Abstract
Fats that are rich in palmitic or stearic acids can be interesterified to increase their applicability for the production of certain foods. When compared with palmitic acid, stearic acid lowers low-density lipoprotein (LDL)-cholesterol, which is a well-known risk factor for coronary heart disease (CHD), but its effects on other cardiometabolic risk markers have been studied less extensively. In addition, the positional distribution of these two fatty acids within the triacylglycerol molecule may affect their metabolic effects. The objective was to compare the longer-term and postprandial effects of (interesterified) fats that are rich in either palmitic or stearic acids on cardiometabolic risk markers in humans. Two searches in PubMed/Medline, Embase (OVID) and Cochrane Library were performed; one to identify articles that studied effects of the position of palmitic or stearic acids within the triacylglycerol molecule and one to identify articles that compared side-by-side effects of palmitic acid with those of stearic acid. The interesterification of palmitic or stearic acid-rich fats does not seem to affect fasting serum lipids and (apo) lipoproteins. However, substituting palmitic acid with stearic acid lowers LDL-cholesterol concentrations. Postprandial lipemia is attenuated if the solid fat content of a fat blend at body temperature is increased. How (the interesterification of) palmitic or stearic acid-rich fats affects other cardiometabolic risk markers needs further investigation.
Keywords: palmitic acid, stearic acid, positional distribution, interesterification, longer-term, postprandial, lipids, lipoproteins, cardiometabolic risk markers, coronary heart disease
1. Introduction
During the last decades, many studies have been carried out to gain more insight into the effects of dietary fat intake on risk markers for cardiovascular disease (CVD), such as disturbances in lipid metabolism, glucose-insulin homeostasis, the hemostatic system, or low-grade systemic inflammation. A well-accepted risk factor for coronary heart disease (CHD) is low-density lipoprotein (LDL)-cholesterol (LDL-C), which is increased by diets that are rich in saturated and trans fatty acids. Therefore, guidelines to prevent CHD are focused on the exchange of dietary saturated and trans fats for unsaturated fats [1]. However, saturated fat is a collective term for different saturated fatty acids that exert different metabolic effects. In the Western diet, palmitic acid (C16:0) and stearic acid (C18:0) are the most commonly consumed saturated fatty acids [2]. It is generally believed that palmitic acid is more cholesterol-raising than stearic acid [3,4]. However, the effects of palmitic and stearic acids on other cardiometabolic risk markers are less well established. Besides chain length of saturated fatty acids, the positional distribution of fatty acids within the triacylglycerol (TAG) molecule might also be important for their metabolic effects [5]. TAG molecules consist of a glycerol backbone to which three fatty acids are esterified. The positional distribution of these fatty acids within the TAG molecule, the so-called TAG structure, can be specified by stereospecific numbering (sn) as sn-1, sn-2, and sn-3. With interesterification, a chemical or enzymatic process used by the food industry, fatty acid positions can be exchanged within and between TAG molecules, thereby creating new TAG structures. This structure determines the physical properties of a fat, including its melting behavior, which in turn determines the suitability of the fat for the food industry; solid fats are, for instance, more suitable for baked goods and certain types of margarines than oils. Some vegetable oils, such as palm oil, contain relatively high amounts of palmitic and/or stearic acid predominantly at the outer sn-1 and -3 positions [6]. The interesterification of these oils increases the amounts of palmitic or stearic acids at sn-2, which will increase their melting points. Since no trans fatty acids are generated by interesterification, this process seems to be a good alternative for partially hydrogenated trans fats. However, the positional distribution of fatty acids might affect their metabolic fate, also because the dietary fatty acid at the sn-2 position is largely retained when incorporated into chylomicron TAG molecules [7]. Given that fats rich in palmitic and/or stearic acid are often used for interesterification, it is important that we thoroughly understand their impact on metabolic health. Therefore, we have systematically reviewed the current knowledge on the longer-term and postprandial effects on cardiometabolic risk markers of 1) the effect of interesterification of either palmitic acid- or stearic acid-rich fats and 2) the difference between palmitic acid- and stearic acid-rich fats.
2. Methods
The databases PubMed/Medline, Embase (OVID), and Cochrane Library were searched for papers published until December, 2019. Two searches were performed; one to identify articles that studied effects of the position of palmitic acid or stearic acid on the TAG molecule and one to identify articles that compared the side-by-side effects of palmitic acid with those of stearic acid. For the effect of TAG structure, the following search strategies were used: ((interesterified[All Fields] OR "esterification"[MeSH Terms] OR "TAG structures"[All Fields] OR "triglycerides/administration and dosage"[MeSH Terms]) AND ("palmitic acid"[All Fields] OR "stearic acid"[All Fields])) for PubMed, ((triglyceride structure/ OR *triacylglycerol/ OR interesterification.mp.) AND (stearic acid/ OR palmitic acid/)) with ‘article’ as filter for Embase, and ((esterification [MeSH descriptor] OR triglycerides [MeSH descriptor with qualifier administration and dosage] OR TAG structures OR interesterified) AND (palmitic acid OR stearic acid)) in Cochrane Library. For the comparison of palmitic acid with stearic acid, the following search strategies were used: (("palmitic acid"[All Fields] OR "palmitate"[All Fields] OR "hexadecanoic acid"[All Fields] OR "C16:0"[All Fields]) AND ("stearic acid"[All Fields] OR "octadecanoic acid"[All Fields] OR "stearate"[All Fields] OR "C18:0"[All Fields])) AND "clinical study"[Publication Type] for Pubmed, (*palmitic acid/ and *stearic acid/ and human.mp) for Embase, and (palmitic acid AND stearic acid) for the Cochrane Library.
Studies were eligible if they met the following inclusion criteria: human dietary intervention trial comparing diets or meals containing either palmitic or stearic acid mainly at sn-1 and -3 with diets or meals containing higher amounts of palmitic or stearic acid at the sn-2 position or comparing diets or meals that are rich in palmitic acid with diets or meals rich in stearic acid; diets or meals had comparable contents of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs); subjects were ≥18 years and apparently healthy; cardiometabolic risk markers (lipids and lipoproteins, hematological markers, glucose-insulin homeostasis, endothelial function markers, and/or inflammation markers) were assessed; the articles were published in English and available as full text.
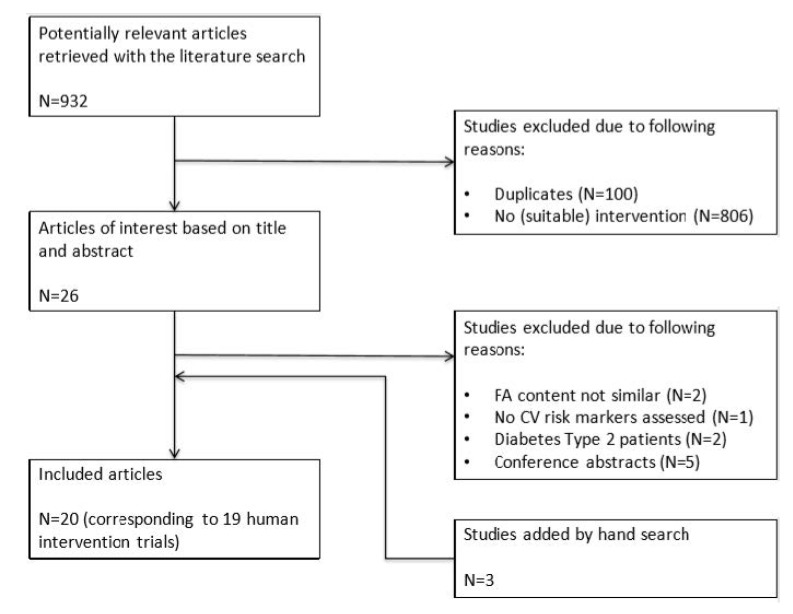
The search for the effect of the position of either palmitic or stearic acid within the TAG molecule resulted in a total of 932 records (248 from PubMed, 646 from Embase, 38 from Cochrane), of which 100 records were duplicates. Twenty-six records from the remaining 832 were considered to be of interest based on their titles and abstracts. After the screening of the full texts, two articles were excluded because the fatty-acid contents of the experimental fats were not comparable, one because no cardiometabolic risk markers were assessed, one because subjects had type 2 diabetes, and five because they were conference abstracts. The reference lists of all eligible papers were searched for additional studies, which resulted in another three articles. In the end, a total of 20 articles corresponding to 19 human intervention trials were included (Figure 1).
Figure 1.
Flow chart of studies on the effects of interesterification of palmitic acid- or stearic acid-rich fats on cardiometabolic risk markers. Abbreviations: FA, fatty acid; CV, cardiovascular.
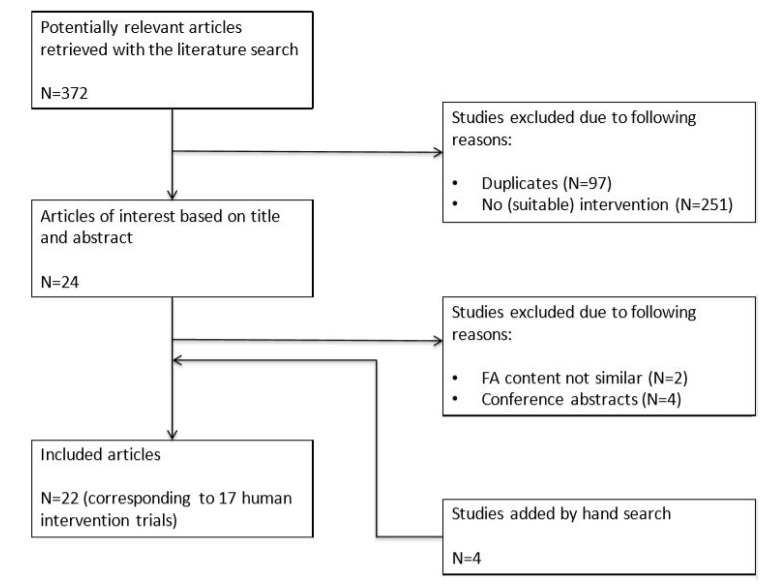
The search for palmitic acid versus stearic acid resulted in a total of 372 records (111 from PubMed/Medline, 125 from Embase, and 136 from Cochrane), of which 97 records were duplicates. Twenty-four records from the remaining 275 were considered to be of interest based on their titles and abstracts. After screening of the full texts, two articles were excluded because the experimental fats differed not only in palmitic acid and stearic acid contents, but also in other fatty acids and four other articles because they were conference abstracts. The reference lists of all eligible papers and previous reviews were searched for additional studies, which resulted in another four articles. In the end, a total of 22 articles corresponding to 17 human trials were included (Figure 2).
