Abstract
Background: The prevalence of vascular dysfunction increases with advancing age, as does the loss of muscle mass, strength and function. This systematic review explores the association between vascular dysfunction and skeletal muscle health in healthy adults. Methods: EMBASE and MEDLINE were searched for cross-sectional and randomized controlled studies between January 2009 and April 2019, with 33 out of 1246 studies included based on predefined criteria. Assessments of muscular health included muscle mass, strength and function. Macrovascular function assessment included arterial stiffness (pulse wave velocity or augmentation index), carotid intima-media thickness, and flow-mediated dilation. Microvascular health assessment included capillary density or microvascular flow (contrast enhanced ultrasound). Results: All 33 studies demonstrated a significant association between vascular function and skeletal muscle health. Significant negative associations were reported between vascular dysfunction and -muscle strength (10 studies); -mass (9 studies); and -function (5 studies). Nine studies reported positive correlations between muscle mass and microvascular health. Conclusions: Multiple studies have revealed an association between vascular status and skeletal muscle health in healthy adults. This review points to the importance of screening for muscle health in adults with vascular dysfunction with a view to initiating early nutrition and exercise interventions to ameliorate functional decline over time.
Keywords: vascular dysfunction, skeletal muscle mass, healthy adults
1. Introduction
The aging process is responsible for a variety of detrimental physiological changes within the human body, including losses of skeletal muscle mass, strength and function (termed sarcopenia) [1,2]. Over time, there is a progressive decline in the number and size of muscle fibers resulting in a total decrease in muscle mass of around 40% between the ages of 25 and 80 years [3]. The numerous negative consequences of sarcopenia are well-established and include an increased risk of falls, fractures, hospitalization, frailty, decreased quality of life and even death [4,5]. As such, sarcopenia has been shown to pose a significant economic burden to health care systems [6]. The prevalence of sarcopenia in community dwelling older adults is estimated to range from 7 to 73.3% in long-term care homes and between 22% and 87% in assisted-living facilities [7,8]. In addition, the progression of sarcopenia is complex and multifactorial, with environmental influences such as physical inactivity, nutrient deficiencies and oxidative stress all postulated to have a role [1,9].
Aging is also a major risk factor for cardiovascular (CV) disease, accounting for 17.3 million deaths per year and projected to be 23.6 million by 2030 globally [10,11]. Multiple studies show a relationship between low muscle mass and increased risk of CV-related mortality [12,13,14]. CV disease includes diseases associated with both the heart and the vasculature, with vascular disease encompassing both macro- and microvascular dysfunction.
Even in disease-free aging, it is postulated that age-related declines in macrovascular blood flow to appendicular regions could play a substantial role in determining muscle health. Macrovascular flow is generally assessed via conduit artery function by measuring arterial blood flow via Doppler ultrasound, arterial stiffness (measured by pulse wave velocity (PWV) or augmentation index (AI)), carotid intima-media thickness, and/or flow-mediated dilation (FMD), which is an estimate of endothelial function. It has been demonstrated that older individuals exhibit 20–30% reductions in limb conduit artery blood flow under both post-absorptive [15] and postprandial conditions [16] when compared to younger adults, possibly due to endothelial dysfunction [17,18]. Such blunted blood flow responses may contribute to age-related declines in anabolic responses to feeding by reducing the delivery and/or utility of insulin and amino acids (AA) in muscle [19,20]. In addition, more recently, Rodriguez and colleagues examined pulse wave velocity as an indicator of arterial stiffness in a meta-analysis and demonstrated that lower muscle mass is significantly associated with increased arterial stiffness [21].
In addition to macrovascular blood flow, ‘microvascular/capillary’ blood flow or perfusion is a critical mediator of insulin and AA delivery to the muscle [22,23,24]. Microvascular blood flow is not routinely measured in clinical practice, but for research purposes can be assessed using contrast enhanced ultrasound (CEUS) [25]. Capillary density and endothelial function are also good indicators of microvascular function—both have been shown to decline with age and improve in response to exercise training [26,27]. It has been suggested that reduced microvascular blood flow may contribute to the anabolic resistance of muscle [28] which is observed with advancing age [20,29,30], however, data on this is not yet conclusive [31].
This systematic review compiles studies that examine both skeletal muscle and vascular health in healthy adult populations. However, the prevalence of hypertension in otherwise healthy adults is estimated to be ~29% with progressive increases due to age [32]. Consequently, we expanded the search to include hypertensive adults without other health complications. While the included studies demonstrate various relationships between skeletal muscle health and vascular health, it remains unclear as to whether there is a causal relationship between the two. Given that there are some shared risk factors for declines in both systems, the relationship between them should be explored in a systematic way [21]. One previous systematic review attempted to do this but focused specifically on arterial stiffness assessed through PWV and did not explore other indices of macro or microvascular status [21]. Therefore, the aim of this systematic review is to determine if there is a relationship between skeletal muscle health and both macro- and microvascular function.
2. Materials and Methods
2.1. Literature Search
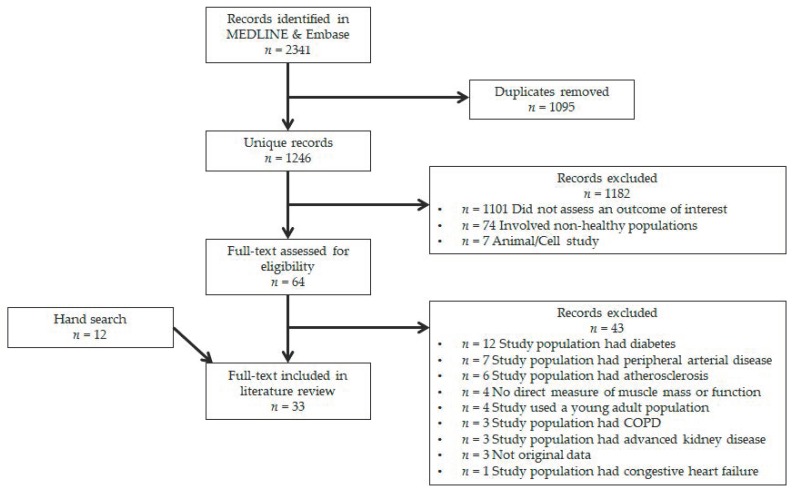
The electronic databases EMBASE and MEDLINE were searched for original articles between January 2009 and April 2019. Evidence from cross-sectional and randomized controlled studies was included. All relevant articles were assessed for pre-defined criteria regarding study outcome, population and design. Database searching identified 2341 records of which 1095 were duplicates, leaving 1246 unique records for title and abstract screening. Following screening, 1182 records were excluded based on lack of assessment of an outcome of interest (n = 1101), inclusion of non-healthy participants (n = 74) and animal/cell studies (n = 7), leaving 64 records for full-text review. Of these, 43 records were excluded primarily due to inclusion of diseased participants (n = 32), leaving a total of 21 records. Through hand-searching full-text articles and references, we identified 12 more records which satisfied all criteria for inclusion. As such, a total of 33 records were included in this systematic review. A flow diagram is illustrated in Figure 1.
Figure 1.
Study selection flow diagram.
2.2. Inclusion Criteria
The primary inclusion criteria were based on the assessment of certain outcomes, specifically validated measurements of vascular function in combination with validated measurements of skeletal muscle health. Detailed descriptions of the included validated markers for skeletal muscle health assessments were split into three general categories: (1) muscle mass, (2) muscle strength and (3) muscle function and are described in Section 2.5. Arterial function measurements and markers that were included are described in Section 2.6.
Animal and cell-culture studies were not considered, nor were human studies involving diseased populations (diabetes, peripheral arterial disease, atherosclerosis, chronic obstructive pulmonary disease, kidney disease, cancer, or heart failure). Due to the prevalence of hypertension in otherwise healthy adults, the inclusion criteria did allow studies involving hypertensive adults with no other disease. Studies with professional athletic populations were not considered.
2.3. Data Extraction
Titles and abstracts resulting from the literature search were evaluated by two independent investigators. Disagreements were resolved by consensus, or by consulting with a third investigator if required. Studies were grouped according to skeletal muscle health assessments and vascular function assessments. Data were extracted and collated on the following study characteristics: reference, study design, participants (human, number of subjects, age, disease status, and gender ratio), skeletal muscle health measurements and vascular function measurements.
2.4. Study Details and Sample Demographics
All 33 studies were either cross-sectional or randomized controlled trials published within the past 10 years (Table 1). Twenty-three studies [20,26,27,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] included healthy mixed-age adults (age range 18–95) and two studies [53,54] included healthy community-dwelling older adults (age range 65–81). Six studies sampled participants with hypertension [55,56,57,58,59,60], one study sampled individuals with diabetes as a comparator to healthy aged population [61], and one study [62] did not report participant demographics. Study populations varied from a minimum of 6 [20] to a maximum of 3356 [42] participants. Eight studies included only men [25,27,37,42,49,50,56,61] and four studies included only women [38,52,53,60]. Most studies reported the age range of the participants, except for two studies with one claiming to study “middle-aged” adults [46] and one using adult participants [62].
