Abstract
Objectives
To describe the pharmacy administration and pharmaceutical care in a module hospital during the coronavirus disease 2019 (COVID-19) epidemic and provide reference for domestic and foreign pharmacists participating in the epidemic prevention and control.
Setting
The study was performed in a Jianghan module hospital constructed at the Wuhan Convention and Exhibition Center in Wuhan, China. This is 1 of the first 3 module hospitals.
Practice description
One thousand eight hundred forty-eight patients were admitted to the Jianghan module hospital, and 1327 cases (71.81% of the total number) were cured and discharged. Pharmacists have successfully completed the tasks of purchase, storage, and free distribution of drugs worth ¥1.03 million (approximately $146,000), reviewed about 20,000 electronic orders, provided one-on-one online medication consultation for 484 patients, and held 5 lectures on rational drug use knowledge, which could help reduce irrational drug use and minimize the risk involved.
Practice innovation
The new COVID-19 “module” pharmaceutical care model is equipped with new features such as pharmacy emergency command group, organizational structure for pharmacy administration, electronic control of drug prescription, and “zero contact” pharmaceutical care relying on the new media platform “WeChat.” This platform provides relevant pharmaceutical care for patients, such as ensuring drug supply, setting up critical care drug trolleys, designing specific drug packaging bags, creating a module radio station to broadcast rational drug use information to the patients, and other aspects.
Evaluation
With the continuous improvement of the module hospital and the progress in in-depth knowledge about COVID-19, some aspects such as patient admission criteria and variety of drugs need to be adjusted depending on the actual situation.
Results
The pharmacists provided pharmaceutical care for 1848 patients with mild COVID-19 disease. They not only ensured the timely supply of the drugs but also reduced the incidence of drug-induced risks through medication review and guidance, thereby improving patient compliance and helping the patients rebuild their confidence in overcoming the disease.
Conclusion
The new COVID-19 module pharmaceutical care model has played an important role in overcoming the epidemic situation of COVID-19 in China and thus can be implemented on a broader scale.
Key Points.
Background
-
•
A novel coronavirus disease 2019 (COVID-19) epidemic occurred in Wuhan, China.
-
•
To block the epidemic, Wuhan took a new major public health initiative by quickly opening a large-spaced, multibed “module hospital” to treat the patients with confirmed mild disease.
-
•
Professional pharmaceutical personnel were urgently needed to provide effective pharmaceutical care for the clinical treatment of the patients.
Findings
-
•
Pharmaceutical care not only ensured the timely supply of the drugs and reduced the drug-induced risk through medication review and medication guidance but also improved the medication compliance of the patients and helped the patients rebuild their confidence in overcoming the disease.
-
•
The new COVID-19 module pharmaceutical care model played an important role in overcoming the epidemic situation of COVID-19 and can be implemented on a broader scale.
-
•
As the module hospital is the first one being used for patients with mild COVID-19, with the continuous improvement of the module hospital, some aspects, such as the patient admission criteria and variety of drugs, need to be adjusted depending on the actual situation.