Figure 2.
Flow chart of studies on the effects of palmitic acid versus stearic acid on cardiometabolic risk markers. Abbreviations: FA, fatty acid.
3. Results
3.1. Longer-Term Effects of sn-2 Content of Palmitic Acid or Stearic Acid on Fasting Cardiometabolic Risk Markers
Six studies have compared side-by-side the effects on fasting cardiometabolic risk markers of diets with high versus low proportions of palmitic acid at the sn-2 position (Table A1) and two studies with high versus low proportions of stearic acid at sn-2 (Table A2). Table 1 summarizes the results. In seven studies, the content of palmitic or stearic acid at sn-2 was increased by the interesterification of experimental fats, while in one study interesterification decreased the sn-2 content of palmitic acid [8]. Studies examining palmitic acid-rich fats used palm oil [9,10], palm olein [11,12], butter [8], or a blend consisting mainly of coconut and palm oil [13]. Two studies have reported the solid fat content at 37 °C; in one study, both the native and interesterified palm oils were liquid [9], while interesterification increased the solid fat content of palm olein from 0 to 6% in the other study [11]. Sources for the stearic acid-rich fats were shea butter [14] and cocoa butter [15]. Interesterification of shea butter increased the solid fat content at 37 °C from 22 to 41% [14]. The melting points of native and interesterified cocoa butter were not measured, but the authors indicated that native cocoa butter was liquid at 37 °C and assumed that the solid fat content of the interesterified fat at 40.5 °C was 19% [15]. Most of the studies had used a randomized cross-over design, except for two studies that used a parallel design [8,12]. The experimental periods varied from 21 to 56 days for studies examining palmitic acid-rich fats and diets provided 1 to 11 energy percent (en%) of palmitic acid. The proportion of palmitic acids at sn-2 was reported in five out of seven studies and it differed between 11 and 60% of total fatty acids. The two studies examining stearic acid-rich fats had interventions periods of 18 and 21 days, and diets provided 10 and 7 en% stearic acid. One study reported proportions of stearic acid at sn-2, and the difference between diets was approximately 20%.
Table 1.
Summary of studies examining the longer-term effects of substituting fats low in palmitic acid (C16:0) or stearic acid (C18:0) sn-2 contents with fats high in C16:0 or C18:0 sn-2 contents, respectively.
| Fasted Lipids and lipoproteins |
High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
Hematological markers | High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
Other markers | High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
|---|---|---|---|---|---|---|---|---|
| Triacyl- glycerol |
0 ↓ 6 = 0 ↑ |
0 ↓ 2 = 0 ↑ |
FVIIa | 0 ↓ 1 = 0 ↑ |
0 ↓ 1 = 0 ↑ |
Glucose | 0 ↓ 3 = 0 ↑ |
0 ↓ 1 = 0 ↑ |
| Non-esterified fatty acids |
0 ↓ 1 = 0 ↑ |
NA | Fibrinogen | 0 ↓ 1 = 0 ↑ |
NA | Insulin | 0 ↓ 2 = 0 ↑ |
0 ↓ 1 = 0 ↑ |
| Total cholesterol |
0 ↓ 6 = * 0 ↑ |
0 ↓ 2 = 0 ↑ |
PAI-1 | 0 ↓ 1 = 0 ↑ |
NA | C-peptide | 0 ↓ 2 = 0 ↑ |
NA |
| LDL- cholesterol |
0 ↓ 6 = * 0 ↑ |
0 ↓ 1 = 0 ↑ |
tPA | 0 ↓ 1 = 0 ↑ |
NA | C-reactive protein | 0 ↓ 1 = 0 ↑ |
NA |
| HDL- cholesterol |
0 ↓ 6 = 0 ↑ |
0 ↓ 1 = 0 ↑ |
vWF | 0 ↓ 1 = 0 ↑ |
NA | |||
| ApoB | 0 ↓ 3 = 0 ↑ |
NA | ||||||
| ApoA1 | 0 ↓ 3 = 0 ↑ |
NA | ||||||
| Lp[a] | 0 ↓ 3 = 0 ↑ |
NA |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C16:0 sn-2 or C18:0 sn-2 contents compared with fats low in C16:0 sn-2 or C18:0 sn-2 contents respectively. *=In men, total and LDL cholesterol concentrations were slightly increased (0.10 mmol/L and 0.08 mmol/L respectively) on the diet with higher C16:0 sn-2 [9]. Abbreviations: apo, apolipoprotein; FVIIa, activated factor VII; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp[a], lipoprotein (a); NA, not available; PAI, plasminogen activator inhibitor; tPA, tissue plasminogen activator; vWF, von Willebrand Factor.
3.1.1. Lipids and (apo) Lipoproteins
The interesterification of palmitic acid-rich fats did not affect concentrations of TAG, total cholesterol (TC), LDL-C, or high-density lipoprotein (HDL)-C [8,9,10,11,12,13]. However, one study reported that men—but not women—showed a small, but statistically significant, increase in TC and LDL-C concentrations in response to the diet with a higher sn-2 content of palmitic acid [9]. No differences were found for non-esterified fatty acid (NEFA) [13], apolipoprotein (apo)B [8,12], apoA1 [8,11,12], and lipoprotein[a] concentrations [11,12,13]. The sn-2 content of stearic acid also had no effects on the concentrations of TAG [14,15], TC [14,15], LDL-C, or HDL-C [14].
3.1.2. Hematological Markers
Only two studies have examined the effects of interesterification on hematological markers. No effects were found of sn-2 content of palmitic acid on concentrations of activated form of coagulation factor VII (FVIIa), fibrinogen, plasminogen activator inhibitor (PAI)-1 antigen, tissue plasminogen activator (tPA) antigen and its activity, and von Willebrand factor (vWF) [13], and of the stearic acid sn-2 content on FVIIa concentrations [14].
3.1.3. Other Markers
The proportion of palmitic acids at sn-2 did not affect the concentrations of glucose [11,12,13], insulin [11,12], C-peptide [11,12], and C-reactive protein (CRP) [13]. Stearic acid sn-2 content also did not affect glucose and insulin concentrations [14].
3.2. Longer-Term Effects of Substituting Palmitic Acid with Stearic Acid on Fasting Cardiometabolic Risk Markers
Eleven studies have compared side-by-side the effects of diets that are rich in palmitic acid with those of diets rich in stearic acid on fasting cardiometabolic risk markers (Table 2 and Table A3). The palmitic acid sources used were palm oil [15,16,17,18,19,20], (interesterified) palm olein [21,22], a blend containing tripalmitin [23], and palm stearin [22]. For stearic acid-rich diets, cocoa butter [15,19,20,24], hydrogenated soybean oil [16,21], shea butter [17,18], hydrogenated canola [22], a blend containing tristearin [23], and an interesterified blend containing fully hydrogenated soybean oil [12] were used. Except for one study [12], all of the studies used a randomized cross-over design. The experimental periods varied from 18 to 56 days and diets provided 4 to 18 en% from palmitic acids or stearic acids. The exchange of palmitic acids with stearic acids between the diets varied between 1 and 15 en%.
Table 2.
Summary of studies examining the longer-term effects of substituting fats high in palmitic acid (C16:0) with fats high in stearic acid (C18:0).
| Fasted Lipids and lipoproteins |
C18:0 vs C16:0 |
Hematological markers | C18:0 vs C16:0 |
C18:0 vs C16:0 |
Other markers |
C18:0 vs C16:0 |
|
|---|---|---|---|---|---|---|---|
| Triacyl- glycerol |
1 ↓ 10 = 0 ↑ |
FVIIc | 1 ↓ 1 = 0 ↑ |
Fibrinogen | 0 ↓ 1 = 0 ↑ |
CETP activity | 1 ↓ 1 = 0 ↑ |
| Total cholesterol | 7 ↓ 4 = 0 ↑ |
Mean platelet volume | 1 ↓ 1 = 0 ↑ |
Plasminogen | 0 ↓ 1 = 0 ↑ |
LCAT activity | 0 ↓ 1 = 0 ↑ |
| VLDL- cholesterol |
0 ↓ 4 = 0 ↑ |
PAI-1 activity | 0 ↓ 1 = 0 ↑ |
White blood cells |
0 ↓ 2 = 0 ↑ |
Glucose | 0 ↓ 2 = 0 ↑ |
| LDL- cholesterol |
5 ↓ 5 = 0 ↑ |
PAI-1 antigen | 0 ↓ 1 = 0 ↑ |
Red blood cells |
0 ↓ 2 = 0 ↑ |
Insulin | 0 ↓ 2 = 0 ↑ |
| HDL- cholesterol |
3 ↓ 7 = 0 ↑ |
tPA activity | 0 ↓ 1 = 0 ↑ |
Hemoglobin | 0 ↓ 2 = 0 ↑ |
C-peptide | 0 ↓ 1 = 0 ↑ |
| ApoB | 1 ↓ 4 = 0 ↑ |
tPA antigen | 0 ↓ 1 = 0 ↑ |
Platelets | 0 ↓ 2 = 0 ↑ |
Various inflammation markers | 0 ↓ 1 = 0 ↑ |
| ApoA1 | 2 ↓ 3 = 0 ↑ |
EFA | 0 ↓ 1 = 0 ↑ |
Antithrombin III | 0 ↓ 1 = 0 ↑ |
||
| Lp[a] | 0 ↓ 1 = 1 ↑ |
Thrombomodulin | 0 ↓ 1 = 0 ↑ |
PTT | 0 ↓ 1 = 0 ↑ |
||
| Prothrombin time | 0 ↓ 1 = 0 ↑ |
APTT | 0 ↓ 1 = 0 ↑ |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 compared with fats high in C16:0. Abbreviations: apo, apolipoprotein; APTT, activated partial thromboplastin time; CETP, cholesteryl ester transfer protein; EFA, euglobulin fibrinolytic activity; FVIIc, Factor VII coagulant activity; LCAT, lecithin-cholesterol acyltransferase; LDL, low-density lipoprotein; Lp[a], lipoprotein (a); HDL, high-density lipoprotein; PAI, plasminogen activator inhibitor; PTT, partial thromboplastin time; tPA, tissue plasminogen activator, VLDL, very-low density lipoprotein.
