Abstract
Background
Epigenetic modifiers are being investigated for a number of CNS malignancies as tumor-associated mutations such as isocitrate dehydrogenase mutations (IDH1/IDH2) and H3K27M mutations, which result in aberrant signaling, are identified. We evaluated the CNS exposure of the DNA methyltransferase inhibitor, 5-azacytidine (5-AZA), in preclinical nonhuman primate (NHP) models to inform its clinical development for CNS tumors.
Methods
5-AZA and 5-AZA+Inulin pharmacokinetics (PK) were evaluated in NHPs (n = 10) following systemic (intravenous [IV]) and intrathecal (intraventricular [IT-V], intralumbar [IT-L], and cisternal [IT-C]) administration. Plasma, cerebrospinal fluid (CSF), cortical extracellular fluid (ECF), and tissues were collected. 5-AZA levels were quantified via ultra-high-performance liquid chromatography with tandem mass spectrometric detection assay and inulin via ELISA. PK parameters were calculated using noncompartmental methods.
Results
After IV administration, minimal plasma exposure (area under the curve [AUC] range: 2.4–3.2 h*µM) and negligible CSF exposure were noted. CSF exposure was notably higher after IT-V administration (AUCINF 1234.6–5368.4 h*µM) compared to IT-L administration (AUCINF 7.5–19.3 h*µM). CSF clearance after IT administration exceeded the mean inulin CSF flow rate of 0.018 ± 0.003 ml/min as determined by inulin IT-V administration. 5-AZA IT-V administration with inulin increased the 5-AZA CSF duration of exposure by 2.2-fold. IT-C administration yielded no quantifiable 5-AZA ECF concentrations but resulted in quantifiable tissue levels.
Conclusions
IT administration of 5-AZA is necessary to achieve adequate CNS exposure. IT administration results in pronounced and prolonged 5-AZA CSF exposure above the reported IC50 range for IDH-mutated glioma cell lines. Inulin administered with 5-AZA increased the duration of exposure for 5-AZA.
Keywords: ependymoma, glioma, intrathecal, pharmacokinetics, primate, 5-azacytidine
Importance of the Study.
A major obstacle to effective therapies for CNS tumors is the delivery of the agent to the tumor site. 5-AZA, a potent cytotoxic and demethylation agent, has demonstrated preclinical efficacy in a murine flank IDH1 anaplastic astrocytoma model. An epigenetic cancer pathway associated with pediatric high-grade gliomas (HGGs) and ependymoma is promoter-associated hypermethylation resulting in genetic transcription silencing that is potentially reversible by 5-AZA. Critical to the development of an efficacious treatment rational for these tumors utilizing 5-AZA is the evaluation of the agent’s CNS penetration following administration. Preclinical NHP pharmacokinetic and microdialysis models were utilized to detail the plasma, CSF, cortical ECF exposure and duration, as well as tissue concentrations, of 5-AZA following systemic and IT administration. These translational findings are fundamental in the development of a meaningful treatment rationale and clinical trial design for HGG and ependymoma with 5-AZA.
Key Points.
In-depth pharmacokinetic study of 5-AZA in a preclinical nontumor bearing model.
CSF exposure of 5-AZA was only accomplished via IT administration.
IT delivery of 5-AZA was well tolerated in the animal model.
A major obstacle to effective therapies for CNS tumors is the delivery of an effective agent to the tumor site. 5-Azacytidine (5-AZA), a potent DNA methyltransferase inhibitor and cytotoxic agent approved for myelodysplastic syndrome and acute myeloid leukemia,1 has demonstrated activity in several preclinical murine CNS tumor models, including a murine flank astrocytoma and glioblastoma model following systemic administration2 and a patient derived xenograft (PDX) glioma IDH2 R132H mutation model in combination with temozolomide (TMZ).3 Isocitrate dehydrogenase mutations (IDH1/IDH2) result in promoter-associated hypermethylation producing genetic transcription silencing.4,5 This epigenetic pathway is associated with multiple tumor types5,6 including adult high-grade glioma (HGG),7 a subset of pediatric HGG8,9 and posterior fossa ependymoma.10,11 A pilot clinical trial in children with recurrent posterior fossa ependymomas has been performed.12
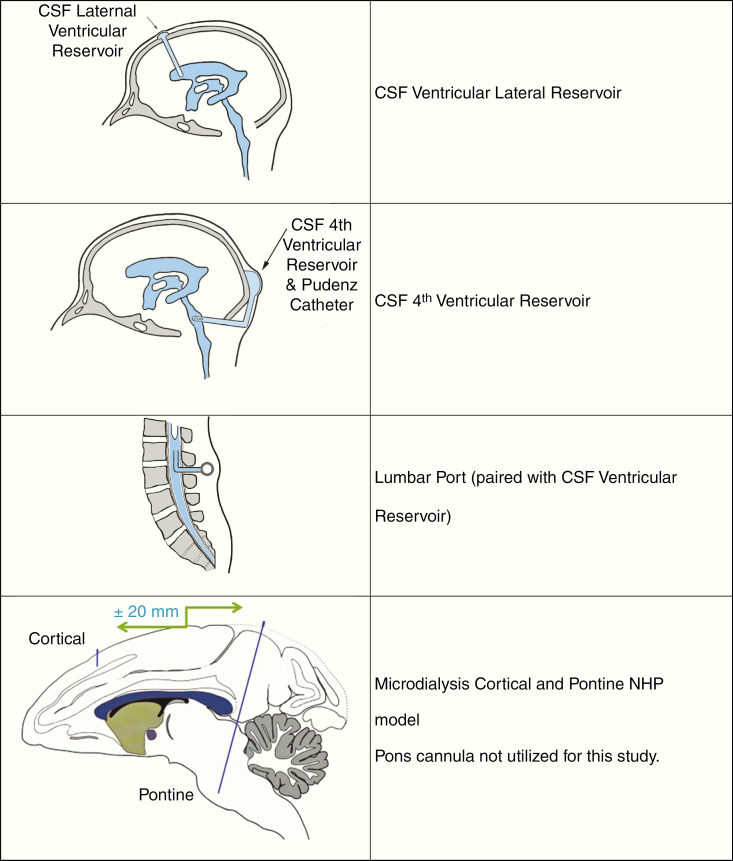
However, the pharmacokinetics (PK) of 5-AZA has not been fully studied to determine the optimal route of administration to maintain effective drug exposure in the CNS. It is known that following administration, 5-AZA is rapidly incorporated into the cell via nucleoside transporters where it is deaminated by cytidine deaminase and phosphorylated by uridine–cytidine kinase becoming a substrate for RNA and DNA.1 Its antitumor effects include multiple mechanisms. A cytotoxic effect is produced via incorporation into RNA, resulting in RNA disruption and cell death.1 De novo pyrimidine synthesis is decreased, limiting cancer cell growth by the inhibition of orotidine 5′ phosphate decarboxylase.1,13–15 Additionally, 5-AZA is a DNA methyltransferase inhibitor resulting in hypomethylation and the elimination of transcription silencing.9,16 To evaluate the potential of 5-AZA as a therapeutic option for CNS tumors including HGG and ependymoma, the cerebrospinal fluid (CSF) and CNS tissue penetration of the agent was assessed in 3 nonhuman primate (NHP) models including Ventricular CSF Reservoir,17 Lumbar Port,18 and Microdialysis (MD)19 models, which have been previously described and are predictive of PK and tolerability in human patients.20
Materials and Methods
RegulatoryThe National Cancer Institute Animal Care and Use Committee approved this study. The NHPs were socially housed, when possible, and cared for in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, eighth edition.21
AnimalsIn total, 10 male rhesus macaques (Macaca mulatta), weighting 8.0–14.1 kg, were utilized for this study; 7 were used for pharmacokinetic studies only and 3 were used for MD/PK with concurrent tissue studies. Where possible, the NHP served as their own control in serial studies.
