] and others did not [69,71].
Recent meta-analyses reviewed three of the four interventional studies cited [68,70] and found that 314 subjects saw no significant benefit in patients treated with vitamin D supplementation [67]. On the other hand, the study by Pettersen suggested an effect of high doses of cholecalciferol (4000 IU/d) in the amelioration of visual memory in heathy adults, particularly among those who had low levels of vitamin D at their enrolment in the trial.
As regards the supplementation of vitamin D together with calcium, a recent randomized controlled clinical trial in healthy older females showed that yogurts fortified with low doses of vitamin D (200 IU) and calcium (400 mg) given twice a day helped in the maintenance of cognitive performance [51].
Taken together, these data suggest that although vitamin D may have a role in the development of the brain and in cognition, data obtained by intervention studies are not sufficient to indicate that the administration of vitamin D, even at high doses, may be useful for patients with impaired cognition. Data on the use of calcium supplements associated with vitamin D are not sufficient to recommend the double supplementation, and further studies are needed to clarify these indications.
3. Conclusions
Progressive increases in life expectancy are associated with a constant increase of chronic diseases associated with age that have a great impact on patients’ quality of life and consistently increase sanitary costs as well as the risks of sarcopenia and dementia.
Hypovitaminosis D is associated with aging and with these chronic diseases. Even though a clear pathogenic mechanism is still not elucidated, association studies have shown a relationship between low vitamin D levels and sarcopenia and dementia. However, interventional studies are not sufficient to recommend treatment with vitamin D to efficiently treat these two diseases.
Acknowledgments
The authors thank Francesco Cattaneo for drawing the old man used in the graphical abstract.
Author Contributions
Conceptualization P.D., bibliographic research P.D. and L.Q.; writing—review and editing, P.D. and L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 2.Ruan Q., Yu Z., Chen M., Bao Z., Li J., He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev. 2015;20:1–10. doi: 10.1016/j.arr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Dodds R.M., Roberts H.C., Cooper C., Sayer A.A. The Epidemiology of Sarcopenia. J. Clin. Densitom. 2015;18:461–466. doi: 10.1016/j.jocd.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayhew A.J., Amog K., Phillips S., Parise G., McNicholas P.D., de Souza R.J., Thabane L., Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48:48–56. doi: 10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- 6.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chumlea W.M.C., Cesari M., Evans W.J., Ferrucci L., Fielding R.A., Pahor M., Studenski S., Vellas B. The Task Force Members International working group on Sarcopenia. J. Nutr. Health Aging. 2011;15:450–455. doi: 10.1007/s12603-011-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.-P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M., et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R., Garry P.J., Lindeman R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Newman A.B., Kupelian V., Visser M., Simonsick E., Goodpaster B., Nevitt M., Kritchevsky S.B., Tylavsky F.A., Rubin S.M., Harris T.B., et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 12.Marty E., Liu Y., Samuel A., Or O., Lane J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone. 2017;105:276–286. doi: 10.1016/j.bone.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Sousa A.S., Guerra R.S., Fonseca I., Pichel F., Ferreira S., Amaral T.F. Financial impact of sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 2016;70:1046–1051. doi: 10.1038/ejcn.2016.73. [DOI] [PubMed] [Google Scholar]
- 14.World Alzheimer Report 2018: The state of the art of dementia research: New frontiers | Alzheimer’s Disease International. [(accessed on 28 January 2020)]; Available online: https://www.alz.co.uk/news/world-alzheimer-report-2018-state-of-art-of-dementia-research-new-frontiers.
