Key Points
Question
Is baseline rhinitis status (allergic, nonallergic, or none) associated with change in nasal congestion when continuous positive airway pressure (CPAP) is used?
Findings
In this cohort study of 102 participants with newly diagnosed obstructive sleep apnea, CPAP was associated with improved subjective nasal congestion on average, but less so in participants with baseline allergic rhinitis.
Meaning
Baseline allergic rhinitis may predict which patients are more vulnerable to potential congestive effects of CPAP.
This cohort study assesses whether continuous positive airway pressure (CPAP) therapy is associated with nasal congestion in patients with allergic rhinitis, nonallergic rhinitis, or no rhinitis.
Abstract
Importance
Nasal congestion occurring after continuous positive airway pressure (CPAP) treatment initiation impairs CPAP adherence. Allergic rhinitis is associated with worsening nasal congestion in patients who are exposed to nonallergic triggers. Use of CPAP presents potential nonallergic triggers (eg, humidity, temperature, pressure, and airflow).
Objective
To compare nasal congestion among CPAP users with allergic rhinitis, nonallergic rhinitis, and no rhinitis. We hypothesize that CPAP patients with baseline allergic rhinitis are more likely to experience a worsening of nasal congestion (or less improvement in nasal congestion) compared with patients with no baseline rhinitis.
Design, Setting, and Participants
This prospective cohort study included consecutive patients newly diagnosed with obstructive sleep apnea in a tertiary sleep center who were using CPAP therapy 3 months after diagnosis. Baseline rhinitis status was assigned as allergic rhinitis, nonallergic rhinitis, or no rhinitis, based on questionnaire responses and past allergy testing. Data were collected from 2004 to 2008 and analyzed from July 2019 to February 2020.
Main Outcomes and Measures
At baseline before CPAP exposure and again 3 months later, subjective nasal congestion was measured with the Nasal Obstruction Symptom Evaluation (NOSE) scale and a visual analog scale (VAS), each scored from 0 to 100 (100 = worst congestion). Changes in nasal congestion were tested over 3 months for the whole cohort, within each rhinitis subgroup (paired t test), and between rhinitis subgroups (multivariate linear regression).
Results
The study cohort comprised 102 participants, of whom 61 (60%) were male and the mean (SD) age was 50 (13). The study included 23 (22.5%) participants with allergic rhinitis, 67 (65.7%) with nonallergic rhinitis, and 12 (11.8%) with no rhinitis. Nasal congestion improved from baseline to 3 months in the whole cohort (mean [SD] NOSE score, 38 [26] to 27 [23], mean [SD] change, −10 [23]; 95% CI, −15 to −6; mean [SD] VAS score, 41 [27] to 32 [28]; mean [SD] change, −10 [26]; 95% CI, [−15 to −4]) and in each rhinitis subgroup. Adjusted improvement in nasal congestion at 3 months was significantly less in the allergic rhinitis subgroup compared with the no rhinitis subgroup (positive difference means less improvement) compared with baseline: NOSE score 14 (95% CI, 1 to 28) and VAS score 15 (95% CI, 0 to 30).
Conclusions and Relevance
Initiation of CPAP was associated with improved subjective nasal congestion, but less improvement in patients with baseline allergic rhinitis. Baseline allergic rhinitis may predict which patients are more vulnerable to potential congestive effects of CPAP.
Introduction
Continuous positive airway pressure (CPAP) is the most commonly used first-line therapy for obstructive sleep apnea in adults and is typically applied nasally.1 Studies of CPAP users have shown that nasal symptoms are common both before and after CPAP initiation, affecting 30% to 50% of patients.2 A variety of nasal symptoms are commonly reported by patients who fail CPAP therapy and are also frequently reported as the reason for failure.3,4 There are few studies testing underlying predictors of change in any specific nasal symptom after CPAP initiation. Anecdotally, some patients experience an improved nasal airway with nasal CPAP use, some experience no change in the nasal airway, and some experience a worsening of the nasal airway.