Models
Pharmacokinetic.
Seven animals that had been previously developed as NHP Ventricular CSF Reservoir and Lumbar Port models were utilized.17,18 All animals had jugular and femoral ports for intravenous (IV) access with CSF access as the following: a lumbar port (n = 1) or CSF reservoir (lateral n = 2) or a combination of CSF reservoir (lateral n = 3, fourth n = 1) and lumbar port as depicted in Figure 1.
Figure 1.
NHP ventricular CSF reservoir, lumbar port, and microdialysis models. NHP, nonhuman primate; CSF, cerebrospinal fluid.
Microdialysis.
Three animals that had previously been developed as the NHP MD model were utilized.19 The model is shown in Figure 1. In this study sampling occurred from cortical cannula alone.
Study clearance and monitoring.
Prior to drug administration, all NHPs were assessed to be physiologically and neurologically within normal limits, as determined by veterinary physical examination, neurological assessment, and blood chemistries with complete blood counts. Following 5-AZA administration, the animals were observed for clinical and neurological complications daily. Clinical chemistries and complete blood counts were collected twice weekly for 2 weeks. A minimum of 2 weeks was required before proceeding to the next study for animals acting as their own control.
Agents.
5-AZA as the FDA-approved formulation, Vidaza (Celgene Corp.) and Inulin, USP grade for injection (Biophysics Assay Laboratory, Inc.).
Pharmacokinetic studies
General: The animals were sedated with ketamine (10 mg/kg, Zetamine; VetOne). The CSF reservoir, lumbar port, and jugular and femoral IV ports were aseptically prepared and utilized for drug administration and sampling. Preadministration plasma and CSF samples were collected. The NHP was recovered to a perched position for restraint via the pole and collar system,22 for drug administration when required, and for sample collection. Environmental enrichment in the form of human interaction, food treats, and television was provided during the restraint period.
Dosage, routes of administration, and sampling.
Dosages, administration and sampling sites, and collection times are outlined in Table 1. Unless otherwise stated, paired serial plasma and CSF samples were collected.
Table 1.
Study Design—Routes of Administration and Sample Times
| Study Type | Administration Route | Evaluable Subjects | Dose (total mg) | Route | Collection Site | Sampling Times (Plasma and CSF) | |
|---|---|---|---|---|---|---|---|
| Plasma | CSF | ||||||
| PK | IV (n = 4) | 1, 2, 3 | 3.75 mg/kg (HED = 75 mg/m2) (36.99–46.5 mg) | IV port— jugular | IV port | Lumbar port or CSF reservoir | 0, 5, 30, 35, and 45 min and 1, 2, 3, 4, 6, 8, 24, and 48 h |
| PK | 5-AZA IT-V (n = 5) | 2, 4, 5, 6 | 10 mg | CSF reservoir | IV port | Lumbar port | 0, 5, and 30 min and 1, 2, 3, 4, 6, 8, 10, and 24 h |
| PK | 5-AZA IT-L (n = 5) | 2, 4, 5 | 10 mg | Lumbar port | IV port | CSF reservoir | 0, 5, and 30 min and 1, 2, 3, 4, 6, 8, 10, and 24 h |
| PK | 5-AZA + inulin IT-V (n = 3) | 4, 5, 6 | 10 mg 5-AZA, 2 mg inulin | CSF reservoir | IV port | Lumbar port | Plasma: 0, 2, 4, 6, 8, and 10 h CSF: 0, 5, and 30 min and 1, 2, 3, 4, 6, 8, 10, and 48 h |
| MD/PK | IT-C (n = 3) | 8, 9,10 | 10 mg | Cisterna magna | Catheter | Subarachnoid catheter | 0, 5, and 30 min and 1, 2, 3, 4 h Cortical ECF 0–4 h Tissue (Brain, liver, and kidney) |
CSF, cerebrospinal fluid; PK, pharmacokinetics; IV, intravenous; HED, human equivalent dose; 5-AZA, 5-azacytidine; MD, microdialysis; IT-V, intraventricular; IT-L, intralumbar; IT-C, intracisternal.
The intrathecal (IT) dosage was 25% of the total mean IV dose rounded to the nearest milligram.
Routes of Administration
Intravenous.
5-AZA was delivered as a 30-min infusion via the IV jugular port. The administration was accomplished while the animals were un-sedated but restrained. Plasma sampling occurred via the IV femoral port. CSF samples were collected from the lumbar port (n = 1) or the CSF ventricular reservoir (lateral n = 3).
Intraventricular (IT-V).
5-AZA was infused over 1 min in a volume of 1.0 ml via the CSF ventricular reservoir (lateral n = 4 and fourth n = 1) during ketamine sedation, following equivalent volume removal of CSF (1.0 ml). The combination of 5-AZA and inulin was delivered in a 1.2 ml volume. Plasma sampling occurred via an IV port. Lumbar CSF samples were collected from the lumbar port.
Intralumbar (IT-L).