3.2.1. Lipids and (apo) Lipoproteins
The concentrations of TAG did not differ between the diets [15,16,17,18,19,20,21,22,23,24], except in one study, where the TAG concentrations were lower after an interesterified stearic acid-rich diet [12]. However, the majority of studies found lower TC concentrations on the stearic acid-rich diet as compared with palmitic acid [15,16,17,18,19,20,23]. In five of these studies, LDL-C concentrations were also decreased [16,17,18,20,23], and in two studies the concentration of LDL-C tended to be lower on stearic acid [12,19]. Lower HDL-C concentrations on the stearic acid-rich diet were found in three studies [17,19,20], while in seven other studies, no significant differences were found [12,16,18,21,22,23,24]. No changes in concentrations of very-low density lipoprotein (VLDL)-C were reported [17,19,20]. Of the studies that measured apoB and apoA1 [12,17,19,23], one observed decreased concentrations of apoB [17] and two of apoA1 [17,19] on the stearic acid-rich diet. Lipoprotein[a] concentrations were higher on the stearic acid-rich diet in one study [25], but no differences were observed in another study [12].
3.2.2. Hematological Markers
One study found decreased factor VII coagulant activity (FVIIc) on the stearic acid-rich diet when compared with palmitic acid [17]. However, FVIIc activities were not different between the diets in another study [22]. The mean platelet volume (MPV) was lower in one study [22], but no difference was observed in another study of the same group [24]. No differences between the diets were reported for other hematological markers [17,20,22,24]. In one study, various inflammation markers were measured and no significant differences were observed [20].
3.2.3. Other Markers
Stearic acid decreased cholesteryl ester transfer protein (CETP) activity when compared with palmitic acid in one study [19] and a similar decrease was observed in another study, although not being significant [23]. No effects on lecithin-cholesterol acyltransferase (LCAT) activity were observed [23]. Three studies examined effects on glucose metabolism. An intravenous glucose tolerance test was performed and a comparable response in glucose and insulin was observed on both of the diets [26]. No differences were observed in the fasting concentrations of glucose [12,20], insulin [12,20], and C-peptide [12].
3.3. Postprandial Effects of sn-2 Content of Palmitic Acid or Stearic Acid on Cardiometabolic Risk Markers
Eight studies have compared side-by-side the postprandial effects of meals with high versus low proportions of palmitic acid at the sn-2 position (Table A4), and four studies with high versus low proportions of stearic acid (Table A5). Table 3 summarizes the results. Most of the studies examining palmitic acid-rich meals have used palm olein. Interesterification of palm olein not only increased the palmitic acid content at sn-2, but also the solid fat content at 37 °C. In one study, lard was used [27], in which interesterification decreased the palmitic acid at sn-2, as well as the solid fat content. Another study used a commonly consumed blend of palm stearin and palm kernel (PSt/PK) [28]. The interesterification of the PSt/PK blend increased palmitic acid at sn-2, but decreased the solid fat content at 37 °C. The stearic acid-rich meals consisted of structured TAG molecules with predominantly stearic and oleic acid (C18:1) [29], cocoa butter [30], shea butter [14], or canola stearin [31]. The interesterification of cocoa and shea butter increased the proportion of stearic acid at sn-2 and the solid fat content at 37 °C, which decreased after interesterification of canola stearin. For palmitic acid-rich meals, the total fat content of the meals varied between 40 and 75 grams, of which 12 to 30 grams originated from palmitic acid. Differences between meals in the proportion of palmitic acids at sn-2 varied between 17.0 and 66.8% of total fatty acids at sn-2. For stearic acid-rich meals, total fat content varied between 50 and 102 grams, including 17 to 30 grams of stearic acid. Two of the four studies reported the proportions of stearic acids at sn-2 and differences between meals were 19.7 and 25.0%. Postprandial follow-up varied between four and eight hours.
Table 3.
Summary of studies examining the postprandial effects of substituting fats low in sn-2 palmitic acid (C16:0) or stearic acid (C18:0) contents with fats high in sn-2 C16:0 or C18:0 contents respectively.
| Postprandial Lipids and lipoproteins |
High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
Hemato-logical markers | High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
Other markers | High vs low C16:0 sn-2 |
High vs low C18:0 sn-2 |
|---|---|---|---|---|---|---|---|---|
| Triacylglycerol | 1 ↓ 6 = 1 ↑ |
2 ↓ 3 = 0 ↑ |
FVIIa | 0 ↓ 1 = 0 ↑ |
1 ↓ 1 = 0 ↑ |
Glucose | 0 ↓ 7 = 0 ↑ |
0 ↓ 3 = 0 ↑ |
| Non-esterified fatty acids | 0 ↓ 6 = 0 ↑ |
0 ↓ 3 = 0 ↑ |
White blood cells | 0 ↓ 1 = 0 ↑ |
0 ↓ 1 = 0 ↑ |
Insulin | 0 ↓ 7 = 0 ↑ |
0 ↓ 3 = 0 ↑ |
| Total cholesterol | 0 ↓ 4 = 0 ↑ |
0 ↓ 3 = 0 ↑ |
C-peptide | 0 ↓ 1 = 0 ↑ |
NA | |||
| VLDL-cholesterol | 0 ↓ 2 = 0 ↑ |
NA | GIP | 1 ↓ 2 = 0 ↑ |
NA | |||
| LDL-cholesterol | 0 ↓ 1 = 0 ↑ |
0 ↓ 3 = 0 ↑ |
Peptide YY | 0 ↓ 2 = 0 ↑ |
NA | |||
| HDL-cholesterol | 0 ↓ 1 = 0 ↑ |
0 ↓ 2 = 0 ↑ |
IL-6 | 0 ↓ 1 = 0 ↑ |
NA | |||
| Chylomicron- cholesterol |
0 ↓ 2 = 0 ↑ |
NA | IL-8 | 0 ↓ 1 = 0 ↑ |
NA | |||
| ApoB48 | 0 ↓ 1 = 0 ↑ |
NA | TNF-α | 0 ↓ 1 = 0 ↑ |
NA | |||
| E-selectin | 0 ↓ 1 = 0 ↑ |
NA |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C16:0 sn-2 or C18:0 sn-2 contents compared with fats low in C16:0 sn-2 or C18:0 sn-2 contents respectively. Abbreviations: apo, apolipoprotein; FVIIa, activated factor VII; GIP, glucose-dependent insulinotropic polypeptide; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; TNF, tumor necrosis factor; VLDL, very-low density lipoprotein.
3.3.1. Lipids and (apo) Lipoproteins
A lower postprandial TAG response—as indicated by the incremental area under the curve (iAUC)—was observed in one study after a meal with higher palmitic acid sn-2 content [32]. The same tendency was found in three other studies [27,33,34], and this was accompanied by a significant lower response in the first four hours after the meal with a higher proportion of palmitic acid at sn-2 in one study [34]. In contrast, one study showed an increased TAG response after a higher palmitic acid sn-2 content [28]. Two other studies found no differences in TAG responses [35,36]. Postprandial responses of NEFAs [7,27,32,34,35,36], TC [7,27,33,34], and HDL-, LDL- [33], VLDL-, and chylomicron- cholesterol [27,32] were comparable. ApoB48 responses were measured in one study and no effect of sn-2 palmitic acid content was observed [7]. For stearic acid, three studies found no changes in the total TAG responses in healthy-weight subjects [14,29,31]. An obese group was included in one of these studies, in which the TAG response was decreased after the high sn-2 stearic acid meal [31]. In addition, in another study, higher sn-2 stearic acid content decreased the TAG response in healthy-weight subjects [30]. The NEFA responses were not differently affected [14,29,31]. In addition, the responses of TC, as well as of LDL-C and HDL-C, were comparable between meals that differed in stearic acid sn-2 content [14,30,31].
3.3.2. Hematological Markers
In one study, no effect of palmitic acid sn-2 content was observed on the FVIIa responses [33]. Interestingly, the effects of stearic acid sn-2 content were different between fat sources, i.e. cocoa butter with a lower stearic acid content at sn-2 increased FVIIa postprandial when compared with cocoa butter with a higher sn-2 content [30], while the amount of stearic acid at the sn-2 position of shea blends had no effect on FVIIa [14].
3.3.3. Other Markers
Postprandial glucose and insulin responses after palmitic acid-rich meals were comparable [27,28,32,33,35,36,37]. However, one study found that the peak value of insulin appeared faster after the meal with higher sn-2 content of palmitic acid (after 60 instead of 90 minutes) [32], while another study observed lower insulin concentrations 30, 90, and 120 minutes after intake of the high sn-2 meal, which was accompanied by a tendency towards a lower total insulin response [33]. Furthermore, one study found lower glucose-dependent insulinotropic polypeptide (GIP) concentrations after the high sn-2 meal [37], while two other studies did not observe any differences [28,35]. Two studies also measured peptide YY (PYY), and no significant differences were found [28,37], although the PYY response tended to be less in women in one study [37]. Only one study examined inflammatory cytokines and the endothelial function marker E-selectin, and no differences were found [7]. Three studies examining stearic acid-rich meals measured postprandial glucose and insulin, and the responses were comparable between the meals [14,29,31]. Furthermore, white blood count (WBC), as measured in one study, was not affected [14].
3.4. Postprandial Effects of Substituting Palmitic Acid with Stearic Acid on Cardiometabolic Risk Markers
Six studies have compared side-by-side postprandial effects of meals high in palmitic acid with those high in stearic acid (Table 4). The fats that were added to enrich meals with palmitic acid were palm oil [38,39], palm olein [7,40], and a blend of tripalmitin with high-oleic sunflower oil (HOSO) [41]. For the stearic acid-rich meals, lard [7,38,40], hydrogenated HOSO [39], and a blend of tristearin with HOSO [41] were used. The fat content of the test meals varied between 50 and 90 grams, from which 9 to 37 grams originated from palmitic or stearic acids. The difference between palmitic and stearic acid in the meals ranged between 5 and 23 en%. Postprandial follow-up varied between four and eight hours (Table A6).
Table 4.
