Abstract
Introduction
COVID-19 may predispose to both venous and arterial thromboembolism due to excessive inflammation, hypoxia, immobilisation and diffuse intravascular coagulation. Reports on the incidence of thrombotic complications are however not available.
Methods
We evaluated the incidence of the composite outcome of symptomatic acute pulmonary embolism (PE), deep-vein thrombosis, ischemic stroke, myocardial infarction or systemic arterial embolism in all COVID-19 patients admitted to the ICU of 2 Dutch university hospitals and 1 Dutch teaching hospital.
Results
We studied 184 ICU patients with proven COVID-19 pneumonia of whom 23 died (13%), 22 were discharged alive (12%) and 139 (76%) were still on the ICU on April 5th 2020. All patients received at least standard doses thromboprophylaxis. The cumulative incidence of the composite outcome was 31% (95%CI 20-41), of which CTPA and/or ultrasonography confirmed VTE in 27% (95%CI 17-37%) and arterial thrombotic events in 3.7% (95%CI 0-8.2%). PE was the most frequent thrombotic complication (n = 25, 81%). Age (adjusted hazard ratio (aHR) 1.05/per year, 95%CI 1.004-1.01) and coagulopathy, defined as spontaneous prolongation of the prothrombin time > 3 s or activated partial thromboplastin time > 5 s (aHR 4.1, 95%CI 1.9-9.1), were independent predictors of thrombotic complications.
Conclusion
The 31% incidence of thrombotic complications in ICU patients with COVID-19 infections is remarkably high. Our findings reinforce the recommendation to strictly apply pharmacological thrombosis prophylaxis in all COVID-19 patients admitted to the ICU, and are strongly suggestive of increasing the prophylaxis towards high-prophylactic doses, even in the absence of randomized evidence.
Keywords: COVID-19, Pulmonary embolism, Deep vein thrombosis, Stroke, Thromboprophylaxis
COVID-19 may predispose to both venous and arterial thromboembolic disease due to excessive inflammation, hypoxia, immobilisation and diffuse intravascular coagulation (DIC) [[1], [2], [3], [4]]. Remarkably, thrombotic complications have hardly been described [[1], [2], [3], [4]]. Precise knowledge of the incidence of thrombotic complications in COVID-19 patients is important for decision making with regard to intensity of thromboprophylaxis, especially in patients admitted to the intensive care unit (ICU) who are at highest thrombotic risk.
We evaluated the incidence of the composite outcome of venous thromboembolism (VTE) and arterial thrombotic complications in all COVID-19 patients admitted to the ICU of 2 Dutch university hospitals and 1 Dutch teaching hospital. Our composite outcome consisted of acute pulmonary embolism (PE), deep-vein thrombosis (DVT), ischemic stroke, myocardial infarction or systemic arterial embolism. Importantly, in all participating hospitals, diagnostic tests were only applied if thrombotic complications were clinically suspected. We calculated the cumulative incidence of the composite outcome, as well as for the venous and arterial thrombotic complications separately. The index date was the moment of ICU admission. Patients were followed until ICU discharge, until they died, or until April 5th 2020, whichever came first. We performed backward conditional regression analyses to identify relevant determinants. The Institutional Review Boards of the participating hospitals waived the need for informed consent due to the observational nature of our study.
We studied 184 patients with proven COVID-19 pneumonia (Table 1 ) admitted to the ICU between March 7th and April 5th 2020. Of those, 23 died (13%), 22 were discharged alive (12%) and 139 (76%) were still on the ICU on April 5th 2020. The median duration of the observation per patient was 7 days (IQR 1-13). All patients received at least standard doses thromboprophylaxis, although regimens differed between hospitals and doses increased over time (Table 2 ). The cumulative incidence of the composite outcome was 31% (95%CI 20-41%; Fig. 1 , Table 3 ), of which CTPA and/or ultrasonography confirmed VTE in 27% (95%CI 17-37%) and arterial thrombotic events in 3.7% (95%CI 0-8.2%). PE was the most frequent thrombotic complication (n = 25, 81%). Age (adjusted hazard ratio (aHR) 1.05/per year, 95%CI 1.004-1.01) and coagulopathy, defined as spontaneous prolongation of the prothrombin time > 3 s or activated partial thromboplastin time > 5 s (aHR 4.1, 95%CI 1.9-9.1), were independent predictors of thrombotic complications.
Table 1.
Characteristics of included patients.
| Age (Mean, standard deviation) | 64 (12) |
| Male sex (number, %) | 139 (76) |
| Body weight (mean, standard deviation) | 87 (16) |
| Active cancer (number, %) | 5 (2.7) |
| Coagulopathy during admissiona (n, %) | 70 (38) |
| Therapeutic anticoagulation at admission (n, %) | 17 (9.2) |
| Renal replacement therapy during admission (n, %) | 23 (13%) |
Defined as: spontaneous prolongation of the prothrombin time (PT) >3 s or activated partial thromboplastin time (APTT) > 5 s.