Administration was accomplished via the lumbar port during ketamine (10 mg/kg intramuscular [IM]) and Dexdomitor/dexmedetomidine hydrochloride (7.5–15 mg/kg IM; Zoetis) sedation, over 1 min following equivalent volume removal of CSF. Post-administration, the animals were placed in the Trendelenburg position for 45–60 min and recovered from sedation with Anti-Sedan/atipamezole hydrochloride (15 mcg/kg IM; Zoetis). Plasma sampling occurred via an IV port. Ventricular CSF samples were collected from the CSF reservoir (lateral n = 3, fourth n = 1).
Sample preservation and handling.
Whole blood (3 ml) and CSF (300 µl) were collected. All blood samples were collected into sodium heparin tubes, immediately spun at 3000 rpm for 5 min, and the resulting plasma volume was decanted into the collection vial.
Collection vials were preloaded with 100 µM tetrahydrouridine (THU), a 5-AZA stabilization agent. THU collection tubes were protected from light and chilled until utilized at each sample collection. Sodium heparin tubes were preloaded with 30 µl and the CSF collection vials with 3 µl THU. Plasma and CSF samples were stored at −80°C until analysis.
MD/pharmacokinetic Studies—intrathecal-cisternal (IT-C) administration
MD cortical probe access: The animals were anesthetized (ketamine [10 mg/kg IM], glycopyrolate [0.01 mg/kg IM; American Regent, Inc.], and propofol [4 mg/kg IV; Fresenius Kabi LLC]) and intubated for surgical access to the MD cannula. The animals were placed in ventral recumbency and the head centered in a stereotactic unit (David Kopf Instruments). Utilizing sterile procedures the cortical cannula was accessed via a skin incision and a sterile CMA 11, 8 mm probe consisting of a cuprophane membrane (Harvard Apparatus) was inserted into the cortical tissue for tissue acclimation, retrodialysis, and MD sample collection.19 For plasma sample collection an IV catheter was placed in the saphenous vein. A catheter was placed contralateral to the MD cannula for the collection of subarachnoid CSF.23
Tissue acclimation.
The cortical tissue was acclimated to the CMA 11 probe by perfusing blank CMA CNS perfusion fluid (M Dialysis) via a Model 106 syringe pump (M Dialysis) at 3 µl/min for 1 h. The resulting extracellular fluid (ECF) dialysate was collected as a presample.
Retrodialysis.
The MD probe was calibrated via retrodialysis. This is an agent-dependent method that determines the in vivo recovery of the probe in the specific tissue for each study. Therefore, the perfusate, at a predetermined 5-AZA concentration of 15 ng/ml, was perfused via the 106 syringe pump at 3 µl/min for 2 h and the resulting ECF dialysate was collected at the end of retrodialysis and frozen at −80°C. The 5-AZA perfusate was also collected. The cortical probe was then flushed at 15 µl/min for 5 min with blank CMA CNS perfusion fluid and the resulting dialysate discarded.
5-AZA administration.
Following the cortical probe flush, the NHP received the agent as a 1.0 ml bolus via cisternal injection following an equivalent volume removal of CSF.
Microdialysis.
Following 5-AZA administration the perfusate was changed to blank CMA CNS perfusion fluid and a collection of a single continuous ECF dialysate sample began from cortex with concurrent plasma and CSF sampling.
Concurrent PK sample collection.
Following 5-AZA administration, plasma and subarachnoid CSF23 were collected concurrently with cortical ECF dialysate.
Tissue collection.
After sampling, the animals were exsanguinated, CSF volume removed via cisternal tap, and the animals euthanized (Euthanasia Solution, 1.0 ml/4.5 kg IV; VetOne). The brain, cortex (sectioned in 12 slices), cerebellum, pons and medulla, liver, and kidney were collected.
MD sample preservation and handling.
The 5-AZA pharmacokinetic samples were collected, preserved, and handled as previously described.
To eliminate volume interference with the smaller Retrodialysis and MD sample size, the collection vials were prepared with 100 µM of dried THU.
Plasma, CSF, and ECF samples were stored at −80°C until analysis. Tissue samples were flash frozen upon collection.
Sample Analysis
Quantification.
5-AZA was quantified via ultra-high-performance liquid chromatography with tandem mass spectrometric detection (uHPLC-MS/MS) assay and validated in plasma and CSF, with a dynamic range of 0.02–4.09 µM and dilution capabilities to 20.47 µM. The range for MD cortical ECF assay was 0.041–40.95 µM with dilution capabilities to 204.74 µM. The range for the tissue sample assay was 100–25 000 pg/mg. Plasma and CSF samples were extracted using a 2-step extraction. Step 1 involved liquid extraction with acetonitrile (ACN) and re-suspension in 0.1 N HCL. Step 2 utilized a cation-exchange solid-phase extraction from the acidified extract of step 1 to further purify the sample prior to measurement by uHPLC-MS/MS. MD samples were extracted using a 1-step protein precipitation extraction with ACN. Tissue samples were weighed then homogenized in ACN and then extracted using a similar 2-step extraction as plasma and CSF. All samples were run alongside a calibration curve, in duplicate, and quality control standards at a low, mid, and high level, each level is done in quintuplet.
Pharmacokinetic analysis.
A noncompartmental analysis was performed to obtain pharmacokinetic parameters for 5-AZA in plasma and CSF using Phoenix WinNonlin 6.4® (Certara). The maximum concentration (Cmax) and time to Cmax (Tmax) were recorded as observed values. The area under the plasma concentration versus time curve was calculated using the linear-up/log-down trapezoidal rule to time infinity (AUCINF). The elimination rate (Kel) was calculated as the slope of the best-fit line drawn through a minimum of 3 terminal concentration points. Half-life (HL) was calculated as the natural log of 2 (ln2) divided by Kel. Plasma and CSF clearance (CL) was calculated as dose/AUCINF. Plasma CL (l/m2/h) was normalized to body surface area (BSA). The BSA was calculated as NHP body weight/Km. A Km of 20 was used to calculate BSA (Km is the mean body weight/estimated surface area;12 kg/0.06 m2).24 As the volume of CSF is not physiologically dependent on body weight, the CSF CL was not normalized to weight.25
Results
The pharmacokinetic value ranges for 5-AZA across studies are given in Table 2.
Table 2.