Summary of studies examining the postprandial effects of substituting fats high in palmitic acid (C16:0) with fats high in stearic acid (C18:0).
| Postprandial Lipids and lipoproteins |
C18:0 vs C16:0 |
Hematological markers | C18:0 vs C16:0 |
Other markers | C18:0 vs C16:0 |
|---|---|---|---|---|---|
| Triacylglycerol | 1 ↓ 4 = 0 ↑ |
FVIIa | 0 ↓ 3 = 0 ↑ |
Glucose | 0 ↓ 1 = 0 ↑ |
| Non-esterified fatty acids | 0 ↓ 2 = 0 ↑ |
FVIIc | 0 ↓ 2 = 0 ↑ |
Insulin | 0 ↓ 2 = 0 ↑ |
| Total cholesterol | 0 ↓ 1 = 0 ↑ |
PAI-1 antigen | 0 ↓ 1 = 0 ↑ |
GIP | 1 ↓ 0 = 0 ↑ |
| VLDL-cholesterol | 0 ↓ 1 = 0 ↑ |
tPA activity | 0 ↓ 1 = 0 ↑ |
Peptide YY | 0 ↓ 1 = 0 ↑ |
| LDL-cholesterol | 0 ↓ 1 = 0 ↑ |
Leptin | 0 ↓ 1 = 0 ↑ |
||
| HDL-cholesterol | 0 ↓ 1 = 0 ↑ |
CETP activity | 0 ↓ 1 = 0 ↑ |
||
| ApoB | 0 ↓ 1 = 0 ↑ |
LPL activity | 0 ↓ 1 = 0 ↑ |
||
| ApoA1 | 0 ↓ 1 = 0 ↑ |
IL-6 | 0 ↓ 1 = 0 ↑ |
||
| Lp[a] | 0 ↓ 1 = 0 ↑ |
TNF-α | 0 ↓ 1 = 0 ↑ |
||
| IL-1β | 0 ↓ 1 = 0 ↑ |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 compared with fats high in C16:0. Abbreviations: apo, apolipoprotein; CETP, cholesteryl ester transfer protein; FVIIa, activated factor VII; FVIIc, Factor VII coagulant activity; GIP, glucose-dependent insulinotropic polypeptide; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; Lp[a], lipoprotein (a); LPL, lipoprotein lipase; PAI, plasminogen activator inhibitor; TNF, tumor necrosis factor; tPA, tissue plasminogen activator; VLDL, very-low density lipoprotein.
3.4.1. Lipids and (apo) Lipoproteins
In two studies, a lower TAG response after the meal rich in stearic acid was observed [7,40] and, in another study, lower TAG concentration three hours after the stearic acid-rich meal [39]. Other studies did not observe any differences [38,41,42]. The postprandial reduction in NEFAs was lower after stearic-acid intake in one study [7], but no differences were observed between the meals in two other studies [40,41]. Postprandial responses of VLDL-C, LDL-C, HDL-C, apoB, and apoA1 were measured in one study, but did not differ over time and between meals [41]. Additionally, the responses in postprandial concentrations of lipoprotein[a] [43], TC, and apoB48 [7] were not differently affected.
3.4.2. Hematological Markers
The postprandial responses of FVIIa after a meal rich in palmitic or stearic acid were comparable [39,42,44]. However, one study observed a non-significant lower response of FVIIa two to six hours after the stearic acid-rich meal with relatively stable FVIIa concentrations between four and eight hours, while FVIIa peaked six hours after palmitic acid and then declined [44]. FVIIc responses were measured in two studies. In one study, no differences between the meals were found [39]. In the other study, however, eight hours after the palmitic acid-rich meal FVIIc had almost returned to baseline, while it reached its highest value eight hours after the stearic acid-rich meal. Nevertheless, no difference was found in total FVIIc response [44].
3.4.3. Other Markers
Postprandial responses of glucose [37,40], insulin [37,38,40], and C-peptide [37] were not differently affected. However, the secretion of GIP was lower after the intake of stearic acid-rich lard [37]. Postprandial changes in concentrations of leptin [38,40], inflammatory cytokines [7,40], E-selectin [7], and PYY [37] were comparable. In addition, changes in CETP and lipoprotein lipase (LPL) activity did not differ between the meals [41].
4. Discussion
Interesterification is widely used by the food industry to modify TAG structures of fats to change their physical characteristics and, thereby, increase their suitability for food applications without the formation of trans fatty acids. The saturated fatty acids within interesterified fats are predominantly palmitic acid and stearic acid. We have systematically reviewed effects of fats rich in either palmitic or stearic acid on cardiometabolic risk markers to better understand metabolic effects of interesterified fats. Focus was on the position of palmitic acid or stearic acid within the TAG molecule and on studies that have compared side-by-side palmitic acid- versus stearic acid-rich fats.
4.1. Longer-Term Effects
Although the exact intakes of interesterified fats are unknown, it has been estimated that—if all trans fats would be replaced with interesterified fats—the mean daily intake in the United States would be approximately 3 en% with an upper limit of 4.8 en% [45]. The daily intakes of interesterified fats as well as the proportions of total and sn-2 palmitic or stearic acids differed widely between studies. However, in most studies, interesterified fat intakes were well above the estimated upper limit of 4.8 en% [45]. Still, no effects of palmitic acid or stearic acid sn-2 content were found. In general, metabolically healthy and relatively young subjects were studied. In the only study that included mildly hypercholesterolemic subjects, no effects of palmitic acid sn-2 content were also observed [10]. Furthermore, studies using stearic acid-rich fats have only been performed in men. It is known that men and women differ in CVD risk [46] and might respond differently to dietary interventions [47]. Indeed, one study observed slightly increased TC and LDL-C in men, but not in women after intake of a fat with a higher palmitic acid sn-2 content [9]. However, the difference between men and women was not statistically significant, but this might be explained by lack of statistical power. Little research has been done on the hemostatic system, inflammation, and glucose-insulin homeostasis, which are all involved in the pathogenesis of CVD [48,49,50]. However, the results so far do not indicate effects of diets enriched with interesterified fats on markers that are involved in these metabolic processes.
Since the use of interesterified fats might increase stearic and/or palmitic acid intakes, we need to thoroughly understand their metabolic effects. The daily intakes of palmitic and stearic acids in the United States are approximately 6 en% and 3 en%, respectively [51]. It is well known that stearic acid lowers concentrations of TC, LDL-C, and HDL-C when compared with palmitic acid [52]. Indeed, the majority of studies showed decreased TC and LDL-C concentrations on the stearic acid-rich diet [16,17,18,23]. In three studies, lower HDL-C concentrations were observed [17,19,20]. Only one out of four studies observed a statistically significant decrease in apoB100 concentrations on the stearic acid-rich diet [17]. However, previous meta-analyses found lower apoB concentrations on stearic acid as compared with palmitic acid [3] and a non-significant increase in apoB when carbohydrates were replaced with palmitic acid but not when replaced with stearic acid [4]. TAG concentrations were comparable between diets, which might suggest that the number of VLDL particles was unchanged. Therefore, it is of interest to examine whether stearic acid induces a shift towards smaller and denser LDL particles. Furthermore, two of the four studies found decreased apoA1 concentrations [17,19]. It is uncertain whether this is associated with less (pre-β) HDL particles, since one HDL particle can contain up to four A1 apolipoproteins [53]. As apoA1 is involved in ATP-binding cassette transporter (ABC) A1-mediated cholesterol efflux from peripheral cells to pre-β-HDL particles, it is of interest to examine whether these decreased apoA1 concentrations result in impaired reverse cholesterol transport. Only a few studies examined effects on hematological markers. The platelet volume decreased when minimally 5 en% palmitic acid was exchanged for stearic acid [22]. Total platelet count was not affected, which suggests smaller platelets that are considered to be less active than larger ones [54]. In addition, FVIIc activity decreased when 14 en% palmitic acid was exchanged for stearic acid [17], but not when 5 en% was exchanged [22]. Furthermore, the first study used shea butter, while the latter used hydrogenated canola oil. It has been suggested that the effects of shea butter may be due to its non-glyceride components instead of its stearic acid content [22]. Hematological markers that were related to fibrinolysis were not affected [17,22]. Remarkably, only one of the longer-term studies included in this review has addressed the effects of palmitic and stearic acids on inflammation [20], and only two studies examined fasting glucose and insulin concentrations [12,20]. In these studies, no differences were observed, but more research is needed to confirm these results.
4.2. Postprandial Effects
The postprandial TAG responses are highly dynamic and they depend on many factors. For example, gender, age, and obesity are known to affect postprandial lipemia [55]. Indeed, the studies that included obese subjects observed higher postprandial TAG responses in obese as compared with healthy-weight subjects [31,38]. In addition, one study observed lower postprandial TAG concentrations in premenopausal women than in men [7]. Normally, TAG concentrations in the blood peak three to five hours after the meal and then return to baseline within six to eight hours [56]. The studies included in this review differed in postprandial follow-up, ranging between four and eight hours. Since not only the peak value of TAG after a meal, but also the time to return to fasting TAG concentrations (the duration of lipemia) is positively related to CVD [55,56], it might be important to follow-up for at least six hours to gain more insights in both peak values and duration of lipemia. In addition, during the day, people generally consume another meal after four to six hours. However, none of the studies included a so-called second meal challenge. Introducing a second fat-rich meal four to six hours after the first meal has been shown to induce the release of chylomicrons that contain fatty acids from the previous meal [57]. Therefore, the composition of the previous meal might affect meal effects. In addition, postprandial impairment of endothelium-dependent vasodilation and oxidative stress are most marked after a second fat-containing meal [58]. Conflicting results have been reported on postprandial TAG responses of native and interesterified palmitic or stearic acid-rich fats. This discrepancy might be explained by the characteristics of the fats used, in particular the solid fat content at 37 °C. In most studies, the solid fat content increased if the proportion of palmitic acid or stearic acid at sn-2 increased. However, in one study, solid fat content was lower for the fat blend high in palmitic acid at sn-2 and the results of this study were opposite to those of other studies, e.g. higher TAG response after the fat with higher sn-2 palmitic acid content [28]. It has been suggested that the solid fat content at body temperature, rather than sn-2 palmitic or stearic acid content, determines the postprandial TAG response [5]. It is hypothesized that a high solid fat content at 37 °C, which is often due to tristearin (SSS) or tripalmitin (PPP) TAG species, impairs micelle formation [14] and reduces accessibility for pancreatic lipase [31], thereby decreasing the rate of absorption by the enterocyte. The FVIIa responses seem to be related to postprandial lipemia, e.g. attenuated lipemia is associated with decreased FVIIa responses [33]. Although no changes in glucose and insulin responses were shown between fats differing in sn-2 palmitic or stearic acid content, the results on postprandial release of gut hormone GIP were less clear. GIP induces insulin secretion and it is released when fatty acids and/or carbohydrates enter the small intestine [59]. GIP has only been measured in studies investigating the sn-2 position of palmitic acid [28,37], and the results differed between these two studies. Palm oil increased GIP more than interesterified palm oil [37], while no difference was observed after the native and interesterified blend of palm stearin and palm kernel [28]. It is likely that this is due to the difference in physical characteristics of the control fats used; fats liquid at body temperature, such as high oleic sunflower oil and palm oil, increase GIP more than fats with solids at body temperature, such as interesterified palm oil and lard [37]. The effects on GIP were possibly attenuated since both the native and interesterified blends of palm stearin and palm kernel were partly solid at body temperature [28]. The only study that has measured the effects of positional distribution within the TAG molecules on postprandial inflammatory cytokines and E-selectin observed no effects of sn-2 palmitic acid content in a meal [7]. Substituting palmitic with stearic acid does not seem to affect the postprandial responses of lipids and (apo) lipoproteins, although two studies observed a lower TAG response after lard when compared with palm olein [40]. However, it is uncertain if this difference is due to the exchange between palmitic and stearic acid or due to differences in sn-2 content of palmitic acid and subsequently physical characteristics; lard has a higher solid fat content at 37 °C. The postprandial effects on hematological markers, glucose-insulin homeostasis, and inflammation require further attention, but, so far, the results do not indicate clear differences between palmitic and stearic acids.