The novel pneumonia coronavirus disease 2019 (COVID-19) epidemic occurred in December 2019, and Wuhan, China, rapidly entered a struggling state to block the epidemic. On January 20, 2020, according to the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases, China National Health Commission incorporated the disease into the management of class B infectious diseases and took class A infectious disease prevention and control measures.1 On the basis of the pathogen, the epidemiology, the clinical characteristics of COVID-19, and the degree of harm to the health of the population, the China National Health Commission incorporated the disease into the management of class B infectious diseases. However, because COVID-19 is mainly transmitted through the respiratory tract and is highly infectious and detrimental for the health of the people, it was necessary to control the epidemic quickly and effectively by taking class A infectious disease prevention and control measures, such as isolation treatment for the patients and medical observation of isolation for close contacts. On February 11, 2020, the “novel coronavirus pneumonia” was officially named “COVID-19” by the World Health Organization, and the International Classification Committee of Viruses officially named the new coronavirus “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), indicating that it is a close relative of SARS coronavirus from a taxonomic perspective.2, 3, 4
With the increasingly severe situation of the epidemic, on February 3, 2020, Wuhan took a new major public health initiative by quickly opening a large-spaced, multibed “module hospital” to treat the patients with confirmed mild disease. This module hospital is different from the mobile field hospital used during wartime or for earthquake relief, and it had not been used in the treatment of acute infectious diseases in China. The module hospital is an innovation of the Wuhan antiepidemic initiative. As the large-scale temporary medical facility was rapidly established to deal with severe epidemic, it provided large capacity and low-cost, simple medical beds on time so that a large number of patients with confirmed mild disease could be treated effectively in a timely manner. Building a module hospital can prevent the exacerbation of mild to severe disease, reduce the bed pressure of designated hospitals, effectively quarantine the source of infection, and prevent the spread of the epidemic. The construction of the module hospital is a practical measure to realize the “as early as possible” treatment of patients with mild disease and a key measure to solve the serious shortage of beds. The module hospital is built by several mobile modules, including a medical function unit, a ward unit, a technical support unit, and other parts, providing multiple functions such as emergency treatment, surgical treatment, clinical examination, and others.5 , 6 Every 50 beds form a medical unit; each unit is equipped with 5 doctors and 20 nurses, and the treatment and rescue areas are equipped with necessary medical facilities and drugs according to the standards for emergency observation rooms of a regular hospital.
The hospital that the authors work for is in charge of the overall operation and management of the Jianghan module hospital—1 of the first 3 module hospitals that has been reconstructed from the Wuhan Convention and Exhibition Center. Because the patients in the module hospital were largely the patients with mild COVID-19, the main treatment method was oral medication. At the same time, there was a serious shortage of frontline medical personnel, and thus, professional pharmaceutical personnel were urgently needed to provide effective pharmaceutical care for clinical treatment. Through continuous exploration, the pharmaceutical department of Wuhan Union Medical College Hospital had innovatively constructed the “pharmaceutical care model of module hospital,” which provided professional pharmaceutical services for the patients in the module hospital.
Objectives
The goal of this project was to introduce pharmaceutical care in a module hospital during the COVID-19 pandemic and to educate health professionals worldwide preparing for public health emergencies. The specific objectives of this project were to (1) describe how to design and implement pharmaceutical care in a module hospital for COVID-19 treatment and (2) document the patient care delivered by the Jianghan module hospital using this design.
Practice description
From 9 PM on February 5, 2020, the Jianghan module hospital with 1564 beds started to receive patients with COVID-19. By March 9, 2020, the total number of patients admitted to the hospital was 1848, of whom 521 (28.2% of the total number) were transferred to the designated hospital, and 1327 (71.81% of the total number) were cured and discharged.7
Criteria for patients who were admitted to the module hospital
The patient admission criteria were as follows: (1) COVID-19 confirmed cases; (2) clinically classified as mild patients; (3) self-care ability and recommended age between 18 and 65 years; (4) no underlying primary diseases in the respiratory system and cardiovascular system and no mental illness; and (5) a negative influenza virus nucleic acid test result.8
Practice innovation
First, we describe the establishment of the organizational structure of the pharmacy administration. Then, we describe the drug dispensing service, including information such as drug allocation, rational drug use, drug use education, and consultation. Six main tasks were performed to develop the organizational structure of the pharmacy administrative team.
Establishment of pharmacy emergency command group
The main task of the pharmacy emergency command group was to perform the functions of the pharmacy administration committee of the module hospital; take general charge of the relevant pharmacy administration work of the module hospital; implement the unified plan and deployment of personnel, drugs, and consumables; and accept the instructions and scheduling of the superior. Specifically, this included a formulation and review of the treatment medication plan and the drug formulary and a review of the types, dosage forms, specifications, quantities, packaging, etc., of the emergency drugs. Any difficulty in supplying drugs, consumables, and disinfectants was reported to the superior in a timely manner with a request for support.