PK Concentration Ranges
| Study | Route | Sample Collected | AUC (h*µM) | HL (h) | C max (µM) | T max (h) | CL |
|---|---|---|---|---|---|---|---|
| 5-AZA PK | IV | Plasma | 2.4–3.2 | 0.25–0.29 | 4.6–7.6 | 0.5 | 95.4–130.0 l/h/ m2 |
| Ventricular CSF | 3 samples quantifiable (n = 2) Not detectable (n = 1) | ||||||
| IT-V | Plasma | 0.17–0.44 | 0.52–1.4 | 0.15–0.33 | 0.08–1.0 | ||
| Lumbar CSF | 1234.6–5368.4 | 0.49–0.91 | 1035.3–3015.7 | 0.45–1.1 | 0.13–0.55 ml/ min | ||
| IT-L | Plasma | 0.24–0.74 | 0.28–1.8 | 0.16–1.54 | 0.08–0.5 | ||
| Ventricular CSF | 7.5–19.3 | 0.52–0.84 | 6.1–9.8 | 0.5–1.1 | 35.3–91.0 ml/ min | ||
| 5-AZA MD/PK | IT-C | Plasma | 0.14–0.18 | 1.1–1.3 | 0.06–0.10 | 0.50 | |
| Subarachnoid CSF | 187.6–1313.6 | 0.7–1.3 | 57.5–753.9 | 0.5–2.0 | 0.52–3.6 ml/ min | ||
| 5-AZA and inulin PK | IT-V | Plasma | 2 samples quantifiable (n = 2) at 2 h Not detectable (n = 1) | ||||
| Lumbar CSF | 1108.91–2747.94 | 0.75–2.2 | 434.0–1820.64 | 0.32–1.0 | 0.25–0.62 ml/ min |
CSF, cerebrospinal fluid; PK, pharmacokinetics; IV, intravenous; AUC, area under the curve; HL, half-life; CL, clearance; 5-AZA, 5-azacytidine; MD, microdialysis; IT-V, intraventricular; IT-L, intralumbar; IT-C, intracisternal.
A mean CSF flow rate of 0.018 ± 0.003 ml/min was determined by IT-V administration of inulin.
PK Studies—All Routes of Administration
Intravenous.
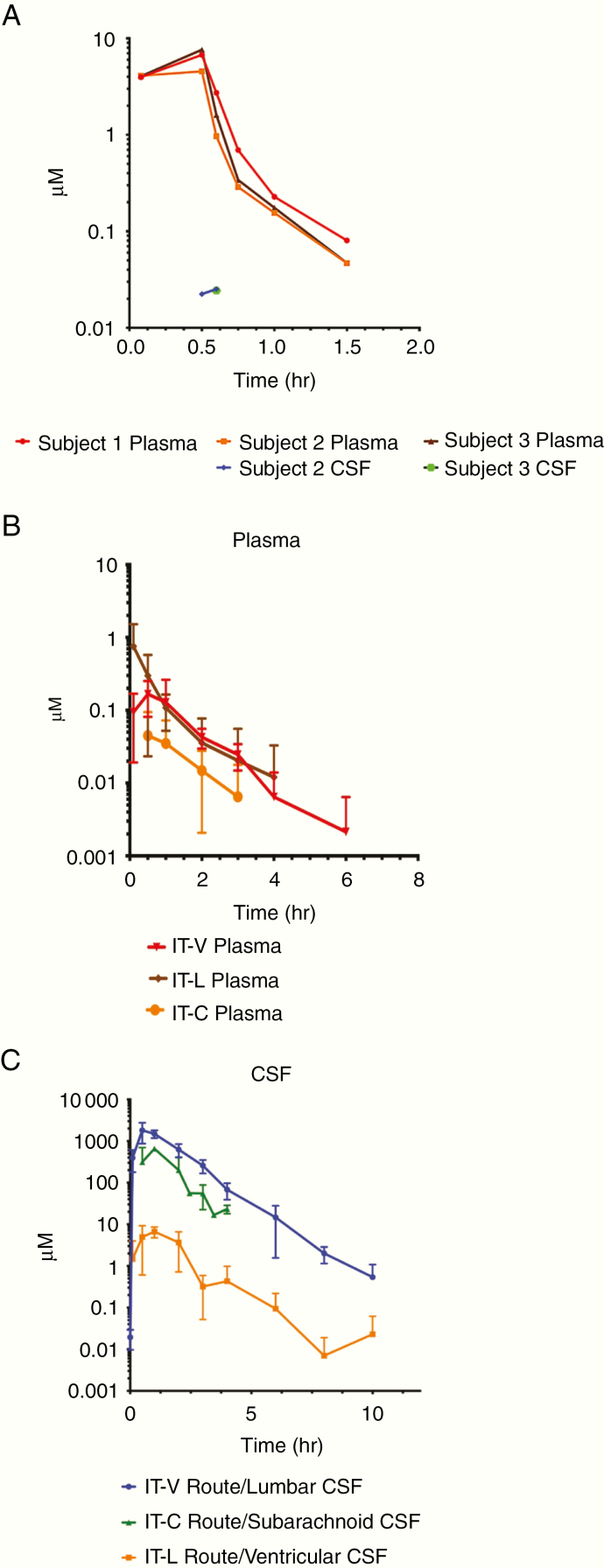
Three of the 4 NHPs who received 5-AZA as a 30-min IV infusion were evaluable. One NHP was unevaluable due to THU degradation in the sample tubes resulting in negligible 5-AZA levels in the plasma. The agent was quantifiable in the plasma of the 3 evaluable studies. The study sample collection time was 0–24 h with a mean duration of quantifiable samples in plasma of 1.5 ± 0.0 h. CSF exposure was indeterminable as concentrations in CSF were below the limit of quantitation in most samples precluding the determination of pharmacokinetic parameters. A total of 3 samples in 2 studies in the 24-h collection period: Subject 2: 30-min end of infusion (EOI)—0.022 µM and 5 min post-EOI—0.025 µM and Subject 3: 30-min EOI—0.024 µM. 5-AZA was not detectable in lumbar CSF for Subject 1. Individual plasma and CSF concentrations are depicted in Figure 2A. The CSF exposure range of 5-AZA following IV administration, at a clinically relevant dose, was negligible and insufficient to allow determination of pharmacokinetic values. To evaluate the CSF exposure and tolerability of 5-AZA with IT administration the agent was studied in the NHP CSF Reservoir with the Lumbar Port model permitting simultaneous access to both ventricular and lumbar CSF.
Figure 2.
(A) Individual subject plasma and CSF 5-AZA concentrations following IV administration. (B and C) Mean plasma and CSF 5-AZA concentrations for all routes of IT administration. 5-AZA, 5-azacytidine; CSF, cerebrospinal fluid.
Intraventricular.
