Abstract
Multifunctional thin films which can display both photocatalytic and antibacterial activity are of great interest industrially. Here, for the first time, we have used aerosol-assisted chemical vapor deposition to deposit highly photoactive thin films of Cu-doped anatase TiO2 on glass substrates. The films displayed much enhanced photocatalytic activity relative to pure anatase and showed excellent antibacterial (vs Staphylococcus aureus and Escherichia coli) ability. Using a combination of transient absorption spectroscopy, photoluminescence measurements, and hybrid density functional theory calculations, we have gained nanoscopic insights into the improved properties of the Cu-doped TiO2 films. Our analysis has highlighted that the interactions between substitutional and interstitial Cu in the anatase lattice can explain the extended exciton lifetimes observed in the doped samples and the enhanced UV photoactivities observed.
Keywords: photocatalysis, antibacterial, thin films, TiO2, CVD, hybrid-DFT
Introduction
Anatase TiO2 is the leading chemically and biologically stable, inexpensive semiconductor with photosensitive properties for applications in photocatalysis and antibacterial surfaces.1−9 For both applications, the general mechanisms for the functional properties are very similar;3,10 when TiO2 is irradiated with photons with energy greater than or equal to the band gap energy, electrons from the valence band are promoted to the conduction band and concomitant holes are formed in the valence band. These electrons and holes can migrate to the surface of the semiconductor and react with water and oxygen to produce reactive oxygen species (ROS).10,11 These ROS can oxidize a wide range of organic species, including pollutants, bacteria, cancer cells, and viruses. In the case of bacteria, the ROS produced cause an oxidative stress on bacterial cells that destroy cell walls, DNA, and proteins.10 This form of antibacterial activity and decontamination of surfaces is innovative as it provides effective relief against bacteria that have grown resistant to many conventional antimicrobial agents, in part due to decades of inappropriate antibiotic use.12,13 Applying such coatings on high contact touch surfaces such as door handles and keyboards in hospital and health care environments has shown a marked reduction of health care-associated infections.14
ROS are destructive not only to bacteria but also to organic pollutants that can be found in air and water. In fact, the primary application of TiO2 as a photocatalyst is as a self-cleaning transparent coating on windows to assist in the breakdown of dirt.1 The incorporation of dopant quantities of Cu into the TiO2 matrix has dual advantages. Not only can Cu be used as an antibacterial agent by itself, through Fenton-type reactions, but TiO2:Cu can also potentially reduce the recombination of charge carriers which are important for both antibacterial and self-cleaning applications of TiO2.15−19
Cu-doped TiO2 has been prepared via solution17−22 and nonsolution23-based routes in nanoparticulate forms mainly for photocatalytic and ferromagnetic applications. Sol–gel techniques24 and physical vapor deposition methods such as magnetron sputtering25 have also been used to fabricate thin films.
In this paper, we demonstrate for the first time the synthesis of Cu-doped TiO2 films using an aerosol-assisted chemical vapor deposition (AACVD) method. AACVD is a facile technique that places only the limitation of solubility on the precursors, that is, there are no volatility concerns as with traditional CVD, thus allowing a wider range of precursors to be employed.26−31 It is also an atmospheric pressure technique that has been used to grow a wide range of films for various applications with the potential for upscale.32−35
Both pure and Cu-doped TiO2 films were prepared via AACVD, and their photocatalytic activity to the degradation of a model organic pollutant, stearic acid, and antibacterial activity to bacteria Escherichia coli and Staphylococcus aureus was examined under 365 nm radiation and compared. Cu-doped TiO2 films showed enhanced photocatalytic [formal quantum efficiency (FQE) of 1.1 × 10–4 molecules photon–1 for 5% Cu-doped TiO2] and antimicrobial activities (destruction of E. coli and S. aureus to almost undetectable levels under 4 h of 365 nm irradiation) compared with pure TiO2. Transient absorption spectroscopy (TAS) studies showed that photogenerated charge carriers were longer-lived in Cu-doped TiO2 films. Hybrid functional ab initio density functional theory (DFT) calculations were also carried out in order to determine the mechanism for the enhancement in photocatalytic activities and charge carrier lifetimes that Cu-doped anatase provides.
Experimental Methodology
All chemicals used were purchased from Sigma-Aldrich Chemical Co. and used as received. Deposition was carried out on an 150 × 45 × 4.5 mm SiO2 (50 nm)-coated float glass (SiO2 acts as a barrier layer preventing the diffusion of ions from within the glass into the deposited film) which has been supplied by Pilkington NSG. Prior to use, the glass substrates were thoroughly cleaned using acetone (99%), isopropanol (99.9%), and distilled water and dried in air.
Deposition Procedure
Deposition was carried out under N2 (BOC Ltd., 99.99% purity) flow using titanium isopropoxide [Ti(OCH(CH3)2)4] (99%), copper nitrate (hydrated), [Cu(NO3)2·3H2O] (99%), and ethyl acetate (99%).
[Ti(OCH(CH3)2)4] (0.5 g, 1.76 mmol) was dissolved in ethyl acetate (30 mL) in a glass bubbler. The resulting solution was atomized using a piezoelectric device (Johnson Matthey Liquifog). For the Cu-doped TiO2 films, [Cu(NO3)2·3H2O] (2, 5, 10, and 20 mol % relative to [Ti(OCH(CH3)2)4]) was added to the [Ti(OCH(CH3)2)4]/ethyl acetate solution. The precursor flow was kept at 1.4 L·min–1. The substrate temperature was 470 °C. After the precursor solution had been transferred, the bubblers were closed, and the substrate was cooled under a flow of N2 to less than 100 °C before it was removed. Coated substrates were handled and stored in air. The coated glass substrate was cut into ca. 1 cm × 1 cm squares for subsequent analysis.
Film Characterization
Powder X-ray diffraction (PXRD) patterns were measured using a modified Bruker-AXS D8 diffractometer with parallel beam optics and a PSD LynxEye silicon strip detector. This instrument uses an unmonochromated Cu Kα source (Kα1, 1.54 Å) operated at 40 kV with a 30 mA emission current. The incident beam angle was set at 1°, and the angular range of the patterns collected was 10° < 2θ < 65° with a step size of 0.05° counted at 1 s/step. Raman spectroscopy was carried out on a Renishaw 1000 spectrometer equipped with a 514.5 nm laser. The Raman system was calibrated using a silicon reference. Scanning electron microscopy (SEM) was performed to determine the surface morphology and film thickness using a JEOL JSM-6301F field emission scanning electron microscope at an accelerating voltage of 5 keV. UV–visible (UV–vis) spectroscopy was performed using a PerkinElmer LAMBDA 950 UV/vis/NIR spectrophotometer over a wavelength range of 300–2500 nm. The spectra were referenced against an air background. X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Kα spectrometer with monochromated Al Kα radiation, a dual beam charge compensation system, and a constant pass energy of 50 eV. Survey scans were collected in the range of 0–1200 eV. High-resolution peaks were used for the principal peaks of Ti (2p), O (2p), Cu (2p), C (1s), and Si (2p). The peaks were modeled using sensitivity factors to calculate the film composition. The area underneath these bands is an indication of the element concentration within the region of analysis (spot size 400 μm). Photoluminescence (PL) spectra from 350 to 800 nm were recorded using an Edinburgh spectrofluorometer equipped with a maximum average power of 5 mW. The excitation wavelength used was 380 nm. The PL signals from 900 to 1600 nm were collected through a Newport Oriel monochromator interfaced with a Newport Merlin lock-in amplifier and detected by a TE-cooled Ge photodetector. The samples were excited using a 532 nm diode-pumped solid-state laser. The excitation power was 70 mW. The PL spectra were recorded under air and at room temperature.
Transient Absorption Spectroscopy
TAS was used to determine exciton lifetimes at room temperature (∼22 °C) from the microsecond to second timescale. Samples were excited using pulsed laser excitation (λ = 355 nm, Opolette 355, pulse width = 6 ns, pulse repetition = 0.8 Hz, laser power = 1.55 mJ·cm–2) through a liquid light guide. A quartz halogen lamp (100 W, Bentham, IL 1) with a stabilized power supply (Bentham, 605) was used as the probe light source. To reduce stray light, scattered light, and sample emission, two monochromators and appropriate optical cutoff filters were placed before and after the sample. The probe light passing through the sample was detected using a Si photodiode (Hamamatsu Photonics, S1722-01). This signal was passed through an amplifier (Costronics Electronics) and then measured using a digital oscilloscope. Decays presented are the average between 100 and 200 laser pulses. Samples were measured inside a gas-tight quartz cell under an argon gas atmosphere.
Functional Testing
The photocatalytic activity was measured by monitoring the photocatalytic decomposition of a model organic pollutant, stearic acid (95%, Sigma-Aldrich). To measure the photocatalytic decomposition of a stearic acid coating, samples were attached to an IR sample holder consisting of an aluminum sheet with a circular hole in the middle. The stearic acid coating was applied from a saturated solution of stearic acid in chloroform (0.05 M) through a dip-coating process. Pilkington NSG Activ glass was used as a benchmark and blank float glass as a control. The breakdown of the C–H bonds in stearic acid was measured using Fourier transform infrared (FTIR) spectroscopy between 2800 and 3000 cm–1 using a PerkinElmer Spectrum RX1 FTIR spectrometer. Measurements were taken at 0, 1, 2, 3, 4, 18, and 23 h intervals with the samples irradiated using a 365 nm UVA lamp.