- 15.Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina A.M., Winblad B., Jönsson L., Liu Z., Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium . In: Dietary Reference Intakes for Calcium and Vitamin D. Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B., editors. National Academies Press (US); Washington, DC, USA: 2011. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 17.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossini M., Adami S., Bertoldo F., Diacinti D., Gatti D., Giannini S., Giusti A., Malavolta N., Minisola S., Osella G., et al. Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo. 2016;68:1–39. doi: 10.4081/reumatismo.2016.870. [DOI] [PubMed] [Google Scholar]
- 19.Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., Guglielmi R., Papini E., Santonati A., Scillitani A., et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients. 2018;10:546. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valcour A., Blocki F., Hawkins D.M., Rao S.D. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J. Clin. Endocrinol. Metab. 2012;97:3989–3995. doi: 10.1210/jc.2012-2276. [DOI] [PubMed] [Google Scholar]
- 21.Bhattoa H.P., Konstantynowicz J., Laszcz N., Wojcik M., Pludowski P. Vitamin D: Musculoskeletal health. Rev. Endocr. Metab. Disord. 2017;18:363–371. doi: 10.1007/s11154-016-9404-x. [DOI] [PubMed] [Google Scholar]
- 22.Priemel M., von Domarus C., Klatte T.O., Kessler S., Schlie J., Meier S., Proksch N., Pastor F., Netter C., Streichert T., et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 23.Reid I.R., Horne A.M., Mihov B., Gamble G.D., Al-Abuwsi F., Singh M., Taylor L., Fenwick S., Camargo C.A., Stewart A.W., et al. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J. Intern. Med. 2017;282:452–460. doi: 10.1111/joim.12651. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald H.M., Reid I.R., Gamble G.D., Fraser W.D., Tang J.C., Wood A.D. 25-Hydroxyvitamin D Threshold for the Effects of Vitamin D Supplements on Bone Density: Secondary Analysis of a Randomized Controlled Trial. J. Bone Miner. Res. 2018;33:1464–1469. doi: 10.1002/jbmr.3442. [DOI] [PubMed] [Google Scholar]
- 25.Sanders K.M., Stuart A.L., Williamson E.J., Simpson J.A., Kotowicz M.A., Young D., Nicholson G.C. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari H.A., Dawson-Hughes B., Orav E.J., Staehelin H.B., Meyer O.W., Theiler R., Dick W., Willett W.C., Egli A. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2016;176:175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 27.Amrein K., Quraishi S.A., Litonjua A.A., Gibbons F.K., Pieber T.R., Camargo C.A., Giovannucci E., Christopher K.B. Evidence for a U-shaped relationship between prehospital vitamin D status and mortality: a cohort study. J. Clin. Endocrinol. Metab. 2014;99:1461–1469. doi: 10.1210/jc.2013-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X., Baylin A., Levy P.D. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br. J. Nutr. 2018;119:928–936. doi: 10.1017/S0007114518000491. [DOI] [PubMed] [Google Scholar]
- 29.Scimeca M., Centofanti F., Celi M., Gasbarra E., Novelli G., Botta A., Tarantino U. Vitamin D Receptor in Muscle Atrophy of Elderly Patients: A Key Element of Osteoporosis-Sarcopenia Connection. Aging Dis. 2018;9:952–964. doi: 10.14336/AD.2018.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bischoff H.A., Borchers M., Gudat F., Duermueller U., Theiler R., Stähelin H.B., Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001;33:19–24. doi: 10.1023/A:1017535728844. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M., Kishimoto K.N., Okuno H., Saito H., Itoi E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve. 2014;49:700–708. doi: 10.1002/mus.23950. [DOI] [PubMed] [Google Scholar]
- 32.Srikuea R., Zhang X., Park-Sarge O.-K., Esser K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 2012;303:C396–C405. doi: 10.1152/ajpcell.00014.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floyd M., Ayyar D.R., Barwick D.D., Hudgson P., Weightman D. Myopathy in chronic renal failure. Q. J. Med. 1974;43:509–524. [PubMed] [Google Scholar]
- 34.de Boland A.R., Massheimer V., Fernandez L.M. 1,25 Dihydroxyvitamin D3 affects calmodulin distribution among subcellular fractions of skeletal muscle. Calcif. Tissue Int. 1988;43:370–375. doi: 10.1007/BF02553281. [DOI] [PubMed] [Google Scholar]