5-AZA was administered as a single agent (n = 5 infused; n = 4 evaluable) as an IT-V bolus into the CSF reservoir and CSF was collected via the lumbar port. One NHP was unevaluable due to a clinical complication unrelated to the study. The study sample collection time was 0–24 h with a mean duration of quantifiable samples of 4 h + 1.41 in plasma and 9 ± 1.15 h in CSF. Mean plasma and CSF concentrations for the evaluable animals are depicted in Figure 2B and C. As expected the CSF exposure range of 5-AZA increased with IT-V administration resulting in an AUC range of 1234.6 to 5368.4 h*µM and HL of 0.52–1.4 h. Plasma concentrations of 5-AZA were quantifiable with a markedly lower exposure than IV administration.
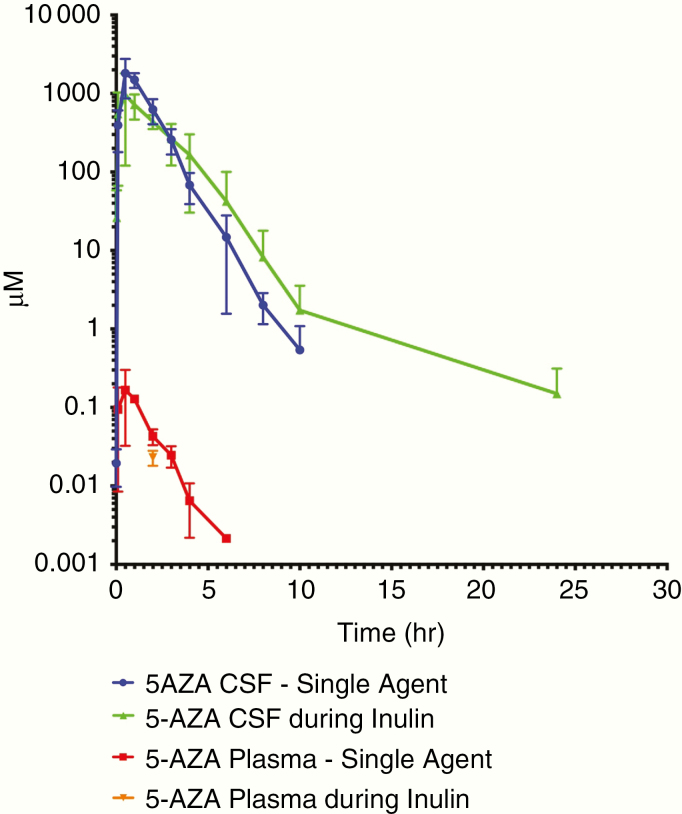
To evaluate the CL of 5-AZA from the ventricular CSF space, 3 NHPs received 5-AZA in combination with inulin, as inulin CL has previously been shown to correlate with CSF flow.26 All animals were evaluable. The mean CSF flow rate as determined by IT-V administration of inulin is 0.018 ± 0.003 ml/min.27 The 5-AZA ranges of CL rates are given in Table 2. Plasma exposure was indeterminable as the majority of plasma concentrations were below the limit of quantitation precluding the determination of pharmacokinetic parameters. Two NHPs had a single quantifiable plasma level at 2 h: Subject 4: 0.038 µM and Subject 6: 0.031 µM. The study CSF sample collection time was 0–48 h with a mean duration of quantifiable CSF samples of 19.3 ± 8.0 h (Figure 3). All pre- and post-neurological assessments were within normal limits for IT-V and IT-L administration. The CSF CL range was minimally changed between 5-AZA administered alone (range 0.13–0.55 ml/min) and 5-AZA in combination with inulin (range 0.25–0.62 ml/min), but notably exceeded the CSF mean flow rate of 0.018 ml/min. The CSF exposure range of 5-AZA was effected by co-administration with inulin. The CSF AUC range was decreased to 1108.91–2747.94 h*µM and the CSF HL was extended to 0.75–2.2 h increasing the duration of a single dose. Additionally, the plasma exposure of 5-AZA became indeterminable with only 2 quantifiable plasma samples in 2 animals.
Figure 3.
Mean plasma and CSF 5-AZA concentrations alone and in combination with inulin following intraventricular administration. 5-AZA, 5-azacytidine; CSF, cerebrospinal fluid.
The PK of 5-AZA during IT-V administration was characterized by increased CSF exposure and tolerability. Of import for this translational study was the evaluation of 5-AZA PK following IT-L administration as this route of administration is frequently utilized for IT drug delivery in patients.
Intralumbar.
Five NHPs received the agent as an IT-L bolus injection via the lumbar port with ventricular CSF sampling via the reservoir; 3 were evaluable. Two NHPs were unevaluable due to a clinical complication unrelated to the study (n = 1) and a malfunction of the lumbar port during administration of the agent (n = 1). The study sample collection time was 0–24 h with a mean duration of quantifiable samples for plasma of 2.33 ± 1.53 h and CSF of 6 ± 2.0 h. Mean plasma and CSF concentration of the evaluable animals are depicted in Figure 2B and C. The CSF exposure range of 5-AZA is higher following IT-L (range 7.5–19.3 h*µM) versus IV administration, but notably lower than IT-V administration. The CSF CL range (35.19–91.0 ml/min), following IT-L administration, is the highest of the 3 IT routes of administration. The plasma exposure range of 5-AZA was similar between IT-L and IT-V administration.
In an effort to correlate the CSF exposure with CNS tissue levels of 5-AZA following IT administration the NHP MD model was utilized to collect and quantify ECF, brain, kidney, and liver tissue levels as well as plasma and CSF in a concurrent PK study.
MD/pharmacokinetic study with IT-C administration.