The C–H bonds in stearic acid absorb at 2958 cm–1 (C–H stretch CH3), 2923 cm–1 (symmetric C–H stretch CH2), and 2853 cm–1 (asymmetric C–H stretch CH2). These peaks can be integrated to give an approximate concentration of stearic acid on the surface using a calibration constant (where 1 A·cm–1 in the integrated area between 2800 and 3000 cm–1 corresponds to approximately 9.7 × 1015 molecules cm–2).36,37 The rate of removal of stearic acid can thus be measured by monitoring the decrease in IR absorbance. The results are typically expressed in terms of FQE, ξ, defined as the number of molecules degraded per incident photon (units, molecule × photon–1). The light source was a black light-bulb lamp, 2 × 8 W (Vilmer-Lourmat). The irradiance of the lamp (I = 3.15 mW cm–2) was measured using a UVX radiometer (UVP).
E. coli (ATCC 25922) and S. aureus (8325-4) strains were maintained by weekly subculture on Brain Heart Infusion (BHI) agar (Oxoid, Basingstoke, UK). Both bacteria were used to inoculate two separate 10 mL aliquots of sterile BHI broths (Oxoid, Basingstoke, UK) and incubated aerobically at 37 °C for 18 h. Bacteria from the overnight culture were harvested by centrifugation at 13,000g for 1 min. The bacteria were then resuspended in phosphate-buffered saline (PBS) (Oxoid, Basingstoke, UK) and again centrifuged at 13,000g for 1 min. Finally, the bacterial pellet was resuspended in PBS before use. The turbidity of the bacterial cell suspension was measured at 600 nm using a spectrophotometer and was adjusted to an optical density, which corresponded to approximately 105 colony-forming units per 25 μL aliquot.
Prior to use, the pure TiO2 and Cu-doped TiO2 slides were cut into 1 × 1 cm sections. A humidity chamber was created to ensure that the suspensions did not dry out. A 25 μL aliquot of the bacterial cell suspension was spread evenly on the surface of each slide and incubated at room temperature (21 ± 2 °C) for the allocated exposure time. For each exposure time (2 and 4 h), triplicate samples were analyzed, and uncoated glass microscope slides were used as a control. The samples were then irradiated for 2 and 4 h using a UVA lamp (Vilber-Lourmat, 2 × 8 W, 365 nm, 0.65 ± 0.23 mW cm–2). A further set of samples (in triplicate) was maintained in the dark for the duration of the irradiation time. Each exposure time was also repeated on two separate occasions.
After incubation, the slides were aseptically transferred to 5 mL of PBS and vortexed for 30 s to release the bacteria into the solution. Serial dilutions of the resulting bacterial suspensions were prepared in PBS, and 100 μL from each dilution was spread onto MacConkey agar (Oxoid, Basingstoke, UK) for E.coli and Mannitol salt agar (MSA, Oxoid Ltd) for S. aureus. Plates were incubated aerobically at 37 °C for 24 h. After incubation, any bacterial colonies were counted, and viable counts of bacteria were calculated.
Theoretical Methodology
All bulk properties, intrinsic defects, and extrinsic dopants together with their respective charge states were simulated using DFT within the plane-wave code VASP.36,38−40 Defect supercells were created from the geometrically optimized bulk parameters of anatase TiO2 using the hybrid HSE06 (Heyd–Scuseria–Ernzerhof)41,42 functional. Hybrid functionals are an improvement on standard DFT functionals, which contain a systematic self-interaction error, thus underestimating band gaps. HSE06 has been shown to provide a reasonably accurate description of the electronic and geometric properties of all the polymorphs of TiO2.2,43−49 In order to describe the interactions between the core and valence electrons, the projector augmented wave (PAW) method50 was used, which mimics an all-electron method but with greater computational efficiency.
A geometry optimization was carried out on the primitive unit cell of bulk anatase TiO2, allowing volume, lattice parameters, and atoms to relax until the forces on all the atoms were less than 0.01 eV Å–1. An accurate geometry and electronic structure was calculated using a 450 eV plane-wave energy cutoff and a Γ-centered 7 × 7 × 5 k-point grid. From the primitive bulk, a 3 × 3 × 2 supercell containing 108 atoms was created, and thus defective variants were made. Geometric optimizations on the supercells were calculated with only the ions allowed to relax until all the forces were less than 0.01 eV Å–1. Γ-centered k-point meshes of 2 × 2 × 2 combined with plane-wave cutoffs of 450 eV were used, and all defect calculations were spin-polarized. It is known that under Ti-rich conditions, Ti2O3 is a limiting phase as seen in other theoretical calculations;51 the geometry and total energy of the relaxed unit cell were calculated using a plane-wave cutoff of 450 eV and a Γ-centered k-point mesh of 8 × 8 × 8. For the Cu chemical potential, three limiting phases were considered: CuO, Cu2O, and Cu where, under O-poor conditions, the chemical potential is limited by the formation of Cu and, under O-rich conditions, by the formation of CuO. A geometry optimization of CuO was calculated using a plane-wave cutoff of 450 eV and a Γ-centered k-point mesh of 6 × 6 × 6, and the lowest energy magnetic configuration was found to be antiferromagnetic, consistent with other first-principles calculations and experiment.52−55 See the Supporting Information for methodology on defect formalism, thermodynamic limits, and optical absorption and emission calculations.
Results and Discussion
Pure and Cu-doped TiO2 films were synthesized from the AACVD reaction of [Ti(OCH(CH3)2)4] and 2, 5, 10, and 20 mol % of [Cu(NO3)2·3H2O] in ethyl acetate at 470 °C with N2 as the carrier gas. All films were translucent with gray/green coloration, particularly the 20 mol % doped film had excellent coverage and well adhered to the substrate, passing the Scotch tape test.56
X-ray Diffraction and Raman Spectroscopy
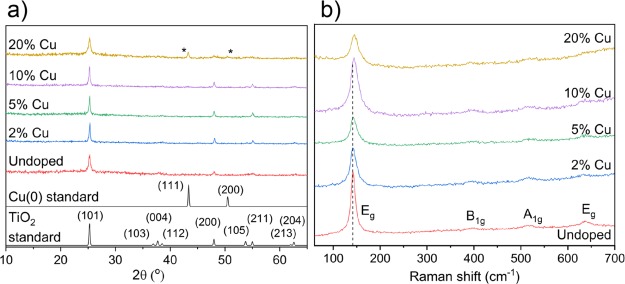
The films were studied using XRD (Figure 1a) to determine whether the desired solid solution had formed. Only reflections for the anatase phase of TiO2 were visible for all films apart from the 20% doped where, as the possible maximum Cu solubility is reached, additional peaks (43.2 and 50.4°) corresponding to the Cu metal were also observed. No noticeable shifting of the anatase Bragg reflections was seen for the Cu-doped films that would be normally expected upon substitutional or interstitial doping because of the difference in ionic radius among Ti(IV) (0.60 Å), Cu(I) (0.77 Å), and Cu(II) (0.73 Å). This does introduce the possibility that the Cu dopant may not be present in the lattice, that is, as a solid solution but actually as a secondary phase(s). However, the lack of TiO2 lattice distortion is explained by the DFT calculations where substitutional Cu doping of anatase is expected to cause minimal distortion of the lattice (see the Defect Thermodynamics section). Raman spectroscopy (Figure 1b) showed only shifts for the tetragonal anatase phase with four active modes visible for all samples including 20%—this is expected as Cu(0) would be Raman inactive. With increasing Cu concentration, the Raman data also showed a small blue shift in the principal Eg vibrational mode (caused by symmetric O–Ti–O stretching vibrations in TiO2 and indicative of unit cell size) of anatase. This indicates an expansion in the anatase unit cell and is in contrast to XRD results where no change was observed. This discrepancy can be explained by the fact that Raman spectroscopy is more surface-sensitive compared to XRD.
Figure 1.
(a) XRD patterns and (b) Raman spectra for the undoped, 2, 5, 10, and 20% Cu-doped TiO2 anatase films grown using AACVD.
Ultraviolet–Visible Spectroscopy and PL
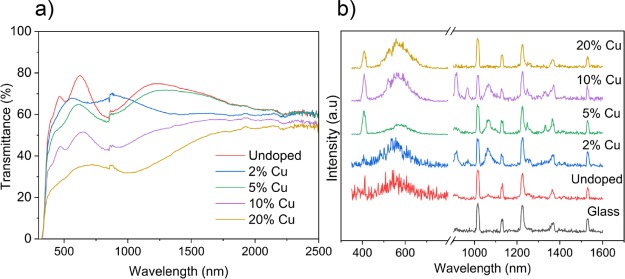
Figure 2a shows the UV–vis spectra for the pure and Cu-doped films. The spectra are quite similar for pure TiO2 and 2% Cu-doped TiO2, with transmittance in the visible region at 70%. The transmittance in the visible region decreases systematically with increasing Cu concentration reaching 30% for the 20% doped film. The indirect optical band gap, calculated using the Tauc plot, remained at 3.3 eV for the pure, 2, 5, and 10% films. For the 20% Cu-doped TiO2 film however, a small shift was observed at 3.2 eV (see the Supporting Information).