5. Conclusions
Interesterification of palmitic acid- or stearic acid-rich fats does not seem to affect fasting serum lipids and (apo) lipoproteins. On the other hand, stearic acid decreases the LDL- and HDL-cholesterol concentrations when compared with palmitic acid. In addition, postprandial lipemia is attenuated if the changes in palmitic acid or stearic acid sn-2 contents increase the solid fat content of the blend at body temperature. No evidence was found that solely substituting palmitic acid with stearic acid affected postprandial lipemia. However, there is a need to further examine the fasting and postprandial effects of (interesterification of) palmitic acid- and stearic acid-rich fats on the hemostatic system, inflammation, and glucose-insulin homeostasis, as well as on emerging cardiometabolic risk markers, such as cholesterol efflux capacity and lipoprotein particle size. In addition, it would be of interest for future studies to specifically examine populations that have a higher risk for CVD, such as elderly or people with obesity, and to examine sex differences.
Appendix A
Table A1.
Longer-term effects of substituting fats low in sn-2 palmitic acid (C16:0) contents with fats high in sn-2 C16:0 contents on fasting cardiometabolic risk. markers.
| First author, Year of publication |
Study population, Age, BMI |
Duration intervention periods, Study design |
Total fat (en%) | C16:0 (en%) |
Source Low sn-2 High sn-2 |
C16:0 sn-2 in fat blends (% 1) |
Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers |
Other markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Nestel, 1995 [10] |
27 men (mildly hyperchol 2) 49 ± 8 y 26.3 ± 2.5 kg/m2 |
21 days Crossover (no WO) |
31 | 6.7 | Palm oil IE palm oil |
8.7 24.7 wt% |
NR | TAG = TC = LDL-C = HDL-C = |
||
| Zock, 1995 [9] |
23 men 37 women 3 29 (19–67) y 22.9 (18.1–30.9) kg/m2 |
21 days Crossover (no WO) |
40 | 11 | Control and IE blend of palm oil blended with sunflower oil | 6.4 66.9 wt% |
0 0 |
TAG = TC = 4 LDL-C = 4 HDL-C = |
||
| Meijer, 1997 [13] |
30 men 30 women ± 35.5 y ± 23.8 kg/m2 |
21 days Crossover 5 (no WO) |
34 | 1 or 25 | Control and IE blend that consisted mainly of coconut and palm oils blended with soybean oil | 7.1 18.0 wt% |
NR | TAG = NEFA = TC = LDL-C = HDL-C = Lp[a] = |
FVIIa = Fibrinogen = PAI-1 antigen = tPA antigen = tPA activity = vWF = |
Glucose = CRP = |
| Christophe, 2000 [8] |
32 men 23–53 y 18.1–23.5 kg/m2 |
28 days Parallel |
NR ± 131 g | NR ± 5 g | IE butter Butter |
NR | NR | TAG = TC = LDL-C= HDL-C= ApoB = ApoA1 = |
||
| Filippou, 2014 [11] |
10 men 31 women ± 29.1 y ± 23.0 kg/m2 |
42 days Crossover (no WO) |
27 | 9 | Palm olein IE palm olein |
9.8 45.9 mol% |
0 5.9 |
TAG = TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = |
Glucose = Insulin = C-peptide = |
|
| Ng, 2018 [12] |
64 women 21 men 20–60 y 21–30 kg/m2 |
56 days Parallel |
35 | 7 | Palm olein CIE palm olein |
11.1 32.4 wt% |
NR | TAG = TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = |
Glucose = Insulin = C-peptide = |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C16:0 sn-2 contents compared with fats low in C16:0 sn-2 contents. 1=% of total fatty acids at sn-2. 2=Subjects were mildly hypercholesterolemic (Average total cholesterol: 6.00 ± 0.78 mmol/L) [10]. 3=Pre- and postmenopausal women were included; however, study was designed in such a way that menstrual cycle or use of oral contraceptives should not have influenced results [9]. 4=In men, total and LDL cholesterol concentrations were slightly increased (0.10 mmol/L and 0.08 mmol/L respectively) on the diet with higher C16:0 sn-2 [9]. 5=Subjects were divided into two parallel groups that were assigned to a diet with either 4 or 8 en% of the blends. Of the 60 subjects in total, 32 (16 men and 16 female) subjects followed the 4 en% diet (age ± 33 years, BMI: ± 24.1 kg/m2) and 28 (14 men and 14 female) subjects the 8 en% diet (age ± 38 years, BMI ± 23.4 kg/m2). The blends provided 1 and 2 en% palmitic acid in the 4 and 8 en% diet respectively, total amount of palmitic acid in the diets was not reported [13]. Abbreviations: apo, apolipoprotein; CIE, chemically interesterified; CRP, C-reactive protein; en%, % of total energy; FVIIa, activated factor VII; HDL-C, high-density lipoprotein cholesterol; IE, interesterified; Lp[a], lipoprotein [a]; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acids; NR, not reported; PAI, plasminogen activator inhibitor; sn, stereospecific numbering; TAG, triacylglycerol; TC, total cholesterol; tPA, tissue plasminogen activator; vWF, von Willebrand Factor; WO, wash out period; wt, weight; y, year.
Table A2.
Longer-term effects of substituting fats low in sn-2 stearic acid (C18:0) contents with fats high in sn-2 C18:0 contents on fasting cardiometabolic risk markers.
| First author, Year of publication |
Study population, Age, BMI |
Duration intervention periods, Study design |
Total fat (en%) | C18:0 (en%) |
Source Low sn-2 High sn-2 |
C18:0 sn-2 in fat blends (% 1) |
Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers |
Other markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Grande, 1970 [15] |
32 men 40–65 y NR |
18 days Latin-square |
34 | 10 | Native or IE cocoa butter 2 blended with safflower oil | NR | NR 3 | TAG = TC = |
||
| Berry, 2007 [14] |
16 men 26.8 ± 8.0 y 23.7 ± 3.7 kg/m2 |
21 days Crossover |
30 g test fat 4 | 74 | Native or IE shea butter blended with sunflower oil | 3.1 22.8 mol% |
22 41 |
TAG = TC = LDL-C = HDL-C = |
FVIIa = | Glucose = Insulin = |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 sn-2 contents compared with fats low in C18:0 sn-2 contents. 1=% of total fatty acids at sn-2 2=the interesterified cocoa butter was a mix of palm oil, totally hydrogenated soybean oil, and olive oil which matched the fatty acid composition of native cocoa butter [15]. 3=Melting points of the blends were not measured. Authors reported that native cocoa butter is normally liquid at 37 °C, while they calculated that IE cocoa butter should have 19% solid fat content at 40.5 °C [15].4=Total daily intake of total fat and stearic acid was not reported. Diets provided 30 grams of test fat and an additional 7 en% (15 grams) of C18:0 per day [14]. Abbreviations: en%, % of total energy; FVIIa, activated factor VII; HDL-C, high-density lipoprotein cholesterol; IE, interesterified; LDL-C, low-density lipoprotein cholesterol; NR, not reported; sn, stereospecific numbering; TAG, triacylglycerol; TC, total cholesterol; y, year.
Table A3.
Longer-term effects of substituting fats high in palmitic acid (C16:0) with fats high in stearic acid (C18:0) on fasting cardiometabolic risk markers.