Chronic rhinitis, whether allergic or nonallergic, is a relatively common nasal condition that may explain at least part of the relationship between CPAP and change in nasal symptoms. Allergic rhinitis is a condition that causes patients a variety of nasal symptoms, including sneezing, nasal itching, nasal drainage, and nasal congestion, in response to allergens. Patients with allergic rhinitis have been found more likely to experience nasal reactions (ie, self-reported symptoms and objectively measured increased nasal resistance) to nonallergic nasal triggers (eg, dilute acetic acid vapor or chlorine gas) compared with individuals without allergic rhinitis.5 Likewise, patients with nonallergic rhinitis react to cold, dry air provocation with self-reported symptoms and objectively measured increased nasal resistance compared with those without rhinitis.6
Use of CPAP presents numerous potential nonallergic nasal triggers including change in humidity, temperature, pressure, mask odors, and airflow rate. If patients with chronic rhinitis overreact (ie, experience nasal congestion) to nonallergic triggers presented by CPAP the same way they do to other nonallergic triggers, then baseline rhinitis status may predispose patients to nasal congestion in response to CPAP exposure or may mitigate the decongestant effect seen in some patients using CPAP.
The aims of this study were to quantify (1) the association between CPAP initiation and change in nasal congestion in general and (2) the association between rhinitis status (allergic, nonallergic, or no rhinitis) and the relative change in nasal congestion after CPAP initiation. We hypothesize that patients using CPAP with allergic rhinitis at baseline are more likely to experience a worsening of nasal congestion—or at least less improvement in nasal congestion—compared with patients with no rhinitis at baseline.
Methods
Study Design
This prospective observational cohort study was reviewed and approved by the University of Washington Institutional Review Board, and all participants gave written, informed consent prior to study enrollment.
Study Participants
Eligible participants were adults with a new polysomnography diagnosis of obstructive sleep apnea diagnosed at the University of Washington Sleep Center at Harborview Medical Center. Diagnosis of obstructive sleep apnea was defined as an apnea-hypopnea index of 5 or more events per hour on either full-night or the diagnostic part of a split-night attended polysomnography using standard scoring criteria.7 Participants were excluded if they did not use CPAP within 4 weeks of the 3-month follow-up. Additional inclusion criteria included age of 18 years or older, ability to give informed consent, ability and willingness to complete the study protocol, and fluency in verbal and written English. Patients were excluded if they had a prior diagnosed sleep disorder, no telephone, or plans to move during the study period.
Data Collection
Study participants were seen the evening of diagnostic polysomnography (baseline visit) just prior to CPAP initiation and 3 months after polysomnography for follow-up. Exposure status (rhinitis status), baseline nasal congestion, and confounder data were obtained at the baseline visit by questionnaire. Body mass index was calculated from measurements obtained by research staff at the baseline visit, and the remainder of the data were elicited by self-report. Status of nasal congestion at 3 months after polysomnography was also obtained by questionnaires.
Variables
Each study participant’s rhinitis status was determined at the baseline visit and categorized as allergic rhinitis, nonallergic rhinitis, or no rhinitis. The allergic rhinitis group was defined as those participants with the combination of both nasal symptoms (ie, sneezing, runny nose, nasal itching, nasal congestion, or previous diagnosis of nasal allergies) and self-report of a positive skin allergen test in the past. Skin allergen testing was not conducted prospectively for this study. The nonallergic rhinitis group was defined as those with the previously mentioned nasal symptoms and no history of positive skin allergen test. The remaining participants (no rhinitis) were participants with no nasal symptoms and no history of positive skin allergen test. The no rhinitis subgroup was the reference group for the analyses. There were no participants with a history of positive skin allergen test and no nasal symptoms.
