Highlights
-
•
Coronoviruses not only affect the respiratory system, but also have deleterious effects on the central nervous system.
-
•
Most neurological diseases could be caused by coronoviruses invasion.
-
•
Coronoviruses cause nerve damage via diverse pathways.
Keywords: Coronaviruses infection, COVID-19, Nervous system, Mechanisms
Abstract
Viral infections have detrimental impacts on neurological functions, and even to cause severe neurological damage. Very recently, coronaviruses (CoV), especially severe acute respiratory syndrome CoV 2 (SARS-CoV-2), exhibit neurotropic properties and may also cause neurological diseases. It is reported that CoV can be found in the brain or cerebrospinal fluid. The pathobiology of these neuroinvasive viruses is still incompletely known, and it is therefore important to explore the impact of CoV infections on the nervous system. Here, we review the research into neurological complications in CoV infections and the possible mechanisms of damage to the nervous system.
1. Introduction
In December 2019, Corona Virus Disease 2019 (COVID-19) epidemic emerged in Wuhan, China, causing global attentions (Thompson, 2020). The virus is known as especially severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was recently documented that, in addition to systemic and respiratory symptoms, 36.4% (78/214) of patients with COVID-19 develop neurological symptoms, including headache, disturbed consciousness, and paresthesia. Severely affected patients are more likely to develop neurological symptoms than patients who have mild or moderate disease (Mao et al., 2020). Additionally, autopsy reports have revealed brain tissue edema and partial neuronal degeneration in deceased patients (Xu et al., 2020). Furthermore, on March 4, 2020, Beijing Ditan Hospital reported for the first time a case of viral encephalitis caused by a novel coronavirus (CoV) attacking the central nervous system (CNS). The researchers confirmed the presence of SARS-CoV-2 in the cerebrospinal fluid by genome sequencing. It illustrated that COVID-19 has potential to cause nervous system damage (Xiang et al., 2020). With the now ongoing COVID-19 pandemic, it is particularly necessary to make clinicians aware of the impact of various CoV infections on the CNS. This article reviews the epidemiology, possible mechanisms of neuroinvasion, and management strategies pertaining to CoV infections with potential nervous system involvement.
2. CoV infections affecting the CNS
Many viral infections can cause serious damage to the structure and function of the nervous system, including severe encephalitis due to viral infections in the CNS, toxic encephalopathy caused by severe systemic viral infections, and severe acute demyelinating lesions developing after viral infections. (Michalicova et al., 2017, Wright et al., 2008). Some viruses are neurotropic and can invade nervous tissues and cause infections of immune-functioning macrophages, microglia, or astrocytes in the CNS (Al-Obaidi et al., 2018, Soung and Klein, 2018).
CoV have an average diameter of 100 nm, and they are spherical or oval. There are large spikes of viral membrane glycoproteins on the surface, and, when observed by electron microscopy, these negatively stained virus particles show a typical crown-like shape. CoV is a positive-sense single-stranded RNA virus, which harbors the largest genome among currently known RNA viruses, with a genome length of about 26–32 kb (Schoeman and Fielding, 2019). The pathogen of the now ongoing novel pneumonia outbreak is the novel CoV 2019 (SARS-CoV-2), which is the seventh known CoV that can infect humans; the remaining six are HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV, and MERS-CoV (Corman et al., 2019). The most common and important types of CoV infections with potential nervous system damage are described below.
2.1. SARS-CoV
Severe acute respiratory syndrome (SARS) is a zoonotic respiratory disease caused by SARS-CoV that started in Asia and spread throughout the world in 2003. It has the characteristics of acute onset and strong infectivity, and is a great threat to human health. The main clinical manifestations of SARS are fever, chills, dry cough, and difficulty breathing. In severe cases, respiratory failure and death may occur (Lai et al., 2004). In addition, SARS-CoV could induce neurological diseases such as polyneuropathy, encephalitis, and aortic ischemic stroke (Tsai et al., 2005). Autopsy studies demonstrated that signs of cerebral edema and meningeal vasodilation could be detected in most cases of SARS. Furthermore, infiltration of monocytes and lymphocytes in the vessel wall, ischemic changes of neurons, demyelinationn of nerve fibers, as well as SARS-CoV virus particles and genome sequences could be detected in the brain (Gu et al., 2005, Zhang et al., 2003).