Figure 2.
(a) UV–vis spectra encompassing the UV, visible, and near-IR wavelengths and (b) PL spectra for the undoped, 2, 5, 10, and 20% Cu-doped TiO2 anatase films grown using AACVD.
Figure 2b shows that the emission of Cu-doped TiO2 films in the UV–vis and IR regions was carried out by PL. PL spectral emission can be used to study charge carrier recombination and to investigate the efficiency of charge carrier trapping, migration, separation, and transfer in semiconductors.57−59 PL emission spectra result from the radiative recombination of photoexcitation, where a higher PL intensity shows a higher degree of radiative recombination.57,60 There have been examples of materials displaying strong PL and high photocatalytic activity.61
PL emission spectra of Cu-doped TiO2 at different Cu-doping concentrations (2, 5, 10, and 20%) in the wavelength range from 350 to 800 and 900 to 1600 nm with excitation wavelengths of 380 and 532 nm (respectively) are presented in Figure 2b. All the samples have a peak at 380–430 nm, which could be due to band gap, defects, or surface trapping of electrons.60 There are broad peaks from around 500 to 650 nm which intensify with increasing Cu doping, and the peaks located at around 520 nm are thought to be due to a self-trapping of electrons by the TiO6 octahedra.60 In the NIR region, emission peaks with wavelengths of 1010, 1128, 1230, 1340, and 1525 nm are found to originate as normal glass emissions. In undoped anatase TiO2, the nonglass peaks exist as low-intensity peaks, in particular peaks around 920 and 1050 nm are likely due to defect-centered emissions. In 2, 5, and 10% Cu-doped anatase, the peaks present in anatase (nonglass) are observed here albeit more pronounced. In 2, 5, and 10% TiO2:Cu, additional peaks exist such as the emission at 960, 1250, and 1400 nm. 20% Cu, however, appears more like undoped anatase, with the glass peaks dominating the spectrum. To understand these results, theoretical calculations on the PL of Cu-doped anatase are given below (dopant-centered optical properties).
X-ray Photoelectron Spectroscopy
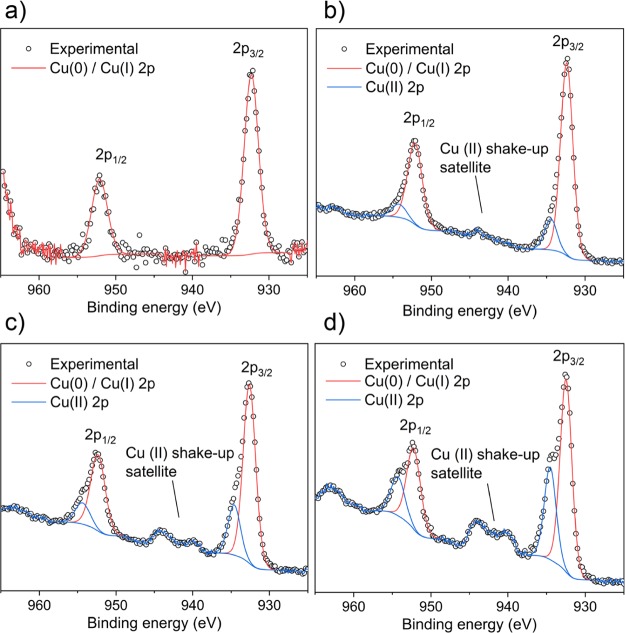
The oxidation state of the Cu dopant on the surface of the films was determined using XPS (Figure 3). In the 2% Cu-doped film, the Cu 2p3/2 peak is centered at 932.3 eV, matching the transition for Cu(0) and/or Cu(I) [because of heavy peak overlap, it is difficult to distinguish between Cu(0) and Cu(I) from the Cu 2p region alone].62,63 The lack of the Cu(II) shake-up satellite peak that is normally found around 945 eV indicates the absence of Cu(II) on this surface. For the higher 5, 10, and 20% doped films, the line shapes of the Cu 2p3/2 and 2p1/2 transitions are asymmetrical and therefore deconvoluted to two different environments. For these films, the principal 2p3/2 peaks are centered at 932.4 eV and matches to Cu(0)/Cu(I). The secondary peaks are centered at 934.5 eV and are assigned to Cu(II).62,63 The presence of Cu(II) here is further supported by the associated satellite peaks at 945 eV. This supports the DFT results which indicate that both Cu(I) and Cu(II) states are expected in both substitutional and interstitial sites in the anatase crystal lattice (see the Defect Thermodynamics section).
Figure 3.
Cu 2p XPS spectra for the (a) 2, (b) 5, (c) 10, and (d) 20% Cu-doped TiO2 anatase films showing the presence of Cu(0), Cu(I), and Cu(II) oxidation states.
The Cu dopant on the surface of the films was determined using the XPS data. For the 0, 2, 5, 10, and 20 mol % solutions used, the concentration of Cu was 0, 22, 30, and 38 at. %, suggesting surface segregation of Cu.
Transient Absorption Spectroscopy
TAS is a form of laser flash spectroscopy that can monitor the generation, recombination, trapping, charge transfer, and so forth of photogenerated charges in semiconductors.64,65 The dynamics of photogenerated charges can be studied by tracking transient changes in absorbance at particular wavelengths,66 where it has been shown that the electrons and holes in anatase TiO2 absorb in the visible region.67,68 The technique has primarily been used to study charge-transfer processes in solar cells (organic–organic or inorganic–organic)69−71 and has also been used to study charge transfers in heterojunction photocatalysts (inorganic–inorganic)72 as well as the kinetics of photocatalytic processes.73−75
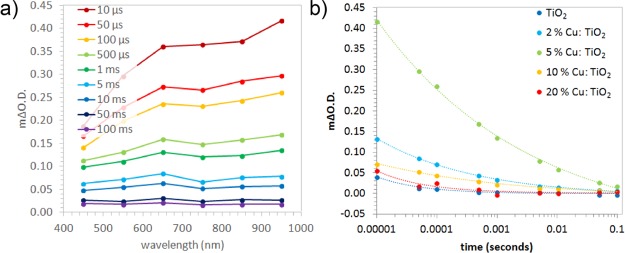
In this study, the kinetics of electron–hole recombination for the series of Cu-doped anatase TiO2 samples were investigated using TAS. Samples were excited using a UVA laser (355 nm, 1.55 mJ·cm–2·per pulse), and the transient changes in absorption were measured from 450 to 950 nm. Previous studies have shown that the photogenerated electrons and holes in anatase show broad absorptions centered at ∼800 and 450 nm, respectively.67 An example TAS spectrum of the 5% Cu-doped TiO2 sample is shown in Figure 4a, which shows an extended absorption across the visible typical of anatase TiO2. The decay kinetics did not change with probe wavelength. This showed that charge-transfer processes were not occurring within the material. For instance, it has typically been cited that phase-segregated metal dopants in TiO2, such as Cu, may act as electron sinks.76 If Cu was indeed acting as an electron sink, our TAS studies would show a difference in decay kinetics in the electron region (∼800 nm) compared with the hole region (∼450 nm). This was not the case for any Cu-doped sample studied herein. As the samples were studied in an argon atmosphere, no photochemistry could occur. This indicated that the transient decay of our absorption signals was due solely to electron–hole recombination. The kinetics of electron–hole recombination were compared across all samples (Figure 4b). These kinetics could be fit to power law decays, typical of the trap/detrapping movement of the charge found in anatase TiO2.77 The rate of recombination was quantified by finding the time where half the initial absorption was lost (t50%). In the 5% Cu-doped TiO2 sample, recombination was the slowest (t50% ≈ 110 ms), followed by the 2% (t50% ≈ 26 ms), 10% (t50% ≈ 19 ms), 20% (t50% ≈ 3 ms), and undoped sample (t50% ≈ 1 ms). In the undoped sample, the timescale of recombination was typical of anatase TiO2. Moreover, our TAS results showed that Cu doping resulted in an enhancement in charge carrier lifetime. Substantial differences in the initial absorption were also observed (Figure 4b). This indicated that there were critical differences in ultrafast timescale recombination between samples, not observable on the timescale of these measurements. It was clear that the 5% Cu-doped TiO2 sample possessed the greatest number of photogenerated charges from 10 μs (followed by the 2, 10, 20%, and undoped TiO2 sample). This trend was analogous to the lifetime of photogenerated charges, where the 5% Cu-doped TiO2 samples showed both the most charges from 10 μs and the slowest rate of electron–hole recombination.
Figure 4.
(a) Transient absorption spectra at select times for the 5% Cu-doped TiO2 thin film. (b) Recombination kinetics at 950 nm for all samples, where the dashed lines represent a fit to a power law decay model [f(t) = a·tb, where t is the time and a and b are variables].
