This nonrandomized phase 2 study assesses the feasibility and safety of durvalumab treatment in adults with advanced solid tumors and HIV-1 infection.
Key Points
Question
Is treatment with durvalumab feasible and safe in patients with HIV-1 infection and advanced cancer?
Findings
In this nonrandomized, open-label, phase 2 study of 20 patients with HIV-1 infection and advanced solid tumors receiving suppressive antiretroviral therapy, treatment with durvalumab was feasible and safe. There were no drug-related grade 3 to 4 adverse events, and the disease control rate was 50%.
Meaning
Patients with cancer with controlled HIV-1 infection should have access to antitumoral immunotherapy with durvalumab.
Abstract
Importance
Therapies targeting the programmed cell death 1 (PD-1) receptor or its ligand (PD-L1), such as the humanized monoclonal antibody durvalumab, have shown durable clinical responses in several tumor types. However, concerns about the safety and feasibility of PD-1/PD-L1 blockade in HIV-1–infected individuals have led to the exclusion of these patients from clinical trials on cancer immunotherapies.
Objective
To evaluate the feasibility and safety of durvalumab treatment in patients with advanced cancer and virologically controlled HIV-1 infection.
Design, Setting, and Participants
The DURVAST study was a nonrandomized, open-label, phase 2 clinical trial in patients with any solid tumor type in which anti–PD-1 or anti–PD-L1 antibodies have approved indications or for which there are data of antitumoral activity with no other available curative therapy. All patients had basal undetectable plasma viremia while undergoing combination antiretroviral therapy.
Interventions
Treatment consisted of intravenous infusion of durvalumab (1500 mg every 4 weeks) until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
Adverse events were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1.
Results
A total of 20 HIV-1–infected patients with advanced cancer were enrolled; 16 (80%) were male, the median (range) age was 54 (30-73) years, and 12 (60%) had progressed with previous cancer treatment lines. A median (range) of 4 (1-16) cycles of durvalumab were administered. Drug-related adverse events were observed in 50% of patients, and all were grade 1 and 2 (mainly diarrhea, asthenia, and arthromyalgia). Four of 16 response-evaluable patients (25%) had a partial response. Five patients (31%) had stable disease, including 4 with durable stable disease (disease control rate of 50%). CD4+ and CD8+ T-cell counts and plasma HIV-1 viremia remained stable throughout the study.
Conclusions and Relevance
Durvalumab treatment was feasible and safe in HIV-1–infected patients with cancer receiving combination antiretroviral therapy. HIV-1–infected patients on suppressive antiretroviral therapy with advanced cancer should have access to cancer immunotherapy treatments.
Trial Registration
ClinicalTrials.gov Identifier: NCT03094286
Introduction
Progresses in human immunodeficiency virus type 1 (HIV-1) therapy have converted the once fatal infection into a chronic condition, yet the search for a widely applicable strategy to cure remains elusive. Individuals with HIV-1 infection have chronic antigenic immune stimulation and inflammation, even with low plasma HIV-1 RNA levels and preserved CD4+ counts, which contributes to increased incidence of non–AIDS-defining cancers,1 such that non–small cell lung cancer (NSCLC) is now the leading cause of cancer deaths in individuals receiving effective combination antiretroviral therapy (cART).2 During infection, HIV-1 is reverse transcribed and integrated as a provirus into the host genome. Although the vast majority of infected cells die with cART, a small percentage survive and harbor as integrated proviruses that comprise the latent reservoir.3
Although therapy with immune checkpoint inhibitors that block inhibitory receptors is now well established for the treatment of different tumor types,4,5,6 in most cancer clinical trials HIV-1 infection is an exclusion criterion because of fears of virus reactivation, immune reconstitution syndrome, or other toxic effects. Programmed cell death 1 (PD-1) receptor confers a selective advantage for latently infected cells persisting during cART and provides a rationale for the assessment of immune checkpoint inhibitor therapy in HIV-1–infected persons with cancer.7 Retrospective analysis8,9 and recent theoretical considerations10 suggest that patients infected with HIV-1 and treated with immune checkpoint inhibitors have good tolerance and antitumoral responses. We conducted a prospective phase 2 study of the humanized anti–programmed cell death ligand 1 (anti–PD-L1) antibody durvalumab in cART-stabilized HIV-1–infected patients with advanced cancer.