Table 1.
Study details and sample demographics.
| Study & Year | Design | Sample | Country | n | Male (%) | Age | Exclusion |
|---|---|---|---|---|---|---|---|
| Barnouin 2017 [33] | Cross-sectional | Healthy adults | UK | 47 | 77 | 22–74 | Cardiovascular, neuromuscular, or respiratory diseases |
| Barrera 2014 [35] | Cross-sectional | Healthy adults | Chile | 259 | 49 | 29–88 | Undernutrition BMI < 18, cancer, autoimmune disease, kidney, liver or cardiac failure, diabetes, cognitive impairment, steroids or HRT |
| Brightwell 2018 [26] | RCT | Healthy adults | USA | 23 | 30 | 65–82 | Diabetes, cancer, smoking, CVD, kidney disease, uncontrolled high blood pressure, low daily protein intake |
| Chung 2018 [36] | Cross-sectional | Healthy adults | Korea | 1590 | 78 | 40–79 | Metabolic syndrome, HRT, any medication |
| den Ouden 2013 [37] | Cross-sectional | Healthy adults | The Netherlands | 403 | 100 | 73–91 | Inability to visit the study center independently |
| Dipla 2017 [55] | Cross-sectional | Healthy and hypertensive adults | Greece | 91 | 60 | 31–55 | CVD, diabetes |
| Fahs 2017 [39] | Cross-sectional | Healthy adults | USA | 71 | 51 | 18–75 | Hypertension, participation in regular exercise, HRT |
| Gonzales 2015 [40] | Cross-sectional | Healthy adults | USA | 45 | 44 | 60–78 | CVD, diabetes, pulmonary disease, HRT, obesity, medication for blood pressure or cholesterol |
| Groen 2014 [61] | Cross-sectional | Healthy and T2DM adults | The Netherlands | 45 | 100 | 23–71 | Impaired renal or liver function, obesity, CVD, hypertension, advanced diabetes, insulin therapy |
| Gueugneau 2016 [56] | Cross-sectional | Healthy and hypertensive adults | France | 37 | 100 | 21–74 | Prior myocardial infarction or stroke, heart failure, atrial fibrillation, diabetes, morbid obesity, Parkinson’s disease |
| Heffernan 2012 [41] | Cross-sectional | Healthy adults | USA | 24 | 46 | 70–85 | Acute/terminal illness, coronary heart disease, myocardial infarction, hypertension, neuromuscular disease, HRT, diabetes, renal disease, BMI > 32.5 |
| Im 2017 [42] | Cross-sectional | Healthy adults | Korea | 3356 | 100 | 40–64 | ABI < 0.9, high WBC count, cancer |
| Khoudary 2015 [38] | Cross-sectional | Healthy adults | USA | 1103 | 0 | 56–62 | Stroke, angina or myocardial infarction, hysterectomy or bilateral oophorectomy, pregnancy, HRT |
| Kohara 2017 [43] | Cross-sectional | Healthy adults | Japan | 1518 | 40 | 60–74 | CVD, PAD, stroke, coronary heart disease, and congestive heart failure |
| Lee 2014 [44] | Cross-sectional | Healthy adults | Korea | 427 | 42 | 52–95 | n/r or unclear |
| Lima-Junior 2018 [57] | Cross-sectional | Hypertensive adults | Brazil | 72 | 28 | 48–68 | <18 years, smoking, diabetics, CVD, inability to perform isometric handgrip, enrolled in physical activity program |
| Mitchell 2013 [25] | RCT | Healthy adults | UK | 36 | 100 | 18–75 | Diabetes, CVD, BMI < 18 or >28 |
| Ochi 2010 [46] | Cross-sectional | Healthy adults | Japan | 496 | 36 | n/r | Stroke, TIA, coronary heart disease and congestive heart failure |
| Phillips 2012 [34] | RCT | Healthy adults | UK | 51 | 57 | 21–72 | Muscle wasting, metabolic or respiratory diseases, CVD, chronic diseases |
| Prior 2016 [47] | Cross-sectional | Healthy adults | USA | 76 | 42 | 45–80 | Coronary artery disease, heart failure, PAD, stroke, liver, kidney or lung disease, smoking |
| Sampaio 2014 [58] | Cross-sectional | Healthy and hypertensive adults | Japan | 175 | 48 | 70–77 | Moderate cognitive impairment, uncontrolled cardiovascular, pulmonary or metabolic diseases, stroke, Parkinson’s disease, PAD, orthopedic disease |
| Sanada 2010 [48] | Cross-sectional | Healthy adults | Japan | 1488 | 29 | 18–85 | CVD, beta-blockers, HRT, athletes |
| Shimizu 2017 [59] | Cross-sectional | Hypertensive adults | Japan | 795 | 57 | 60–89 | Participants without hypertension, BMI < 18.5, high BP, history of stroke |
| Shiotsu 2018 [49] | RCT | Healthy adults | Japan | 45 | 100 | 63–85 | Current participation in structured exercise program, CVD, musculoskeletal disease, diabetes |
| Suwa 2018 [50] | Cross-sectional | Healthy adults | Japan | 1354 | 100 | 35–59 | CVD, history of stroke |
| Timmerman 2012 [20] | RCT | Healthy older adults | USA | 6 | 50 | 67–73 | Obesity, chronic diseases |
| Verdijk 2016 [27] | RCT | Healthy adults | The Netherlands | 30 | 100 | 19–83 | CVD, PAD, diabetes, inability to participate in an exercise program |
| Wong 2018 [60] | RCT | Hypertensive adults | Korea | 41 | 0 | 49–67 | Pre-menopause, CVD, diabetes, HRT, smoking, exercise, endocrine disorders, psychiatric disorders |
| Wüst 2009 [62] | Cross-sectional | Adults | UK | 11 | 45 | n/r | n/r or unclear |
| Yamamoto 2009 [51] | Cross-sectional | Healthy adults | Japan | 526 | 34 | 20–83 | Obesity, chronic diseases, smoking, ABI < 0.9, any medication |
| Yoo 2018 [53] | Cross-sectional | Community-dwelling older adults | Korea | 236 | 0 | 67–79 | CVD, cognitive disorder, malignancy |
| Yoshizawa 2009 [52] | RCT | Healthy adults | Japan | 35 | 0 | 32–59 | Chronic diseases, smoking, any medication |
| Zhang 2019 [54] | Cross-sectional | Community-dwelling older adults | China | 1002 | 42 | 65–81 | n/r or unclear |
Abbreviations: BMI: Body Mass Index; RCT: Randomized Controlled Trial; HRT: Hormone Replacement Therapy; CVD: Cardiovascular Disease; T2DM: Type 2 Diabetes Mellitus; ABI: Ankle-Brachial Index; WBC: White Blood Cells; PAD: Peripheral Artery Disease; TIA: Transient Ischemic Attack; n/r: Not reported.
2.5. Assessment of Skeletal Muscle Health
Muscle mass measurements were obtained by: (i) bioelectrical impedance analysis (BIA), (ii) computed tomography (CT), (iii) dual-energy X-ray absorptiometry (DXA) and (iv). muscle fiber cross sectional area analyses (commonly measured through muscle biopsy analysis). Muscle strength assessments were limited to: (i) hand-grip strength, (ii) torque measurements using an isokinetic dynamometer, and (iii) repetition-maximum exercises. Muscle function assessments were largely varied and included: (i) arm extensibility, (ii) 40-foot walking speed, (iii) sit and reach, (iv) sit-to-stand, (v) timed up and go (TUG), (vi) 12-min walk distance, (vii) perceived fatigue after a fast-pace 400-m walk, (viii) 10-m walking speed and ix. aerobic capacity. Table 2 indicates details of the assessment tools used to estimate skeletal muscle health. Most studies reported a measurement of skeletal muscle strength [26,27,34,35,36,37,39,41,49,53,55,57,59,60]. Thirteen studies assessed muscle mass [20,25,27,34,39,42,43,44,47,48,54,58,61] and six studies assessed muscle fiber cross-sectional area (CSA) [33,46,47,56,61,62]. Seven studies examined muscle function [35,38,40,49,50,51,52], one study assessed muscular power [41] and one study assessed the muscle anabolic response [20]. Body composition was measured as whole body lean mass [27,34,35,38,39,40,42,43,44,47,48,49,51,52,54,58] or as appendicular lean mass [20,25,26,33,36,37,41,46,50,53,55,56,57,59,60,61,62]. To assess skeletal muscle mass various modalities were utilized including BIA [42,43,44,54,58], DXA [25,27,34,39,47,48] and CT [27,43,46,47,61]. Muscle biopsies were obtained from the vastus lateralis muscle to analyze muscle fiber CSA [27,33,56,61,62]. Muscular strength and power were measured using various assessments including hand-grip strength [35,36,37,49,53,55,57,59], leg extension one-repetition maximum (1-RM) [27,39,41,49,52], peak leg torque [26] and leg extension eight-repetition maximum (8-RM) [34,60]. The muscle anabolic response was measured as muscle protein synthesis rate [20]. Muscle function was the most varied assessment and included measurements of sit and reach [49,51], sit-to-stand [38], TUG [35], 12-min walk distance [35], perceived fatigue after a fast-pace 400-m walk [40], 40-foot walking speed [38], 10-m walking speed [49], aerobic capacity [52] and arm extensibility [50] (Table 2).