Subordinate groups perform their duties accordingly
The pharmacy emergency command group consisted of the drug supply assurance group, the drug dispensing group, the quality control group, and the clinical pharmaceutical care group. Each group performed its own duties accordingly.
Drug supply assurance group
The drug supply assurance group was responsible for the purchase, storage, and distribution of drugs and disinfectants and ensured the supply of drugs. This group made purchases and requisition and budget plans according to the inventory and the consumption. Furthermore, it adjusted the inventory in time according to the diagnosis and the treatment plans, the clinical treatment demand, and the drug reserve to fulfill the demand of clinical use. Finally, it submitted the drug applications to the supporting unit and the local government, requested for the drugs in a timely manner, and took good care of the registration and management of donated antiepidemic drugs.
Drug dispensing group
The drug dispensing group was responsible for the daily drug dispensing work of the module hospital. It controlled the drug use trend, provided drug use information for the clinic, ensured the drug supply, and prevented overstocking in a timely manner.
Quality control group
The quality control group checked the quality of the purchased and donated drugs, including legal qualification and validity. It strictly implemented the relevant provisions of the State Food and Drug Administration. The drugs donated and allocated for domestic production must be the ones approved by the State Administration for Production, with an approval number and a quality standard, and the validity period must be more than 6 months from the expiration date. Furthermore, this group immediately handled and reported the problematic drugs once they were discovered.
Clinical pharmaceutical care group
The clinical pharmaceutical care group reviewed the prescriptions and provided online medication consultation and education for the patients through the Internet, telephone, video, and other forms for drug use–related problems. This group communicated with the medical staff of the module hospital on time, gave feedback on the problems encountered while providing pharmaceutical care, paid attention to adverse drug reactions (ADRs), and recorded and filled in the ADR report on time.
In the following section, we describe the characteristics of the module drug dispensing service including information system, module pharmacy, critical care drug trolley, and packaging service.
Module drug dispensing service based on COVID-19 characteristics
Establishment of the module hospital information system
The module hospital information system existed as a subsystem and was connected with the hospital information system in time. The electronic medical record system and outpatient doctor workstation system were used to issue medication orders for the patients. The pharmacy information system was used for account keeping and management, such as drug supply in and out of the warehouse and inventory, drug data collection, drug planning, prescription deployment, and expiration period management. When entering the module hospital, the patients were assigned an identification code, and the patient data were filed and entered into the information system. Each doctor was assigned a unique user code and password so that he or she can complete the work of issuing orders, inspections, and checklists on logging into the electronic system.
Establishment of the module pharmacy to ensure drug supply service
Based on the characteristic of mild symptoms of the patients with COVID-19, together with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (trial version, sixth edition, National Health Council), other documents and literature,9 , 10 the reference list of key therapeutic drugs was summarized, and the structure and quantity of patients’ drug demand were identified to establish a scientific and rational drug formulary by the drug supply assurance group.
Table 1 presents the list of the drugs. According to the classification based on the dosage forms and pharmacologic functions, approximately 50 kinds of drugs were listed. All drugs were stored under the storage conditions specified in the drug instruction manual, and attention was paid to storage temperature and humidity.
Table 1.