Methods
Twenty patients were enrolled and treated with durvalumab at the fixed dose of 1500 mg every 4 weeks until disease progression or unacceptable toxic effects. Eligible patients comprised HIV-1–infected individuals receiving effective cART with advanced solid tumors in which anti–PD-1/PD-L1 antibodies had approved indications or data of antitumoral activity. The complete inclusion and exclusion criteria are provided in the trial protocol (Supplement 1). The protocol was approved by the institutional review boards of the 8 hospitals involved. Written informed consent was obtained from each patient.
The primary objective of the DURVAST study was to explore the feasibility of durvalumab treatment in HIV-1–infected persons with cancer. Feasibility was defined as the ability to receive at least a median number of 4 cycles of durvalumab. Data analysis was performed from January 2019 through June 2019. The complete study objectives, assessments, and statistical analysis are provided in the eMethods in Supplement 2.
Results
A total of 20 HIV-1–infected participants with advanced cancer were enrolled between May 2017 and June 2018 (Table 1; eFigure 1 in Supplement 2); 16 (80%) were male, the median (range) age was 54 (30-73) years, and 12 (60%) had progressed with previous cancer treatment lines. Fourteen patients had NSCLC, 2 had melanoma, 1 had small cell lung cancer, 2 had anal carcinoma, and 1 had bladder carcinoma. All patients were on stable cART with undetectable HIV-1 plasma viremia (Table 1). At the time of the data cutoff, the median duration of follow-up was 12.7 months. Patients received a median (range) of 4 (1-16) study treatment cycles. Eight patients were still on treatment at the time of the data cutoff, with a median (range) number of 11 (5-16) cycles (eFigure 1 in Supplement 2).
Table 1. Baseline Characteristics of All Treated Patients.
| Characteristic | No. (%) (N = 20) |
|---|---|
| Age, median (range), y | 54 (30-73) |
| Sex | |
| Male | 16 (80) |
| Female | 4 (20) |
| ECOG performance status | |
| 0-1 | 19 (95) |
| 2 | 1 (5) |
| No. of prior systemic therapies | |
| 0 | 8 (40) |
| 1 | 4 (20) |
| ≥2 | 8 (40) |
| PD-L1 (TPS) | |
| Negative (<1%) | 11 (55) |
| Low (1%-49%) | 1 (5) |
| High (≥50%) | 3 (15) |
| HIV-1 group transmission | |
| IDUs | 8 (40) |
| MSM | 6 (30) |
| Heterosexual individuals | 4 (20) |
| Unknown | 2 (10) |
| Duration of HIV-1 infection, median (range), y | 16.0 (3.0-32.9) |
| Duration of cART, median (range), y | 10 (2-20) |
| Basal CD4+ count, cells/mm3 | |
| 100-199 | 1 (5) |
| 200-350 | 8 (40) |
| >350 | 11 (55) |
| cART therapy | |
| NRTIs + INSTIs | 14 (70) |
| NRTIs + non-INSTIs | 6 (30) |
Abbreviations: cART, combination antiretroviral therapy; ECOG, Eastern Cooperative Oncology Group; IDU, injection drug user; INSTI, integrase strand-transfer inhibitor; MSM, men who have sex with men; NRTI, nucleoside reverse transcriptase inhibitor; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score.
There were no grade 3 or 4 drug-related adverse events (AEs). The most common AEs were diarrhea (grade 1 and 2, both in 2 patients [10%]), arthromyalgia (grade 1 and 2 in 11 patients [55%] and 2 patients [10%], respectively), and asthenia (grade 1 and 2 in 9 patients [45%] and 2 patients [10%], respectively). No AEs with a potential immunologic cause, such as pneumonitis, hypothyroidism, or hypophysitis, occurred. All AEs are provided in Table 2 and eTable 1 in Supplement 2. There were 4 early deaths, not drug related (additional data are provided in eResults in Supplement 2). Finally, there were no AEs related to HIV-1 reactivation. Both the CD4+ and CD8+ T-cell counts remained stable throughout the course of treatment (Figure, A and B), including 1 patient with a long-lasting response (>14 months) with basal CD4+ T-cell counts of 164 cells/mm3 and a CD4+/CD8+ T-cell ratio of 0.67. Similarly, the plasma viremia remained undetectable (Figure, C).