The Nasal Obstruction Symptom Evaluation (NOSE) scale and a visual analog scale (VAS) rating of nasal congestion were used to measure nasal congestion at baseline and again 3 months later. The NOSE scale is a 5-item instrument measuring subjective nasal obstruction that has been shown to be valid, reliable, and responsive to change in nasal congestion.8 Participants rate items such as “trouble breathing through my nose” according to how much of a problem they have had over the previous month on a 5-point Likert-type scale ranging from “not a problem” (item score = 0) to “severe problem” (item score = 4). The NOSE scale total score is the sum of items multiplied by 5 ranging from 0 to 100 points, with 100 representing a severe problem with nasal congestion. The minimal clinically important difference is 4-6 by the distribution method and 19 by the clinical anchor method in a sample of participants.8,9
Nasal congestion was also assessed by VAS rating. Participants were asked to rate their degree of difficulty breathing though their nose on a 100-mm VAS ranging from none (0 mm) to severe (100 mm). The nasal congestion VAS score is the measurement in millimeters with a potential range of 0 to 100, with 100 representing severe nasal congestion. The VAS is a validated and widely used measurement modality10,11 and is well suited to measuring unidimensional outcomes such as nasal congestion. The nasal congestion VAS has also been shown to correlate with the NOSE score.8,12 The NOSE score was our primary outcome because it has had more rigorous psychometric validation than the nasal congestion VAS.
Potential confounding variables selected a priori were age, sex, body mass index, smoking status (current use or not), baseline nasal congestion, history of nasal surgery, and medical treatment for rhinitis at baseline, including nasal steroid use, nasal antihistamine use, nasal decongestant use, oral antihistamine use, or oral decongestant use.
Statistical Analysis
Prior to analysis, all data were checked for errors and possible inconsistencies, which were resolved by reviewing the raw data. This analysis revealed an outlier in both NOSE score and nasal congestion VAS score in the no rhinitis group. Review of this participant’s 3-month questionnaire revealed extreme responses to numerous questions across other categories of outcomes collected for different analyses. For example, the respondent answered with the most extreme responses for all questions related to daily functioning, social activities, sleep quality, and emotional functioning. Emotional functioning questions included questions regarding depression, irritability, anger, and inability to cope. Because of concerns with the validity of these responses, this individual was removed from the analyses.
Continuous variables were reported as means and SDs, and dichotomous variables were reported as counts and percentages. Nasal congestion change scores were calculated for each study participant by subtracting the baseline nasal congestion scores from the 3-month nasal congestion scores, with negative change scores representing reduced nasal congestion. The Cohen d effect sizes for each group were calculated by dividing the change scores by the baseline SDs. Changes in nasal congestion were summarized with means and SDs, 95% CIs, and Cohen d effect sizes for the whole cohort and for each rhinitis status subgroup. A Cohen d effect size 0.2 was considered a small but important effect, 0.5 was considered medium, and 0.8 or greater was considered large.13
Differences in the nasal congestion change scores were calculated between subgroups as the differences in mean change scores. Cohen d effect sizes for differences between subgroups were calculated by dividing the differences by the SD of reference subgroup (no rhinitis). Differences in the nasal congestion change scores between subgroups were summarized with means and SDs, 95% CIs, and Cohen d effect sizes.
Differences in the nasal congestion change scores between subgroups were also assessed with multivariate linear regression adjusting for potential confounders. Each confounder was tested for inclusion in the multivariate model. Confounders were included in the final model if their inclusion appreciably altered (≥10%) the point estimates of differences in nasal congestion change between allergic rhinitis or nonallergic rhinitis relative to no rhinitis, a validated method of adjusting for confounders.14 Age, sex, baseline nasal congestion score (baseline NOSE score for NOSE model and baseline VAS score for VAS model), and oral antihistamine use were found to appreciably alter (>10%) the point estimates and were included in the multivariate analyses. All analyses were performed with Stata/SE version 15 (College Station, TX).
Results
We initially enrolled 229 participants in the cohort, of whom 21 were retroactively excluded due to lack of sleep apnea on polysomnography, 52 were excluded because they were not exposed to CPAP within 4 weeks of their follow-up, 12 withdrew, and 42 did not return their questionnaire at 3 months. This resulted in a cohort of 102 study participants (23 with allergic rhinitis, 67 with nonallergic rhinitis, and 12 with no rhinitis). As detailed in Table 1, the cohort was mostly middle-aged and predominantly male with obesity. The 3 groups were different by several of the measured characteristics, including those hypothesized to be potential confounders.