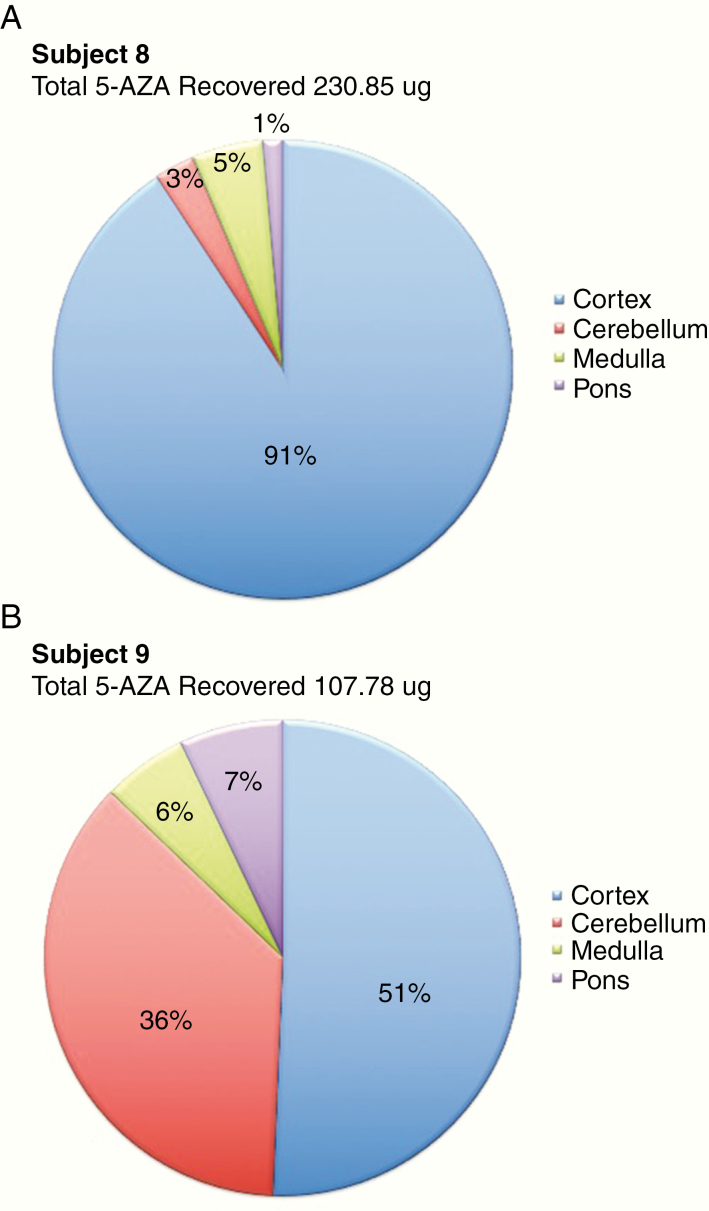
Three NHPs received 5-AZA via cisternal bolus injection (IT-C) with subarachnoid CSF sampling via a catheter. All animals were evaluable for MD and concurrent PK. For the MD analysis, the cortical pre-perfusate and dialysate ECF concentrations collected during tissue acclimation of the probe were undetectable for the agent. The mean retrodialysis perfusate analyzed concentration was 15.36 ng/ml; a 97.7% accuracy of the targeted retrodialysis perfusate concentration of 15 ng/ml. There were no detectable concentrations of the agent in the cortex for the retrodialysis (2-h collection period) or MD dialysate ECF (4-h collection period). For the pharmacokinetic analysis, the study sample collection was 0–4 h with a mean duration of quantifiable samples for plasma of 2.33 ± 0.58 h and CSF of 4 ± 0.0 h. The mean plasma and CSF concentration are shown in Figure 2B and C. Brain, cortex (mean weight 6.46 ± 0.26 g/section), cerebellum (mean weight 16.9 ± 0.59 g), medulla (mean weight 2.8 ± 0. 31 g) and pons (mean weight 5.31 ± 0.46 g), kidney (180 ± 3.6 g), and liver were collected for 2 NHPs and were evaluable for 5-AZA concentrations. Four hours post-administration, a mean percentage of 1.69% ± 0.87% of total 5-AZA dose of 10 mg was recovered in the brain. 5-AZA concentrations in the liver and kidney were not detectable. The individual tissue concentration percentages for the 2 NHPs are depicted in Figure 4A and B. The plasma and CSF pharmacokinetic value ranges for 5-AZA for IT-C administration are given in Table 2. The plasma exposure range was indeterminable being without sufficient quantifiable concentrations to determine a pharmacokinetic value. Two plasma samples were quantifiable at 2 h in 2 of the 3 animals. The CSF exposure range continued to be higher with IT-C (187.6–1313.6 h*µM) versus IV administration and resulted in a middle range between IT-V and IT-L administration.
Figure 4.
Percentage of recovered 5-AZA in brain tissue concentrations per subject at 4 h post-intracisternal administration: (A) Subject 8 and (B) Subject 9. 5-AZA, 5-azacytidine.
Observed clinical events
Where possible, all adverse events were graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE v4.03).28
5-AZA Single Agent Administration
IV and IT-L.
No adverse clinical events occurred.
Intraventricular.
Three NHPs experienced emesis 25 min to 48 h post-5-AZA administration and a moderate transient CSF pleocytosis was noted at 24–48 h. Two NHPs had Grade 1 somnolence; 1 NHP had accompanying Grade 2 perioral cyanosis which resolved in 2.5 h. All animals responded to clinical treatment and toxicities resolved to normal. No neurological complications were observed.
5-AZA + Inulin Combination Administration
Intraventricular.
Three NHPs experienced emesis 25 min to 24 h post-administration. Two NHPs had a moderate transient CSF pleocytosis at 48 h. One NHP had a Grade 1 somnolence. All animals responded to clinical treatment and resolved to normal. No neurological complications occurred.
All animals tolerated IT-V and IT-L administration of 5-AZA moderately well.
Discussion
This translational study provides information critical in optimizing clinical trial design for 5-AZA administration to patients with CNS tumors. An in-depth analysis of the PK of 5-AZA, administered systemically and intrathecally, was done in a preclinical NHP model proven, in previous studies, to be predictive of PK in humans.20 We demonstrated that IT delivery is required to achieve pronounced and prolonged, yet tolerable, exposure of 5-AZA in the CNS where CSF drug concentrations were utilized as a surrogate of CNS tissue penetration and validated by cortical ECF and tissue sampling.
Plasma pharmacokinetic values following a 30-min IV infusion of 5-AZA in this animal model are similar to the human plasma pharmacokinetic values following a 10-min IV infusion in patients with myelodysplastic syndrome.29 In this human pharmacokinetic study, the IV administration of 5-AZA also resulted in plasma exposure of short duration (2 h), and similarly, the duration of the agent’s exposure in NHP plasma was 1.5 h following IV administration. The corresponding NHP CSF concentrations, available via this model, demonstrated that IV administration of 5-AZA results in no detectable CSF penetration.