Table 2. Adverse Events.
| Type of adverse event | No. (%) (N = 20) | ||||
|---|---|---|---|---|---|
| Grade | |||||
| 1 | 2 | 3 | 4 | 5 | |
| Any | 15 (75) | 10 (50) | 1 (5)a | 1 (5)a | 2 (10)a |
| Respiratory infection | 1 (5) | 1 (5) | 1 (5)a | 0 | 1 (5)a |
| Neurological disorders | 0 | 0 | 0 | 0 | 1 (5)a |
| Arterial ischemia | 0 | 0 | 0 | 1 (5)a | 0 |
| Hypotension | 0 | 3 (15) | 0 | 0 | 0 |
| Fever | 2 (10) | 2 (10) | 0 | 0 | 0 |
| Arthromyalgia | 11 (55) | 2 (10) | 0 | 0 | 0 |
| Asthenia | 9 (45) | 2 (10) | 0 | 0 | 0 |
| Nausea and vomiting | 5 (25) | 0 | 0 | 0 | 0 |
| Constipation | 2 (10) | 1 (5) | 0 | 0 | 0 |
| Dysphagia | 2 (10) | 1 (5) | 0 | 0 | 0 |
| Diarrhea | 2 (10) | 2 (10) | 0 | 0 | 0 |
| Skin adverse events | 3 (15) | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 (5) | 0 | 0 | 0 |
Adverse event was not treatment related.
Figure. T-cell Count and Plasma Viremia.
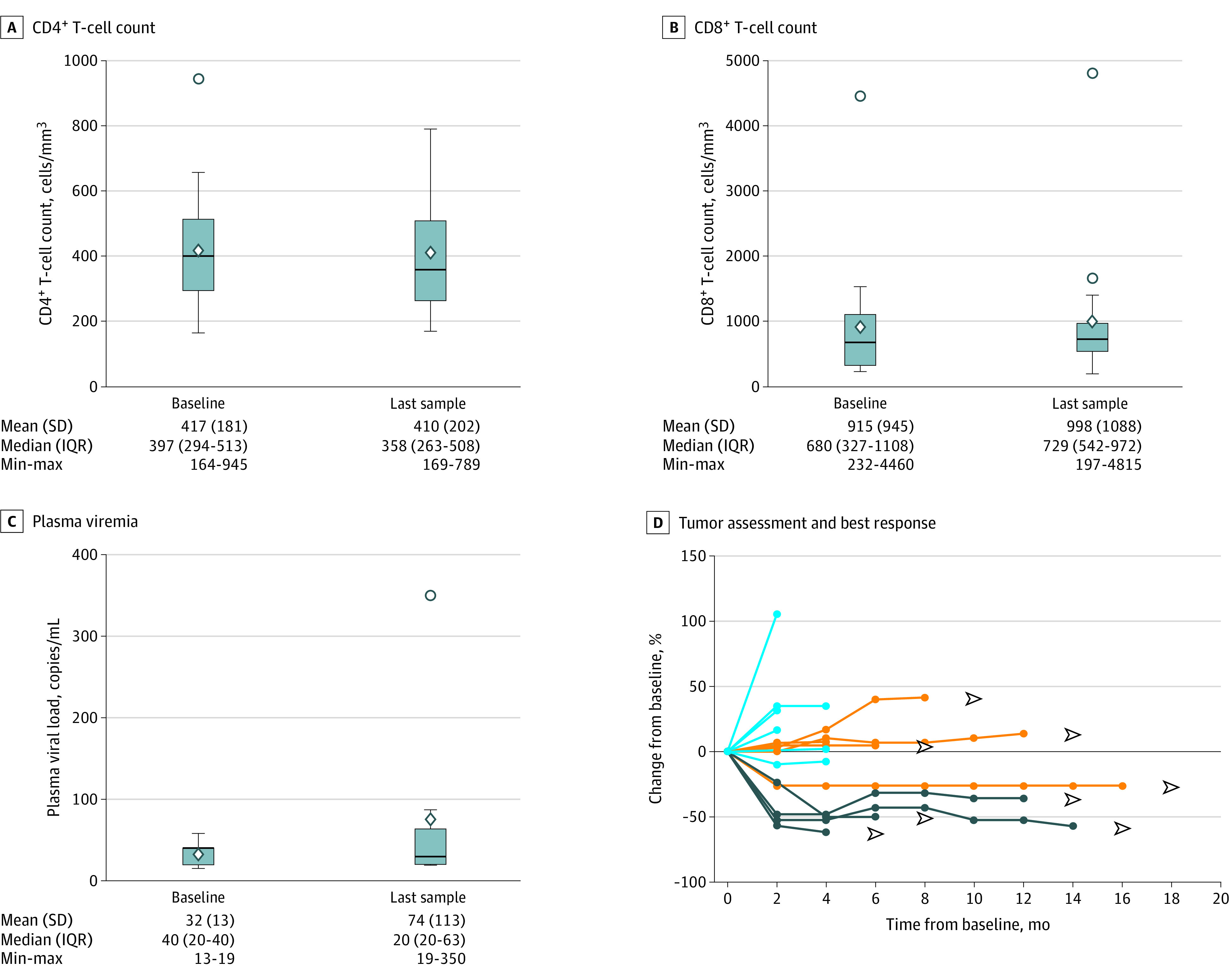
There were no significant changes in CD4+ T-cell counts (A), CD8+ T-cell counts (B), or plasma viral load (C) when comparing pretreatment samples with last samples after treatment. D, Four patients achieved a partial response (dark blue lines); 5 patients achieved stable disease (orange lines) that in 4 cases continued for more than 24 weeks; and 7 patients had progressive disease (light blue lines) as their best response. IQR indicates interquartile range.