Table 1. Cohort Characteristics.
| Characteristic | Rhinitis | ||
|---|---|---|---|
| Allergic (n = 23) | Nonallergic (n = 67) | No rhinitis (n = 12) | |
| Demographic characteristics | |||
| Age, mean (SD), y | 50 (12) | 50 (13) | 53 (13) |
| Male, No. (%) | 11 (48) | 40 (60) | 9 (75) |
| BMI, mean (SD)a | 33 (7) | 34 (8) | 37 (8) |
| Current smoker, No. (%) | 4 (19) | 6 (9) | 1 (9) |
| Nasal characteristics | |||
| Baseline score, mean (SD)b | |||
| NOSE | 44 (23) | 38 (26) | 32 (33) |
| VAS | 48 (29) | 37 (26) | 45 (31) |
| Basal nasal medication use, No. (%) | 12 (52) | 17 (25) | 3 (25) |
| Steroid use, No. (%) | 11 (48) | 16 (24) | 3 (25) |
| Antihistamine use, No. (%) | 2 (9) | 1 (2) | 0 |
| Decongestant use, No. (%) | 2 (9) | 3 (5) | 0 |
| History of nasal surgery, No. (%) | 2 (9) | 6 (9) | 0 |
| Oral antihistamine, No. (%) | 7 (30) | 7 (10) | 1 (8) |
| Oral decongestant use, No. (%) | 5 (22) | 3 (4) | 0 |
| OSA characteristics | |||
| Apnea Hypopnea Index, events/h, mean (SD) | 52 (33) | 48 (28) | 62 (26) |
| Lowest desaturation, % saturation, mean (SD) | 80 (8) | 82 (8) | 71 (14) |
| Baseline Epworth Sleepiness Scale Score, mean (SD) | 10 (5) | 10 (5) | 10 (7) |
| CPAP use, h/night, mean (SD) | 4.2 (2.7) | 3.9 (3.1) | 6.0 (1.8) |
Abbreviations: BMI, body mass index; CPAP, continuous positive airway pressure therapy; NOSE, Nasal Obstruction Symptom Evaluation score; OSA, obstructive sleep apnea; VAS, visual analog scale score.
Calculated as weight in kilograms divided by height in meters squared.
NOSE and VAS scores range from 0 to 100, where 100 indicates worst congestion.
Change in Nasal Congestion
As a whole, the cohort showed more improvement than worsening in nasal congestion with CPAP exposure. The mean (SD) NOSE score improved from 38 (26) to 27 (23) corresponding to a NOSE score change of −10 (95% CI, −15 to −6) and Cohen d effect size −0.4 (95% CI, −0.6 to −0.2). Using the distribution definition of minimal important change (6 points), 54 participants (52.9%) had important improvement in NOSE score, 26 participants (25.5%) had no important change, and 22 (21.6%) had important worsening. Using the anchor definition of minimal important change (19 points), 30 participants (29.4%) had important improvement, 64 participants (62.7%) had no important change, and 8 participants (7.8%) had important worsening. The mean (SD) nasal congestion VAS improved from 41 (27) to 32 (28) corresponding to a VAS score change of −10 (26) (95% CI, −15 to −4) and Cohen d effect size −0.4 (95% CI, −0.6 to −0.1).
Each subgroup (allergic rhinitis, nonallergic rhinitis, and no rhinitis) had more improvement than worsening in nasal congestion with CPAP exposure (Table 2). The effect sizes for the allergic rhinitis and nonallergic rhinitis subgroups were small to medium, whereas the effect sizes for the no rhinitis subgroup were medium to large (Table 2). After adjusting for potential confounders, each subgroup had improvement in nasal congestion scores (Table 3). However, using the anchor-based minimal clinically important difference in NOSE score (19-point change), only the no rhinitis subgroup had a clinically important change (improvement) in the NOSE score with 95% CI (Table 3).