Table 2.
Assessment of skeletal muscle health.
| Study & Year | Parameter | Region | Modality | Device |
|---|---|---|---|---|
| Brightwell 2018 * [26] | Muscular strength | Appendicular | Peak leg torque | Biodex isokinetic dynamometer |
| Chung 2018 [36] | Muscular strength | Appendicular | Hand-grip strength | Hand-grip dynamometer |
| den Ouden 2013 [37] | Muscular strength | Appendicular | Hand-grip strength | JAMAR hand-grip dynamometer |
| Dipla 2017 [55] | Muscular strength | Appendicular | Hand-grip strength | Biopac hand-grip dynamometer |
| Lima-Junior 2018 [57] | Muscular strength | Appendicular | Hand-grip strength | Hand-grip dynamometer |
| Shimizu 2017 [59] | Muscular strength | Appendicular | Hand-grip strength | Smedley hand-grip dynamometer |
| Wong 2018 [60] | Muscular Strength | Appendicular | 8-RM | Cybex dynamometer |
| Yoo 2018 [53] | Muscular strength | Appendicular | Hand-grip strength | T.K.K hand-grip dynamometer |
| Im 2017 [42] | Muscle mass | Whole body | BIA | Inbody |
| Kohara 2017 [43] | Muscle mass | Whole body | BIA, CT | Omron, GE |
| Lee 2014 [44] | Muscle mass | Whole body | BIA | InBody |
| Mitchell 2013 * [25] | Muscle mass | Appendicular | DXA | Lunar |
| Sampaio 2014 [58] | Muscle mass | Whole body | BIA | n/r |
| Sanada 2010 [48] | Muscle mass | Whole body | DXA | Hologic |
| Timmerman 2012 * [20] | Muscle anabolic response | Appendicular | Muscle biopsy | Bergström needle |
| Zhang 2019 [54] | Muscle mass | Whole body | BIA | InBody |
| Barnouin 2017 * [33] | Muscle CSA | Appendicular | Muscle biopsy | Bergström needle |
| Gueugneau 2016 * [56] | Muscle CSA | Appendicular | Muscle biopsy | Bergström needle |
| Ochi 2010 [46] | Muscle CSA | Appendicular | CT | GE |
| Wüst 2009 * [62] | Muscle CSA | Appendicular | Muscle biopsy | Percutaneous needle |
| Gonzales 2015 [40] | Muscular function | Whole body | 400 m walk | Polar monitor (to track HR during walk) |
| Khoudary 2015 [38] | Muscular function | Whole body | 40-foot walking speed, sit-to-stand test | n/r |
| Suwa 2018 [50] | Muscular function | Appendicular | Arm extensibility test | n/r |
| Yamamoto 2009 [51] | Muscular function | Whole body | Sit and reach test | Takei Scientific digital flexibility testing device |
| Yoshizawa 2009 [52] | Muscular function | Whole body | 1-RM, aerobic capacity | Selectorized weight machines, cycle ergometer |
| Barrera 2014 [35] | Muscular strength and function | Whole body | 12-min walk, TUG, hand-grip strength | Digital force transducer and hand-grip dynamometer |
| Fahs 2017 [39] | Muscular strength and muscle mass | Whole body | 1-RM, DXA | Selectorized weight machines, Hologic |
| Groen 2014 * [61] | Muscle mass and CSA | Appendicular | CT, muscle biopsy | n/r, percutaneous needle |
| Heffernan 2012 [41] | Muscle strength and power | Appendicular | 1-RM | Keiser Sports |
| Phillips 2012 [34] | Muscle mass and strength | Whole body | DXA, leg extension 75% 1-RM | Lunar, Leisure Lines |
| Prior 2016 * [47] | Muscle mass and CSA | Whole body | DXA, CT | Lunar, Siemens |
| Shiotsu 2018 [49] | Muscular strength and function | Whole body | 1-RM, hand-grip strength, 10-m walk, sit and reach test | Leg press/curl, chest/shoulder press, seated row, hand-grip dynamometer |
| Verdijk 2016 * [27] | Muscular strength and muscle mass | Whole body | 1-RM, CT, DXA, muscle biopsy | Technogym, Phillips Medical, GE, percutaneous needle |
* Microvascular only assessment (no asterisk indicates macrovascular only); Abbreviations: RM: Repetition Maximum; BIA: Bioelectrical Impedance Analysis; CT: Computed Tomography; DXA: Dual-Energy X-ray Absorptiometry; CSA: Muscle Fiber Cross-Sectional Area; TUG: The Timed Up and Go Test; n/r: Not reported.
2.6. Assessment of Vascular Health
Common validated markers of arterial function were: (i) pulse wave velocity, (ii) augmentation index, (iii) carotid intima-media thickness and (iv). flow-mediated dilation. Table 3 indicates details of the assessment tools used to measure vascular health. Macrovascular and/or microvascular health was assessed in the included studies, with the majority examining macrovascular blood flow. Macrovascular health was primarily assessed as arterial stiffness using multiple measurements of either pulse wave velocity (PWV) [36,39,40,42,43,46,48,49,51,52,54,58,60], radial augmentation index [41,44,57], carotid intima-media thickness (CIMT) [35,37,38,50,59] or flow-mediated dilation (FMD) [41]. CIMT was assessed using ultrasound [35,37,38,50,59] and radial augmentation index was measured using applanation tonometry [41,44,57]. For the studies assessing PWV, seven studies assessed brachial-ankle PWV [36,43,46,48,51,54,60], four studies assessed carotid-femoral PWV [39,40,49,52] and two studies assessed carotid-ankle PWV [42,58]. PWV was measured using a volume-plethysmographic apparatus [36,43,46,48,51,52,54,60], an oscillometric apparatus [42,49,58] and using applanation tonometry [39,40]. FMD was assessed using ultrasound [34,41] and applanation tonometry [53]. Microvascular health was assessed primarily through histology staining to determine capillary density in a vastus lateralis muscle biopsy [26,27,33,47,56,61,62] and through microvascular blood flow using contrast-enhanced ultrasound (CEUS) with Sonovue microbubbles [25]. One study assessed muscle perfusion using a muscle oxygenation apparatus [55].
Table 3.
Assessment of vascular health.
| Study & Year | Parameter | Vascular Site | Method | Device |
|---|---|---|---|---|
| Chung 2018 [36] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Fahs 2017 [39] | PWV | Carotid-femoral | Applanation tonometry | SphygmoCor |
| Gonzales 2015 [40] | PWV | Carotid-femoral | Applanation tonometry | SphygmoCor |
| Im 2017 [42] | PWV | Carotid-ankle | Oscillometric | Fukuda Denshi |
| Kohara 2017 [43] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Ochi 2010 [46] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Sampaio 2014 [58] | PWV | Carotid-ankle | Oscillometric | Fukuda Denshi |
| Sanada 2010 [48] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Shiotsu 2018 [49] | PWV | Carotid-femoral | Oscillometric | Fukuda Denshi |
| Wong 2018 [60] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Colin Medical |
| Yamamoto 2009 [51] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Yoshizawa 2009 [52] | PWV | Carotid-femoral | Volume-plethysmographic apparatus | Colin Medical |
| Zhang 2019 [54] | PWV | Brachial-ankle | Volume-plethysmographic apparatus | Omron |
| Barrera 2014 [35] | CIMT | Carotid | Ultrasound | GE |
| den Ouden 2013 [37] | CIMT | Carotid | Ultrasound | ATL Ultramark IV |
| Khoudary 2015 [38] | CIMT | Carotid | Ultrasound | Teratech Corp |
| Shimizu 2017 [59] | CIMT | Carotid | Ultrasound | GE |
| Suwa 2018 [50] | CIMT | Carotid | Ultrasound | Aplio |
| Heffernan 2012 [41] | Aix | Radial | Applanation tonometry | Omron |
| Lee 2014 [44] | Aix | Radial | Applanation tonometry | SphygmoCor |
| Lima-Junior 2018 [57] | Aix | Radial | Applanation tonometry | EndoPAT |
| Dipla 2017 [55] | FMD | Brachial | Muscle oxygenation apparatus | NIRS Artinis |
| Yoo 2018 [53] | FMD | Brachial | Applanation tonometry | EndoPAT |
| Phillips 2012 [34] | LBF | Femoral | Doppler ultrasound | Toshiba |
| Barnouin 2017 * [33] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Brightwell 2018 * [26] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Groen 2014 * [61] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Gueugneau 2016 * [56] | C: F Ratio | Femoral | Immunohistochemistry | Bergström needle |
| Prior 2016 * [47] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Verdijk 2016 * [27] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Wüst 2009 * [62] | C: F Ratio | Femoral | Immunohistochemistry | Percutaneous needle |
| Mitchell 2013 * [25] | MBF | Femoral | Contrast-enhanced ultrasound | Sonovue |
| Timmerman 2012 * [20] | MBF | Femoral | Doppler ultrasound | Philips ATL |
* Microvascular only assessment (no asterisk indicates macrovascular only); Abbreviations: PWV: Pulse Wave Velocity; CIMT: Carotid Intima Media Thickness; Aix: Radial Augmentation Index; FMD: Flow Mediated Dilation; LBF: Leg Blood Flow; C: F Ratio: Capillary to Fiber Ratio; MBF: Microvascular Blood Flow.