Four of 16 response-evaluable patients (25%) had a partial response with a duration of response of more than 4 months, 6 months, 12 months, and 14 months (Figure, D). Five of the 16 patients (31%) had stable disease (Figure, D), including 4 patients with durable stable disease (>6 months, >8 months, >12 months, and >16 months), with a disease control rate of 50% (Figure, D). Additional results of antitumoral activity are provided in the eResults in Supplement 2.
Discussion
Patients infected with HIV-1 who are receiving suppressive cART have a near-normal life expectancy, and now cancer, mainly NSCLC, is one of the leading causes of mortality.2 Previous retrospective data supported the safety of treatment with anti–PD-1/PD-L1 antibodies in HIV-1–infected patients.8,9 However, until recently, most cancer clinical trials have excluded HIV-1–infected patients.11,12
The DURVAST study demonstrates that immunotherapy with durvalumab in HIV-1–infected people is feasible and safe, with no grade 3 or 4 drug-related AEs. These results complement previous data reported from the phase 1 study conducted by Uldrick et al13 in 30 patients with cancer with HIV-1 infection treated with pembrolizumab. Similar to our results, pembrolizumab was well tolerated without unexpected toxic effects and no signs of viral reactivation. Our results are also consistent with previous studies of durvalumab treatment in pretreated HIV-1–uninfected people with NSCLC, in whom the incidence of grade 3 AEs ranged from 8% to 18% and grade 4 AEs appeared in less than 1%.5
Moreover, the antitumoral activity of durvalumab in our study was higher than anticipated. Among 16 patients evaluable for response, the disease control rate was 50%, including long-lasting responses (Figure, D). Although the number of patients was small, it is not possible to rule out the fact that HIV-1 infection by itself, or the cART treatment, was positively associated with antitumoral activity. Our results suggest a longer duration of clinical benefit in patients treated with integrase strand-transfer inhibitors (eTable 2 in Supplement 2). We speculate the possibility that integrase strand-transfer inhibitors could be associated with antitumoral immune response of durvalumab. Conversely, the antitumoral activity found with pembrolizumab in the study from Uldrick et al13 was low, with only 1 of 19 patients with non–AIDS-defining cancers having a partial response, probably owing to the inclusion in this study of nonimmunosensitive tumor types, such as cholangiocarcinoma, breast cancer, and pancreatic cancer.
In agreement with the Uldrick et al study,13 our results confirm that treatment with durvalumab is also safe regarding sustained control of HIV-1 infection. All patients continued cART treatment, and plasma viremia remained undetected. In addition, CD4+ and CD8+ T-cell counts were stable throughout durvalumab treatment. Although other studies exclude patients with low basal CD4+ T-cell counts, we found no correlation of clinical benefit with basal CD4+ or CD8+ T-cell counts (eTable 6 in Supplement 2). Of note, 1 patient with NSCLC with a CD4+ T-cell count of less than 200 cells/mm3 had no side effects and a long-lasting partial response, suggesting that treating patients with low basal CD4+ T-cell counts might be safe. Although an increase in viral transcription could be hypothetically associated with PD-1 blockade in CD4+ T lymphocytes infected by HIV-1, the maintenance of cART precludes the reactivation of the viral reservoir (eFigure 7 in Supplement 2).7,14 Thus, reinvigoration of CD8+ PD-1+ T lymphocytes on induction of viral reactivation could in fact be associated with reduced viral reservoirs.15
Limitations
This study is limited by the small sample size, which does not allow completely ruling out the existence of unexpected complications owing to the use of durvalumab to treat HIV-1–infected individuals with cancer. Nevertheless, data from previous studies and retrospective case series also support the feasibility and safety of treatment with anti–PD-1/PD-L1 antibodies in this context.