Drug formulary in module pharmacy
| Drug class | Drug products | Drug products, n (%)a |
|---|---|---|
| COVID-19 western medicine treatment | ||
| Antiviral drugs | Abidol hydrochloride tablets, oseltamivir phosphate capsules | 2 (4) |
| Antibacterial drugs | Moxifloxacin tablets (2 brands), levofloxacin tablets, cefdinir capsules | 4 (8) |
| Antipyretic and analgesic drugs | Ibuprofen tablets, diclofenac sodium sustained release tablets | 2 (4) |
| Antitussive, antiasthmatic, and expectorant drugs | Aminophylline sustained release tablets, aminophylline injection, acetylcysteine tablets, fudosteine tablets | 4 (8) |
| Digestive system drugs | Esomeprazole enteric coated tablets, bacillus licheniformis capsules, montmorillonite powder, ketoprol | 4 (8) |
| Chronic disease drugs | ||
| Cardiovascular and cerebrovascular drugs | Nifedipine sustained release tablets, nifedipine controlled release tablets, nitroglycerin injection, amlodipine besylate tablets, levamlodipine besylate tablets, perindopril tablets, clopidogrel bisulfate tablets, metoprolol succinate sustained release tablets, amiodarone injection, furosemide injection | 10 (20) |
| Lipid-lowering drugs | Atorvastatin calcium tablets | 1 (2) |
| Hypoglycemic drugs | Metformin hydrochloride tablets | 1 (2) |
| Antithyroid drugs | Thiamazole tablets | 1 (2) |
| Hepatoprotective drugs | Inosine tablets | 1 (2) |
| Others | ||
| Sedative and hypnotic drugs | Estazolam tablets | 1 (2) |
| Antiallergic drugs | Desloratadine tablet, chlorphenamine tablet, promethazine injection | 3 (6) |
| Chinese patent drugs | Jinhua qinggan granule, lianhua qingwen capsule, huoxiang zhengqi oral liquid, qiangli loquat dew, compound glycyrrhizic acid tablet, suhuang zhike capsule, niuhuang zhiqing suppository | 7 (14) |
| Critical care drugs | Dopamine hydrochloride injection, adrenaline hydrochloride injection, isoprenaline hydrochloride injection, atropine sulfate injection, deacetylated hairy anthocyanin injection, pedicel alkaloid injection, Nikethamide injection, hydroxyethyl starch 130/0.4 electrolyte injection, dexamethasone injection | 9 (18) |
Abbreviation used: COVID-19, coronavirus disease 2019.
Denominator equals 50 unique drugs.
Drug structure analysis
The main treatment was oral medicine. Table 1 shows that there were 37 kinds of oral preparations (74%) and 13 kinds of injections (26%). The reason for the treatment with mainly oral medicine was that the module hospital mainly hosted the patients who had COVID-19 with mild symptoms.
In addition to COVID-19 targeted treatment, the drug formulary also focused on patients’ mental state and underlying primary diseases and also covered the Chinese patent medicine and emergency medicine. As indicated in Table 1, (1) the drug formulary covered the antiviral, antibacterial, antipyretic, analgesic, antitussive, antiasthmatic, expectorant, and gastrointestinal drugs. These drugs were screened out as related to the treatment of COVID-19 (32% of total drugs) according to the Novel Coronavirus Pneumonia Treatment Plan, the characteristic action of SARS-CoV-2 on the respiratory and intestinal tracts, and the mechanism and the clinical manifestations of the disease; (2) sedative and hypnotic drugs were prepared (2% of total drugs) because some patients can be highly nervous; (3) for a few patients with underlying primary diseases, the drug formulary covered drugs for chronic diseases, such as antihypertensive, hypoglycemic, and lipid-lowering drugs (28% of total drugs); (4) the combination of traditional Chinese and Western medicine was conducive to the rehabilitation of the patients with COVID-19. Therefore, the drug formulary also included 7 kinds of Chinese patent medicines (14% of total drugs); (5) considering that some patients with mild symptoms may develop more severe symptoms, 18 kinds of critical care drugs were included (18% of total drugs).
Setting up the critical care trolley
Based on the course characteristics of COVID-19 and considering that some patients with mild disease may suddenly develop severe or critical disease,9 the drug dispensing group set up 4 critical care trolleys (Figure 1 ) loaded with all kinds of critical care drugs (Table 1) and supplies, which could be provided on-call anytime. The trolley was divided into 3 layers. The first layer was a box with a cover, which was used to load the entire box of critical care drugs, and the back of the box cover was designed with a separate bag for loading scattered drugs. The second and third layers were designed with a left drawer and a right cabinet with a door. The drawer was for placing syringes and other consumables, and the cabinet was for placing large infusions. The entire body of the critical care trolley was made of stainless steel with a pulley and handle design, making it convenient for emergency movement. Once a patient developed a critical condition such as shock, respiratory failure, heart failure, etc., the medical staff visited the patient with the critical care drug trolley to immediately administer the critical care medicine. According to the patient’s condition, the medical staff decided about transferring the patient to the designated hospital.