Table 2. Change in Nasal Congestion Bivariate Analysisa.
| Measure | Rhinitis | ||
|---|---|---|---|
| Allergic (n = 23) | Nonallergic (n = 67) | No rhinitis (n = 12) | |
| NOSE score | |||
| Baseline, mean (SD) | 44 (23) | 37 (26) | 32 (33) |
| 3-mo follow-up, mean (SD) | 35 (22) | 27 (23) | 17 (20) |
| Change (95% CI)b | −9 (−20 to 2) | −10 (−12 to −8) | −15 (−31 to 1) |
| Cohen d (95% CI)b | −0.4 (−0.9 to 0.1) | −0.4 (−0.5 to −0.3) | −0.5 (−0.9 to 0) |
| Clinically meaningful change, No. (%)c | |||
| Improved | 5 (22) | 21 (31) | 4 (33) |
| No change | 15 (65) | 42 (62) | 7 (58) |
| Worse | 4 (6) | 4 (6) | 1 (8) |
| Nasal congestion VAS score | |||
| Baseline, mean (SD) | 47 (29) | 39 (26) | 45 (31) |
| 3-mo, mean (SD) | 35 (28) | 33 (28) | 19 (21) |
| Change (95% CI)b | −12 (−20 to 0) | −6 (−13 to 1) | −26 (−36 to −16) |
| Cohen d (95% CI)b | −0.4 (−0.7 to 0) | −0.2 (−0.5 to 0) | −0.8 (−1.2 to −0.5) |
Abbreviations: NOSE, Nasal Obstruction Symptom Evaluation score; VAS, visual analog scale score.
NOSE and VAS scores range from 0 to 100, where 100 indicates worst congestion.
Change in nasal congestion at 3 months (negative is improvement).
Clinically meaningful change based on the anchor method (NOSE change 19).
Table 3. Adjusted Nasal Change Scores by Rhinitis Statusa.
| Adjusted change | Rhinitis, mean (95% CI) score | ||
|---|---|---|---|
| Allergic | Nonallergic | No rhinitis | |
| NOSE | −3 (−12 to 7) | −10 (−17 to −4) | −17 (−28 to −6) |
| VAS | −9 (−21 to 2) | −9 (−16 to −2) | −24 (−36 to −12) |
Abbreviations: NOSE, Nasal Obstruction Symptom Evaluation; VAS, visual analog scale.
Adjusted for age, sex, baseline nasal medications, oral antihistamine use, and baseline nasal congestion score (baseline NOSE score in NOSE change model and baseline nasal congestion VAS score in VAS change model). Data presented as adjusted mean (95% CI) using multivariate linear regression. Negative mean change scores represent improved nasal congestion.
Adjusted Differences in Nasal Congestion Between Subgroups
Relative to the no rhinitis subgroup, the allergic rhinitis subgroup had less improvement in nasal congestion change scores (NOSE and VAS) (Table 4). The 95% CIs of the adjusted difference in the NOSE score change between allergic rhinitis and no rhinitis subgroups did not cross 0 and had an upper bound of 28, well above the minimal clinically important difference for NOSE (19 points by anchor method and 4 to 6 points by distribution method). Therefore, these results suggest that the improvement in nasal congestion with CPAP use in patients with allergic rhinitis is likely smaller by an important amount compared with improvement in patients with no rhinitis. Similar results were seen with VAS scores.
Table 4. Adjusted Difference in Nasal Change Scores Relative to No Rhinitisa.
| Adjusted difference | Rhinitis, mean (95% CI) score | ||
|---|---|---|---|
| Allergic | Nonallergic | No rhinitis | |
| NOSE | 14 (1 to 28) | 7 (−4 to 18) | 0 [Reference] |
| VAS | 15 (0 to 30) | 15 (3 to 28) | 0 [Reference] |
Abbreviations: NOSE, Nasal Obstruction Symptom Evaluation; VAS, visual analog scale.
Adjusted for age, sex, baseline nasal medications, oral antihistamine use, and baseline nasal congestion score (baseline NOSE score in NOSE change model and baseline nasal congestion VAS score in VAS change model). Data presented as adjusted difference in mean change (95% CI) using multivariate linear regression. Positive differences in mean change scores represent worse (or less improved) nasal congestion relative to the no rhinitis subgroup.