| First author, Year of publication |
Study population, Age, BMI |
Duration intervention period, Study design |
Total fat (en%) | C16:0 C18:0 (en%) |
Difference between diets C16:0 C18:0 (en%) |
Main source C16:0 C18:0 |
Lipids and lipoproteins | Hematological markers | Other markers |
|---|---|---|---|---|---|---|---|---|---|
| Grande, 1970 [15] |
32 men 40–65 y NR |
18 days Latin-square |
34 | 15 10 |
6 8 |
Palm oil Cocoa butter |
TAG = TC ↓ |
||
| Bonanome, 1988 [16] |
11 men 64 ± 4.0 y 24 ± 1.7 kg/m2 |
21 days Cross-over (no WO) |
40 | 18 17 |
15 | Palm oil Hydrogenated soybean oil |
TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C = |
||
| Tholstrup, 1994 [17] + 1995 [25] |
15 men 24.9 (22–30) y 23.2 (20.4–26.4) kg/m2 |
21 days Cross-over |
40 | 16 1 14 |
14 | Palm oil Shea butter |
TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C ↓ ApoB ↓ ApoA1 ↓ Lp[a] ↑ |
FVIIc ↓ PAI-1 activity = PAI-1 antigen = tPA activity = tPA antigen = EFA = |
|
| Dougherty, 1995 [18] | 10 men 37.4 ± 6.6 y 25.2 ± 2.5 kg/m2 |
40 days Cross-over (no WO) |
27-29 | 7 | 5 6 |
Palm oil Shea butter |
TAG = TC ↓ LDL-C ↓ HDL-C = |
||
| Schwab, 1996 [19] + 1997 [26] |
12 women 2 (premenopausal) 23.5 ± 3.1 y 22.1 ± 2.4 kg/m2 |
28 days Cross-over |
37 | 12 7 |
3 5 |
Palm oil, butter Cocoa butter |
TAG = NEFA =2 TC ↓ VLDL-C = LDL-C = HDL-C ↓ ApoB = ApoA1 ↓ |
CETP activity ↓ Glucose = 2 Insulin = 2 |
|
| Nestel, 1998 [21] |
15 subjects (mildly hyperchol men and women 3) 51 ± 7 y 26.2 ± 3.9 kg/m2 |
35 days Cross-over (no WO) |
41-42 | 8 4 | ±5 |
Palm olein Fully hydrogenated soybean oil |
TAG = TC = LDL-C = HDL-C = |
||
| Snook, 1999 [23] |
16 women (premenopausal) 28 ± 6 y NR |
35 days 3x3 cross-over |
40 | 13 | 10 11 |
Tripalmitin Tristearin |
TAG = TC ↓ LDL-C ↓ HDL-C = ApoB = ApoA1 = |
CETP activity = LCAT activity = |
|
| Kelly, 2001 [22] |
13 men 35 ± 12 y 26 ± 3.3 kg/m2 |
28 days Cross-over |
27–28 | 8 7 |
6 5 |
Palm stearin and/or palm olein Hydrogenated canola |
TAG = TC = LDL-C = HDL-C = |
FVIIc = MPV ↓ Fibrinogen = Plasminogen = WBC = RBC = Hb = PLT = APTT = ATIII = |
|
| Kelly, 2002 [24] |
9 men 39 ± 10 y 25 ± 2.5 kg/m2 |
21 days Cross-over |
28–29 | 7 4 |
1 2 |
Potato crisps, shortbread biscuits, muesli bars Milk chocolate |
TAG = TC = LDL-C = HDL-C = |
MPV = WBC = RBC = Hb = PLT = |
|
| Ng, 2018 [12] |
64 women 21 men 20–60y 21–30 kg/m2 |
56 days Parallel |
35 | 7 8 |
5 7 |
IE Palm olein IE hydrogenated soybean oil |
TAG ↓ TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = |
Glucose = Insulin = C-peptide = |
|
| Meng, 2019 [20] |
20 postmenopausal women (mildly hyperchol 5) 64 ± 7 y 26.4 ± 3.4 kg/m2 |
35 days Cross-over |
30 | 14 10 |
8 9 |
Palm oil Cocoa butter |
TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C ↓ ApoB = ApoA1 = Lp[a] = |
PT = PTT = |
Glucose = Insulin = CRP = TNF-a = IL-6 = SAA-1 = sICAM-1 = sICAM-3 = sVCAM-1 = E-selectin = P-selectin = Thrombomodulin = |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 compared with fats high in C16:0. 1Total dietary intake of C16:0 and C18:0 was not reported, values represent intakes from the blends. Blends provided 90% of total fat intake [17]. 2The measurements of glucose, insulin, and non-esterified fatty acids were performed in a sub study with 8 of the 12 participants. Glucose and insulin were assessed with an intravenous glucose tolerance test and due to technical reasons the results of only 6 subjects were available on both diets [26]. 3Number of men and women that completed the study was not reported but 12 men and 8 women were screened. Not defined if women were pre- or postmenopausal. Subjects were mildly hypercholesterolemic (Average total cholesterol: 6.13 ± 0.80 mmol/L) [21]. 4Total dietary intake of C16:0 and C18:0 was not reported, values represent intakes from the blends. Blends provided 55% of total fat intake [21]. 5Mildly hypercholesterolemic based on LDL-cholesterol concentrations (Average LDL-cholesterol: 3.5 ± 0.7 mmol/L, total cholesterol: 5.6 ± 0.8 mmol/L) [20]. Abbreviations: apo, apolipoprotein; APTT, activated partial thromboplastin time; ATIII, antithrombin III; CETP, cholesteryl ester transfer protein; CRP, C-reactive protein; EFA, euglobulin fibrinolytic activity; en%, % of total energy; FVIIc, Factor VII coagulant activity; HDL-C, high-density lipoprotein cholesterol; Hb, hemoglobin; HOSO, high oleic acid sunflower oil; IE, interesterified; IL, interleukin; LCAT, lecithin-cholesterol acyltransferase; Lp[a], lipoprotein [a]; LDL-C, low-density lipoprotein cholesterol; MPV, mean platelet volume; NR, not reported; PAI, plasminogen activator inhibitor; PLT, platelets; PT, prothrombin time; PTT, partial thromboplastin time; RBC, red blood cells; SAA, serum amyloid A; sICAM, soluble intercellular adhesion molecule; sn, stereospecific numbering; sVCAM, soluble vascular cell adhesion molecule; TAG, triacylglycerol; TC, total cholesterol; TNF, tumor necrosis factor; tPA, tissue plasminogen activator; VLDL, very low-density lipoprotein; WBC, white blood cells; WO, wash out period; wt, weight; y, year.
Table A4.
Postprandial effects of substituting fats low in palmitic acid (C16:0) sn-2 contents with fats high in C16:0 sn-2 contents on cardiometabolic risk markers.
| First author, Year of publication |
Population, Age, BMI, Follow-up |
Total energy (kcal) | Total fat in grams (en%) |
C16:0 content in grams (en%) |
Source Low sn-2High sn-2 |
C16:0 sn-2 in fat blends (% 1) |
Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers |
Other markers |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zampelas, 1994 [35] | 16 men 24.8±2.6 y 22.7±2.4 kg/m2 6 h |
662 | 40 (54 en%) |
12 (16 en%) |
Palm olein IE blend of palm stearine with sunflower oil |
5.9 72.7 wt% |
NR | TAG = NEFA = |
Glucose = Insulin = GIP = |
||
| Summers, 1998 [36] |
2 men 6 women (pre- and postmenopausal) 30.5 (18–55) y 24 (19–30) kg/m2 6 h |
932 | 60 (58 en%) |
18 (17 en%) |
NR | 5.9 67.8 mol% |
NR | TAG = NEFA = |
Glucose = Insulin = |
||
| Yli-Jokipii, 2001 [32] |
10 women (premenopausal) 26.9 ± 2.56 y 18.5–25 kg/m2 6 h |
NR | 55 g/m2 body surface area | 17 g/m2 body surface area | Palm oil IE palm oil |
9 31 mol% |
0 0 |
TAG ↓ NEFA = VLDL-C = CM-C = |
Glucose = Insulin = |
||
| Yli-Jokipii, 2003 [27] |
2 men 7 women (premenopausal) 24 ± 3 y 21.5 ± 2.5 kg/m2 8 h |
NR | 55 g/m2 body surface area | 17 g/m2 body surface area | IE Lard Lard |
52 69 mol% |
11.0 2 12.5 |
TAG = 3 NEFA = TC = |
Glucose = Insulin = |
||
| Berry, 2007 [33] |
20 men 28.8 ± 10.3 y 23.2 ± 2.6 kg/m2 6 h |
853 | 50 (53 en%) |
14 (15 en%) |
Palm oil IE palm oil |
7.2 37.2 mol% |
3.6 15.2 |
TAG = TC = LDL-C = HDL-C = |
FVIIa = WBC = |
Glucose = Insulin = |
|
| Sanders, 2011 [7] Filippou, 2014 [37] |
25 men 25 women (premenopausal) ± 24.8 y ± 23.5 kg/m2 8 h |
846 | 50 (53 en%) |
20 (22 en%) |
Palm olein IE palm olein |
9.2 39.1 mol% |
0 4.7 |
TAG = NEFA = TC = ApoB48 = |
Glucose = Insulin = C-peptide = GIP ↓ PYY= |
IL-6 = IL-8 = TNF-α = E-selectin = |
|
| Hall, 2014 [34] |
11 men 50 ± 7 y 27.6 ± 3.1 kg/m2 6 h |
1047 | 75 (64 en%) |
30 (26 en%) |
Palm olein IE palm olein |
9.8 45.9 mol% |
NR |
TAG = 4 NEFA = TC = |
|||
| Hall, 2017 [28] |
12 men 20.5 ± 1.1 y 22.4 ± 2.8 kg/m2 4 h |
832 | 52 56 en% |
26 28 en% |
PSt/PK IE PSt/PK |
36.0 54.7 mol% |
24 5 21 |
TAG ↑ |
Glucose = Insulin = GIP = PYY = |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C16:0 sn-2 contents compared with fats low in C16:0 sn-2 contents. 1=% of total fatty acids at sn-2. 2= 12.5% Lard and 11.0% IE lard was solid at 35 °C, and 8.3% and 6.5% at 40 °C respectively. No values reported for 37 °C [27]. 3=iAUC of VLDL-TAG was smaller after lard [27]. 4=TAG iAUC of 0 to 4 h after IE palm olein was lower than after palm olein (p=0.024). Chylomicron TAG was lower at 4 h after IE palm olein compared to palm olein (p=0.038) [34]. 5= 24% PSt/PK and 21% IE PSt/PK was solid at 35 °C, and 17 and 11% at 40 °C respectively. No values for 37 °C [28]. Abbreviations: apo, apolipoprotein; CM-C, chylomicron cholesterol; en%, % of total energy; FVIIa, activated factor VII; GIP, glucose-dependent insulinotropic polypeptide; HDL-C, high-density lipoprotein cholesterol; IE, interesterified; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acids; NR, not reported; PSt/PK, palm stearin blended with palm kernel; PYY, peptide YY; TAG, triacylglycerol; TC, total cholesterol; TNF, tumor necrosis factor; VLDL, very low-density lipoprotein; WBC, white blood cells; wt, weight; y, year.
Table A5.
Postprandial effects of substituting fats low in stearic acid (C18:0) sn-2 contents with fats high in C18:0 sn-2 contents on cardiometabolic risk markers.
| First author, Year of publication |
Population, Age, BMI, Follow-up |
Total energy (kcal) | Total fat in grams (en%) |
C18:0 content in grams (en%) |
Source Low sn-2 High sn-2 |
C18:0 sn-2 in fat blends (% 1) |
Solid at 37 °C (%) | Lipids and lipoproteins |
Hematological markers | Other markers |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Summers, 1999 [29] |
14 women 49 (29–70) y 27.5 (20.6–52.8) kg/m2 6 h |
932 | 60 (58 en%) |
18 (18 en%) |
NR | NR 83.3 |
NR | TAG = NEFA = |
Glucose = Insulin = |
||
| Sanders, 2003 [30] |
17 men 38.2 ± 11.1 y 24.5 ± 2.9 kg/m2 6 h |
749 | 50 (60 en%) |
17 (20 en%) |
Cocoa butter IE cocoa butter |
NR | NR 2 | TAG ↓ | TC = LDL-C = |
FVIIa ↓ | |
| Berry, 2007 [14] |
16 men 26.8 ± 8.0 y 23.7 ± 3.7 kg/m2 8 h |
853 | 50 (53 en%) | 26 (28 en%) |
Native or IE shea butter blended with HOSO | 3.1 22.8 mol% |
22.2 41.2 |
TAG = NEFA = |
TC = LDL-C = HDL-C = |
FVIIa = WBC = |
Glucose = Insulin = |
| Robinson, 2009 [31] |
10 non-obese men (55.8 ± 7.0y, 26.6 ± 2.5 kg/m2) 11 obese men (59.3 ± 6.0y, 32.9 ± 4.3 kg/m2), 6 h |
NR | 86-102 (76 en%) (1 g/kg body mass) |
25-30 (21 en%) |
Canola stearin (EIE, CIE, native) blended with HOSO | 0.5 0.6 25.5 wt% |
5.4 5.6 18.6 |
Non-obese: TAG = Obese: TAG ↓ 3 |
Both: NEFA = TC = LDL-C = HDL-C |
Both: Glucose = Insulin = |
|
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 sn-2 contents compared with fats low in C18:0 sn-2 contents. 1=% of total fatty acids at sn-2. 2=Melting points were 35 and 50 °C for native and randomized cocoa butter, respectively [30]. 3=The native blend (high C18:0 sn-2) had a lower TAG response compared to the chemically interesterified blend (low C18:0 sn-2) but not compared to the enzymatically interesterified blend [31]. Abbreviations: CIE, chemically interesterified; en%, % of total energy; EIE, enzymatically interesterified; FVIIa, activated factor VII; HDL-C, high-density lipoprotein cholesterol; HOSO, high oleic sunflower oil; IE, interesterified; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acids; NR, not reported; TAG, triacylglycerol; TC, total cholesterol; WBC, white blood cells; wt, weight; y, year.