- 35.Santillán G., Katz S., Vazquez G., Boland R.L. TRPC3-like protein and vitamin D receptor mediate 1alpha,25(OH)2D3-induced SOC influx in muscle cells. Int. J. Biochem. Cell Biol. 2004;36:1910–1918. doi: 10.1016/j.biocel.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Buitrago C., Vazquez G., De Boland A.R., Boland R.L. Activation of Src kinase in skeletal muscle cells by 1, 1,25-(OH(2))-vitamin D(3) correlates with tyrosine phosphorylation of the vitamin D receptor (VDR) and VDR-Src interaction. J. Cell. Biochem. 2000;79:274–281. doi: 10.1002/1097-4644(20001101)79:2<274::AID-JCB100>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 37.Morelli S., de Boland A.R., Boland R.L. Generation of inositol phosphates, diacylglycerol and calcium fluxes in myoblasts treated with 1,25-dihydroxyvitamin D3. (Pt 3)Biochem. J. 1993;289:675–679. doi: 10.1042/bj2890675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buitrago C., González Pardo V., de Boland A.R. Nongenomic action of 1 alpha,25(OH)(2)-vitamin D3. Activation of muscle cell PLC gamma through the tyrosine kinase c-Src and PtdIns 3-kinase. Eur. J. Biochem. 2002;269:2506–2515. doi: 10.1046/j.1432-1033.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 39.Berchtold M.W., Brinkmeier H., Müntener M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 40.Endo I., Inoue D., Mitsui T., Umaki Y., Akaike M., Yoshizawa T., Kato S., Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 41.Bhat M., Kalam R., Qadri S.S., Madabushi S., Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. 2013;154:4018–4029. doi: 10.1210/en.2013-1369. [DOI] [PubMed] [Google Scholar]
- 42.Sleeman I., Aspray T., Lawson R., Coleman S., Duncan G., Khoo T.K., Schoenmakers I., Rochester L., Burn D., Yarnall A. The Role of Vitamin D in Disease Progression in Early Parkinson’s Disease. J. Parkinsons Dis. 2017;7:669–675. doi: 10.3233/JPD-171122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girgis C.M., Clifton-Bligh R.J., Turner N., Lau S.L., Gunton J.E. Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin. Endocrinol. 2014;80:169–181. doi: 10.1111/cen.12368. [DOI] [PubMed] [Google Scholar]
- 44.Lappe J.M., Binkley N. Vitamin D and Sarcopenia/Falls. J. Clin. Densit. 2015;18:478–482. doi: 10.1016/j.jocd.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Verde Z., Giaquinta A., Sainz C.M., Ondina M.D., Araque A.F. Bone Mineral Metabolism Status, Quality of Life, and Muscle Strength in Older People. Nutrients. 2019;11:2748. doi: 10.3390/nu11112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aspell N., Laird E., Healy M., Lawlor B., O’Sullivan M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength And Physical Performance In Community-Dwelling Older Adults: Findings From The English Longitudinal Study Of Ageing. Clin. Interv. Aging. 2019;14:1751–1761. doi: 10.2147/CIA.S222143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agergaard J., Trøstrup J., Uth J., Iversen J.V., Boesen A., Andersen J.L., Schjerling P., Langberg H. Does vitamin-D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? - a randomized controlled trial. Nutr. Metab. 2015;12:32. doi: 10.1186/s12986-015-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabrizi R., Hallajzadeh J., Mirhosseini N., Lankarani K.B., Maharlouei N., Akbari M., Asemi Z. The effects of vitamin D supplementation on muscle function among postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2019;18:591–603. doi: 10.17179/excli2019-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goswami R., Vatsa M., Sreenivas V., Singh U., Gupta N., Lakshmy R., Aggarwal S., Ganapathy A., Joshi P., Bhatia H. Skeletal muscle strength in young Asian Indian females after vitamin D and calcium supplementation: a double-blind randomized controlled clinical trial. J. Clin. Endocrinol. Metab. 2012;97:4709–4716. doi: 10.1210/jc.2012-2340. [DOI] [PubMed] [Google Scholar]
- 50.Saha S., Goswami R., Ramakrishnan L., Vishnubhatla S., Mahtab S., Kar P., Srinivasan S., Singh N., Singh U. Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: Randomized control trial. Clin. Endocrinol. 2018;88:217–226. doi: 10.1111/cen.13507. [DOI] [PubMed] [Google Scholar]
- 51.Beauchet O., Launay C.P., Galery K., Vilcocq C., Dontot-Payen F., Rousseau B., Benoit V., Allali G. Effects of Vitamin D and Calcium Fortified Yogurts on Gait, Cognitive Performances, and Serum 25-Hydroxyvitamin D Concentrations in Older Community-Dwelling Females: Results from the GAit, MEmory, Dietary and Vitamin D (GAME-D2) Randomized Controlled Trial. Nutrients. 2019;11:2880. doi: 10.3390/nu11122880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcion E., Wion-Barbot N., Montero-Menei C.N., Berger F., Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002;13:100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 53.Taghizadeh M., Djazayery A., Salami M., Eshraghian M.R., Zavareh S.A.T. Vitamin-D-free regimen intensifies the spatial learning deficit in Alzheimer’s disease. Int. J. Neurosci. 2011;121:16–24. doi: 10.3109/00207454.2010.523132. [DOI] [PubMed] [Google Scholar]