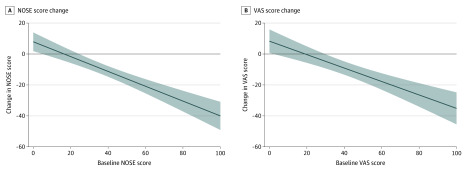
Relative to the no rhinitis group, the nonallergic rhinitis subgroup also had less improvement in nasal congestion scores (NOSE and VAS) (Table 4). However, the difference was smaller than that seen with allergic rhinitis, and the 95% CI for the NOSE change did not suggest a clinically important difference. In each model, baseline oral antihistamine use and baseline nasal congestion score were associated with change in nasal congestion scores (data not shown). Higher baseline NOSE score was associated with greater change (improvement) in NOSE score after adjusting for rhinitis status (Figure).
Figure. Regression Estimate of Change in Nasal Obstruction Symptom Evaluation (NOSE) Score and Visual Analog Scale (VAS) Score vs Baseline Score.
Both NOSE (A) and VAS (B) scores range from 0 to 100, where 100 indicates worst outcomes. Negative change in either score represents an improvement of nasal congestion.
Discussion
This study shows overall improvement of self-rated nasal congestion in all diagnostic subgroups (allergic rhinitis, nonallergic rhinitis, and no rhinitis) after use of CPAP for 3 months. Those with allergic rhinitis had less improvement than those with no rhinitis by a clinically important difference. These results suggest that the use of CPAP can improve baseline nasal congestion, especially in people who do not have baseline allergic rhinitis. These findings are consistent with the hypothesis that baseline allergic rhinitis is associated with less improvement in nasal congestion by CPAP, independent of baseline congestion.
We chose to use 3 rhinitis exposure groups in this analysis to clarify the difference between each of these potentially unique groups as well as to have an appropriate reference for comparison with the allergic rhinitis group. Allergic rhinitis is clinically diagnosed by a combination of clinical symptoms (primarily nasal symptoms) and the presence of an immunoglobulin E–mediated allergic reactivity. Nonallergic rhinitis is another disorder of nasal symptoms without immunoglobulin E–mediated allergy. Nonallergic rhinitis may also be an important predictor of the nasal response to the stimuli from CPAP. Indeed, a 2010 study6 found that participants with nonallergic rhinitis predicted increased objective nasal congestion, as measured by nasal airway resistance, in response to cold air provocation.
There have been limited studies testing for change in nasal congestion with CPAP initiation. One such study measured nasal symptoms with a subsection of the Rhinoconjunctivitis Quality of Life Questionnaire.15 This small study found that nasal symptoms did not change with CPAP initiation in those with allergic rhinitis (n = 15) or nonallergic rhinitis (n = 16). The current study improves on this previous study in sample size and by focusing on a specific nasal symptom by using 2 validated nasal congestion instruments.
A study by Aguilar et al16 evaluated for change in nasal symptoms and other nasal metrics before and after CPAP initiation. This small study (n = 36) showed worsening nasal dryness in those using CPAP for more than 4 hours per night. Those with less than 4 hours of CPAP use did not have a change in nasal measures. Eosinophils were additionally increased in nasal mucosa among patients using CPAP for more than 4 hours per night. These findings confirmed the importance of nasal symptoms after initiating CPAP treatment but did not seek to identify predictors of worsening nasal symptoms. Identifying patients with obstructive sleep apnea who may be at risk of worsening nasal symptoms may help identify those in need of close follow-up or nasal treatments to help ensure CPAP tolerance.
While the differential change in nasal congestion may not seem to be of clinical relevance to the treatment of obstructive sleep apnea, a reduced nasal airway caliber is associated with reduced CPAP use. For instance, baseline nasal obstruction, as measured by minimal cross-sectional area by acoustic rhinometry, has been shown to be associated with reduced CPAP use.17,18 Additionally, nasal surgery has been shown to improve CPAP use among individuals with nasal obstruction.19,20 Allergic or nonallergic rhinitis may predict, above and beyond baseline nasal congestion alone, individuals who will have less tolerance to CPAP. If those with allergic rhinitis have lower CPAP use, this will present a possible target of future interventions to improve CPAP use in this prevalent group of patients. Research has shown, consistently and across outcomes, a dose-response relationship of improved outcomes with increasing CPAP use.21,22,23,24 Improving CPAP use would be of benefit to these patients. Further work should look into the association between allergic rhinitis, nonallergic rhinitis, and CPAP use.