Figure 1.

Critical care drug trolley set up in the module hospital.
Packaging service of the designated drugs
The drugs used in the module hospital were the same as those used in the ordinary hospital. However, for the special case of COVID-19, specific outer packaging of the drugs was required.11 Considering that the patients with mild disease needed mainly the oral drugs, we adopted transparent plastic bags that could be sealed, labeled them with the patient’s name and bed number using a tag, and marked them with the name and quantity of the drugs with a black oily pen, as shown in Figure 2 . The advantages of the package are as follows: (1) the medicine in the transparent bag is clear at a glance with a clear label, which is convenient for handover and can be checked by different personnel during drug distribution: pharmacist check—nurse distribute—patient inform; (2) moisture-proof, dustproof, and sealable features ensure the convenience and cleanliness of the drug storage; and (3) disposable plastic bags are cheap and economical and can be sterilized and destroyed after use.
Figure 2.
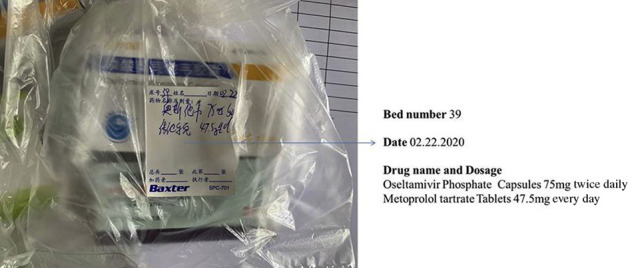
Designated drug packaging bags.
Rational drug use based on the pharmacists’ expertise to ensure safety
Based on several clinical medication practice analyses, the National Health Commission edited the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (sixth trial edition, revised version, National Health Council), which recommended a combination of Chinese and Western medicine to treat COVID-19. Most of the patients in the module hospital were older people (older than 50 years), and some of them had chronic primary diseases. Therefore, the combined use of Chinese and Western medicine, the use of multiple Chinese patent drugs, or the combined use of Chinese patent drugs and Chinese herbal decoctions was common. Most of the doctors participating in the diagnosis and treatment in the module hospital were Western medicine doctors; hence, it may be necessary for the pharmacists of the clinical pharmaceutical care group to actively participate in evaluating drug compatibility, interactions, and adverse reactions to ensure the safety of the patients. Taking the Jianghan module hospital as an example, possible problems of drug compatibility are summarized in Appendix 1.
The module pharmacy was placed in a clean area. After the doctor processed the order on the computer, the order information was immediately transmitted to the pharmacy. Pharmacists examined electronic prescriptions and checked with doctors when a prescription was called into question. Approximately 20,000 electronic orders of the doctors were reviewed online by clinical pharmacists, which reduced the irrational drug use and minimized the risk involved. After reviewing the prescriptions, the qualified drugs were dispensed, packaged, and handed over to the patients by the pharmacists in the designated areas. To reduce and avoid the severe ADRs or adverse drug events (ADEs), the clinical pharmacists informed and educated the patients about reasonable and safe medication. When ADRs or ADEs were observed in the patients, the doctors or nurses consulted with the clinical pharmacists and filled in the ADR report on the ADR or ADE online reporting platform within time.
Make full use of the information technology and new media to provide patients with medication consultation and personalized pharmaceutical care in a timely manner
Considering the particularity of the COVID-19 infection, making full use of information technology to provide pharmaceutical care not only reduced direct contact with the patients and avoided unnecessary cross infections but also saved resources such as protective clothing, so that limited resources could be used in places of great need.