Although prior studies in an IDH1 R132H mutant glioma PDX mouse model demonstrated a response after a systemic intraperitoneal injection of non-pharmacy grade 5-AZA alone and in combination with TMZ,3 this nontumor bearing NHP model, with plasma PK similar to humans, indicates a lack of CSF exposure following IV administration of pharmacy grade 5-AZA. A potential explanation for the dichotomy of results between the 2 preclinical models is the PDX mouse model is tumor bearing whereas the NHP CSF penetration model is nontumor bearing. While the presence of tumor may increase the permeability of the blood-brain barrier (BBB) in the PDX mouse model permitting higher agent concentrations at the tumor site, CSF penetration data generated with a BBB unaffected by tumor in the NHP model are critical to optimal clinical trial design as it is clear that not all gliomas have BBB disruption.30,31 Additionally, patients with glioblastoma and other diffusely infiltrating gliomas32 demonstrate an extended tumor burden beyond the enhancing component.30
5-AZA, in vitro, has reduced proliferation of IDH1-mutated and IDH1 wt glioma cell lines. An IC50 range of 4.4–15.7 µM has been reported for IDH-mutated and 2.7–10.5 µM for IDH1 wt,3 but in vivo studies with 5-AZA in mouse IDH1 and IDH1 wt PDX models, activity was demonstrated against the PDX IDH-mutated tumor alone. Since activity was demonstrated for the IDH1 wt glioma cells in vitro, with a higher inhibitory effect than in the IDH1-mutated glioma cells, this implies a failure of efficacy for 5-AZA in the IDH1 wt model was due to insufficient drug exposure at the tumor site. While other factors may have affected the response of 5-AZA, in the IDH1 wt tumor model versus the PDX IDH1-mutated, a non-disrupted BBB must be considered.
Additionally, BBB function differs between species.33 While murine tumor-bearing models demonstrate efficacy in their species-specific capacity, data comparison with an NHP model, a more predictive higher species, provides a greater understanding of the necessary treatment regimen and potential clinical trial outcome. 5-AZA may have higher CSF penetration with systemic administration in the presence of a disrupted BBB, but systemic administration alone will not result in sufficient exposure of 5-AZA. IT administration of 5-AZA at 25% of the total IV dose yielded concentrations in the IC50 range (IT-L) or well above (IT-V) in this NHP model.
This study investigated the IT administration of 5-AZA in differing CSF spaces or locations as the direction of CSF flow may affect drug concentrations at different levels. Of the IT routes of administration studied, IT-V delivery via the CSF ventricular reservoir achieved the highest CSF exposure, which was greater than IT-L administration via the lumbar port, and greater than IT-C administration.
In addition to CSF flow, CSF turnover may also differ by location. This study demonstrates that the CL of 5-AZA delivered intrathecally is greater than the inulin-determined CSF flow and is not equivalent across the CSF space (ventricular, lumbar, and subarachnoid). The CL of 5-AZA from ventricular CSF during IT-L administration was the highest of the 3 IT routes. The CL of the agent from subarachnoid CSF during cisternal administration was markedly less than CL from the IT-L route of administration. The lowest CSF CL rate from IT delivery resulted from lumbar CSF during IT-V administration. The increased CSF CL of 5-AZA above the CSF flow rate during IT administration may be attributed to cytidine deamination and rapid cellular uptake mediated by human nucleoside transporters: concentrative nucleoside transporter (hCNT) and equilibrative nucleoside transporter (hENT). 5-AZA is transported into the cell by hENT1, 2, 3, and 4 and hCNT2 and 3, with emphasis on hCNT3.34,35 The nucleoside transporter hENT3 is found predominately in the cortex and lateral ventricles.36 The human choroid plexus had been found to express hENT1, 2, and 3 as well as hCNT3.37 Additionally, nucleoside transporters have been identified in the choroid plexus of rhesus animals.38 The MD study with IT-C administration of the agent supports this conclusion, as 5-AZA was not quantifiable in the ECF of the cortex during the retrodialysis calibration of the agent or MD following cisternal administration of the agent. Concurrently sampled subarachnoid CSF and plasma, as well as neural tissue, had quantifiable 5-AZA. The NHP models utilized for these studies confirm that CSF drug penetration is a surrogate for CNS drug tissue penetration for 5-AZA.
The 5-AZA tissue levels are representative of the concentrations at 4 h after cisternal administration. There is intra-animal variability that is attributed to differing times of tissue collection and preservation following euthanasia and necropsy. Concentrations varied markedly in the cerebellum yet independent of the intra-animal variability, the greatest % concentration of 5-AZA was detected in the cortex.
IT administration of inulin with 5-AZA had the unanticipated effect of a marginal decrease in the maximum concentration and total exposure while notably increasing the HL and duration of exposure of 5-AZA by 2-fold. Inulin was administered with 5-AZA as an inert tracer to track the flow of CSF from the ventricles to the lumbar space determining the rate of CSF flow and the mechanism by which this pharmacokinetic effect occurred is unclear and will continue to be studied.
Treatment with 5-AZA has been associated with neurodegeneration in mice39 in this study, we observed only minor acute clinical complications that responded to treatment, resolving to normal with no neurological complications. A recent clinical trial of 5-AZA IT treatment in children with posterior fossa ependymoma concluded that 5-AZA could be delivered via the fourth ventricle without causing neurological toxicity.12
In conclusion, IT delivery, preferably via the IT-V route for tumors involving the cortex, is necessary to yield sufficient CNS exposure and is safe and tolerable in this animal model. Optimizing clinical trial design requires the use of both pharmacokinetic and pharmacodynamic preclinical animal models.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Conflict of interest statement. The authors have no conflict of interest to report.
Authorship Statement:
Conception and design: C.L.M. and K.E.W. Development of methodology: C.L.M., M.L.T., L.T.R., and C.J.P. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C.L.M., R.C., L.T.R., and C.J.P. Analysis and interpretation of data: C.L.M., L.T.R., C.J.P., and K.E.W. Writing, review, and revision of manuscript: C.L.M., L.T.R., R.C., M.L.T., C.J.P., W.D.F., and K.E.W. Administrative, technical, or material support: (ie, reporting or organization data, constructing databases): C.L.M., R.C., L.T.R., and C.J.P. Study supervision: K.E.W.