2.2. MERS-CoV
Middle East Respiratory Syndrome (MERS), caused by MERS-CoV, originates from bats, and the intermediate host is camel. Patients with MERS-CoV infection usually present with pneumonia-related symptoms, such as fever, myalgia, cough, and dyspnea. Severe cases can lead to acute respiratory distress syndrome (ARDS), septic shock, multiple organ failure, and death (WHO MERS-Cov Research, 2013). MERS-CoV is known to be potentially neuroinvasive, and that a retrospective study found that 25.7% of patients with MERS can develop insanity and 8.6% of patients have seizures (Saad et al., 2014). Kim et al. also reported that almost 1/5 of patients with MERS-CoV infection show neurological symptoms during the infection process, including but not limited to disturbance of consciousness, paralysis, ischemic stroke, Guillain-Barre syndrome and other poisoning or infectious neuropathy. Interestingly, their neurological complications are not accompanied by respiratory symptoms, but delayed by 2–3 weeks (Kim et al., 2017).
2.3. SARS-CoV-2
The genetic similarity between SARS-CoV-2 and SARS-CoV is 79.5%, and its similarity to bat coronavirus is as high as 96% (Wu et al., 2020). Patients infected with SARS-CoV-2 have symptoms of varying degrees, ranging from fever or a mild cough to pneumonia and extensive involvement of multiple organ functions with a mortality rate of 2% to 4%. At present, clinical data have revealed that some patients with COVID-19 have symptoms similar to intracranial infections such as headache, epilepsy, and disturbed consciousness. Moreover, a growing number of COVID-19 patients report a sudden loss of smell or taste. It is therefore likely that anosmia and dysgeusia might be observed in patients with COVID-19 (Giacomelli et al., 2020, Ryan, 2020, Hopkins and Kumar, 2020). In fact, some even develop COVID-19-related symptoms only after showing neurologic symptoms (Mao et al., 2020). Recently, Beijing Ditan Hospital reported for the first time a case of viral encephalitis caused by the novel CoV attacking the CNS. The researchers confirmed the presence of SARS-CoV-2 in cerebrospinal fluid by genome sequencing, adding support to the theory this new pneumonia virus can also cause nervous system damage (Xiang et al., 2020). It is therefore likely that other pathogenic bacteria, such as bacteria, may destroy the blood–brain barrier, and secondary intracranial infections may cause headaches, projectile vomiting, visual loss, and limb convulsions in patients with severe COVID-19 symptoms.
3. Nervous system diseases related to CoV infections
3.1. Viral encephalitis
Encephalitis refers to inflammatory lesions in the brain parenchyma caused by pathogens, including neuronal damage and nerve tissue lesions. It is characterized by acute onset, and common symptoms include headache, fever (mainly high fever), vomiting, convulsions, and consciousness disorders (Ellul and Solomon, 2018). Early diagnosis of viral encephalitis is critical. In the ongoing pneumonia epidemic, the treatment team of Beijing Ditan Hospital confirmed the presence of SARS-CoV-2 in the cerebrospinal fluid of patients with COVID-19 by genome sequencing, thereby clinically verifying viral encephalitis (Xiang et al., 2020). This provided a solid basis for CoV causing the encephalitis.