Table A6.
Postprandial effects of substituting fats high in palmitic acid (C16:0) with fats high in stearic acid (C18:0) on cardiometabolic risk markers.
| First author, year of publication | Population, Age, BMI, Postprandial follow-up |
Total energy (kcal) | Total fat in grams (en%) | Content C16:0 C18:0 (g) |
Content C16:0 C18:0 (en%) |
Source C16:0 C18:0 |
Lipids and lipoproteins | Hematological markers |
Other markers |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Mennen, 1998 [42] |
91 women (postmenopausal) 75.7 ± 5.2 y 27.7 ± 4.1 kg/m2 6–7 h |
948– 889 |
55.7–49.3 (53–50 en%) |
22 19 |
21 19 |
NR | TAG = | FVIIa = | ||
| Jensen, 1999 [38] |
15 women (premenopausal) 8 normal-weight (27 ± 2 y, 19.2–23.7 kg/m2) 7 overweight (29 ± 3 y, 28.8–47.5 kg/m2) 8 h |
406kcal/m2 body surface area | 29 g/m2 (65 en%) |
12 g/m2 5 g/m2 |
27 10 |
Palm oil Lard |
Both: TAG = |
Both: Insulin = Leptin = |
||
| Sanders, 2000 [39] |
11 men5 women (premenopausal) 25.5 (18–32) y 23.2 (20.1–27.8) kg/m2 7 h |
1242 | 90 (65 en%) |
37 36 |
27 26 |
Palm oil Hydrogenated and IE HOSO |
TAG = | FVIIa = FVIIc = |
||
| Tholstrup, 2001 [41] + 2003 [44] + 2004 [43] |
16 men 23.4 ± 2.4 y 23 ± 2 kg/m2 8 h |
1672 1 | 75 1 (50.6 en%2) | 32 1 34 1 |
17 18 |
IE blend of tripalmitin or tristearin with HOSO | TAG = NEFA = VLDL-C = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = |
FVIIa = FVIIc = PAI-1 antigen = tPA activity = |
CETP activity = LPL activity = |
|
| Teng, 2011 [40] |
10 men 21.9 ± 0.7 y 21.0 ± 1.6 kg/m2 4 h |
754 | 50 (60 en%) |
17 9 |
21 10 |
Palm olein Lard |
TAG ↓ NEFA = |
Glucose = Insulin = Leptin = |
IL-6 = TNF-α = IL-1ß = |
|
| Sanders, 2011 [7] Filippou, 2014 [37] |
25 men 25 women (premenopausal) ± 24.8y, ± 23.5 kg/m2 8 h |
846 | 50 (53 en%) |
20 9 |
22 9 |
Palm olein Lard |
TAG ↓ NEFA ↓ TC = ApoB48 = |
Glucose = Insulin = C-peptide = GIP ↓ PYY= |
IL-6 = IL-8 = TNF-α = E-selectin = |
Markers are significantly lower (↓), higher (↑) or not significantly different (=) after intake of fats high in C18:0 compared with fats high in C16:0. 1=per 75 kg body weight. Range of fat intake was 65-85 grams [41]. 2=50.6 en% was reported. However, our calculations indicate 40.4 en% [41].Abbreviations: apo, apolipoprotein; CETP, cholesteryl ester transfer protein; en%, % of total energy; FVIIa, activated factor VII; FVIIc, Factor VII coagulant activity; GIP, glucose-dependent insulinotropic polypeptide; HDL-C, high-density lipoprotein cholesterol; HOSO, high oleic sunflower oil; IE, interesterified; IL, interleukin; Lp[a], lipoprotein [a]; LPL, lipoprotein lipase; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acids; NR, not reported; PAI, plasminogen activator inhibitor; PYY, peptide YY; TAG, triacylglycerol; TC, total cholesterol; TNF, tumour necrosis factor; tPA, tissue plasminogen activator; VLDL-C, very low-density lipoprotein cholesterol; wt, weight; y, year.
Author Contributions
M.A.v.R. and R.P.M. contributed equally to all aspects of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.FAO . Fats and Fatty Acids in Human Nutrition. Volume 91. FAO; Rome, Italy: 2010. [PubMed] [Google Scholar]
- 2.Ervin R.B., Wright J.D., Wang C.Y., Kennedy-Stephenson J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data. 2004;348:1–6. [PubMed] [Google Scholar]
- 3.Fattore E., Bosetti C., Brighenti F., Agostoni C., Fattore G. Palm oil and blood lipid-related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014;99:1331–1350. doi: 10.3945/ajcn.113.081190. [DOI] [PubMed] [Google Scholar]
- 4.Mensink R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 5.Berry S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009;22:3–17. doi: 10.1017/S0954422409369267. [DOI] [PubMed] [Google Scholar]
- 6.Alfieri A., Imperlini E., Nigro E., Vitucci D., Orrù S., Daniele A., Buono P., Mancini A. Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health. Int. J. Mol. Sci. 2018;19:104. doi: 10.3390/ijms19010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders T.A., Filippou A., Berry S.E., Baumgartner S., Mensink R.P. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am. J. Clin. Nutr. 2011;94:1433–1441. doi: 10.3945/ajcn.111.017459. [DOI] [PubMed] [Google Scholar]
- 8.Christophe A.B., De Greyt W.F., Delanghe J.R., Huyghebaert A.D. Substituting enzymatically interesterified butter for native butter has no effect on lipemia or lipoproteinemia in Man. Ann. Nutr. Metab. 2000;44:61–67. doi: 10.1159/000012822. [DOI] [PubMed] [Google Scholar]
- 9.Zock P.L., de Vries J.H., de Fouw N.J., Katan M.B. Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. Am. J. Clin. Nutr. 1995;61:48–55. doi: 10.1093/ajcn/61.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Nestel P.J., Noakes M., Belling G.B., McArthur R., Clifton P.M. Effect on plasma lipids of interesterifying a mix of edible oils. Am. J. Clin. Nutr. 1995;62:950–955. doi: 10.1093/ajcn/62.5.950. [DOI] [PubMed] [Google Scholar]
- 11.Filippou A., Teng K.T., Berry S.E., Sanders T.A. Palmitic acid in the sn-2 position of dietary triacylglycerols does not affect insulin secretion or glucose homeostasis in healthy men and women. Eur. J. Clin. Nutr. 2014;68:1036–1041. doi: 10.1038/ejcn.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng Y.T., Voon P.T., Ng T.K.W., Lee V.K.M., Mat Sahri M., Mohd Esa N., Ong S.H., Ong A.S.H. Interesterified palm olein (IEPalm) and interesterified stearic acid-rich fat blend (IEStear) have no adverse effects on insulin resistance: A randomized control trial. Nutrients. 2018;10:1112. doi: 10.3390/nu10081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijer G.W., Weststrate J.A. Interesterification of fats in margarine: Effect on blood lipids, blood enzymes, and hemostasis parameters. Eur. J. Clin. Nutr. 1997;51:527–534. doi: 10.1038/sj.ejcn.1600437. [DOI] [PubMed] [Google Scholar]
- 14.Berry S.E.E., Miller G.J., Sanders T.A.B. The solid fat content of stearic acid-rich fats determines their postprandial effects. Am. J. Clin. Nutr. 2007;85:1486–1494. doi: 10.1093/ajcn/85.6.1486. [DOI] [PubMed] [Google Scholar]
- 15.Grande F., Anderso J.T.N., Keys A. Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man. Am. J. Clin. Nutr. 1970;23:1184–1193. doi: 10.1093/ajcn/23.9.1184. [DOI] [PubMed] [Google Scholar]
- 16.Bonanome A., Grundy S.M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Engl. J. Med. 1988;318:1244–1248. doi: 10.1056/NEJM198805123181905. [DOI] [PubMed] [Google Scholar]
- 17.Tholstrup T., Marckmann P., Jespersen J., Sandström B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. Am. J. Clin. Nutr. 1994;59:371–377. doi: 10.1093/ajcn/59.2.371. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty R.M., Allman M.A., Iacono J.M. Effects of diets containing high or low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. Am. J. Clin. Nutr. 1995;61:1120–1128. doi: 10.1093/ajcn/61.5.1120. [DOI] [PubMed] [Google Scholar]
- 19.Schwab U.S., Maliranta H.M., Sarkkinen E.S., Savolainen M.J., Kesäniemi Y.A., Uusitupa M.I. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metab. Clin. Exp. 1996;45:143–149. doi: 10.1016/S0026-0495(96)90044-X. [DOI] [PubMed] [Google Scholar]
- 20.Meng H., Matthan N.R., Wu D., Li L., Rodríguez-Morató J., Cohen R., Galluccio J.M., Dolnikowski G.G., Lichtenstein A.H. Comparison of diets enriched in stearic, oleic, and palmitic acids on inflammation, immune response, cardiometabolic risk factors, and fecal bile acid concentrations in mildly hypercholesterolemic postmenopausal women-randomized crossover trial. Am. J. Clin. Nutr. 2019;110:305–315. doi: 10.1093/ajcn/nqz095. [DOI] [PubMed] [Google Scholar]
- 21.Nestel P.J., Pomeroy S., Kay S., Sasahara T., Yamashita T. Effect of a stearic acid-rich, structured triacylglycerol on plasma lipid concentrations. Am. J. Clin. Nutr. 1998;68:1196–1201. doi: 10.1093/ajcn/68.6.1196. [DOI] [PubMed] [Google Scholar]
- 22.Kelly F.D., Sinclair A.J., Mann N.J., Turner A.H., Abedin L., Li D. A stearic acid-rich diet improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur. J. Clin. Nutr. 2001;55:88–96. doi: 10.1038/sj.ejcn.1601122. [DOI] [PubMed] [Google Scholar]
- 23.Snook J.T., Park S., Williams G., Tsai Y.H., Lee N. Effect of synthetic triglycerides of myristic, palmitic, and stearic acid on serum lipoprotein metabolism. Eur. J. Clin. Nutr. 1999;53:597–605. doi: 10.1038/sj.ejcn.1600815. [DOI] [PubMed] [Google Scholar]
- 24.Kelly F.D., Sinclair A.J., Mann N.J., Turner A.H., Raffin F.L., Blandford M.V., Pike M.J. Short-term diets enriched in stearic or palmitic acids do not alter plasma lipids, platelet aggregation or platelet activation status. Eur. J. Clin. Nutr. 2002;56:490–499. doi: 10.1038/sj.ejcn.1601332. [DOI] [PubMed] [Google Scholar]