The emerging media platform “WeChat” has the following characteristics: group or private chat, text or voice message, remote online video call or voice call, and other functions, which can meet the needs of different patients and facilitate pharmacists to remotely view patients’ conditions, medication status, and possible ADRs. We set up a WeChat group for medication consultation and pasted the quick response (QR) code in each ward of the module hospital with the help of the nurses to facilitate the patients to join the group by scanning the QR code. The group provided medication consultation for patients. There was 1 QR code and 1 WeChat group for every 150 beds. Patients were free to join or quit the WeChat group. More than 20 clinical pharmacists joined all the WeChat groups and were quickly divided into groups according to their specialties, provided medication consultation anytime and anywhere, answered patients’ different kinds of questions, and appeased patients’ anxiety. Figure 3 shows pharmacists in the module hospital using WeChat to provide medication consultation to the patients. Among them, Figure 3A shows the WeChat QR code pasted to the module ward, and Figures 3B and 3C show that the pharmacists provided personalized one-on-one medication consultation services for the patients.
Figure 3.
Providing medication consultation for patients using WeChat.
Establish a module radio station to broadcast the knowledge about rational drug use
Because most patients were seniors, who tend to use smartphones and new technologies less frequently, the knowledge about rational drug use was distributed in a timely manner by a community module radio station to relieve the patients’ panic and tension about the COVID-19 disease. Pharmacists of the clinical pharmaceutical care group oversaw the module radio station, which informed all the patients in the module about the medication, rational nutrition and diet suggestions for COVID-19, and self-protection and medication guidance after discharge. The series “rational medication to fight against the epidemic” of the Jianghan module radio station’s information emission covered the following subjects: (1) education about the antiviral drugs, antibacterial drugs, Chinese patent drugs, and symptomatic treatment drugs (antipyretic, antitussive, etc.) at the module hospital; (2) nutrition and diet for the prevention and treatment of the COVID-19 and living habit suggestions; and (3) self-management and medication guidance after discharge. The lecture was broadcasted at a fixed time every day, with the start time and content announced in the public area of the module hospital. The same lectures were looped again after 3 days. Figure 4 shows clinical pharmacists broadcasting and spreading the knowledge about rational drug use.
Figure 4.

Pharmacist broadcasting rational medication knowledge for the patients.
Evaluation
The pharmacy emergency command group was established for the standardized management of the pharmaceutical care for patients who have COVID-19 with mild symptoms in the Jianghan module hospital.
According to the principle of “early detection, early diagnosis, early reporting, early isolation, and early treatment,” the module hospital was the first one to have been used for patients who have COVID-19 with mild symptoms. With the continuous improvement of the module hospital, some aspects such as patient admission criteria and variety of drugs need to be adjusted depending on the actual situation. For early stage treatment, 50 kinds of drugs were determined, whereas in the later stage, the quantity and variety of drugs had been adjusted and supplemented according to the actual situation.
For some patients with chronic basic diseases, the treatment needed to be altered, such as using insulin preparations for the patients with diabetes. With the progress of in-depth knowledge about COVID-19, some antiviral drugs should be added in the drug formulary to be used against COVID-19 according to the newer editions of the Novel Coronavirus Pneumonia Treatment Plan based on medical evidence.
Results
Patient characteristics
The sex and the age of 1312 patients in the module hospital were analyzed, and the results are presented in Table 2 , showing that the proportion of infected men (54.8%) was larger than that of women. The age of patients ranged from 16 to 74 years. Among these patients, the 50- to 59-year age group accounted for the largest proportion (36.59%), followed by the 40- to 49-year age group (20.12%) and 30- to 39-year age group (18.37%). A total of 34 patients (24 male patients and 10 female patients) had a history of smoking (2.6% of the total). The results showed that the proportion of patients older than 50 years reached 55.27%, indicating that pharmacists should pay attention to the patients’ compliance with medication, especially older patients who need to take multiple drugs orally. The rate of smoking in the patients was very low, perhaps because the module hospital mainly hosted patients who had COVID-19 with mild symptoms.