3.2. Infectious toxic encephalopathy
Infectious toxic encephalopathy, also known as acute toxic encephalitis, refers to a type of reversible brain dysfunction syndrome caused by factors such as systemic toxemia, metabolic disorders, and hypoxia during the process of acute infection (Mizuguchi et al., 2007, Tauber et al., 2017, Young, 2013). The basic pathological changes in this disease include cerebral edema, with no evidence of inflammation on cerebrospinal fluid analysis. Its clinical symptoms are complex and diverse. Patients with a mild course of the disease may develop headache, dysphoria, mental disorder, and delirium. Seriously affected patients may experience disorientation, loss of consciousness, coma, and paralysis (Dobbs, 2011, Mizuguchi et al., 2007). Acute viral infection is also an important cause of this disease, exemplified by a respiratory infection caused by CoV. Patients with COVID-19 often suffer from severe hypoxia and viremia (Guo et al., 2020), which has the potential to cause toxic encephalopathy. Moreover, almost 40% of patients with COVID-19 develop headache, disturbed consciousness, and other brain dysfunction symptoms (Mao et al., 2020), and that an autopsy study reported that edema has been detected in brain tissue of COVID-19 patients (Xu et al., 2020). Collectively, these findings provide the evidence that COVID-19 could cause infectious toxic encephalopathy, although detailed studies are greatly required.
3.3. Acute cerebrovascular disease
A considerable amount of evidence indicates that especially respiratory-related infection is an independent risk factor for acute cerebrovascular disease (Elkind, 2007, Warren-Gash et al., 2018). Data from the use of experimental mouse models suggests that influenza virus can aggravate ischemic brain injury by triggering a cytokine cascade and increase the risk of cerebral hemorrhage after treatment with tissue-type plasminogen activator (Muhammad et al., 2011). The infection of CoV, especially SARS-CoV-2, has been widely reported to cause cytokine storm syndromes, which may be one of the factors that CoV cause acute cerebrobasilar disease (Mehta et al., 2020, Chen et al., 2020). In addition, critically ill patients with severe SARS-CoV-2 infections often show elevated levels of D-dimer and severe platelet reduction, which may render these patients prone to acute cerebrovascular events (Wang et al., 2020). It is therefore likely that during CoV infections, patients at risk of developing cerebrovascular disease should be alerted with regard to the occurrence of acute cerebrovascular events.
4. Mechanisms of CoV infections on the nervous system damage
4.1. Direct infection injury
The genetic material and even proteins of various viruses can often be detected in nervous system tissue samples (such as cerebrospinal fluid or brain), suggesting that viruses can directly invade the nervous system and cause nerve damage (Koyuncu et al., 2013, Leber et al., 2016).
4.1.1. Blood circulation pathway
A typical virus entering the CNS through the blood circulation is the JE virus, which multiplies in the vascular cells of the skin area affected by the mosquito bite. It is subsequently released into the blood to reproduce in mononuclear macrophages throughout the body. The secondary release into the blood may increase the permeability of the blood–brain barrier through the produced cytokines, thereby promoting the virus to enter the brain and causing viral encephalitis (Unni et al., 2011). Although there is rare evidence that CoV, especially SARS-CoV-2, invade the nervous system via the blood circulation pathway (Koyuncu et al., 2013, Desforges et al., 2019), subsequent studies are expected.
4.1.2. Neuronal pathway
Neuronal pathway is important vehicles for neurotropic viruses to enter the CNS. Viruses can migrate by infecting sensory or motor nerve endings, achieving retrograde or anterograde neuronal transport through the motor proteins, dynein and kinesins (Swanson and McGavern, 2015). An example of a neuronal pathway is that of olfactory neuron transport. The unique anatomical organization of olfactory nerves and the olfactory bulb in the nasal cavity and forebrain effectively makes it a channel between the nasal epithelium and the CNS (Koyuncu et al., 2013). As a consequence, CoV can enter the brain through the olfactory tract in the early stages of infection or nasal vaccination (Desforges et al., 2019, Mori, 2015). For example, after CoV infects nasal cells, it can reach the entire brain and cerebrospinal fluid through the olfactory nerve and olfactory bulb within 7 days and cause inflammation and demyelinating reaction. However, removal of the olfactory bulb in the mice, resulted in a restricted invasion of CoV into the CNS (Bohmwald et al., 2018). Gu et al. also detected SARS virus particles and genome sequences in brain neurons (Gu et al., 2005). The observations mentioned here indicate that CoV can invade the CNS from the periphery through neural pathways.