Limitations
The study cohort specifically included CPAP users, which may explain why mean improvements in nasal congestion were observed in all subgroups. It is possible that potential participants who experienced worsened nasal congestion with initial CPAP use decided to discontinue CPAP early and were excluded. We measured follow-up nasal congestion at 3 months after polysomnography, so any changes in nasal congestion before this time point would not be identified in this study. This constraint also prevented us from testing the association between CPAP and worsening nasal symptoms in patients who discontinued CPAP within 2 months. Ideally, a future study would measure nasal congestion shortly after CPAP initiation to capture patients who have an adverse nasal reaction to CPAP. We hypothesize that allergic rhinitis is associated with greater worsening of nasal congestion in the early CPAP failure group.
Our study may also have limited ability to detect a change in nasal congestion after CPAP use because the follow-up time point was fixed at 3 months, whereas inclusion as a CPAP user could occur at any time within 4 weeks of the end point. Some study participants may have attempted to use CPAP early in this 4-week window and stopped using the device. These participants would be included in our analyses, but their change in nasal congestion may have waned after stopping CPAP use. If participants with allergic rhinitis are overrepresented in these early CPAP failures (which we hypothesize but is unknown in this cohort), the results would be biased toward the null hypothesis and may have blunted the association between allergic rhinitis status and change in nasal congestion with CPAP initiation.
The nonallergic rhinitis group in our study may include individuals with allergic rhinitis misclassified as having nonallergic rhinitis because they did not have a previous allergy test. Prospective allergy testing was beyond the scope of this noninvasive observational cohort study. The degree of misclassification bias will depend on whether those who had previously pursued skin allergy testing are different in their response to CPAP from those with allergic rhinitis but who were not yet tested. If those with previous allergy testing had more severe allergic rhinitis and had a more extreme worsening in nasal congestion (or less improvement in nasal congestion) associated with CPAP initiation, the bias might have exaggerated the results. If those with previous allergy testing were not different, the misclassification would have a conservative bias (ie, it would tend to make the groups appear more similar). The baseline nasal congestion was worse in participants with allergic rhinitis compared with participants with nonallergic rhinitis, which would be expected if allergy testing were a sign of worse nasal congestion and possibly worse sensitivity to nonallergic triggers; however, it would also be consistent with the fact that patients with allergic rhinitis tend to have worse nasal symptoms than patients with nonallergic rhinitis independent of CPAP as a nasal trigger. The proportion of patients with allergic rhinitis in our study, 23%, is similar to the prevalence in a large population-based study, 19%,25 which suggests there might be relatively little misclassification of allergic rhinitis. Future studies would benefit from prospective allergy testing in all patients to delineate allergic and nonallergic rhinitis more definitively.
Conclusions
In conclusion, it appears that patients with no rhinitis experience significant improvement in nasal congestion with CPAP initiation, while those with allergic rhinitis and possibly nonallergic rhinitis experience relative worsening or less improvement in nasal congestion with CPAP initiation. Allergic rhinitis may predict who will have difficulty with CPAP therapy because of less favorable changes in nasal congestion and may present a possible target for early intervention to improve CPAP effectiveness in the care of sleep apnea.