Photocatalysis Testing
The photocatalytic activities of the as-synthesized films were evaluated against the degradation of a model organic pollutant, octadecanoic (stearic) acid, under ultraviolet (UVA) illumination (I = 3.15 mW cm–2). Stearic acid is highly stable under UV light (in the absence of an underlying effective photocatalyst) and can be easily monitored on transparent materials via infrared spectroscopy. Its photocatalytic degradation can be monitored following the disappearance of characteristic C–H modes at 2958, 2923, and 2853 cm–1 (Figure 5b). The photocatalytic rates were estimated from the linear regression of the initial steps (30–40%) of the curve of integrated area versus illumination time. The corresponding rates were expressed as FQEs, defined as molecules of stearic acid degraded over incident photons (units, molecule × photon–1) (Figure 5a). The variation in FQE values was not attributed to differences in physical properties of the films as all films investigated showed comparable thicknesses and crystallinity based on XRD and SEM analyses (Figure 1a and Supporting Information). In addition, a blank piece of glass (as a control) and Pilkington Activ self-cleaning glass (15 nm TiO2 anatase coating) were also tested for comparison.
Figure 5.
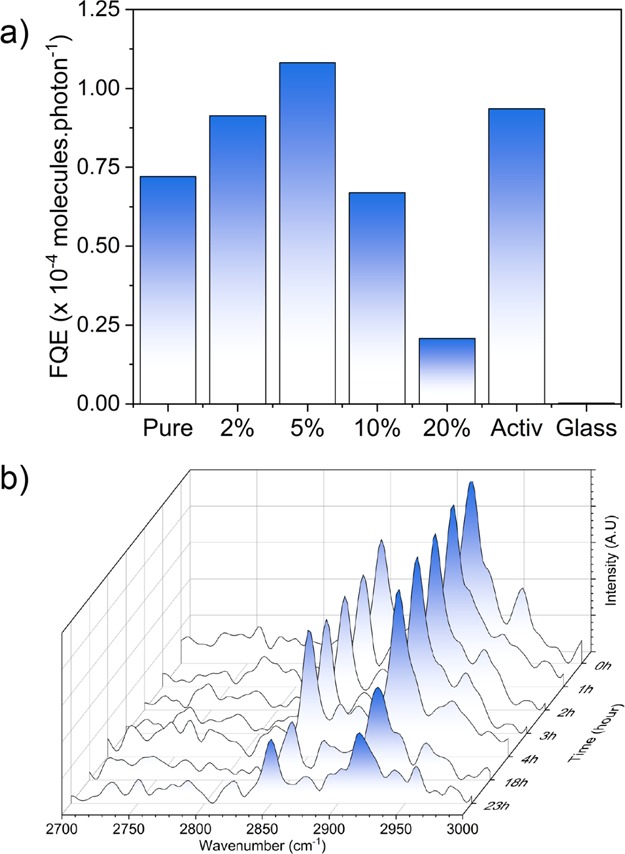
(a) FQEs obtained during the degradation of stearic acid under the UVA irradiation of Cu:TiO2 and undoped thin films. Blank glass and Activ samples are included for comparison. (b) IR spectra of stearic acid upon UVA illumination (I = 3.15 mW·cm–2) on a typical Cu-doped TiO2, 5% Cu:TiO2.
The pure TiO2 sample showed a destruction rate of 7.2 × 10–5 molecules·photon–1; upon doping 2 and 5% of Cu, the rate increases to 9.1 × 10–5 and 1.1 × 10–4 molecules·photon–1 (a higher rate than Activ, 9.4 × 10–5 molecules·photon–1), respectively. At higher concentrations of Cu (10 and 20%), the stearic acid destruction rate decreases to 6.7 × 10–5 and 2.1 × 10–5 molecules·photon–1, respectively. The film photocatalytic activities observed (Figure 5) could be correlated directly with photogenerated charge carrier lifetimes (Figure 4), where high lifetime resulted in high photocatalytic activity. These observations were attributed to the kinetics of the typical processes associated with photocatalysis on TiO2. It is commonly accepted that photocatalysis on TiO2 occurs through the reaction of (i) holes with surface H2O producing highly reactive hydroxyl radicals that subsequently degrade nearby organics and (ii) electrons with O2 forming highly reactive superoxide radicals that also degrade nearby organics.71 These two processes occur on different timescales, where it has been demonstrated that holes can react with H2O within ∼2 μs and electrons react with O2 from 10 to 900 μs.72 Our TAS studies showed that the sample with the longest-lived photogenerated charge carrier was 5% Cu-doped TiO2, which showed both the slowest rate of recombination and the highest number of photogenerated charges.
Antibacterial Testing
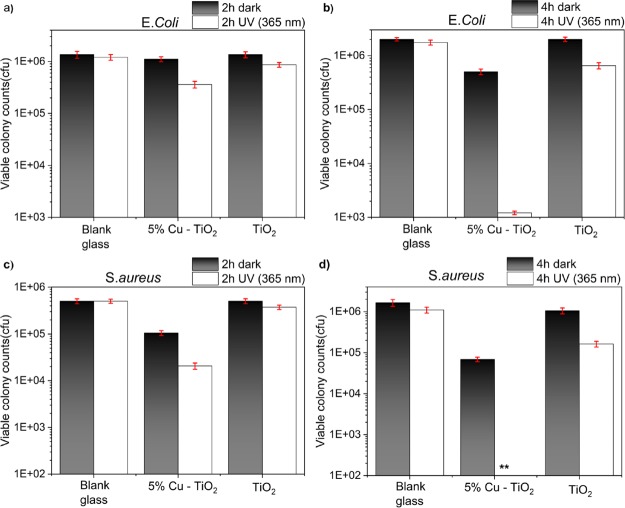
Because the 5% Cu-doped TiO2 sample showed the best photocatalytic results, this sample was chosen along with pure TiO2 for antimicrobial activity testing against a Gram-negative bacterium, E. coli, and a Gram-positive bacterium, S. aureus, in the dark and under UVA-irradiated conditions. In the case of E. coli, there was no significant reduction in the colony counts for the pure TiO2 sample in the dark from 2 to 4 h (Figure 6a,b). A small reduction was observed under UVA irradiation, which is likely due to the oxidative stress caused by the generation of ROS. This was also observed when the pure TiO2 sample was tested against S. aureus (Figure 6c,d). However, for the Cu-doped sample, there was a small but significant decrease in the colony counts of both E. coli and S. aureus in the dark, which is possibly due to the cytotoxicity of Cu0, Cu+, and Cu2+. Under UVA irradiation of 4 h, there was a rapid decrease in bacterial numbers, to below or almost below detectable levels, for S. aureus and E. coli, respectively (Figure 6b,d). We attribute this to the generation of ROS that attack organic matter when TiO2 is irradiated with energy above its band gap as well as the cytotoxicity of Cu species. The activity was higher in the Cu-doped TiO2 film compared to that in the undoped film, as previously observed from TAS measurements and discussed, because of the Cu states acting as trapping sites that can reduce charge carrier recombination. The lifetimes of the charge carrier are important as they have to travel to the surface of the semiconductor before they can undergo reactions with water and oxygen to produce ROS that then proceed to destroy bacterial cells. The secondary reason for the superior activity of the doped samples over the undoped samples is due to the presence of Cu that has its own antibacterial properties via Fenton-type reactions as discussed in the Introduction. The reasons for the higher killing efficacy for the Gram-positive bacteria S. aureus over the Gram-negative E. coli are not fully understood, but it may be due to their increased permeability toward cytotoxic Cu ions of the Gram-positive bacteria cell envelope that is composed of a cell membrane and a highly permeable cell wall. Although the cell envelope is thinner for Gram-negative bacteria, it contains two membrane layers of which the outer membrane is constructed of mostly tightly packed lipopolysaccharide molecules that are successful permeability barriers.78
Figure 6.
Antimicrobial test results for pure and 5% doped films in the dark and under UVA conditions for 2 (a,c) and 4 h (b,d) against Gram-negative E. coli (a,b) and Gram-positive S. aureus (c,d) bacteria.
Defect Thermodynamics
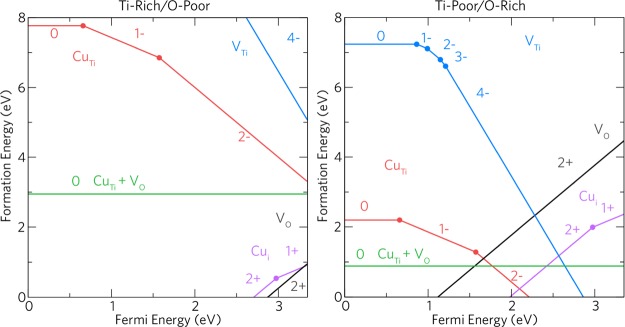
In order to rationalize the increased performance of the Cu-doped TiO2 films over the undoped films, we have carried out a detailed hybrid DFT study of the defect chemistry of Cu-doping anatase TiO2. Figure 7 displays the thermodynamic transition levels for all the intrinsic and extrinsic defects considered in this study under Ti-rich/O-poor and Ti-poor/O-rich conditions.
Figure 7.
Transition level diagram under both Ti-rich/O-poor (left) and Ti-poor/O-rich (right) growth regimes. The Fermi energy ranges from the VBM (0 eV) to the CBM (∼3.35 eV).