4.2. Hypoxia injury
When a virus proliferates in lung tissue cells, it causes diffuse alveolar and interstitial inflammatory exudation, edema, and the formation of transparent membranes. This, in turn, leads to alveolar gas exchange disorders causing hypoxia in the CNS, increasing anaerobic metabolism in the mitochondria of brain cells (Abdennour et al., 2012). The accumulation of acid can cause cerebral vasodilation, swelling of brain cells, interstitial edema, obstruction of cerebral blood flow, and even headache due to ischemia and congestion (Abdennour et al., 2012). If the hypoxia continues unabated, cerebral edema and the cerebral circulation disorder may worsen sharply. With intracranial hypertension, the brain function gradually deteriorates, and drowsiness, bulbar conjunctival edema, and even coma can be observed (Abdennour et al., 2012). In addition, for patients at particular risk of developing cerebrovascular disease, hypoxia may also induce the occurrence of acute cerebrovascular disease such as acute ischemic stroke. Owing to the fact that the patients with COVID-19 often suffer from severe hypoxia (Guo et al., 2020), hypoxia injury may cause subsequent nervous system damage.
4.3. Immune injury
Nervous system damage caused by viral infection may be mediated by the immune system (Klein et al., 2017). The pathology of severe viral infections is closely linked to the development of a systemic inflammatory response syndrome (SIRS). SIRS could be abnormally initiated in severe pneumonia caused by CoV infection, while early anti-inflammatory intervention effectively prevent immune damage and reduce the risk of injury in the nervous system (Mehta et al., 2020, Fu et al., 2010). Furthermore, SARS and COVID-19 have resulted in a large number of fatalities, most of which have been due to multiple organs failure (MOF) caused by virus-induced SIRS or SIRS-like immune disorders (Yin et al., 2004, Chen et al., 2020). The persistence of CoV infections and its ability to infect macrophages, microglia, and astrocytes in the CNS are particularly important. A neurotropic virus can activate glial cells and induce a pro-inflammatory state (Li et al., 2004). interleukin (IL)-6, an important member of the cytokine storm, is positively correlated with the severity of COVID-2019 symptoms (Wan et al., 2020). Additionally, experiments have confirmed that primary glial cells cultured in vitro secrete a large amount of inflammatory factors such as IL-6, IL-12, IL-15, and TNF-α after being infected with CoV (Bohmwald et al., 2018). Furthermore, activation of immune cells in the brain will cause chronic inflammation and brain damage.
4.4. Angiotensin-converting enzyme 2
Angiotensin-converting enzyme 2 (ACE2) is a cardio-cerebral vascular protection factor existing in a variety of organs, including the nervous system and skeletal muscles, playing a major role in regulating blood pressure and anti-atherosclerosis mechanisms (Miller and Arnold, 2019). Meanwhile, ACE2 is also an important target for various CoV and influenza viruses (Turner et al., 2004, Wrapp et al., 2020, Yang et al., 2014). Binding to ACE2 receptors, the above-mentioned viruses may cause abnormally elevated blood pressure and increase the risk of cerebral hemorrhage. In addition, given that SARS-CoV-2 spike protein could interact with ACE2 expressed in the capillary endothelium, the virus may also damage the blood–brain barrier and enter the CNS by attacking the vascular system (Baig et al., 2020).