3. Results
3.1. Macrovascular Studies
Macrovascular studies focus on dysfunction at the level of the large conduit arteries, which transport blood away from the heart. Most studies included in this review focused on macrovascular analyses with detailed descriptions of the included macrovascular studies presented in Table 4.
Table 4.
Association between skeletal muscle and macrovascular blood flow.
| Study & Year | Sample | Age | Muscle and Vascular Association | Type of Association | Finding |
|---|---|---|---|---|---|
| Barrera 2014 [35] | Healthy adults | 29–88 | Muscular strength/function and CIMT | Difference between groups (p < 0.05) † | In older adults, CIMT is negatively associated with muscular strength and function |
| Chung 2018 [36] | Healthy adult men | 40–79 | Muscular strength and PWV | Difference between groups (p < 0.05) † | In middle-aged and older adults, arterial stiffness is negatively associated with muscular strength and function |
| den Ouden 2013 [37] | Healthy older men | 73–91 | Muscular strength and CIMT | Correlation (r = −0.17; p < 0.05) | In older men, CIMT is negatively associated with muscular strength |
| Dipla 2017 # [55] | Healthy and hypertensive adults | 31–55 | Muscular strength and muscle perfusion | Difference between groups (p < 0.05) † | Hypertensive adults have reduced tissue oxygen saturation compared to healthy controls; to produce same amount of torque compared to healthy controls requires a two-fold increase in BP |
| Fahs 2017 [39] | Healthy adults | 18–75 | Muscular strength and PWV | Correlation (r = −0.230/−0.484; p < 0.05) | In adults, arterial stiffness is negatively correlated with absolute and relative muscular strength |
| Gonzales 2015 [40] | Healthy older adults | 60–78 | Muscular function and PWV | Beta coefficient (p < 0.05) | In older adults, arterial stiffness is positively correlated with muscle fatigue |
| Heffernan 2012 [41] | Healthy older adults | 70–85 | Muscular power and augmentation index | Correlation (r = -0.54; p < 0.05) | In older adults, arterial stiffness is negatively associated with muscular power |
| Im 2017 [42] | Healthy adult men | 40–64 | Muscle mass and PWV | Correlation (p < 0.05) | In middle-aged men, arterial stiffness is negatively correlated with muscle mass |
| Khoudary 2015 [38] | Healthy older women | 56–62 | Muscle function and CIMT | Beta coefficient (0.028; p < 0.05) | In older women, CIMT is negatively associated with muscle function |
| Kohara 2017 [43] | Healthy older adults | 60–74 | Muscle mass and PWV | Correlation (r = −0.24; p < 0.05) | In older adults, arterial stiffness is negatively correlated with muscle mass |
| Lee 2014 [44] | Healthy older adults | 52–95 | Muscle mass and augmentation index | Beta coefficient (p < 0.05) | In older adults, arterial stiffness is negatively associated with muscle mass |
| Lima-Junior 2018 # [57] | Hypertensive older adults | 48–68 | Muscular strength and augmentation index | Beta coefficient (−0.49; p < 0.05) | In older adults with hypertension, arterial stiffness is negatively associated with muscular strength |
| Ochi 2010 [46] | Healthy adults | n/r | Muscle CSA and PWV | Correlation (r = −0.34; p < 0.05) | In men, arterial stiffness is negatively associated with muscle mass |
| Phillips 2012 * [34] | Healthy adults—resistance exercise | 21–72 | Muscle mass/strength and leg blood flow | Difference between groups (p < 0.05) † | Following resistance exercise training, adults experience increases in leg blood flow, muscle mass and strength regardless of age in response to feeding |
| Sampaio 2014 # [58] | Healthy and hypertensive older adults | 70–77 | Muscle mass and PWV | Odds ratio (1.82; p < 0.05) | In healthy and hypertensive older adults, arterial stiffness is negatively associated with muscle mass |
| Sanada 2010 [48] | Healthy adults | 41–71 | Muscle mass and PWV | Difference between groups (p < 0.05) † | Women with sarcopenia have higher arterial stiffness compared to healthy controls |
| Shimizu 2017 # [59] | Hypertensive older adults | 60–89 | Muscular strength and CIMT | Difference between groups (p < 0.05) † | In older adults with hypertension, CIMT is negatively associated with muscular strength |
| Shiotsu 2018 * [49] | Healthy older men—resistance exercise | 63–85 | Muscular strength/function and PWV | Difference between groups (p < 0.05) † | Following resistance exercise training, older men experience a decrease in arterial stiffness and an increase in muscular strength/function |
| Suwa 2018 [50] | Healthy adult men | 35–59 | Muscular function and CIMT | Beta coefficient (−0.189; p < 0.05) | In middle-aged adults, CIMT is negatively associated with arm flexibility |
| Wong 2018 *# [60] | Hypertensive older women—stair climbing exercise | 49–67 | Muscular strength and PWV | Correlation (r = −0.47; p < 0.05) | Following stair climbing training, hypertensive older women experience a decrease in arterial stiffness and an increase in muscular strength |
| Yamamoto 2009 [51] | Healthy adults | 40–83 | Muscular function and PWV | Correlation (r = 0.17/0.45; p < 0.05) | In middle-aged and older adults, arterial stiffness is negatively correlated with flexibility |
| Yoo 2018 [53] | Older women | 67–79 | Muscular strength and endothelial function | Correlation (r = 0.176; p < 0.05) | After adjusting for comorbidities, in older women, endothelial function is positively correlated with muscular strength |
| Yoshizawa 2009 * [52] | Healthy women—aerobic exercise | 32–59 | Muscular function and PWV | Difference between groups (p < 0.05) † | Following aerobic training, middle-aged women experience a decrease in arterial stiffness and an increase in muscular function |
| Zhang 2019 [54] | Older adults | 65–81 | Muscle mass and PWV | Odds ratio (1.11; p < 0.05) | After adjusting for comorbidities, in older adults, arterial stiffness is negatively associated with muscle mass |
* Exercise intervention study; # hypertensive population; † correlation coefficient not reported; Abbreviations: CIMT: Carotid Intima-Media Thickness; PWV: Pulse Wave Velocity; CSA: Mid-thigh Muscle Cross-sectional Area; n/r: Not reported.
Body composition, specifically muscle mass, appears to have a strong association with arterial health. Given that PWV is the most commonly used assessment of arterial stiffness, six studies used PWV as a measurement of arterial health. These studies were conducted in middle-aged and older adults and revealed a negative correlation between PWV and muscle mass [42,43,46,48,54,58]. Using the radial augmentation index as a measurement of arterial stiffness, a single study found an inverse relationship between limb muscle mass and arterial function [44]. These correlations are indicative of greater arterial stiffness in individuals with lower muscle mass. These publications collectively suggest that skeletal muscle mass has an association with arterial health, specifically arterial wall elasticity.