- 54.Grimm M.O.W., Lehmann J., Mett J., Zimmer V.C., Grösgen S., Stahlmann C.P., Hundsdörfer B., Haupenthal V.J., Rothhaar T.L., Herr C., et al. Impact of Vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in Alzheimer’s disease. Neurodegener. Dis. 2014;13:75–81. doi: 10.1159/000355462. [DOI] [PubMed] [Google Scholar]
- 55.Yu J., Gattoni-Celli M., Zhu H., Bhat N.R., Sambamurti K., Gattoni-Celli S., Kindy M.S. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J. Alzheimers Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landel V., Millet P., Baranger K., Loriod B., Féron F. Vitamin D interacts with Esr1 and Igf1 to regulate molecular pathways relevant to Alzheimer’s disease. Mol. Neurodegener. 2016;11:22. doi: 10.1186/s13024-016-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito S., Ohtsuki S., Nezu Y., Koitabashi Y., Murata S., Terasaki T. 1α,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1-40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS. 2011;8:20. doi: 10.1186/2045-8118-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y.-X., He L.-Y., Zhang M., Wang F., Liu F., Peng W.-X. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. doi: 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 59.Gezen-Ak D., Atasoy I.L., Candaş E., Alaylioglu M., Yılmazer S., Dursun E. Vitamin D Receptor Regulates Amyloid Beta 1-42 Production with Protein Disulfide Isomerase A3. ACS Chem. Neurosci. 2017;8:2335–2346. doi: 10.1021/acschemneuro.7b00245. [DOI] [PubMed] [Google Scholar]
- 60.DeLuca G.C., Kimball S.M., Kolasinski J., Ramagopalan S.V., Ebers G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013;39:458–484. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- 61.Almeras L., Eyles D., Benech P., Laffite D., Villard C., Patatian A., Boucraut J., Mackay-Sim A., McGrath J., Féron F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics. 2007;7:769–780. doi: 10.1002/pmic.200600392. [DOI] [PubMed] [Google Scholar]
- 62.Eyles D., Almeras L., Benech P., Patatian A., Mackay-Sim A., McGrath J., Féron F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J. Steroid Biochem. Mol. Biol. 2007;103:538–545. doi: 10.1016/j.jsbmb.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 63.Van Schoor N.M., Comijs H.C., Llewellyn D.J., Lips P. Cross-sectional and longitudinal associations between serum 25-hydroxyvitamin D and cognitive functioning. Int. Psychogeriatr. 2016;28:759–768. doi: 10.1017/S1041610215002252. [DOI] [PubMed] [Google Scholar]
- 64.Gschwind Y.J., Bischoff-Ferrari H.A., Bridenbaugh S.A., Härdi I., Kressig R.W. Association between serum vitamin D status and functional mobility in memory clinic patients aged 65 years and older. Gerontology. 2014;60:123–129. doi: 10.1159/000355667. [DOI] [PubMed] [Google Scholar]
- 65.Annweiler C., Maby E., Meyerber M., Beauchet O. Hypovitaminosis D and executive dysfunction in older adults with memory complaint: A memory clinic-based study. Dement. Geriatr. Cogn. Disord. 2014;37:286–293. doi: 10.1159/000356483. [DOI] [PubMed] [Google Scholar]
- 66.Maddock J., Zhou A., Cavadino A., Kuźma E., Bao Y., Smart M.C., Saum K.-U., Schöttker B., Engmann J., Kjærgaard M., et al. Vitamin D and cognitive function: A Mendelian randomisation study. Sci. Rep. 2017;7:13230. doi: 10.1038/s41598-017-13189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodwill A.M., Szoeke C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017;65:2161–2168. doi: 10.1111/jgs.15012. [DOI] [PubMed] [Google Scholar]
- 68.Dhesi J.K., Jackson S.H.D., Bearne L.M., Moniz C., Hurley M.V., Swift C.G., Allain T.J. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 69.Przybelski R., Agrawal S., Krueger D., Engelke J.A., Walbrun F., Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19:1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 70.Dean A.J., Bellgrove M.A., Hall T., Phan W.M.J., Eyles D.W., Kvaskoff D., McGrath J.J. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults--a randomised controlled trial. PLoS ONE. 2011;6:e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettersen J.A. Does high dose vitamin D supplementation enhance cognition? A randomized trial in healthy adults. Exp. Gerontol. 2017;90:90–97. doi: 10.1016/j.exger.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 72.Rosendahl-Riise H., Spielau U., Ranhoff A.H., Gudbrandsen O.A., Dierkes J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017;30:3–15. doi: 10.1111/jhn.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smedshaug G.B., Pedersen J.I., Meyer H.E. Can vitamin D supplementation improve grip strength in elderly nursing home residents? A double-blinded controlled trial. Scand. J. Food Nutr. 2007;51:74–78. doi: 10.1080/03461230701422528. [DOI] [Google Scholar]
- 74.Moreira-Pfrimer L.D.F., Pedrosa M.A.C., Teixeira L., Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann. Nutr. Metab. 2009;54:291–300. doi: 10.1159/000235874. [DOI] [PubMed] [Google Scholar]