4.5. Others
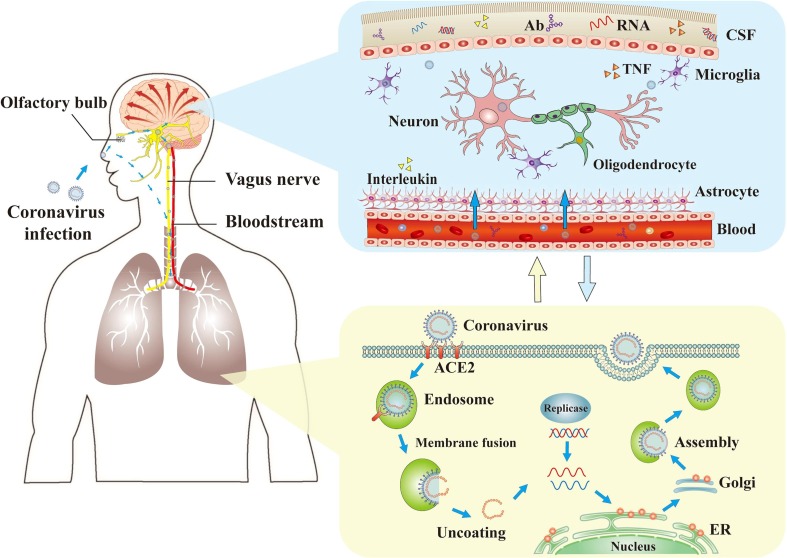
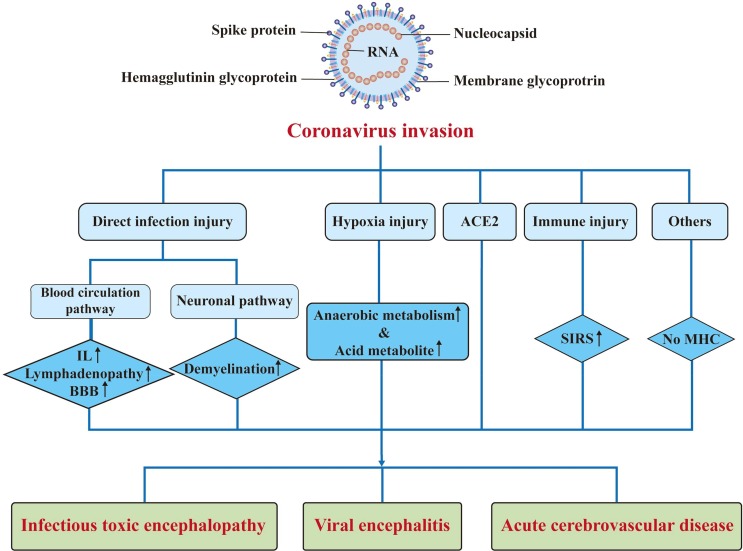
The biological properties of the CNS may facilitate exacerbation of the neurological damage caused by CoV infections. The CNS has a dense parenchymal structure and the usual lack of permeability of its blood vessels is a barrier to virus invasion. However, if a virus gains access to the CNS, it is difficult to remove (Reinhold and Rittner, 2017). Due to the lack of major histocompatibility complex antigens in nerve cells, the elimination of viruses in nerve cells depends solely on the role of cytotoxic T cells; however, the apoptosis of mature neurons after virus infection also has a relatively protective effect (Wuthrich et al., 2015). Furthermore, the homeostasis characteristics of the cells in the CNS also contribute to the continued existence of the virus (Reinhold and Rittner, 2017) (Fig. 1 ; Fig. 2 ).
Fig. 1.
The mechanisms of coronaviruses infections and neurological damage caused by coronaviruses. The coronaviruses can cause nerve damage through direct infection pathways (blood circulation pathways and neuronal pathways), hypoxia, immune injury, ACE2, and other mechanisms. Meanwhile, the coronaviruses have detrimental effects to attack the lung tissue, and causes a series of lung lesions such as hypoxia. Furthermore, the coronaviruses can enter the nervous system directly through the olfactory nerve, and also enter the nervous system through blood circulation and neuronal pathways, resulting in neurological disorders. Ab: antibody; ACE2: angiotensin-converting enzyme 2; CSF: cerebrospinal fluid; ER: endoplasmic reticulum; TNF: tumor necrosis factor.
Fig. 2.