In addition to skeletal muscle mass, skeletal muscle strength is an important indicator of overall muscle health. Hand-grip strength has been correlated with changes in muscle function and joint disability scores [63], disease activity states [64] and even all-cause mortality [65]. Multiple studies in this review reported that increased CIMT, a common measurement of arterial stiffness, is associated with impaired hand-grip strength [35,37,59]. Using other measurements of arterial stiffness, such as radial augmentation index [57] and pulse wave velocity [36], other studies show the same association between higher arterial stiffness and lower grip strength. One included study demonstrated that higher PWV is associated with lower absolute and relative muscle strength assessed using selected weight machines [39]. This observation is important as it directly demonstrates whole body strength loss and not solely hand-grip strength impairment. One included study demonstrated that decreased muscle strength is associated with endothelial dysfunction [53]. The authors of this study suggest that endothelial function plays an important role in overall muscle health. Additionally, increased arterial stiffness measured through radial augmentation index is associated with impaired leg power during leg press exercise [41]. These studies provide evidence for an association between skeletal muscle strength and arterial dysfunction.
Impaired skeletal muscle function also shows an association with macrovascular dysfunction. Various mobility tests are predictive of the onset of disability, hospitalization and mortality [66,67,68,69,70]. This association is important given that many included studies in this review demonstrate the correlation between arterial stiffness and poor performance on tests of muscle function. For example, increased PWV is associated with increased fatigue during a 400-m walk test [40] and increased CIMT is associated with poor performance on the 40-foot walking speed test [38], 12-min walk test [35] and the timed up and go test [35]. In addition, studies show impairments in muscle flexibility with arterial dysfunction. Increased arterial stiffness is associated with poor performance on multiple flexibility tests including sit and reach [51], and arm extensibility tests [50]. Furthermore, increased arterial stiffness is associated with poor performance on a common test of muscle function, the sit-to-stand test [38]. These studies suggest that the overall muscle function is impaired with arterial dysfunction.
Most included studies examined healthy adult populations. Nonetheless, the prevalence of hypertension in otherwise healthy adults is estimated to be ~29% [32] and increases as people age. Therefore, we examined relevant studies that included hypertensive participants [55,56,57,58,59,60]. All six studies involved middle-aged and older adults. Five of the six studies reported that arterial stiffness had a significant negative association with muscular strength [57,59,60] and muscle mass [58]. These studies suggest an additional burden of muscle dysfunction in hypertensive populations.
Exercise interventions that increase muscle mass, strength and function were also found to increase overall macrovascular health. One study included a group of middle-aged women that were subjected to 12 weeks of aerobic training [52]. Following the training program, participants experienced a concomitant decrease in arterial stiffness and increase in muscular function through increased strength on various leg exercises and increased aerobic capacity on the cycle ergometer [52]. One group combined both aerobic and resistance exercise into 2 times per week workouts lasting 10 weeks [49]. Upon completion of the study, participants experienced a decrease in arterial stiffness and an increase skeletal muscle strength through significant increases in leg press, chest press, shoulder press, leg curl and seated row exercises [49]. One study subjected young, middle-aged and older participants to progressive resistance exercise training three times a week for twenty weeks [34]. Upon completion of the training, researchers reported significant improvements in skeletal muscle mass and strength, as well as leg blood in response to feeding and exercise [34]. Lastly, a 12-week stair climbing exercise intervention in hypertensive older women increased skeletal muscle strength measured by 8-RM on a leg extension machine and decreased arterial stiffness [60]. This study is noteworthy as it demonstrates that it is possible to improve skeletal muscle health together with macrovascular health despite the presence of hypertension. Overall, these studies show the concomitant positive response of the skeletal muscle and arterial function to exercise, suggesting an intimate connection between the two.
3.2. Microvascular Studies
Arteries branch into arterioles which further branch into capillaries, which are primarily responsible for distributing blood carrying nutrients and oxygen to muscle tissues beds. Thus, it is critical to examine the relationship between the muscle microvasculature and skeletal muscle health. Both higher capillary density and/or greater microvascular flow within the muscle (muscle perfusion) offers potential for greater diffusion of substrates, oxygen, hormones, and nutrients, thereby enhancing skeletal muscle mass and function. Detailed descriptions of the included microvascular studies are presented in Table 5.
Table 5.
Association between skeletal muscle and microvascular blood flow.
| Study & Year | Sample | Age | Muscle and Vascular Association | Type of Association | Finding |
|---|---|---|---|---|---|
| Barnouin 2017 [33] | Healthy adults | 22–74 | Muscle fiber CSA and capillary density | Correlation (R2 = 0.46; p < 0.05) | In young and older adults, capillary-to-fiber ratio is positively correlated with muscle mass |
| Brightwell 2018 * [26] | Healthy older adults—aerobic exercise | 65–82 | Muscular strength and capillary density | Difference between groups (p < 0.05) † | Following aerobic training, older adults experience an increase in capillary density and an increase in muscular strength |
| Groen 2014 [61] | Healthy adult men | 23–71 | Muscle fiber CSA and capillary density | Difference between groups (p < 0.05) † | Older adults have reduced capillary-to-fiber ratio and muscle mass compared to young controls |
| Gueugneau 2016 # [56] | Healthy and hypertensive older men | 72–74 | Muscle fiber CSA and capillary density | Difference between groups (p < 0.05) † | Older adults with hypertension have lower capillary-to-fiber ratio and muscle mass compared to healthy older controls |
| Mitchell 2013 [25] | Healthy adult men | 18–75 | Muscle mass and microvascular blood flow | Difference between groups (p < 0.05) † | Young adults have higher muscle mass and have higher microvascular blood flow in response to feeding compared to healthy older adults |
| Prior 2016 [47] | Healthy adults | 45–80 | Muscle mass and capillary density | Correlation (r = 0.30–0.37; p < 0.05) | In adults, capillary-to-fiber ratio is positively correlated with muscle mass |
| Timmerman 2012 [20] | Healthy older adults—aerobic exercise | 67–73 | Muscle protein synthesis and microvascular blood flow | Difference between groups (p < 0.05) † | Following aerobic exercise, older adults experience improved microvascular flow and muscle protein synthesis |
| Verdijk 2016 * [27] | Healthy older adults—resistance exercise | 65–83 | Muscle fiber CSA/strength and capillary density | Difference between groups (p < 0.05) † | Following resistance training, older adults experience an increase in capillary-to-fiber ratio and an increase in muscle mass and strength |
| Wüst 2009 [62] | Adults | n/r | Muscle fiber CSA and capillary density | Correlation (r = 0.62; p < 0.05) | In adults, capillary-to-fiber ratio is positively correlated with muscle mass |
* Exercise intervention study; # hypertensive population; † correlation coefficient not reported; Abbreviations: CSA: Mid-Thigh Muscle Cross-sectional area.
Capillary density, a common marker of microvascular health, appears to have an association with skeletal muscle mass regardless of age. Most studies examined this association through muscle biopsy analysis. These studies demonstrate that increased capillary density is correlated with increased muscle mass across age groups [33,47,62], as well as with increase muscle strength [26,27]. Correspondingly, decrease capillary density was observed in sarcopenic individuals along with concomitant decline in exercise capacity [47,61]. Microvascular flow and function were also found to decline in populations with functional impairments such as aging and chronic disease [43,61].
Two studies in this review included microvascular blood flow analysis in hypertensive participants. One study showed that hypertensive older adults have significantly lower capillary to fiber ratio and muscle mass compared to healthy older controls [56]. Furthermore, adults with hypertension have a reduced tissue oxygen saturation compared to healthy controls and require a two-fold increase in blood pressure to produce equal amount of muscle torque compared to controls [55]. These studies suggest a microvascular impairment in hypertensive populations.
Exercise interventions that increase muscle mass, strength and function were also found to increase microvascular flow and capillary density. In addition to the included cross-sectional studies, several randomized controlled trials that involved an exercise intervention were included. These studies are important as they demonstrate the intimate relationship between vascular health and skeletal muscle health. Two studies examined microvascular function via capillary density and reported significant improvements following both resistance [27] and aerobic [26] training. Following 24 weeks of aerobic exercise, older adults experienced a concomitant increase in skeletal muscle strength and capillary density [26]. Another study demonstrated that older adults following 12 weeks of resistance exercise training experienced an increase in skeletal muscle mass, strength and capillary density [27]. One study examined nutrient delivery to the muscle following a bout of aerobic exercise and reported significant improvements in muscle protein synthesis as well as microvascular blood flow [20]. These data suggest that various types of exercise can significantly impact both the skeletal muscle function and microvascular health of the participants.
4. Discussion
Sarcopenia, the progressive loss of muscle mass, muscle function and physical performance, was previously associated with cardiovascular disease [12,13,14]. Given that many of the risk factors for sarcopenia and cardiovascular disease are shared, it is not surprising that there is an association between measures of vascular dysfunction and muscle health. Existing reviews solely target arterial stiffness as an indicator of vascular dysfunction and its’ association with skeletal muscle health [21]. This systematic review takes a broader approach and examines other validated indices of vascular function including microvascular function. The focus of the review is on relatively healthy adults and we observed an inverse association between skeletal muscle health and vascular dysfunction. Our data indicate that the inverse relationship between vascular dysfunction and skeletal muscle is consistently observed in the hypertensive populations as well. Given the high prevalence of hypertension (63.1%) in adults over the age of 60 [32], these data suggest that it may be important to start to screen for and address muscle health issues in adults with hypertension. Not unexpectedly, exercise interventions demonstrated both macrovascular and microvascular health benefits, in addition to improving indices of skeletal muscle health, indicating the importance of habitual exercise for healthy aging.
