In response to the coronavirus disease 2019 (COVID-19) pandemic, the delivery of medical services has been fundamentally altered. Transthoracic echocardiography (TTE) plays a prominent role in identifying cardiac complications of COVID-19, but TTE places sonographers within 2 feet of patients' faces, well within the droplet zone, for ≥20 min. Consequently, cardiac sonographers have among the highest exposures to COVID-19 among health care providers and consume scarce personal protective equipment with each examination. We sought to investigate the impact of the COVID-19 pandemic on the utilization and appropriateness of TTE at a busy academic medical center in an urban endemic area.
We reviewed adult TTE utilization at University of Chicago Medicine (UCM) from January 19, 2020, the week of the first reported case of COVID-19 in the state of Illinois (and second in the United States), though March 31, 2020.1
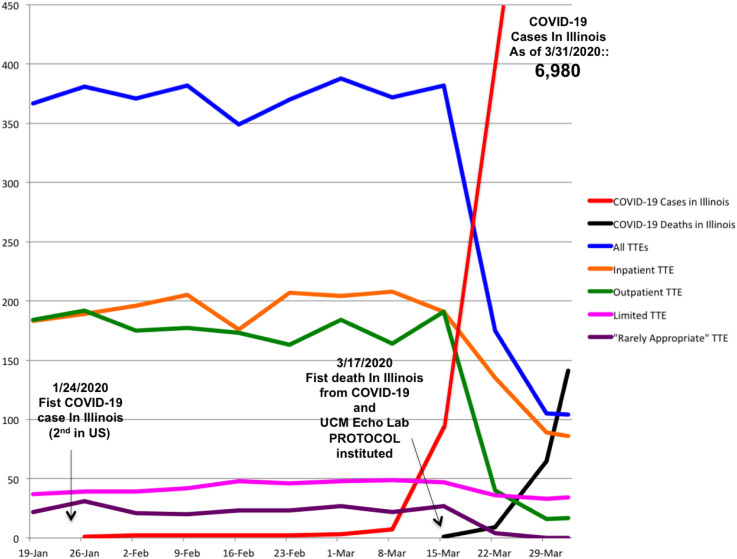
For each transthoracic echocardiographic study, complete versus limited status and appropriate use criteria designation as “appropriate,” “maybe appropriate,” or “rarely appropriate” was established.2 TTE utilization and appropriateness were compared before and after hospital- and state-issued pandemic guidelines required the UCM echocardiography laboratory to enact a new work-flow protocol to guide the practice of TTE. UCM and Illinois directives required cessation of elective procedures, elimination of nonessential personnel, and strict social distancing.1 The work-flow protocol consisted of the following: All requests for inpatient and outpatient TTE underwent physician review before study performance, with approval based on the standards of “medical necessity and time sensitivity.” Each patient's novel coronavirus infection status was considered, though given rapidly evolving testing availability and diagnostic algorithms during this period, all prospective TTE patients were treated as potentially coronavirus positive. This protocol was enacted on March 17, 2020, the day of the first death from COVID-19 in Illinois, when there were 160 reported cases statewide. At study conclusion 14 days later, there were 6,980 cases and 141 deaths in Illinois from COVID-19.1 No member of the UCM echocardiography laboratory staff tested positive for the novel coronavirus.
A total f 3,642 transthoracic echocardiographic examinations were performed in the 2.5-month study period, including 1,983 inpatient (55%) and 1,659 outpatient (45%) studies. Of all these studies, 13% were limited TTE, including 17% of inpatient and 4% of outpatient studies. Among the 3,275 (90%) studies for which appropriate use criteria designation could be determined, 88% were appropriate and 6% were rarely appropriate.
Compared with the weeks prior, the new work-flow protocol resulted in a 66% decline in the overall weekly TTE volume, including 48% among inpatient and 84% among outpatient studies (Figure 1 ). The percentage of limited TTE significantly increased after protocol implementation among all transthoracic echocardiographic studies (12% vs 28%, P < .001) and more than doubled among inpatient studies (15% vs 34%, P < .001). The rate of appropriate examinations significantly increased (87% vs 96%, P < .001) after protocol implementation, while rarely appropriate studies were almost completely eliminated (7% vs 1%, P < .001).