In this study, the dominant acceptor and donor defects present in anatase TiO2 are considered. These have been shown in previous theoretical studies to be the oxygen vacancy (VO) and the titanium vacancy (VTi).46,49,51,79−81 Ti-rich/O-poor growth conditions typically favor the formation of n-type defects, thus VO is lowest in formation energy under these conditions (ΔHf (VO0) = ∼1.19 eV). Under Ti-poor/O-rich conditions, the formation of an oxygen vacancy rises to ∼4.71 eV. VO acts as a resonant two-electron donor with the 2+/0 transition level occurring ∼0.12 eV above the conduction band maximum (CBM). This is in contrast to other wide band gap metal oxides such as ZnO,82−85 SnO2,85−88 and BaSnO389,90 where VO acts as a deep donor and is not expected to contribute largely to the intrinsic conductivity of the material. Our results are consistent with previous theoretical studies on anatase.49,51,89−92
The titanium vacancy acts as an ultradeep acceptor with the 0/1– transition level occurring ∼0.87 eV above the valence band maximum (VBM). Under typically p-type favorable conditions (Ti-poor/O-rich), the formation energy of the neutral charge state is ∼7.23 eV and almost doubles under Ti-rich/O-poor growth conditions (ΔHf (VTi0) = ∼14.26 eV). All holes are localized on adjacent oxygens around the defect as shown in previous calculations.93 Under a Ti-poor/O-rich regime, VTi4– begins to compensate VO2+ ∼2.28 eV above the VBM trapping the Fermi energy around this point.
Under Ti-poor/O-rich conditions, CuTi acts as a relatively low formation energy deep acceptor. The formation energy of CuTi0 is ∼2.20 eV, and the 0/1– transition level occurs ∼0.65 eV above the VBM. The 1–/2– transition level occurs ∼1.57 eV above the VBM, and the 3– charge state is not seen over the entirety of the band gap. Copper is therefore incorporated as Cu(II) when in a substitutional configuration, which explains the prevalence of this oxidation state in the experiment.21,22,94−96Figure 8a–c shows the hole localization on and around the CuTi defect in the neutral (7c), 1– (7b), and 2– (7c) charge states. In CuTi, one hole is localized in a d-orbital on Cu and the other two are delocalized on the six joining oxygens. The evolution to the 1– charge state fills a hole with an electron leaving one localized in the Cu d-orbital and the other delocalized as before. In the 2– charge state, (7c), the remaining hole is still mostly localized in the Cu d-orbital but with some density on two equatorial (to the plane of the page) oxygen p-orbitals. Despite being a larger cation than Ti,97 Cu substitution results in a minimal distortion of the crystal lattice. The Cu atom itself remains in the original Ti position over all charge states, and the adjacent oxygen ions hardly shift from their positions in the neutral, 1–, and 3– charge states. The CuTi2– case displays a small shift of one of the O ions by ∼10%, as seen in Figure 8c. CuTi2– begins to be compensated for by VO2+ ∼1.67 eV above the VBM (∼1.68 eV below the CBM) under Ti-poor/O-rich conditions trapping the Fermi energy at this point. Under n-type favorable conditions, Ti-rich/O-poor, CuTi0 has a very high formation energy of ∼7.76 eV and is thus likely to form in negligible quantities.
Figure 8.
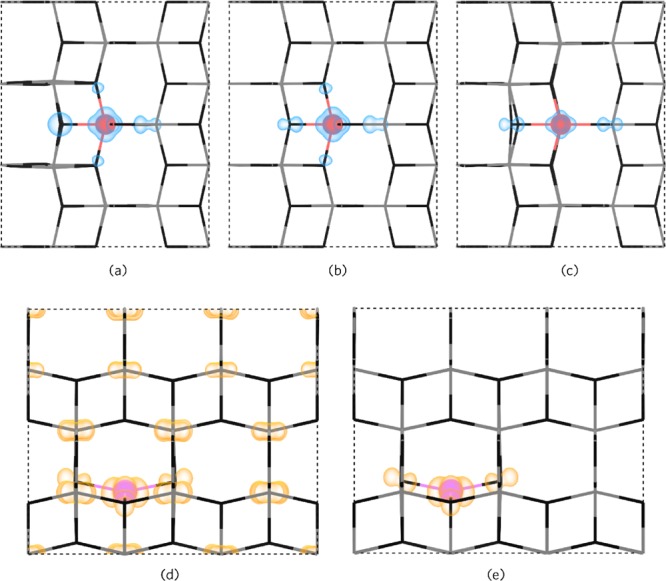
Partial charge densities for the Cu-related defects. (a–c) Hole charge density for CuTi0, CuTi–, and CuTi2– defects as viewed along the (010) direction. (d,e) Electron charge density for Cui and Cui+, respectively, as viewed down the (100) direction. The hole density is shown in blue and the electron density in orange 0–0.015 eV Å–1.
Our calculations show that the formation energy of CuTi is reduced when adjacent to an oxygen vacancy ([CuTi + VO]). Under O-poor and O-rich conditions, this defect cluster possesses formation energies of ∼2.94 and ∼0.89 eV, respectively. In [CuTi + VO], Cu relaxes slightly away from VO compared to Cu in CuTi2– as expected. These results indicate however that the presence of this defect cluster is unlikely to appear in large quantities under both Ti-poor/O-rich conditions and that Cu doping will not likely induce oxygen vacancies in anatase TiO2 consistent with the work by Mathew et al.98
Cui acts as a resonant one-electron donor under both growth regimes and has a quite low formation energy under Ti-rich/O-poor conditions (ΔHf (Cui0) = ∼0.96 eV), which rises to ∼2.41 eV under Ti-poor/O-rich conditions. From our calculations, it is likely that interstitial Cu incorporates as Cu(I) with the 2+/1+ transition level occurring ∼0.37 eV below the CBM. In the literature, Cu is seen as both Cu(II) and Cu(I), and it is likely that both Cui and CuTi coexist in the anatase lattice, as indicated by our calculations. Despite being a resonant donor, the 1+/0 transition level occurs ∼0.05 eV above the CBM, indicating that increasing the copper incorporation into anatase will not lead to high carrier concentrations. When in the neutral charge state (CuTi), Cu is expected to exist as Cu2+, thus when the Fermi level is very high, that is, with increased Cu incorporation, Cu2+ is expected to be seen (likewise with lower concentrations, Cu+ may be seen). From our calculations, Cui moves from the perfect octahedral interstitial position to a distorted square planar position where it bonds to four oxygens. This can be seen in Figure 8d,e. This behavior is not surprising considering that Cu2+ is well known to be Jahn–Teller active, thus preferring a distorted square planar configuration. Under all charge states, Cu remains in the same position and the Cu–O distances remain at 2.00 Å similar to the average Ti–O bond length of 1.98 Å in anatase. The neighboring four Ti ions move slightly away by an average of 4% from the Cui defect and remain in the same position over all the charge states. In Figure 8d,e, the partial charge density is shown for the neutral (Figure 8d) and 1+ (Figure 8e) charge states. In the neutral charge state, both electrons are delocalized over the Ti 3d orbitals that make up the CBM [indicating Cu(II)], and in the 1+ charge state, there is a localization of the electron density on and around Cu [indicating Cu(I)].
Dopant-Centered Optical Properties
The optical transitions for all defects were evaluated and are shown for the intrinsic defects, VTi and VO, in Figure 9 and substitutional and interstitial Cu in Figure 10.
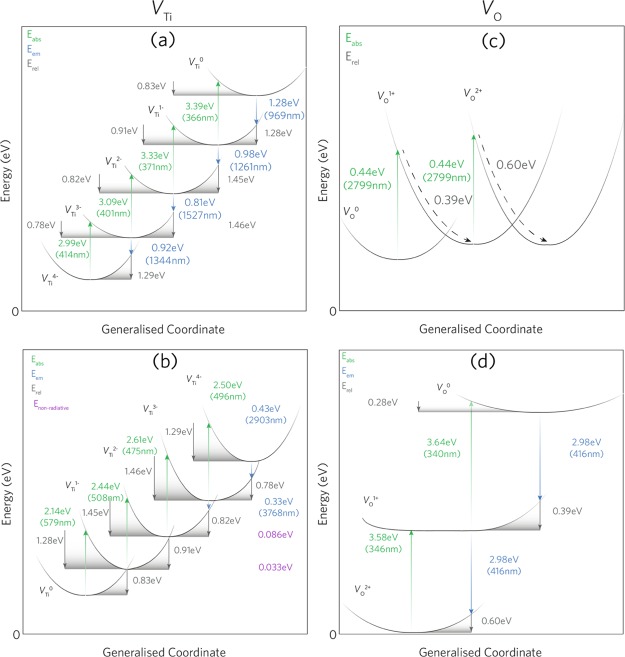
Figure 9.
Configurational coordinate diagrams for VTi (a,b) and VO (c,d). (a,c) Absorption and subsequent emission of an electron to/from the CBM and (b,d) capture of an electron and subsequent emission from/to the VBM.