Given that most of the included studies are cross-sectional, the mechanism of the association between vascular dysfunction and muscle strength and function is not clear. Rodriguez and colleagues completed an extensive review of the musculoskeletal system and vasculature and proposed a conceptual disease model [71]. On the microsystemic level, systemic inflammation, local inflammation, low calcium and low vitamin D intake, as well as impaired glucose metabolism promote cellular stress and damage. If the cellular stress and damage is chronic, it is manifested in damage to both the vascular system, impacting endothelial function and eventually microvascular health and the musculoskeletal system [71]. Arterial stiffness and perhaps microvascular dysfunction would occur in combination with sarcopenia on the macrosystemic level. It has been shown that muscle perfusion, an indicator of peripheral microvascular health, decline with age [25]. This decline can lead to a decrease in ‘nutritive’ flow to the muscle, impacting the availability of nutrients need for muscle function [22,23,24]. Reduced ‘nutritive’ blood flow may contribute to the anabolic resistance of muscle observed with ageing [29,30], eventually leading to loss of muscle mass, strength and function. What is not known is if the relationship between vascular dysfunction and muscle health is bi-directional and this needs to be further explored in prospective studies.
There are some important limitations of the included studies that must be considered. First, there is a heterogeneity in assessment of arterial health. For example, arterial dysfunction in the included studies is measured through pulse-wave velocity, CIMT, augmentation index and flow-mediated dilation. This variation makes it difficult to complete a meta-analysis, though we felt it was important to include these studies to demonstrate the relationship between arterial dysfunction and skeletal muscle health regardless of outcome measurement. Second, like the variation in arterial function assessments, there is a heterogeneity in assessment of skeletal muscle health and function. While muscle mass and strength assessments are relatively consistent, muscle function assessments varied greatly from 40-foot walking speed tests [38] to arm extensibility tests [50]. Lastly, the number of studies evaluating microvascular health is comparatively low and more research is needed in this field.
The exclusion criteria for each study varied which impacts several variables including nutrition status, daily physical activity and use of medications. Though a few of the studies excluded participants based on a low BMI [4,25,59] and low daily protein intake [26], the rest of the studies did not examine the nutrition status of the participants. Nutrition has been suggested to play an important role in arterial stiffness, specifically through the dietary intake of vitamin D and calcium. An observational study conducted on 131 participants suggested that insufficient intake of vitamin D is associated with increased arterial stiffness [72]. A large study of 12,097 men and women determined that higher calcium intake from food was associated with decreased risk for stroke and non-fatal cardiovascular disease [73]. Despite these data, a detailed meta-analysis of vitamin D supplementation and the impact on arterial stiffness determined that there is inconsistent evidence to suggest a connection between the two factors, which was attributed to large heterogeneity in study design [74]. Similarly, other studies demonstrated no relationship between calcium supplementation and markers of vascular disease [75,76]. Most studies included in this systematic review did not measure daily physical activity. Given the importance of physical activity for maintaining skeletal muscle mass and improving cardiovascular function [77,78], an accurate measurement of daily activity is important for a complete assessment of the participants. Lastly, the use of medications was poorly reported in most studies and this can impact a variety of factors related to vascular dysfunction including blood pressure, blood triglycerides and blood cholesterol levels.
Although the focus of this systematic review has been on relatively healthy subjects, the association between skeletal muscle health and vascular dysfunction has been demonstrated in other populations. A recent meta-analysis examined macrovascular function through pulse wave velocity and demonstrated that lower muscle tissue is associated with higher arterial stiffness in populations with diabetes and kidney disease [21]. This association has been demonstrated in healthy children as well, in which researchers determined that arterial stiffness (measured by CIMT) is negatively associated with muscular strength [79]. Furthermore, following isometric handgrip training, young healthy adults improved their brachial artery flow-mediated dilation [80]. Overall, the connection between skeletal muscle function and vascular dysfunction is observed in other populations and may be exacerbated in populations with disease.
5. Conclusions
In conclusion, we described over 30 studies that demonstrate an inverse relationship between vascular dysfunction and skeletal muscle health. This association is observed both on the macrovascular and the microvascular levels. Given the cross-sectional nature of most of the studies included, it is impossible to say if impaired vascular health causes skeletal muscle dysfunction or vice versa. Nevertheless, we included some exercise intervention trials that demonstrate concurrent improvements in skeletal muscle health and vascular function [26,27,49,52,60]. More studies are necessary to determine if vascular dysfunction is predictive of skeletal muscle dysfunction. Furthermore, the field requires a standardized assessment of macrovascular dysfunction and should also start to include assessment of microvascular dysfunction. The clinical significance of this association between vascular health and muscle health cannot be overlooked, considering the heavy clinical and economic burden of vascular dysfunction and sarcopenia, independently. If skeletal muscle health impairment is predictive of future cardiovascular events or vice versa, early screenings will allow for early preventative interventions to help improve long-term outcomes as the population ages. It is possible that interventions targeting vascular dysfunction may have a long-term benefit on muscle health or vice versa, but this needs to be systematically tested in prospective randomized clinical studies.
Acknowledgments
The authors thank Deborah Hustead for her assistance with possible statistical analysis for this review.
Author Contributions
Conceptualization, S.L.P., S.J., J.C.L.-B., B.E.P. and P.J.A.; methodology, S.D., J.C.L.-B., S.L.P.; validation, S.D., J.C.L.-B., S.L.P.; formal analysis, S.D.; investigation, S.D.; data curation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, S.L.P., S.J., J.C.L.-B., B.E.P. and P.J.A.; supervision, J.C.L.-B. and S.L.P.; funding acquisition, S.L.P. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Abbott Nutrition.
Conflicts of Interest
Abbott employees (S.L.P., J.C.L.-B. and S.J.) were involved in the study design, preparation and review of the manuscript, and in the decision to publish. S.D. received funding as part of an internship with Abbott towards this manuscript development. B.E.P. and P.J.A. declare no conflicts of interest.