Table 2.
Patient characteristics (N = 1312)
| Characteristic | Patients, n (%) |
|---|---|
| Sex | |
| Male | 719 (54.8) |
| Female | 593 (45.2) |
| Age, y | |
| 70–74 | 9 (0.69) |
| 60–69 | 236 (17.99) |
| 50–59 | 480 (36.59) |
| 40–49 | 264 (20.12) |
| 30–39 | 241 (18.37) |
| 20–29 | 76 (5.79) |
| 16–20 | 6 (0.46) |
| A history of smoking | |
| Male | 24 (1.83) |
| Female | 10 (0.76) |
Completed pharmaceutical care tasks
By March 9, 2020, the Jianghan module hospital was closed. Up until then, the pharmacists had worked for 34 days in the module hospital for patients who had mild COVID-19. The pharmaceutical care task completed by pharmacists is shown in Table 3 .
Table 3.
Completed pharmaceutical care task
| Task no. | Pharmaceutical care |
|---|---|
| 1 | Purchase, storage, and free distribution of drugs worth ¥1.03 million ($146,000) for patients. No mistakes and accidents in drug dispensing. |
| 2 | Online review of approximately 20,000 electronic doctor’s orders. |
| 3 | No serious adverse drug reactions or events were noted. |
| 4 | Provided 1-to-1 online medication consultation for 484 patients successively. Reduced the burden of the medical staff in the module hospital. |
| 5 | Held 5 lectures on rational drug use knowledge. Greatly relieved the psychological stress of patients. |
| 6 | Provided medication and health education on time to most of the patients in the WeChat group. |
Practice implications
The module hospital studied in this article was the first one to have been used in the prevention and control of COVID-19 infectious disease. The practice of pharmacy administration and pharmaceutical care in the hospital were explored for the first time. In treating 1848 patients with mild COVID-19 disease, the pharmacy department of Wuhan Union Medical College Hospital quickly launched the emergency mode, established a pharmacy emergency command group, built an organizational structure for pharmacy administration, and constantly improved the medication management in the module hospital. We also innovatively used information technology and a new media platform, WeChat, to provide pharmaceutical care; we created a new COVID-19 module pharmaceutical care model to provide pharmaceutical care for the patients by ensuring drug supply, setting up critical care trolleys, designing specific drug packaging bags, and creating a module radio station to inform about rational medication knowledge, and achieved excellent outcome. This model can be applied to other module hospitals in China to fight against COVID-19 and can also provide reference for international public health emergencies.
Conclusion
The pharmacy emergency command group compiled the “work manual for pharmacists in a module hospital” quickly, which helped to standardize the medication management. The drug formulary of the module hospital needs continuous readjustment to adapt to the clinical demands according to the characteristics of the patients admitted. As the professional pharmaceutical care for the patients was effective, the pharmacists have become an important part of the medical staff in the module hospital. Owing to the high risk of infection, “noncontact” medication consultation and education should also be provided, which can improve the patients’ compliance and relieve patients’ psychological pressure.
Biographies
XiaoLi Hua, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology and Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
Ming Gu, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
Fang Zeng, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
Huiping Hu, Hubei Key Laboratory of Nature Medicinal Chemistry and Resource Evaluation, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
Yu Zhang, PhD, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology and Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
Chen Shi, PhD, Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology and Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
Footnotes
Authors Hua and Gu have contributed equally to this work.
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81603037 to CS) and the National Key Research and Development Plan of China (2017YFC0909900).
Contributor Information
Yu Zhang, Email: whxhzy@163.com.
Chen Shi, Email: 29136909@qq.com.
Supplementary data. Appendix 1
-
1.