References
- 1. Leone G, D’Alò F, Zardo G, Voso MT, Nervi C. Epigenetic treatment of myelodysplastic syndromes and acute myeloid leukemias. Curr Med Chem. 2008;15(13):1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borodovsky A, Salmasi V, Turcan S, et al. 5-azacytidine reduces methylation, promotes differentiation and induces tumor regression in a patient-derived IDH1 mutant glioma xenograft. Oncotarget. 2013;4(10):1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamashita AS, da Costa Rosa M, Borodovsky A, Festuccia WT, Chan T, Riggins GJ. Demethylation and epigenetic modification with 5-azacytidine reduces IDH1 mutant glioma growth in combination with temozolomide. Neuro Oncol. 2018;21(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mack SC, Hubert CG, Miller TE, Taylor MD, Rich JN. An epigenetic gateway to brain tumor cell identity. Nat Neurosci. 2016;19(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellor J, Rainier S. IDH1: linking metabolism and epigenetics. Front Genet. 2018;9:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen YC, Gotea V, Margolin G, Elnitski L. Significant associations between driver gene mutations and DNA methylation alterations across many cancer types. PLoS Comput Biol. 2017;13(11):e1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016;18(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanan MI, Underhill DA, Eisenstat DD. Targeting epigenetic pathways in the treatment of pediatric diffuse (high grade) gliomas. Neurotherapeutics. 2017;14(2):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alvarez CM, Starke RM, Komotar RJ, Connolly ES. Reduced H3K27me# is a new epigenetic biomarker for pediatric posterior fossa ependymomas. Neurosurgery. 2017;81(1):N7–N8. [DOI] [PubMed] [Google Scholar]
- 11. Rogers HA, Kilday JP, Mayne C, et al. Supratentorial and spinal pediatric ependymomas display a hypermethylated phenotype which includes the loss of tumor suppressor genes involved in the control of cell growth and death. Acta Neuropathol. 2012;123(5):711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandberg DI, Yu B, Patel R, et al. Infusion of 5-azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior fossa ependymoma: a pilot clinical trial. J Neurooncol. 2019;141(2):449–457. [DOI] [PubMed] [Google Scholar]
- 13. Chiak A. Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30(5):405–422. [DOI] [PubMed] [Google Scholar]
- 14. Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem Rev. 2009;109(7):2880–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poirier S, Samami S, Mamarbachi M, et al. The epigenetic drug 5-azacytidine interferers with cholesterol and lipid metabolism. J Biol Chem. 2014;289(27):18736–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5(2):e9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lester McCully CM, Bacher J, MacAllister RP, et al. Development of a cerebrospinal fluid lateral reservoir model in rhesus monkeys (Macaca mulatta). Comp Med. 2015;65(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 18. MacAllister RP, Lester McCully CM, Bacher J, et al. Minimally invasive lumbar port system for the collection of cerebrospinal fluid from rhesus macaques (Macaca mulatta). Comp Med. 2016;66(4):349–352. [PMC free article] [PubMed] [Google Scholar]
- 19. McCully CM, Pastakia D, Bacher J, et al. Model for concomitant microdialysis sampling of the pons and cerebral cortex in rhesus macaques (Macaca mulatta). Comp Med. 2013;63(4):355–360. [PMC free article] [PubMed] [Google Scholar]
- 20. Baratz E, Lester McCully C, Shih J, Warren KE. Comparison of pharmacokinetic parameters between non-human primates and human patients. Neuro Oncol. 2018;20(Suppl 2):i57. [Google Scholar]
- 21. Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th ed Washington, DC: National Academies Press; 2011. [Google Scholar]
- 22. McCully CL, Godwin KS. The collar and snaphook restraint system for rhesus monkey: a new approach to pole and collar training and access port presentation. Contemp Top. 1992;31(5):14–16. [Google Scholar]
- 23. Bacher JD, Balis FM, McCully CL, Godwin KS. Cerebral subarachnoid sampling of cerebrospinal fluid in the rhesus monkey. Lab Anim Sci. 1994;44(2):148–152. [PubMed] [Google Scholar]
- 24. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerr JZ, Berg S, Blaney SM. Intrathecal chemotherapy. Crit Rev Oncol Hematol. 2001;37(3):227–236. [DOI] [PubMed] [Google Scholar]
- 26. Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5(2):202–207. [DOI] [PubMed] [Google Scholar]
- 27. Lester McCully CM, Rodgers L, Cruz R, Thomas MT III, Peer C, Figg WD, Warren KE. Rhesus macaque (Macaca mulatta) cerebrospinal fluid flow rate and volume determined by plasma and cerebrospinal fluid pharmacokinetics following intraventricular administration of inulin #P228. Abstracts of Scientific Presentations 2018 AALAS National Meeting Baltimore, Maryland. J Am Assoc Lab Anim Sci. 2018;57(5):534–642. [PMC free article] [PubMed] [Google Scholar]
- 28. National Cancer Institute. Common Terminology Criteria for Adverse Events. NIH Publication No 09-5410; Revised June 2010. [Google Scholar]
- 29. Marcucci G, Silverman L, Eller M, Lintz L, Beach CL. Bioavailability of azacitidine subcutaneous versus intravenous in patients with the myelodysplastic syndromes. J Clin Pharmacol. 2005;45(5):597–602. [DOI] [PubMed] [Google Scholar]
- 30. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warren KE. Beyond the blood:brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S. Morphologic patterns of noncontrast-enhancing tumor in glioblastoma correlate with IDH1 mutation status and patient survival. J Clin Neurosci. 2018;47:168–173. [DOI] [PubMed] [Google Scholar]
- 33. Nicolas JM. Species Differences and Impact of Disease State on BBB. In: Di, L, Kerns, EH, eds. Blood–Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs. John Wiley & Sons, Inc.; 2015. [Google Scholar]
- 34. Damaraju VL, Mowles D, Yao S, et al. Role of human nucleoside transporters in the uptake and cytotoxicity of azacitidine and decitabine. Nucleosides Nucleotides Nucleic Acids. 2012;31(3): 236–255. [DOI] [PubMed] [Google Scholar]
- 35. Hummel-Eisenbeiss J, Hascher A, Hals PA, et al. The role of human equilibrative nucleoside transporter 1 on the cellular transport of the DNA methyltransferase inhibitors 5-azacytidine and CP-4200 in human leukemia cells. Mol Pharmacol. 2013;84(3):438–450. [DOI] [PubMed] [Google Scholar]
- 36. Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters—a review. Nucleosides Nucleotides Nucleic Acids. 2017;36(1):7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Redzic ZB, Malatiali SA, Grujicic D, Isakovic AJ. Expression and functional activity of nucleoside transporters in human choroid plexus. Cerebrospinal Fluid Res. 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Washington CB, Giacomini KM, Brett CM. Nucleoside transport in isolated human and rhesus choroid plexus tissue slices. Pharm Res. 1998;15(7):1145–1147. [DOI] [PubMed] [Google Scholar]
- 39. Subbanna S, Nagre NN, Shivakumar M, Basavarajappa BS. A single day of 5-azacytidine exposure during development induces neurodegeneration in neonatal mice and neurobehavioral deficits in adult mice. Physiol Behav. 2016;167:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]