References
- 1.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019 doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M., et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deschenes M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 4.Beaudart C., Rizzoli R., Bruyere O., Reginster J.Y., Biver E. Sarcopenia: Burden and challenges for public health. Arch. Public Health. 2014;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott D., Daly R.M., Sanders K.M., Ebeling P.R. Fall and Fracture Risk in Sarcopenia and Dynapenia With and Without Obesity: The Role of Lifestyle Interventions. Curr. Osteoporos. Rep. 2015;13:235–244. doi: 10.1007/s11914-015-0274-z. [DOI] [PubMed] [Google Scholar]
- 6.Goates S., Du K., Arensberg M.B., Gaillard T., Guralnik J., Pereira S.L. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J. Frailty Aging. 2019;8:93–99. doi: 10.14283/jfa.2019.10. [DOI] [PubMed] [Google Scholar]
- 7.Locquet M., Beaudart C., Petermans J., Reginster J.Y., Bruyere O. EWGSOP2 Versus EWGSOP1: Impact on the Prevalence of Sarcopenia and Its Major Health Consequences. J. Am. Med. Dir. Assoc. 2019;20:384–385. doi: 10.1016/j.jamda.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Rejon A.I., Ruiz-Lopez M.D., Wanden-Berghe C., Artacho R. Prevalence and Diagnosis of Sarcopenia in Residential Facilities: A Systematic Review. Adv. Nutr. 2019;10:51–58. doi: 10.1093/advances/nmy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dent E., Morley J.E., Cruz-Jentoft A.J., Arai H., Kritchevsky S.B., Guralnik J., Bauer J.M., Pahor M., Clark B.C., Cesari M., et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 10.Laslett L.J., Alagona P., Jr., Clark B.A., III, Drozda J.P., Jr., Saldivar F., Wilson S.R., Poe C., Hart M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012;60:S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Smith S.C., Jr., Collins A., Ferrari R., Holmes D.R., Jr., Logstrup S., McGhie D.V., Ralston J., Sacco R.L., Stam H., Taubert K., et al. Our time: A call to save preventable death from cardiovascular disease (heart disease and stroke) J. Am. Coll. Cardiol. 2012;60:2343–2348. doi: 10.1016/j.jacc.2012.08.962. [DOI] [PubMed] [Google Scholar]
- 12.Atkins J.L., Whincup P.H., Morris R.W., Lennon L.T., Papacosta O., Wannamethee S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014;62:253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang S.Y., Chang H.Y., Lee M.S., Chia-Yu Chen R., Pan W.H. Skeletal muscle mass and risk of death in an elderly population. Nutr. Metab. Cardiovasc. Dis. 2014;24:784–791. doi: 10.1016/j.numecd.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Spahillari A., Mukamal K.J., DeFilippi C., Kizer J.R., Gottdiener J.S., Djousse L., Lyles M.F., Bartz T.M., Murthy V.L., Shah R.V. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr. Metab. Cardiovasc. Dis. 2016;26:1039–1047. doi: 10.1016/j.numecd.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato A.J., Uberoi A., Wray D.W., Nishiyama S., Lawrenson L., Richardson R.S. Differential effects of aging on limb blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 16.Skilton M.R., Lai N.T., Griffiths K.A., Molyneaux L.M., Yue D.K., Sullivan D.R., Celermajer D.S. Meal-related increases in vascular reactivity are impaired in older and diabetic adults: Insights into roles of aging and insulin in vascular flow. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1404–H1410. doi: 10.1152/ajpheart.00484.2004. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer D.S., Sorensen K.E., Spiegelhalter D.J., Georgakopoulos D., Robinson J., Deanfield J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 18.Seals D.R., Moreau K.L., Gates P.E., Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp. Gerontol. 2006;41:501–507. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Clark M.G., Wallis M.G., Barrett E.J., Vincent M.A., Richards S.M., Clerk L.H., Rattigan S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman K.L., Dhanani S., Glynn E.L., Fry C.S., Drummond M.J., Jennings K., Rasmussen B.B., Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am. J. Clin. Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez A.J., Karim M.N., Srikanth V., Ebeling P.R., Scott D. Lower muscle tissue is associated with higher pulse wave velocity: A systematic review and meta-analysis of observational study data. Clin. Exp. Pharmacol. Physiol. 2017;44:980–992. doi: 10.1111/1440-1681.12805. [DOI] [PubMed] [Google Scholar]
- 22.Clark M.G. Impaired microvascular perfusion: A consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am. J. Physiol. Endocrinol. Metab. 2008;295:E732–E750. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark M.G., Rattigan S., Barrett E.J. Nutritive blood flow as an essential element supporting muscle anabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:185–189. doi: 10.1097/01.mco.0000222097.90890.c2. [DOI] [PubMed] [Google Scholar]
- 24.Durham W.J., Casperson S.L., Dillon E.L., Keske M.A., Paddon-Jones D., Sanford A.P., Hickner R.C., Grady J.J., Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010;24:4117–4127. doi: 10.1096/fj.09-150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell W.K., Phillips B.E., Williams J.P., Rankin D., Smith K., Lund J.N., Atherton P.J. Development of a new Sonovue contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013;1:e00119. doi: 10.1002/phy2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brightwell C.R., Markofski M.M., Moro T., Fry C.S., Porter C., Volpi E., Rasmussen B.B. Moderate-intensity aerobic exercise improves skeletal muscle quality in older adults. Transl. Sports Med. 2019;2:109–119. doi: 10.1002/tsm2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdijk L.B., Snijders T., Holloway T.M., Van Kranenburg J., Van Loon L.J. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med. Sci. Sports Exerc. 2016;48:2157–2164. doi: 10.1249/MSS.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 28.Rennie M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 2009;34:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P.M., Rennie M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 30.Wilkes E.A., Selby A.L., Atherton P.J., Patel R., Rankin D., Smith K., Rennie M.J. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 2009;90:1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- 31.Phillips B.E., Atherton P.J., Varadhan K., Limb M.C., Williams J.P., Smith K. Acute cocoa flavanol supplementation improves muscle macro- and microvascular but not anabolic responses to amino acids in older men. Appl. Physiol. Nutr. Metab. 2016;41:548–556. doi: 10.1139/apnm-2015-0543. [DOI] [PubMed] [Google Scholar]
- 32.Fryar C.D., Ostchega Y., Hales C.M., Zhang G., Kruszon-Moran D. NCHS Data Brief. National Center for Health Statistics; Hyattsville, MD, USA: 2017. Hypertension Prevalence and Control among Adults: United States, 2015–2016; pp. 1–8. [PubMed] [Google Scholar]
- 33.Barnouin Y., McPhee J.S., Butler-Browne G., Bosutti A., De Vito G., Jones D.A., Narici M., Behin A., Hogrel J.Y., Degens H. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J. Cachexia Sarcopenia Muscle. 2017;8:647–659. doi: 10.1002/jcsm.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips B., Williams J., Atherton P., Smith K., Hildebrandt W., Rankin D., Greenhaff P., Macdonald I., Rennie M.J. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J. Appl. Physiol. 2012;112:347–353. doi: 10.1152/japplphysiol.01031.2011. [DOI] [PubMed] [Google Scholar]
- 35.Barrera G., Bunout D., de la Maza M.P., Leiva L., Hirsch S. Carotid ultrasound examination as an aging and disability marker. Geriatr. Gerontol. Int. 2014;14:710–715. doi: 10.1111/ggi.12146. [DOI] [PubMed] [Google Scholar]
- 36.Chung J., Kim M., Jin Y., Kim Y., Hong J. Fitness as a determinant of arterial stiffness in healthy adult men: A cross-sectional study. J. Sports Med. Phys. Fit. 2018;58:150–156. doi: 10.23736/s0022-4707.17.06767-6. [DOI] [PubMed] [Google Scholar]
- 37.den Ouden M.E., Schuurmans M.J., Arts I.E., Grobbee D.E., Bots M.L., van den Beld A.W., Lamberts S.W., van der Schouw Y.T. Atherosclerosis and physical functioning in older men, a longitudinal study. J. Nutr. Health Aging. 2013;17:97–104. doi: 10.1007/s12603-012-0424-2. [DOI] [PubMed] [Google Scholar]
- 38.El Khoudary S.R., Chen H.Y., Barinas-Mitchell E., McClure C., Selzer F., Karvonen-Gutierrez C., Jackson E.A., Ylitalo K.R., Sternfeld B. Simple physical performance measures and vascular health in late midlife women: The Study of Women’s Health across the nation. Int. J. Cardiol. 2015;182:115–120. doi: 10.1016/j.ijcard.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahs C.A., Thiebaud R.S., Rossow L.M., Loenneke J.P., Bemben D.A., Bemben M.G. Relationships between central arterial stiffness, lean body mass, and absolute and relative strength in young and older men and women. Clin. Physiol. Funct. Imaging. 2018;38:676–680. doi: 10.1111/cpf.12467. [DOI] [PubMed] [Google Scholar]