Conclusions
In summary, treatment with durvalumab in patients with advanced tumors and HIV-1 infection is feasible and safe. Larger studies are needed to validate the suggested favorable antitumoral activity of durvalumab in HIV-1–infected people. HIV-1–infected patients with advanced cancer should have access to cancer therapies with immune checkpoint inhibitors.
Trial Protocol and Statistical Analysis Plan
eMethods.
eResults.
eTable 1. Drug related adverse events (AEs).
eTable 2. Outcome to durvalumab based on the type of cART.
eTable 3. PD-L1 expression and response.
eTable 4. Molecular classification by NGS of tumors.
eTable 5. Pre-treatment most differentially expressed genes between patients with- and without clinical benefit to durvalumab.
eTable 6. Correlation of basal CD4+, CD8+ T cell counts and antitumoral efficacy.
eReferences.
eFigure 1. Flowchart of HIV-1-infected cancer patients enrolled in the DURVAST study.
eFigure 2. Tumor response.
eFigure 3. A. Progression free survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study. B. Overall survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study.
eFigure 4. A. Progression free survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study, by PD-L1 status. B. Overall survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study, by PD-L1 status.
eFigure 5. Volcano plot with differentially expressed genes in patients without- versus with clinical benefit.
eFigure 6. Signature scores in patients with and without clinical benefit.
eFigure 7. Restoring CD8 T cells and latency reversal “Shock and kill strategy.”
References
- 1.Franceschi S, Lise M, Clifford GM, et al. ; Swiss HIV Cohort Study . Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103(3):416-422. doi: 10.1038/sj.bjc.6605756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winstone TA, Man SFP, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest. 2013;143(2):305-314. doi: 10.1378/chest.12-1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with ant–HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479-484. doi: 10.1038/s41586-018-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garassino MC, Cho BC, Kim JH, et al. ; ATLANTIC Investigators . Durvalumab as third-line or later treatment for advanced non–small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat Commun. 2019;10(1):814. doi: 10.1038/s41467-019-08798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Cao M, Martinez-Picado J, Karachaliou N, Rosell R, Meyerhans A. Cancer immunotherapy of patients with HIV infection. Clin Transl Oncol. 2019;21(6):713-720. doi: 10.1007/s12094-018-1981-6 [DOI] [PubMed] [Google Scholar]
- 9.Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5(7):1049-1054. doi: 10.1001/jamaoncol.2018.6737 [DOI] [PubMed] [Google Scholar]
- 10.Zheltkova V, Argilaguet J, Peligero C, Bocharov G, Meyerhans A. Prediction of PD-L1 inhibition effects for HIV-infected individuals. PLoS Comput Biol. 2019;15(11):e1007401. doi: 10.1371/journal.pcbi.1007401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744. doi: 10.1200/JCO.2017.73.7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research HIV Working Group. J Clin Oncol. 2017;35(33):3774-3780. doi: 10.1200/JCO.2017.73.7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. ; Cancer Immunotherapy Trials Network (CITN)-12 Study Team . Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer—a phase 1 study. JAMA Oncol. 2019;(Jun):2. doi: 10.1001/jamaoncol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanch-Lombarte O, Gálvez C, Revollo B, et al. Enhancement of antiviral CD8+ T-cell responses and complete remission of metastatic melanoma in an HIV-1–infected subject treated with pembrolizumab. J Clin Med. 2019;8(12):E2089. doi: 10.3390/jcm8122089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198-1202. doi: 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods.
eResults.
eTable 1. Drug related adverse events (AEs).
eTable 2. Outcome to durvalumab based on the type of cART.
eTable 3. PD-L1 expression and response.
eTable 4. Molecular classification by NGS of tumors.
eTable 5. Pre-treatment most differentially expressed genes between patients with- and without clinical benefit to durvalumab.
eTable 6. Correlation of basal CD4+, CD8+ T cell counts and antitumoral efficacy.
eReferences.
eFigure 1. Flowchart of HIV-1-infected cancer patients enrolled in the DURVAST study.
eFigure 2. Tumor response.
eFigure 3. A. Progression free survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study. B. Overall survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study.
eFigure 4. A. Progression free survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study, by PD-L1 status. B. Overall survival of HIV-1-infected cancer patients treated with durvalumab in the DURVAST study, by PD-L1 status.
eFigure 5. Volcano plot with differentially expressed genes in patients without- versus with clinical benefit.
eFigure 6. Signature scores in patients with and without clinical benefit.
eFigure 7. Restoring CD8 T cells and latency reversal “Shock and kill strategy.”