Figure 1.
Weekly Transthoracic Echocardiography (TTE) Utilization and Appropriateness at University of Chicago Medicine (UCM), and State of Illinois COVID-19 Infections and Deaths, during the COVID-19 Pandemic.
These findings illustrate the impact of the early stages of the COVID-19 pandemic on the practice of TTE at an academic medical center in an urban COVID-19 “hot spot.” Response to the pandemic requires striving for the optimal balance between best patient care and the safety of providers. This is particularly challenging for TTE practice, given its core role in the diagnosis of cardiac complications of COVID-19 and the uniquely high exposure risk for sonographers performing TTE. In addition, hospital and state COVID-19 pandemic mandates have required fundamental alterations to the practice of TTE. At our institution, these factors necessarily required a new work flow involving physician review of all echocardiography referrals. This focused intervention resulted in a marked decrease in TTE volume and significant improvements in the appropriateness of the studies performed. The rate of rarely appropriate transthoracic echocardiographic examinations performed in our laboratory is at the low end of the published experience, but nonetheless a “physician-level review” of echocardiography orders essentially eliminated rarely appropriate examinations.3, 4, 5 Although the context of a pandemic may limit applicability, this finding is relevant in light of the recent Centers for Medicare and Medicaid Services mandate requiring appropriate use criteria determinations for “advanced” imaging modalities. Echocardiography is not subject to this mandate, but our findings support the conclusion that more scrutiny at the point of order is beneficial.6
Limited TTE utilization as a percentage of all transthoracic echocardiographic studies has also markedly increased during the pandemic. The primary cardiac complications of COVID-19 involve myocardial injury, with troponin elevations, including myocarditis and acute coronary syndromes.7 Thus left ventricular performance is of primary importance, and it can be effectively evaluated using limited two-dimensional TTE.5 Limited TTE also reduces sonographer imaging time and thus minimizes this high-risk exposure.
Cardiac point-of-care ultrasound offers similar benefits and may allow rapid focused diagnostic assessment by providers already at the bedside.8 However, the lack of uniform formal reporting and image storage for later review may be limiting.
In summary, the practice of TTE has acutely adapted to the COVID-19 pandemic. Our findings suggest that a strategy of more active physician review and increased use of limited TTE can eliminate unnecessary examinations, reduce consumption of personal protective equipment, and minimize high-risk sonographer exposure to the virus.
References
- 1.State of Illinois. Coronavirus (COVID-19) response. https://coronavirus.illinois.gov/s/ Available at:
- 2.American College of Cardiology Foundation Appropriate Use Criteria Task Force. American Society of Echocardiography. American Heart Association. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229–267. doi: 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Rameh V., Kossaify A. Appropriate use criteria in echocardiography: an observational institutional study with the perspective of a quality improvement project. Clin Med Insights Cardiol. 2016;10:23–28. doi: 10.4137/CMC.S36504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R., Negishi K., Otahal P., Marwick T.H. Temporal changes in appropriateness of cardiac imaging. J Am Coll Cardiol. 2015;65:763–773. doi: 10.1016/j.jacc.2014.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu A.T., Parizo J., Moradi-Ragheb N., Heidenreich P.A. Association between offering limited left ventricular ejection fraction echocardiograms and overall use of echocardiography. JAMA Intern Med. 2018;178:1270–1272. doi: 10.1001/jamainternmed.2018.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid Services Appropriate use criteria program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Appropriate-Use-Criteria-Program Available at:
- 7.Madjid M., Safavi-Naeini P., Solomon S., Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. In press. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick J.N., Grimm R., Johri A.M., Kimura B.J., Kort S., Labovitz A.J. Recommendations for echocardiography laboratories participating in cardiac point of care cardiac ultrasound (POCUS) and critical care echocardiography training: report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:409–422. doi: 10.1016/j.echo.2020.01.008. [DOI] [PubMed] [Google Scholar]