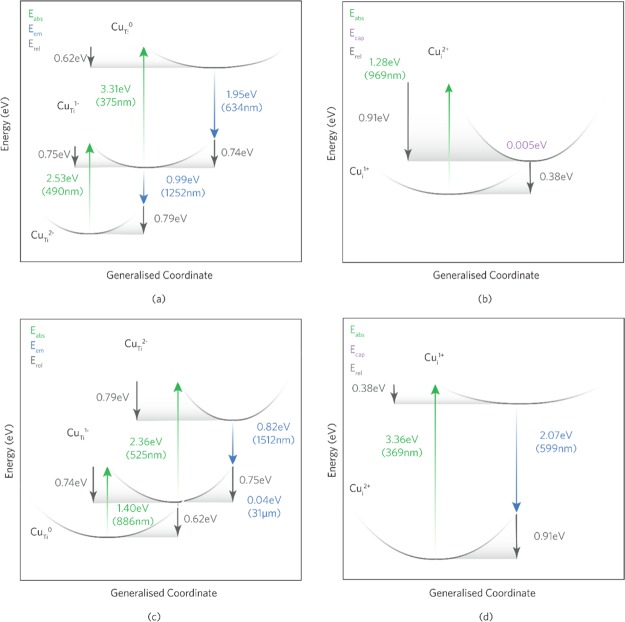
Figure 10.
One-dimensional configurational coordinate diagram for (a,c) CuTi and (b,d) Cui under electron and hole ionization, respectively. In each example, the absorption and emission energies are independent of the chemical potentials and therefore the growth conditions.
Absorption
VTi undergoes optical absorption from both the excitation of an electron to the conduction band and the ionization of a hole in the valence band and is shown in Figure 9a,b, respectively. For the excitation of an electron to the conduction band, VTi undergoes four transitions with 414, 401, 371, and 366 nm for VTi4– → VTi3–, VTi3– → VTi2–, VTi2– → VTi–, and VTi– → VTi0, respectively. These absorptions are likely to exist within the absorption edge of anatase and thus will be indistinguishable; however, these may be present in fine structure absorption spectra peaks.99 VTi can also undergo the capture of an electron (or hole ionization) from the valence band, resulting in four more absorptions with wavelengths of 579, 508, 475, and 496 nm for VTi → VTi–, VTi– → VTi2–, VTi2– → VTi3–, and VTi3– → VTi4–, respectively. It is likely that these peaks will be seen with the increasing concentration of VTi and that a greater absorption of visible light will be observed.
Figure 9c,d shows the optical absorption values for VO. Despite VO being thermodynamically resonant within the conduction band, the optical excitation of electrons can still occur. The optical excitation to the conduction band from VO0 → VO+ and VO+ → VO2+ is a two-photon process with a very low absorption energy of 2799 nm whose observation is not likely. A similar two-photon absorption is observed for the capture of an electron from the valence band with much larger absorption energies of around 340 nm.
Figure 10a shows that CuTi undergoes optical absorption from CuTi2– to CuTi– and from CuTi– to CuTi0 in the near-UV (490 nm) and the UV (375 nm) regions, respectively, for the excitation of an electron to the conduction band. These absorptions will occur within the absorption edge of anatase TiO2 and is thus not expected to be distinguishable experimentally. CuTi can also undergo hole ionization from the VBM as shown in Figure 10c where the absorptions occur in the NIR (886 nm; CuTi → CuTi–) and the green region (525 nm; CuTi– → CuTi2–) of the visible spectrum. These results can be seen in the transmission data in (Figure 2a) by the dip around the absorption edge and ∼525 nm compared to undoped pure anatase and is shown to be quite pronounced in the 10% Cu samples, signifying larger concentrations of substitutional Cu.
Figure 10b,d displays the electron and hole ionization transitions for interstitial Cu (Cui), respectively. Figure 10b shows that the optical excitation of an electron to the CBM results in the absorption of a photon in the NIR region (969 nm) for Cui+ → Cui2+. From the transmission data in (Figure 2a), broad troughs are seen ∼900–1200 nm for the 10 and 20% Cu samples, suggesting that an increased incorporation of interstitial Cu is present. The broad troughs are indicative of a large relaxation energy100 (Erel) which for Cui+ to Cui2+ is ∼0.91 eV. A small dip in the transmission occurs for 5% Cu and none at all for 2% Cu, leading to the conclusion that the 2+ → 1+ transition may be suppressed. It is not expected that an optical transition will occur from the 1+ to the 0 charge states as thermal ionization occurs in the conduction band and has thus been omitted from this diagram. The capture of an electron by Cui2+ (from the VBM) facilitates the absorption of a photon in the near-UV with a wavelength of which, as with CuTi, is not expected to be distinguishable experimentally because of its position in the absorption edge of TiO2.
Emission
The intrinsic defect emissions for VTi and VO are shown in Figure 9. Infrared PL also occurs under both electron excitation and capture. Under electron excitation from VTi to the conduction band, it is expected for the transitions: VTi0 → VTi–, VTi– → VTi2–, VTi2– → VTi3–, and VTi3– → VTi4– that emissions of 969, 1261, 1527, and 1344 nm will be seen. Under the emission of an electron to the VBM (Figure 9b), two IR emissions are observed at 2903 and 3768 nm for VTi4– → VTi3– and VTi3– → VTi2–, respectively. The other transitions are likely to be nonradiative as they occur at very small energies (0.033–0.086 eV). Most experimental PL studies are carried out in the UV–vis region of the electromagnetic spectrum and (<900 nm) and are thus not accounted for in the scientific literature. One study on TiO2 nanoribbons, however, shows a PL peak of around 969 nm.101 The Stokes shifts for each transition (difference between absorption and emission) are fairly large and as such indicate a broad shift between the absorption and emission. The total Stokes shifts for each transition: VTi4– ↔ VTi3–, VTi3– ↔ VTi2–, VTi2– ↔ VTi–, and VTi– ↔ VTi are 2.07, 2.28, 2.36, and 2.11 eV, respectively. Within the PL of Cu-doped TiO2 in Figure 2b, the peaks at 969, 1250, and 1340 nm correspond to those by VTi. It is likely that the glass emission around 1531 nm masks the 1527 nm peak from the VTi2– → VTi3– transition.
VO only undergoes PL via the release of an electron to the VBM. This is a two-photon process with a wavelength of 416 nm; thus, the peak intensity is expected to be larger than that of a single photon process. Numerous room-temperature studies observe a peak of around 427–417 nm (where a shift is expected at increasing temperature) which Kernazhitsky et al. identify as an oxygen vacancy-related peak.99,102−106 In Figure 2b, the peak at 416 nm matches well to that calculated here. The intensity of the peak rises with increasing Cu doping, indicating the formation of oxygen vacancies. The total Stokes shift for the two-electron process is calculated as 1.26 eV, in good agreement with the study of Wang et al., which shows a Stokes shift of 1.36 eV.102
The emission energies present for electron (Figure 10a) and hole (Figure 10c) ionization of CuTi range from the mid-IR region to the red region of the visible spectrum. In Figure 10a, the transition of CuTi0 → CuTi– and the subsequent transition of CuTi– to CuTi2– give rise to PL peaks at ∼ 634 and ∼1252 nm, respectively. Upon the release of a hole to the VBM, two PL peaks are expected around 1512 nm (CuTi2– → CuTi–) and ∼31 μm (CuTi– → CuTi). The latter absorption is expected to be nonradiative because of the very small emission energy and will thus be dissipated through the anatase lattice as phonons.107 Similar behavior is seen in acceptor defects such as VZn in ZnO108 as well as VGa in GaN.107 It is expected that these peaks will be broad because of the relatively large Erel values (∼0.7 eV). Because of the glass emission at 1525 nm in Figure 2b, it is hard to tell whether this peak is present in the AACVD-made Cu-doped TiO2 thin films.
Cui will only photoluminesce after the capture of an electron from the valence band. Figure 10b depicts this with a value of 599 nm and a broad peak (Erel = 0.91 eV). This is seen in the room-temperature PL data in (Figure 2b) from ∼450 to 625 nm. The highest intensity PL occurs in the 20% Cu sample, which, together with the transmission data in (Figure 2a), indicates that the presence of interstitial Cu is more substantial. Figure 10b shows that Cui will not photoluminesce after electron ionization because of the thermodynamic resonance of Cui+ in the conduction band. Instead, it is likely that Cui+ will readily capture an electron with an energy barrier of 0.005 eV to return to the 2+ charge state.
The presence of interstitial Cu in the anatase lattice together with substitutional Cu is likely beneficial to both the photocatalytic activity and the electron-carrier separation lifetimes. The mechanism of this is possibly due to the synergistic effect of deep acceptor/shallow donor pairs whereby an excited electron from CuTi can be readily accepted by an interstitial Cu defect, thereby increasing the separation. Electron capture by substitutional Cu occurs in the visible/NIR region of the spectrum (CuTi0 → CuTi–; Eabs = 886 nm), and electron excitation of Cui also occurs in the visible/NIR region (Cui+ → Cui2+; Eabs = 969 nm) aiding the visible light-enhanced photocatalytic activity. It is possible that competition between these defects may arise with increased Cu incorporation favoring the formation of interstitial Cu over substitutional Cu, thereby losing the beneficial synergistic effect. This is evidenced through the detrimental effect to the carrier separation lifetimes in Figure 4 and to the decrease in photocatalytic activity (Figure 5a) toward higher doping levels where the CuTi:Cui ratio is suggested to be higher (10 and 20%).