- 25.Tholstrup T., Marckmann P., Vessby B., Sandström B. Effect of fats high in individual saturated fatty acids on plasma lipoprotein[a] levels in young healthy men. J. Lipid Res. 1995;36:1447–1552. [PubMed] [Google Scholar]
- 26.Schwab U., Niskanen L., Uusitupa M. Palmitic and stearic acid enriched diets have similar effects on glucose metabolism in healthy young females. Nutr. Metab. Cardiovasc. Dis. 1997;7:315. [Google Scholar]
- 27.Yli-Jokipii K.M., Schwab U.S., Tahvonen R.L., Kurvinen J.P., Mykkänen H.M., Kallio H.P.T. Chylomicron and VLDL TAG structures and postprandial lipid response induced by lard and modified lard. Lipids. 2003;38:693–703. doi: 10.1007/s11745-003-1117-6. [DOI] [PubMed] [Google Scholar]
- 28.Hall W.L., Iqbal S., Li H., Gray R., Berry S.E.E. Modulation of postprandial lipaemia by a single meal containing a commonly consumed interesterified palmitic acid-rich fat blend compared to a non-interesterified equivalent. Eur. J. Nutr. 2017;56:2487–2495. doi: 10.1007/s00394-016-1284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers L.K.M., Fielding B.A., Herd S.L., Ilic V., Clark M.L., Quinlan P.T., Frayn K.N. Use of structured triacylglycerols containing predominantly stearic and oleic acids to probe early events in metabolic processing of dietary fat. J. Lipid Res. 1999;40:1890–1898. [PubMed] [Google Scholar]
- 30.Sanders T.A., Berry S.E., Miller G.J. Influence of triacylglycerol structure on the postprandial response of factor VII to stearic acid-rich fats. Am. J. Clin. Nutr. 2003;77:777–782. doi: 10.1093/ajcn/77.4.777. [DOI] [PubMed] [Google Scholar]
- 31.Robinson D.M., Martin N.C., Robinson L.E., Ahmadi L., Marangoni A.G., Wright A.J. Influence of interesterification of a stearic acid-rich spreadable fat on acute metabolic risk factors. Lipids. 2009;44:17–26. doi: 10.1007/s11745-008-3253-7. [DOI] [PubMed] [Google Scholar]
- 32.Yli-Jokipii K., Kallio H., Schwab U., Mykkänen H., Kurvinen J.P., Savolainen M.J., Tahvonen R. Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J. Lipid Res. 2001;42:1618–1625. [PubMed] [Google Scholar]
- 33.Berry S.E., Woodward R., Yeoh C., Miller G.J., Sanders T.A. Effect of interesterification of palmitic acid-rich triacylglycerol on postprandial lipid and factor VII response. Lipids. 2007;42:315–323. doi: 10.1007/s11745-007-3024-x. [DOI] [PubMed] [Google Scholar]
- 34.Hall W.L., Brito M.F., Huang J., Wood L.V., Filippou A., Sanders T.A., Berry S.E. An interesterified palm olein test meal decreases early-phase postprandial lipemia compared to palm olein: A randomized controlled trial. Lipids. 2014;49:895–904. doi: 10.1007/s11745-014-3936-1. [DOI] [PubMed] [Google Scholar]
- 35.Zampelas A., Williams C.M., Morgan L.M., Wright J., Quinlan P.T. The effect of triacylglycerol fatty acid positional distribution on postprandial plasma metabolite and hormone responses in normal adult men. Br. J. Nutr. 1994;71:401–410. doi: 10.1079/BJN19940147. [DOI] [PubMed] [Google Scholar]
- 36.Summers L.K., Fielding B.A., Ilic V., Quinlan P.T., Frayn K.N. The effect of triacylglycerol-fatty acid positional distribution on postprandial metabolism in subcutaneous adipose tissue. Br. J. Nutr. 1998;79:141–147. doi: 10.1079/BJN19980025. [DOI] [PubMed] [Google Scholar]
- 37.Filippou A., Berry S.E., Baumgartner S., Mensink R.P. Sanders, T.A. Palmitic acid in the sn-2 position decreases glucose-dependent insulinotropic polypeptide secretion in healthy adults. Eur. J. Clin. Nutr. 2014;68:549–554. doi: 10.1038/ejcn.2014.49. [DOI] [PubMed] [Google Scholar]
- 38.Jensen J., Bysted A., Dawids S., Hermansen K., Hølmer G. The effect of palm oil, lard, and puff-pastry margarine on postprandial lipid and hormone responses in normal-weight and obese young women. Br. J. Nutr. 1999;82:469–479. doi: 10.1017/S0007114599001725. [DOI] [PubMed] [Google Scholar]
- 39.Sanders T.A., de Grassi T., Miller G.J., Morrissey J.H. Influence of fatty acid chain length and cis/trans isomerization on postprandial lipemia and factor VII in healthy subjects (postprandial lipids and factor VII) Atherosclerosis. 2000;149:413–420. doi: 10.1016/S0021-9150(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 40.Teng K.T., Nagapan G., Cheng H.M., Nesaretnam K. Palm olein and olive oil cause a higher increase in postprandial lipemia compared with lard but had no effect on plasma glucose, insulin and adipocytokines. Lipids. 2011;46:381–388. doi: 10.1007/s11745-010-3516-y. [DOI] [PubMed] [Google Scholar]
- 41.Tholstrup T., Sandström B., Bysted A., Hølmer G. Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. Am. J. Clin. Nutr. 2001;73:198–208. doi: 10.1093/ajcn/73.2.198. [DOI] [PubMed] [Google Scholar]
- 42.Mennen L., de Maat M., Meijer G., Zock P., Grobbee D., Kok F., Kluft C., Schouten E. Factor VIIa response to a fat-rich meal does not depend on fatty acid composition: a randomized controlled trial. Arter. Thromb Vasc Biol. 1998;18:599–603. doi: 10.1161/01.ATV.18.4.599. [DOI] [PubMed] [Google Scholar]
- 43.Tholstrup T., Samman S. Postprandial lipoprotein(a) is affected differently by specific individual dietary fatty acids in healthy young men. J. Nutr. 2004;134:2550–2555. doi: 10.1093/jn/134.10.2550. [DOI] [PubMed] [Google Scholar]
- 44.Tholstrup T., Miller G.J., Bysted A., Sandström B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am. J. Clin. Nutr. 2003;77:1125–1132. doi: 10.1093/ajcn/77.5.1125. [DOI] [PubMed] [Google Scholar]
- 45.Lefevre M., Mensink R.P., Kris-Etherton P.M., Petersen B., Smith K., Flickinger B.D. Predicted changes in fatty acid intakes, plasma lipids, and cardiovascular disease risk following replacement of trans fatty acid-containing soybean oil with application-appropriate alternatives. Lipids. 2012;47:951–962. doi: 10.1007/s11745-012-3705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bots S.H., Peters S.A.E., Woodward M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2:e000298. doi: 10.1136/bmjgh-2017-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mensink R.P., Katan M.B. Effect of monounsaturated fatty acids versus complex carbohydrates on high-density lipoproteins in healthy men and women. Lancet. 1987;1:122–125. doi: 10.1016/S0140-6736(87)91965-9. [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golia E., Limongelli G., Natale F., Fimiani F., Maddaloni V., Pariggiano I., Bianchi R., Crisci M., D’Acierno L., Giordano R., et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr. Atheroscler Rep. 2014;16:435. doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 50.Lowe G., Rumley A. The relevance of coagulation in cardiovascular disease: What do the biomarkers tell us? Thromb. Haemost. 2014;112:860–867. doi: 10.1160/TH14-03-0199. [DOI] [PubMed] [Google Scholar]
- 51.Kris-Etherton P.M., Griel A.E., Psota T.L., Gebauer S.K., Zhang J., Etherton T.D. Dietary stearic acid and risk of cardiovascular disease: intake, sources, digestion, and absorption. Lipids. 2005;40:1193–1200. doi: 10.1007/s11745-005-1485-y. [DOI] [PubMed] [Google Scholar]
- 52.Mensink R.P., Sanders T.A., Baer D.J., Hayes K.C., Howles P.N., Marangoni A. The Increasing Use of Interesterified Lipids in the Food Supply and Their Effects on Health Parameters. Adv. Nutr. 2016;7:719–729. doi: 10.3945/an.115.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kido T., Kurata H., Kondo K., Itakura H., Okazaki M., Urata T., Yokoyama S. Bioinformatic Analysis of Plasma Apolipoproteins A-I and A-II Revealed Unique Features of A-I/A-II HDL Particles in Human Plasma. Sci. Rep. 2016;6:31532. doi: 10.1038/srep31532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu S.G., Becker R.C., Berger P.B., Bhatt D.L., Eikelboom J.W., Konkle B., Mohler E.R., Reilly M.P., Berger J.S. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J. Thromb. Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson K.G., Poppitt S.D., Minihane A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220:22–33. doi: 10.1016/j.atherosclerosis.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Pirillo A., Norata G.D., Catapano A.L. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 2014;30:1489–1503. doi: 10.1185/03007995.2014.909394. [DOI] [PubMed] [Google Scholar]
- 57.Jackson K.G., Robertson M.D., Fielding B.A., Frayn K.N., Williams C.M. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am. J. Clin. Nutr. 2002;76:942–949. doi: 10.1093/ajcn/76.5.942. [DOI] [PubMed] [Google Scholar]
- 58.Tushuizen M.E., Nieuwland R., Scheffer P.G., Sturk A., Heine R.J., Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J. Thromb. Haemost. 2006;4:1003–1010. doi: 10.1111/j.1538-7836.2006.01914.x. [DOI] [PubMed] [Google Scholar]
- 59.Vollmer K., Holst J.J., Baller B., Ellrichmann M., Nauck M.A., Schmidt W.E., Meier J.J. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]