Table 2.
Local protocol for thromboprophylaxis in participating centres for patients admitted to the intensive care unit during the study period.
| Site | |
|---|---|
| Leiden University Medical Center | nadroparin 2850 IU sc per day or 5700 IU per day if body weight > 100 kg |
| Erasmus University Medical Center | Nadroparin 5700 IU per day; nadroparin 5700 IU sc twice daily from April 4th 2020 and onwards |
| Amphia Hospital Breda | Nadroparin 2850 IU sc per day or 5700 IU per day if body weight > 100 kg; nadroparin 5700 IU sc per day from March 30th 2020 and onwards |
Fig. 1.
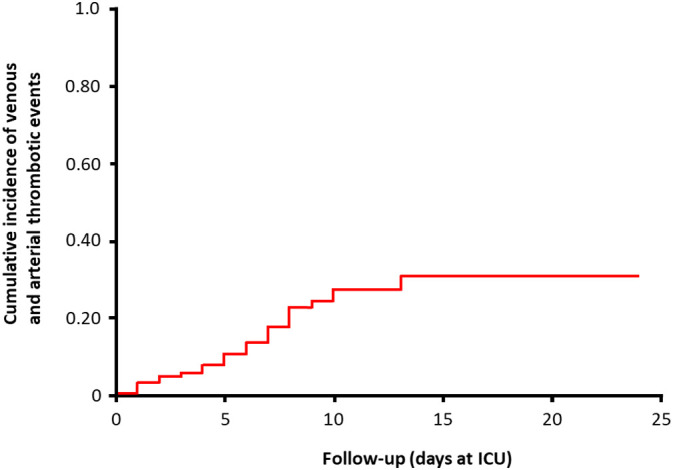
Cumulative incidence of venous and arterial thrombotic complications during the course of intensive care unit admission of patients with proven COVID-19 pneumonia.
Table 3.
Description of thrombotic complications.
| Type of event | Number of cases | Relevant details |
|---|---|---|
| Pulmonary embolism | 25 |
|
| Other venous thromboembolic events | 3 |
|
| Arterial thrombotic events | 3 |
|
Note: acute pulmonary embolism was diagnosed with CT-pulmonary angiography, deep vein thrombosis/upper extremity vein thrombosis was diagnosed with ultrasonography, strokes were diagnosed with CT.
Despite systematic thrombosis prophylaxis, the 31% incidence of thrombotic complications in ICU patients with COVID-19 infections is remarkably high and well comparable to the VTE incidence in other patient categories with overt DIC [5]. Notably, none of our patients actually developed DIC.
What are the consequences of our observations? First, they represent a conservative estimation, because the majority of patients was still on the ICU and therefore at risk after the described observation period. Also, VTE is more difficult to recognize in intubated patients with a higher threshold to perform diagnostic imaging tests because of strict isolation. If VTE screening had been applied, the incidence could have been even higher. We have to acknowledge that due to the design of the study, we could not adjust our findings for the actual administered doses of nadroparin, nor study the effect of the changes in the local protocols for thromboprophylaxis indicated in Table 2. In view of this, our findings reinforce the recommendation to strictly apply pharmacological thrombosis prophylaxis in all COVID-19 patients admitted to the ICU, and are suggestive of increasing the prophylaxis towards high-prophylactic doses, e.g. going from enoxaparin 40 mg OD to 40 mg BID, even in the absence of randomized evidence.
Finally, we propose that, rather than treating all patients with COVID-19 infections at the ICU with therapeutic anticoagulation, physicians should be vigilant for signs of thrombotic complications, and order appropriate diagnostic tests at a low threshold [5].
Author contributions
FAK, MJHAK and MWH designed the study based on the observations of HE and DAMPJG. FAK, MJHAK, FHJK, MAMS, NJMvdM and KMK gathered data. FAK and MVH performed the analyses drafted the first version of the manuscript. All authors revised the manuscript critically for important intellectual content and agree with the final version.
Acknowledgments
Acknowledgements
None.
Disclosures
Frederikus Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, MSD and Actelion, the Dutch Heart foundation and the Dutch Thrombosis association, all outside the submitted work. Menno Huisman reports grants from ZonMW Dutch Healthcare Fund, and grants and personal fees from Boehringer-Ingelheim, Pfizer-BMS, Bayer Health Care, Aspen, Daiichi-Sankyo, all outside the submitted work. Marieke Kruip reports unrestricted research grants from Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, Pfizer, Sobi, and The Netherlands Organisation for Health Research and Development (ZonMW). The other authors having nothing to disclose.
References
- 1.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. China; Jama: 2020. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., Scully M. How I treat disseminated intravascular coagulation. Blood. 2018;131:845–854. doi: 10.1182/blood-2017-10-804096. [DOI] [PubMed] [Google Scholar]