Conclusions
AACVD provides a facile route to highly photoactive and antimicrobial Cu-doped TiO2 thin films. From experimental analysis, the 5% TiO2:Cu film in particular displayed the greatest exciton lifetimes, photocatalytic activity (in the degradation of stearic acid), and antibacterial activity (against E. coli and S. aureus) under 365 nm irradiation. Using hybrid DFT calculations, this was demonstrated to be due to a synergistic effect from interstitial and substitutional Cu within the anatase lattice and confirmed by optical transmission and PL experiments. Effective dopant selection and concentration control are therefore key to providing the maximum efficiency in terms of carrier lifetimes for migration to the surface for the necessary reactions to take place for photocatalysis and antibacterial activity.
Acknowledgments
The authors would like to thank Professor James R. Durrant for useful discussions and Dr Steve Firth and Dr Tom Gregory for useful discussion on SEM. The authors are grateful to King Abdulaziz City for Science and Technology (KACST), Saudi Arabia, for the provision of a PhD studentship to Abdullah Alotaibi and for financial support from the Saudi Cultural Bureau in London. The authors are also grateful to the UK Materials and Molecular Modelling Hub for computational resources, which are partially funded by EPSRC (EP/P020194/1), and to UCL for the provision of the Legion, Myriad and Grace supercomputers. Via our membership of the UK’s HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202, EP/R029431), this work was carried out under the ARCHER UK National Supercomputing Service (http://www.archer.ac.uk). A.K. thanks Imperial College for a Junior Research Fellowship, the EPSRC for a Capital Award Emphasising Support for Early Career Researchers, and the Royal Society for an Equipment Grant (RSG\R1\180434).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.9b22056.
Defect formalism, thermodynamic limits, and optical calculations and SEM of the Cu-doped TiO2 films (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Linsebigler A. L.; Lu G.; Yates J. T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. 10.1021/cr00035a013. [DOI] [Google Scholar]
- Scanlon D. O.; Dunnill C. W.; Buckeridge J.; Shevlin S. A.; Logsdail A. J.; Woodley S. M.; Catlow C. R. A.; Powell M. J.; Palgrave R. G.; Parkin I. P.; Watson G. W.; Keal T. W.; Sherwood P.; Walsh A.; Sokol A. A. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798. 10.1038/nmat3697. [DOI] [PubMed] [Google Scholar]
- McCullagh C.; Robertson J. M. C.; Bahnemann D. W.; Robertson P. K. J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: a review. Res. Chem. Intermed. 2007, 33, 359–375. 10.1163/156856707779238775. [DOI] [Google Scholar]
- Hashimoto K.; Irie H.; Fujishima A. TiO2 photocatalysis: a historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. 10.1143/jjap.44.8269. [DOI] [Google Scholar]
- Georgekutty R.; Seery M. K.; Pillai S. C. A highly efficient Ag-ZnO photocatalyst: synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. 10.1021/jp802729a. [DOI] [Google Scholar]
- Morrison S. R.; Freund T. Chemical role of holes and electrons in ZnO photocatalysis. J. Chem. Phys. 1967, 47, 1543–1551. 10.1063/1.1712115. [DOI] [Google Scholar]
- Ali T. T.; Narasimharao K.; Parkin I. P.; Carmalt C. J.; Sathasivam S.; Basahel S. N.; Bawaked S. M.; Al-Thabaiti S. A. Effect of pretreatment temperature on the photocatalytic activity of microwave irradiated porous nanocrystalline ZnO. New J. Chem. 2015, 39, 321–332. 10.1039/c4nj01465k. [DOI] [Google Scholar]
- Promdet P.; Quesada-Cabrera R.; Sathasivam S.; Li J.; Jiamprasertboon A.; Guo J.; Taylor A.; Carmalt C. J.; Parkin I. P. High Defect Nanoscale ZnO Films with Polar Facets for Enhanced Photocatalytic Performance. ACS Appl. Nano Mater. 2019, 2, 2881–2889. 10.1021/acsanm.9b00326. [DOI] [Google Scholar]
- Hassan I. A.; Sathasivam S.; Nair S. P.; Carmalt C. J. Antimicrobial properties of copper-doped ZnO coatings under darkness and white light illumination. ACS Omega 2017, 2, 4556–4562. 10.1021/acsomega.7b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Zhang W.; Niu J.; Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- Chadwick N. P.; Glover E. N. K.; Sathasivam S.; Basahel S. N.; Althabaiti S. A.; Alyoubi A. O.; Parkin I. P.; Carmalt C. J. Photo-activity and low resistivity in N/Nb Co-doped TiO2 thin films by combinatorial AACVD. J. Mater. Chem. A 2016, 4, 407–415. 10.1039/c5ta07922e. [DOI] [Google Scholar]
- Musat V.; Teixeira B.; Fortunato E.; Monteiro R. C. C.; Vilarinho P. Al-doped ZnO thin films by sol–gel method. Surf. Coating. 2004, 180–181, 659–662. 10.1016/j.surfcoat.2003.10.112. [DOI] [Google Scholar]
- Matsubara K.; Fons P.; Iwata K.; Yamada A.; Sakurai K.; Tampo H.; Niki S. ZnO transparent conducting films deposited by pulsed laser deposition for solar cell applications. Thin Solid Films 2003, 431–432, 369–372. 10.1016/s0040-6090(03)00243-8. [DOI] [Google Scholar]
- Page K.; Palgrave R. G.; Parkin I. P.; Wilson M.; Savin S. L. P.; Chadwick A. V. Titania and silver–titania composite films on glass—potent antimicrobial coatings. J. Mater. Chem. 2007, 17, 95–104. 10.1039/b611740f. [DOI] [Google Scholar]
- Wu B.; Huang R.; Sahu M.; Feng X.; Biswas P.; Tang Y. J. Bacterial responses to Cu-doped TiO2 nanoparticles. Sci. Total Environ. 2010, 408, 1755–1758. 10.1016/j.scitotenv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Jaimy K. B.; Safeena V. P.; Ghosh S.; Hebalkar N. Y.; Warrier K. G. K. Photocatalytic activity enhancement in doped titanium dioxide by crystal defects. Dalton Trans. 2012, 41, 4824–4832. 10.1039/C2DT12018F. [DOI] [PubMed] [Google Scholar]
- Choi W.; Termin A.; Hoffmann M. R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. A 1994, 98, 13669–13679. 10.1021/j100102a038. [DOI] [Google Scholar]
- Hassan I. A.; Parkin I. P.; Nair S. P.; Carmalt C. J. Antimicrobial activity of copper and copper(I) oxide thin films deposited via aerosol-assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. 10.1039/C4TB00196F. [DOI] [PubMed] [Google Scholar]
- Ruparelia J. P.; Chatterjee A. K.; Duttagupta S. P.; Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- You M.; Kim T. G.; Sung Y.-M. Synthesis of Cu-doped TiO2 nanorods with various aspect ratios and dopant concentrations. Cryst. Growth Des. 2010, 10, 983–987. 10.1021/cg9012944. [DOI] [Google Scholar]
- Colón G.; Maicu M.; Hidalgo M. C.; Navío J. A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal., B 2006, 67, 41–51. 10.1016/j.apcatb.2006.03.019. [DOI] [Google Scholar]
- Choudhury B.; Dey M.; Choudhury A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. 10.1186/2228-5326-3-25. [DOI] [Google Scholar]
- Park H. S.; Kim D. H.; Kim S. J.; Lee K. S. The photocatalytic activity of 2.5 wt% Cu-doped TiO2 nano powders synthesized by mechanical alloying. J. Alloys Compd. 2006, 415, 51–55. 10.1016/j.jallcom.2005.07.055. [DOI] [Google Scholar]
- Celik E.; Gokcen Z.; Ak Azem N. F.; Tanoglu M.; Emrullahoglu O. F. Processing, characterization and photocatalytic properties of Cu doped TiO2 thin films on glass substrate by sol–gel technique. Mater. Sci. Eng., B 2006, 132, 258–265. 10.1016/j.mseb.2006.03.038. [DOI] [Google Scholar]
- Zhang W.; Li Y.; Zhu S.; Wang F. Copper doping in titanium oxide catalyst film prepared by dc reactive magnetron sputtering. Catal. Today 2004, 93–95, 589–594. 10.1016/j.cattod.2004.06.009. [DOI] [Google Scholar]
- Sathasivam S.; Arnepalli R. R.; Kumar B.; Singh K. K.; Visser R. J.; Blackman C. S.; Carmalt C. J. Solution Processing of GaAs Thin Films for Photovoltaic Applications. Chem. Mater. 2014, 26, 4419–4424. 10.1021/cm501280e. [DOI] [Google Scholar]
- Sathasivam S.; Arnepalli R. R.; Singh K. K.; Visser R. J.; Blackman C. S.; Carmalt C. J. A solution based route to GaAs thin films from As (NMe 2) 3 and GaMe 3 for solar cells. RSC Adv. 2015, 5, 11812–11817. 10.1039/c4ra13902j. [DOI] [Google Scholar]