Pathogenesis of nervous system injury caused by coronaviruses. ACE2: angiotensin-converting enzyme 2; BBB: blood brain barrier; IL: interleukin; MHC: major histocompatibility complexes; SIRS: systemic inflammatory response syndrome.
5. Conclusion
CoV infections can affect the nervous system, and it is currently believed that CoV in concert with host immune mechanisms may turn these infections into persistent infections that may lead to neurological diseases. Therefore, patients with CoV infections should be evaluated early for neurological symptoms, including headache, consciousness disorder, paresthesia, and other pathological signs. Timely analysis of cerebrospinal fluid and awareness and management of infection-related neurological complications are key to improving the prognosis of critically ill patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81703482 and 81974171 to C.Y.) and the Science and Technology Support (Social Development) Project of Bureau of Science and Technology of Changzhou (No. CE20195044 to L.Y.).
References
- Abdennour L., Zeghal C., Deme M., Puybasset L. Interaction brain-lungs. Ann. Fr. Anesth. Reanim. 2012;31(6):e101–107. doi: 10.1016/j.annfar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Al-Obaidi M.M.J., Bahadoran A., Wang S.M., Manikam R., Raju C.S., Sekaran S.D. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018;62(1):16–27. doi: 10.4149/av_2018_102. [DOI] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang X.R., Ju Z.Y., He W.F. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Lienau J., Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist (Berl) 2019;60(11):1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dube M. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs M.R. Toxic encephalopathy. Semin. Neurol. 2011;31(2):184–193. doi: 10.1055/s-0031-1277989. [DOI] [PubMed] [Google Scholar]
- Elkind M.S. Why now? Moving from stroke risk factors to stroke triggers. Curr. Opin. Neurol. 2007;20(1):51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- Ellul M., Solomon T. Acute encephalitis - diagnosis and management. Clin. Med. (Lond) 2018;18(2):155–159. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., Cheng, Y., Wu, Y., 2020. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools [published online ahead of print, 2020 Mar 3]. Virologica Sinica. [DOI] [PMC free article] [PubMed]
- Giacomelli, A., Pezzati, L., Conti, F., Bernacchia, D., Siano, M., Oreni, L., et al., 2020. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study [published online ahead of print, 2020 Mar 26]. Clin Infect Dis ciaa330. [DOI] [PMC free article] [PubMed]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C., Kumar, N., 2020. Loss of sense of smell as marker of COVID-19 infection. Retrieved from https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf. [DOI] [PMC free article] [PubMed]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.S., Garber C., Howard N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017;18(2):132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu O.O., Hogue I.B., Enquist L.W. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.N., Tsang K.W., Seto W.H., Ooi C.G. Clinical, Laboratory, and Radiologic Manifestation of SARS. Curr. Infect Dis. Rep. 2004;6(3):213–219. doi: 10.1007/s11908-004-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber A.L., Everhart K., Balada-Llasat J.M., Cullison J., Daly J., Holt S. Multicenter evaluation of biofire filmarray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J. Clin. Microbiol. 2016;54(9):2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78(7):3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M.D., Chen S.H., He Q.W., Chang J., Hong C.D., et al., 2020. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv 2020.02.22.20026500.
- Mehta P, McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., 2020. COVID-19: consider cytokine storm syndromes and immunosuppression [published online ahead of print, 2020 Mar 16]. Lancet S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed]
- Michalicova A., Bhide K., Bhide M., Kovac A. How viruses infiltrate the central nervous system. Acta Virol. 2017;61(4):393–400. doi: 10.4149/av_2017_401. [DOI] [PubMed] [Google Scholar]
- Miller A.J., Arnold A.C. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Auton. Res. 2019;29(2):231–243. doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M., Yamanouchi H., Ichiyama T., Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol. Scand. Suppl. 2007;186:45–56. [PubMed] [Google Scholar]
- Mori I. Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol. 2015;59(4):338–349. doi: 10.4149/av_2015_04_338. [DOI] [PubMed] [Google Scholar]
- Muhammad S., Haasbach E., Kotchourko M., Strigli A., Krenz A., Ridder D.A. Influenza virus infection aggravates stroke outcome. Stroke. 2011;42(3):783–791. doi: 10.1161/STROKEAHA.110.596783. [DOI] [PubMed] [Google Scholar]
- Reinhold A.K., Rittner H.L. Barrier function in the peripheral and central nervous system-a review. Pflugers. Arch. 2017;469(1):123–134. doi: 10.1007/s00424-016-1920-8. [DOI] [PubMed] [Google Scholar]
- Ryan W.M., 2020. There's a new symptom of coronavirus, doctors say: Sudden loss of smell or taste. Retrieved from https://www.usatoday.com/story/news/health/2020/03/24/coronavirus-symptoms-loss-smell-taste/2897385001/.
- Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung A., Klein R.S. Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol. Med. 2018;24(11):950–962. doi: 10.1016/j.molmed.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber S.C., Eiffert H., Bruck W., Nau R. Septic encephalopathy and septic encephalitis. Expert Rev. Anti. Infect. Ther. 2017;15(2):121–132. doi: 10.1080/14787210.2017.1265448. [DOI] [PubMed] [Google Scholar]
- Thompson R. Pandemic potential of 2019-nCoV. Lancet. Infect. Dis. 2020;20(3):280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan. 2005;14(3):113–119. [PubMed] [Google Scholar]
- Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni S.K., Ruzek D., Chhatbar C., Mishra R., Johri M.K., Singh S.K. Japanese encephalitis virus: from genome to infectome. Microbes Infect. 2011;13(4):312–321. doi: 10.1016/j.micinf.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wan S.X., Yi Q.J., Fan S.B., Lv J.L., Zhang X.X., Guo L., et al., 2020. Characteristics of lymphocyte subsets and cytokines inperipheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv 2020.02.10.20021832.
- Wang, Y., Wang, Y., Chen, Y., Qin, Q., 2020. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures [published online ahead of print, 2020 Mar 5]. J Med Virol. [DOI] [PMC free article] [PubMed]
- Warren-Gash C., Blackburn R., Whitaker H., McMenamin J., Hayward A.C. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur. Respir. J. 2018;51(3) doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO MERS-Cov Research, G., 2013. State of Knowledge and Data Gaps of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Humans. PLoS Curr 5. [DOI] [PMC free article] [PubMed]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E.J., Brew, B.J., Wesselingh, S.L., 2008. Pathogenesis and diagnosis of viral infections of the nervous system. Neurol Clin 26 (3), 617-633, vii. [DOI] [PubMed]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich C., Batson S., Koralnik I.J. Lack of Major Histocompatibility complex class I upregulation and restrictive infection by JC virus hamper detection of neurons by T lymphocytes in the central nervous system. J. Neuropathol. Exp. Neurol. 2015;74(8):791–803. doi: 10.1097/NEN.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang P., Xu X.M., Gao L.L., Wang H.Z., Xiong H.F., Li R.H. First case of 2019 novel coronavirus disease with Encephalitis. ChinaXiv. 2020;T202003:00015. [Google Scholar]
- Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al., 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published online ahead of print, 2020 Feb 18]. Lancet Respir Med. [DOI] [PMC free article] [PubMed]
- Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C.H., Wang, C., Tang, Z., Wen, Y., Zhang, S.W., Wang, B.E., 2004. [Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 16 (11), 646-650. [PubMed]
- Young G.B. Encephalopathy of infection and systemic inflammation. J. Clin. Neurophysiol. 2013;30(5):454–461. doi: 10.1097/WNP.0b013e3182a73d83. [DOI] [PubMed] [Google Scholar]
- Zhang, Q.L., Ding, Y.Q., Hou, J.L., He, L., Huang, Z.X., Wang, H.J., et al., 2003. [Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization]. Di Yi Jun Yi Da Xue Xue Bao 23 (11), 1125-1127. [PubMed]