Decoction of traditional Chinese medicine: Hansi yufei formula, Yidu bifei formula, Neibi waituo formula, and Pifei qixu formula are not recommended to be used in combination with traditional Chinese medicine Huoxiang zhengqi. If it must be used in combination, it is necessary to adjust the dosage.
-
2.
It is not recommended to use Shengxuebao mixture in combination with all decoction and patent drugs indicated in the national guide formulary because it may deteriorate the disease.
-
3.
Yunnan Baiyao contains Aconitum kusnezoffii, which is not recommended to be used with the prescriptions of Huoxiang Zhengqi and Pifei Qixu formula containing Pinellia ternata, because it will induce 18 counter reactions.
-
4.
It is not recommended to use the Hanshi Yufei formula together with antipyretic and analgesic drugs in Western medicine, such as paracetamol and aspirin, which may cause excessive sweating or even collapse. Special monitoring must be done.
-
5.
Powerful loquat dew (containing about 0.15 mg morphine per mL), when used in combination with Western medicine, should be avoided to be used in combination with linazolamide and other monoamine oxidase inhibitors, cimetidine, anticholinergics, diuretics, and coumarins. Special monitoring must be done.
-
6.
Xuebijing injection is composed of traditional Chinese medicine for promoting blood circulation and removing blood stasis. It is not recommended to be used in combination with anticoagulants. If combined, special monitoring must be done. In addition, this drug has the risk of inducing anaphylactic shock and should be monitored in the first 30 min of infusion.
-
7.
Patients with hypertension should be monitored when taking Hanshi Yufei formula, Yidu Bifei decoction, Jinhua Qinggan granules, and Lianhua Qingwen pills. All four drugs all contain Ephedra, which can promote hypertension.
References
- 1.National Health Commission of the People’s Republic of China Announcement no. 1 of 2020 by the National Health Commission of the People’s Republic of China. http://www.gov.cn/xinwen/2020-01/21/content_5471158.htm Available at:
- 2.World Health Organization WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 Available at:
- 3.Gorbalenya A.E., Baker S.C., Baric R.S. Severe acute respiratory syndrome-related coronavirus: the species and its viruses–a statement of the Coronavirus Study Group. bioRxiv. 2020 [Google Scholar]
- 4.International Committee on Taxonomy of Viruses Naming the 2019 coronovirus. https://talk.ictvonline.org/ Available at: Accessed February 11, 2020.
- 5.Sugalski G., Murano T., Fox A., Rosania A. Development and use of mobile containment units for the evaluation and treatment of potential Ebola virus disease patients in a United States hospital. Acad Emerg Med. 2015;22(5):616–622. doi: 10.1111/acem.12664. [DOI] [PubMed] [Google Scholar]
- 6.Cheng B., Shi R.F., Du D.Y. Mobile emergency (surgical) hospital: development and application in medical relief of “4.20” Lushan earthquake in Sichuan Province, China. Chin J Traumatol. 2015;18(1):5–9. doi: 10.1016/j.cjtee.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Weibo Wuhan has the largest number of people in the square cabin hospital officially closed. https://m.weibo.cn/status/4480624184472967?wm=3333_2001&from=timeline&sourcetype=weixin Available at:
- 8.Medical Administration Bureau of National Health Commission of the People's Republic of China: Work manual of the module hospital (Edition 3). Available at: https://xw.qq.com/cmsid/20200223A0HZF300. Accessed February 23, 2020.
- 9.General Office of National Health Commission of the People’s Republic of China. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial version 6). (National Health Office Medical Letter [2020]145)[EB/OL]. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed February 19, 2020.
- 10.Wang Y.-M., Cao M., Huang F., Wang J. Shelter hospital medicine support in medical support of MOOTW. Chinese Med Equip J. 2014;35(9):133–134. [Google Scholar]
- 11.Yang B., Fan H.-J., Shi L. Medicine packaging improvement based on PAP shelter hospital requirements. Chinese Med Equip J. 2017;38(5):29–35. [Google Scholar]