- 40.Gonzales J.U., Wiberg M., Defferari E., Proctor D.N. Arterial stiffness is higher in older adults with increased perceived fatigue and fatigability during walking. Exp. Gerontol. 2015;61:92–97. doi: 10.1016/j.exger.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Heffernan K.S., Chale A., Hau C., Cloutier G.J., Phillips E.M., Warner P., Nickerson H., Reid K.F., Kuvin J.T., Fielding R.A. Systemic vascular function is associated with muscular power in older adults. J. Aging Res. 2012;2012:386387. doi: 10.1155/2012/386387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im I.J., Choi H.J., Jeong S.M., Kim H.J., Son J.S., Oh H.J. The association between muscle mass deficits and arterial stiffness in middle-aged men. Nutr. Metab. Cardiovasc. Dis. 2017;27:1130–1135. doi: 10.1016/j.numecd.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Kohara K., Okada Y., Ochi M., Ohara M., Nagai T., Tabara Y., Igase M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J. Cachexia Sarcopenia Muscle. 2017;8:557–566. doi: 10.1002/jcsm.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.W., Youm Y., Kim C.O., Lee W.J., Choi W., Chu S.H., Park Y.R., Kim H.C. Association between skeletal muscle mass and radial augmentation index in an elderly Korean population. Arch. Gerontol. Geriatr. 2014;59:49–55. doi: 10.1016/j.archger.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochi M., Kohara K., Tabara Y., Kido T., Uetani E., Ochi N., Igase M., Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Prior S.J., Ryan A.S., Blumenthal J.B., Watson J.M., Katzel L.I., Goldberg A.P. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2016;71:1096–1101. doi: 10.1093/gerona/glw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanada K., Miyachi M., Tanimoto M., Yamamoto K., Murakami H., Okumura S., Gando Y., Suzuki K., Tabata I., Higuchi M. A cross-sectional study of sarcopenia in Japanese men and women: Reference values and association with cardiovascular risk factors. Eur. J. Appl. Physiol. 2010;110:57–65. doi: 10.1007/s00421-010-1473-z. [DOI] [PubMed] [Google Scholar]
- 49.Shiotsu Y., Watanabe Y., Tujii S., Yanagita M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp. Gerontol. 2018;111:27–34. doi: 10.1016/j.exger.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Suwa M., Imoto T., Kida A., Yokochi T., Iwase M., Kozawa K. Association of body flexibility and carotid atherosclerosis in Japanese middle-aged men: A cross-sectional study. BMJ Open. 2018;8:e019370. doi: 10.1136/bmjopen-2017-019370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto K., Kawano H., Gando Y., Iemitsu M., Murakami H., Sanada K., Tanimoto M., Ohmori Y., Higuchi M., Tabata I., et al. Poor trunk flexibility is associated with arterial stiffening. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1314–H1318. doi: 10.1152/ajpheart.00061.2009. [DOI] [PubMed] [Google Scholar]
- 52.Yoshizawa M., Maeda S., Miyaki A., Misono M., Saito Y., Tanabe K., Kuno S., Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32–59 years. Br. J. Sports Med. 2009;43:615–618. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- 53.Yoo J.I., Kim M.J., Na J.B., Chun Y.H., Park Y.J., Park Y., Hah Y.S., Ha Y.C., Park K.S. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J. Cachexia Sarcopenia Muscle. 2018;9:1034–1041. doi: 10.1002/jcsm.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Guo Q., Feng B.L., Wang C.Y., Han P.P., Hu J., Sun X.D., Zeng W.F., Zheng Z.X., Li H.S., et al. A Cross-Sectional Study of the Association between Arterial Stiffness and Sarcopenia in Chinese Community-Dwelling Elderly Using the Asian Working Group for Sarcopenia Criteria. J. Nutr. Health Aging. 2019;23:195–201. doi: 10.1007/s12603-018-1147-9. [DOI] [PubMed] [Google Scholar]
- 55.Dipla K., Triantafyllou A., Koletsos N., Papadopoulos S., Sachpekidis V., Vrabas I.S., Gkaliagkousi E., Zafeiridis A., Douma S. Impaired Muscle Oxygenation and Elevated Exercise Blood Pressure in Hypertensive Patients: Links With Vascular Stiffness. Hypertension. 2017;70:444–451. doi: 10.1161/HYPERTENSIONAHA.117.09558. [DOI] [PubMed] [Google Scholar]
- 56.Gueugneau M., Coudy-Gandilhon C., Meunier B., Combaret L., Taillandier D., Polge C., Attaix D., Roche F., Feasson L., Barthelemy J.C., et al. Lower skeletal muscle capillarization in hypertensive elderly men. Exp. Gerontol. 2016;76:80–88. doi: 10.1016/j.exger.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Lima-Junior D., Farah B.Q., Germano-Soares A.H., Andrade-Lima A., Silva G.O., Rodrigues S.L.C., Ritti-Dias R. Association between handgrip strength and vascular function in patients with hypertension. Clin. Exp. Hypertens. 2018;41:692–695. doi: 10.1080/10641963.2018.1539096. [DOI] [PubMed] [Google Scholar]
- 58.Sampaio R.A., Sewo Sampaio P.Y., Yamada M., Yukutake T., Uchida M.C., Tsuboyama T., Arai H. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2014;14(Suppl. 1):109–114. doi: 10.1111/ggi.12206. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu Y., Sato S., Koyamatsu J., Yamanashi H., Nagayoshi M., Kadota K., Kawashiri S.Y., Inoue K., Nagata Y., Maeda T. Handgrip strength and subclinical carotid atherosclerosis in relation to platelet levels among hypertensive elderly Japanese. Oncotarget. 2017;8:69362–69369. doi: 10.18632/oncotarget.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong A., Figueroa A., Son W.M., Chernykh O., Park S.Y. The effects of stair climbing on arterial stiffness, blood pressure, and leg strength in postmenopausal women with stage 2 hypertension. Menopause. 2018;25:731–737. doi: 10.1097/GME.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 61.Groen B.B., Hamer H.M., Snijders T., van Kranenburg J., Frijns D., Vink H., van Loon L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014;116:998–1005. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 62.Wust R.C., Gibbings S.L., Degens H. Fiber capillary supply related to fiber size and oxidative capacity in human and rat skeletal muscle. Adv. Exp. Med. Biol. 2009;645:75–80. doi: 10.1007/978-0-387-85998-9_12. [DOI] [PubMed] [Google Scholar]
- 63.Bodur H., Yilmaz O., Keskin D. Hand disability and related variables in patients with rheumatoid arthritis. Rheumatol. Int. 2006;26:541–544. doi: 10.1007/s00296-005-0023-1. [DOI] [PubMed] [Google Scholar]
- 64.Escalante A., Haas R.W., del Rincon I. Measurement of global functional performance in patients with rheumatoid arthritis using rheumatology function tests. Arthritis Res. Ther. 2004;6:R315–R325. doi: 10.1186/ar1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leong D.P., Teo K.K., Rangarajan S., Lopez-Jaramillo P., Avezum A., Jr., Orlandini A., Seron P., Ahmed S.H., Rosengren A., Kelishadi R., et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 66.Abellan van Kan G., Rolland Y., Andrieu S., Bauer J., Beauchet O., Bonnefoy M., Cesari M., Donini L.M., Gillette Guyonnet S., Inzitari M., et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 67.Afilalo J., Eisenberg M.J., Morin J.F., Bergman H., Monette J., Noiseux N., Perrault L.P., Alexander K.P., Langlois Y., Dendukuri N., et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 68.Blain H., Carriere I., Sourial N., Berard C., Favier F., Colvez A., Bergman H. Balance and walking speed predict subsequent 8-year mortality independently of current and intermediate events in well-functioning women aged 75 years and older. J. Nutr. Health Aging. 2010;14:595–600. doi: 10.1007/s12603-010-0111-0. [DOI] [PubMed] [Google Scholar]
- 69.Ries J.D., Echternach J.L., Nof L., Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys. Ther. 2009;89:569–579. doi: 10.2522/ptj.20080258. [DOI] [PubMed] [Google Scholar]
- 70.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M., Brach J., Chandler J., Cawthon P., Connor E.B., et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez A.J., Scott D., Ebeling P.R. Exploring the Links Between Common Diseases of Ageing—Osteoporosis, Sarcopenia and Vascular Calcification. Clin. Rev. Bone Miner. Metab. 2019;17:23. doi: 10.1007/s12018-018-9251-2. [DOI] [Google Scholar]
- 72.Andrade J., Er L., Ignaszewski A., Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin. J. Am. Soc. Nephrol. 2008;3:1800–1806. doi: 10.2215/CJN.00900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan B., Nowson C.A., Daly R.M., English D.R., Hodge A.M., Giles G.G., Ebeling P.R. Higher Dietary Calcium Intakes Are Associated With Reduced Risks of Fractures, Cardiovascular Events, and Mortality: A Prospective Cohort Study of Older Men and Women. J. Bone Miner. Res. 2015;30:1758–1766. doi: 10.1002/jbmr.2515. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez A.J., Scott D., Srikanth V., Ebeling P. Effect of vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin. Endocrinol. 2016;84:645–657. doi: 10.1111/cen.13031. [DOI] [PubMed] [Google Scholar]
- 75.Anderson J.J., Kruszka B., Delaney J.A., He K., Burke G.L., Alonso A., Bild D.E., Budoff M., Michos E.D. Calcium Intake From Diet and Supplements and the Risk of Coronary Artery Calcification and its Progression Among Older Adults: 10-Year Follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J.H., Yoon J.W., Kim K.W., Lee E.J., Lee W., Cho S.H., Shin C.S. Increased dietary calcium intake is not associated with coronary artery calcification. Int. J. Cardiol. 2012;157:429–431. doi: 10.1016/j.ijcard.2012.03.171. [DOI] [PubMed] [Google Scholar]
- 77.Nocon M., Hiemann T., Muller-Riemenschneider F., Thalau F., Roll S., Willich S.N. Association of physical activity with all-cause and cardiovascular mortality: A systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 78.Sofi F., Capalbo A., Cesari F., Abbate R., Gensini G.F. Physical activity during leisure time and primary prevention of coronary heart disease: An updated meta-analysis of cohort studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 79.Melo X., Santa-Clara H., Santos D.A., Pimenta N.M., Minderico C.S., Fernhall B., Sardinha L.B. Independent Association of Muscular Strength and Carotid Intima-Media Thickness in Children. Int. J. Sports Med. 2015;36:624–630. doi: 10.1055/s-0034-1398678. [DOI] [PubMed] [Google Scholar]
- 80.Badrov M.B., Freeman S.R., Zokvic M.A., Millar P.J., McGowan C.L. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur. J. Appl. Physiol. 2016;116:1289–1296. doi: 10.1007/s00421-016-3366-2. [DOI] [PubMed] [Google Scholar]