References
- 1.Polo O, Berthon-Jones M, Douglas NJ, Sullivan CE. Management of obstructive sleep apnoea/hypopnoea syndrome. Lancet. 1994;344(8923):656-660. doi: 10.1016/S0140-6736(94)92089-3 [DOI] [PubMed] [Google Scholar]
- 2.Hoffstein V, Viner S, Mateika S, Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure: patient compliance, perception of benefits, and side effects. Am Rev Respir Dis. 1992;145(4, pt 1):841-845. doi: 10.1164/ajrccm/145.4_Pt_1.841 [DOI] [PubMed] [Google Scholar]
- 3.Brander PE, Soirinsuo M, Lohela P. Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome: effect of nasal CPAP treatment. Respiration. 1999;66(2):128-135. doi: 10.1159/000029354 [DOI] [PubMed] [Google Scholar]
- 4.Pépin JL, Leger P, Veale D, Langevin B, Robert D, Lévy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome: study of 193 patients in two French sleep centers. Chest. 1995;107(2):375-381. doi: 10.1378/chest.107.2.375 [DOI] [PubMed] [Google Scholar]
- 5.Shusterman D, Murphy MA. Nasal hyperreactivity in allergic and non-allergic rhinitis: a potential risk factor for non-specific building-related illness. Indoor Air. 2007;17(4):328-333. doi: 10.1111/j.1600-0668.2007.00482.x [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Oh YS, Kim KJ, Jang TY. Use of cold dry air provocation with acoustic rhinometry in detecting nonspecific nasal hyperreactivity. Am J Rhinol Allergy. 2010;24(4):260-262. doi: 10.2500/ajra.2010.24.3488 [DOI] [PubMed] [Google Scholar]
- 7.Epstein LJ, Kristo D, Strollo PJ Jr, et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276. doi: 10.5664/jcsm.27497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157-163. doi: 10.1016/j.otohns.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Stewart MG, Smith TL, Weaver EM, et al. . Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130(3):283-290. doi: 10.1016/j.otohns.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Huskisson EC. Measurement of pain. Lancet. 1974;2(7889):1127-1131. doi: 10.1016/S0140-6736(74)90884-8 [DOI] [PubMed] [Google Scholar]
- 11.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184. doi: 10.1016/0304-3959(76)90113-5 [DOI] [PubMed] [Google Scholar]
- 12.Lam DJ, James KT, Weaver EM. Comparison of anatomic, physiological, and subjective measures of the nasal airway. Am J Rhinol. 2006;20(5):463-470. doi: 10.2500/ajr.2006.20.2940 [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; 1988. [Google Scholar]
- 14.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923-936. doi: 10.1093/oxfordjournals.aje.a116813 [DOI] [PubMed] [Google Scholar]
- 15.Shadan FF, Jalowayski AA, Fahrenholz J, Kline LE, Dawson A. Nasal cytology: a marker of clinically silent inflammation in patients with obstructive sleep apnea and a predictor of noncompliance with nasal CPAP therapy. J Clin Sleep Med. 2005;1(3):266-270. doi: 10.5664/jcsm.26342 [DOI] [PubMed] [Google Scholar]
- 16.Aguilar F, Cisternas A, Montserrat JM, et al. . Effect of nasal continuous positive pressure on the nostrils of patients with sleep apnea syndrome and no previous nasal pathology: predictive factors for compliance. Article in Spanish. Arch Bronconeumol. 2016;52(10):519-526. doi: 10.1016/j.arbres.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Li HY, Engleman H, Hsu CY, et al. . Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep. 2005;28(12):1554-1559. doi: 10.1093/sleep/28.12.1554 [DOI] [PubMed] [Google Scholar]
- 18.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol. 2006;20(2):133-137. doi: 10.1177/194589240602000202 [DOI] [PubMed] [Google Scholar]
- 19.Nakata S, Noda A, Yagi H, et al. . Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43(4):296-299. [PubMed] [Google Scholar]
- 20.Powell NB, Zonato AI, Weaver EM, et al. . Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope. 2001;111(10):1783-1790. doi: 10.1097/00005537-200110000-00023 [DOI] [PubMed] [Google Scholar]
- 21.Meslier N, Lebrun T, Grillier-Lanoir V, et al. . A French survey of 3,225 patients treated with CPAP for obstructive sleep apnoea: benefits, tolerance, compliance and quality of life. Eur Respir J. 1998;12(1):185-192. doi: 10.1183/09031936.98.12010185 [DOI] [PubMed] [Google Scholar]
- 22.Blondet MC, Perez J, Rodriguez W. Continuous positive airway pressure and obstructive sleep apnea in an Hispanic population. Sleep Breath. 2001;5(3):109-114. doi: 10.1055/s-2001-17435 [DOI] [PubMed] [Google Scholar]
- 23.Antic NA, Catcheside P, Buchan C, et al. . The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111-119. doi: 10.1093/sleep/34.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver TE, Maislin G, Dinges DF, et al. . Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711-719. doi: 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaaban R, Zureik M, Soussan D, et al. . Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372(9643):1049-1057. doi: 10.1016/S0140-6736(08)61446-4 [DOI] [PubMed] [Google Scholar]