- Alotaibi A. M.; Sathasivam S.; Nair S. P.; Parkin I. P. Antibacterial properties of Cu–ZrO 2 thin films prepared via aerosol assisted chemical vapour deposition. J. Mater. Chem. B 2016, 4, 666–671. 10.1039/c5tb02312b. [DOI] [PubMed] [Google Scholar]
- Bhachu D. S.; Moniz S. J. A.; Sathasivam S.; Scanlon D. O.; Walsh A.; Bawaked S. M.; Mokhtar M.; Obaid A. Y.; Parkin I. P.; Tang J.; Carmalt C. J. Bismuth oxyhalides: synthesis, structure and photoelectrochemical activity. Chem. Sci. 2016, 7, 4832–4841. 10.1039/c6sc00389c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick N.; Sathasivam S.; Kafizas A.; Bawaked S. M.; Obaid A. Y.; Al-Thabaiti S.; Basahel S. N.; Parkin I. P.; Carmalt C. J. Combinatorial aerosol assisted chemical vapour deposition of a photocatalytic mixed SnO2/TiO2 thin film. J. Mater. Chem. A 2014, 2, 5108–5116. 10.1039/C4TA00545G. [DOI] [Google Scholar]
- Sathasivam S.; Kafizas A.; Ponja S.; Chadwick N.; Bhachu D. S.; Bawaked S. M.; Obaid A. Y.; Al-Thabaiti S.; Basahel S. N.; Carmalt C. J.; Parkin I. P. Combinatorial Atmospheric Pressure CVD of a Composite TiO2/SnO2 Thin Film. Chem. Vap. Depos. 2014, 20, 69–79. 10.1002/cvde.201307081. [DOI] [Google Scholar]
- Ponja S. D.; Sathasivam S.; Parkin I. P.; Carmalt C. J. Transparent conductive aluminium and fluorine co-doped zinc oxide films via aerosol assisted chemical vapour deposition. RSC Adv. 2014, 4, 49723–49728. 10.1039/c4ra09997d. [DOI] [Google Scholar]
- Knapp C. E.; Carmalt C. J. Solution based CVD of main group materials. Chem. Soc. Rev. 2016, 45, 1036–1064. 10.1039/c5cs00651a. [DOI] [PubMed] [Google Scholar]
- Alotaibi A. M.; Sathasivam S.; Williamson B. A. D.; Kafizas A.; Sotelo-Vazquez C.; Taylor A.; Scanlon D. O.; Parkin I. P. Chemical vapor deposition of photocatalytically active pure brookite TiO2 thin films. Chem. Mater. 2018, 30, 1353–1361. 10.1021/acs.chemmater.7b04944. [DOI] [Google Scholar]
- Ponja S. D.; Williamson B. A. D.; Sathasivam S.; Scanlon D. O.; Parkin I. P.; Carmalt C. J. Enhanced electrical properties of antimony doped tin oxide thin films deposited via aerosol assisted chemical vapour deposition. J. Mater. Chem. C 2018, 6, 7257–7266. 10.1039/c8tc01929k. [DOI] [Google Scholar]
- Kresse G.; Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B: Condens. Matter Mater. Phys. 1993, 47, 558. 10.1103/physrevb.47.558. [DOI] [PubMed] [Google Scholar]
- Mills A.; Wang J. Simultaneous monitoring of the destruction of stearic acid and generation of carbon dioxide by self-cleaning semiconductor photocatalytic films. J. Photochem. Photobiol., A 2006, 182, 181–186. 10.1016/j.jphotochem.2006.02.010. [DOI] [Google Scholar]
- Kresse G.; Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 49, 14251. 10.1103/physrevb.49.14251. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 11169. 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- Paier J.; Marsman M.; Hummer K.; Kresse G.; Gerber I. C.; Ángyán J. G. Screened hybrid density functionals applied to solids. J. Chem. Phys. 2006, 124, 154709. 10.1063/1.2187006. [DOI] [PubMed] [Google Scholar]
- Heyd J.; Scuseria G. E.; Ernzerhof M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. 10.1063/1.1564060. [DOI] [Google Scholar]
- Buckeridge J.; Butler K. T.; Catlow C. R. A.; Logsdail A. J.; Scanlon D. O.; Shevlin S. A.; Woodley S. M.; Sokol A. A.; Walsh A. Polymorph engineering of TiO2: demonstrating how absolute reference potentials are determined by local coordination. Chem. Mater. 2015, 27, 3844–3851. 10.1021/acs.chemmater.5b00230. [DOI] [Google Scholar]
- Çelik V.; Mete E. Range-separated hybrid exchange-correlation functional analyses of anatase TiO2 doped with W, N, S, W/N, or W/S. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 86, 205112. 10.1103/physrevb.86.205112. [DOI] [Google Scholar]
- Huy H. A.; Aradi B.; Frauenheim T.; Deák P. Calculation of carrier-concentration-dependent effective mass in Nb-doped anatase crystals of TiO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 83, 155201. 10.1103/physrevb.83.155201. [DOI] [Google Scholar]
- Janotti A.; Varley J.; Rinke P.; Umezawa N.; Kresse G.; Van de Walle C. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 81, 085212. 10.1103/physrevb.81.085212. [DOI] [Google Scholar]
- Bhachu D. S.; Sathasivam S.; Sankar G.; Scanlon D. O.; Cibin G.; Carmalt C. J.; Parkin I. P.; Watson G. W.; Bawaked S. M.; Obaid A. Y.; Al-Thabaiti S.; Basahel S. N. Solution processing route to multifunctional titania thin films: Highly conductive and photcatalytically active Nb: TiO2. Adv. Funct. Mater. 2014, 24, 5075–5085. 10.1002/adfm.201400338. [DOI] [Google Scholar]
- Matsubara M.; Saniz R.; Partoens B.; Lamoen D. Doping anatase TiO 2 with group Vb and VI-b transition metal atoms: a hybrid functional first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 1945–1952. 10.1039/c6cp06882k. [DOI] [PubMed] [Google Scholar]
- Boonchun A.; Reunchan P.; Umezawa N. Energetics of native defects in anatase TiO2: a hybrid density functional study. Phys. Chem. Chem. Phys. 2016, 18, 30040–30046. 10.1039/C6CP05798E. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 50, 17953. 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- Morgan B. J.; Watson G. W. Intrinsic n-type defect formation in TiO2: a comparison of rutile and anatase from GGA+ U calculations. J. Phys. Chem. C 2010, 114, 2321–2328. 10.1021/jp9088047. [DOI] [Google Scholar]
- Rocquefelte X.; Schwarz K.; Blaha P. Theoretical investigation of the magnetic exchange interactions in copper (II) oxides under chemical and physical pressures. Sci. Rep. 2012, 2, 759. 10.1038/srep00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuz’menko A. B.; Van Der Marel D.; Van Bentum P. J. M.; Tishchenko E. A.; Presura C.; Bush A. A. Phonon anomalies versus magnetic ordering in CuO. Phys. B 2000, 284–288, 1396–1397. 10.1016/s0921-4526(99)02557-0. [DOI] [Google Scholar]
- Scanlon D. O.; Morgan B. J.; Watson G. W.; Walsh A. Acceptor levels in p-type Cu 2 O: rationalizing theory and experiment. Phys. Rev. Lett. 2009, 103, 096405. 10.1103/physrevlett.103.096405. [DOI] [PubMed] [Google Scholar]
- Scanlon D. O.; Morgan B. J.; Watson G. W. Modeling the polaronic nature of p-type defects in Cu 2 O: The failure of GGA and GGA+ U. J. Chem. Phys. 2009, 131, 124703. 10.1063/1.3231869. [DOI] [PubMed] [Google Scholar]
- Mittal K. L. Adhesion measurement of thin films. Act. Passive Electron. Components 1976, 3, 21–42. 10.1155/apec.3.21. [DOI] [Google Scholar]
- Lim S. P.; Pandikumar A.; Lim H. N.; Ramaraj R.; Huang N. M. Boosting photovoltaic performance of dye-sensitized solar cells using silver nanoparticle-decorated N, S-Co-doped-TiO2 photoanode. Sci. Rep. 2015, 5, 11922. 10.1038/srep11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. K. S.; Gajbhiye N. S. Room temperature magnetic properties of Cu-doped titanate, TiO2 (B) and anatase nanorods synthesized by hydrothermal method. Mater. Chem. Phys. 2012, 132, 175–179. 10.1016/j.matchemphys.2011.11.020. [DOI] [Google Scholar]
- Hasan M. R.; Suhaimya S. H. M.; Matb A. N. C. A sol–gel derived, copper-doped, titanium dioxide–reduced graphene oxide nanocomposite electrode for the photoelectrocatalytic reduction of CO2 to methanol and formic acid. RSC Adv. 2015, 5, 77803. 10.1039/c5ra12525a. [DOI] [Google Scholar]
- Karunakaran C.; Abiramasundari G.; Gomathisankar P.; Manikandan G.; Anandi V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. 10.1016/j.jcis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Liqiang J.; Xiaojun S.; Baifu X.; Baiqi W.; Weimin C.; Honggang F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. 10.1016/j.jssc.2004.05.064. [DOI] [Google Scholar]
- Biesinger M. C.; Lau L. W. M.; Gerson A. R.; Smart R. S. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. 10.1016/j.apsusc.2010.07.086. [DOI] [Google Scholar]
- Deroubaix G.; Marcus P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. 10.1002/sia.740180107. [DOI] [Google Scholar]
- Pesci F. M.; Cowan A. J.; Alexander B. D.; Durrant J. R.; Klug D. R. Charge carrier dynamics on mesoporous WO3 during water splitting. J. Phys. Chem. Lett. 2011, 2, 1900–1903. 10.1021/jz200839n. [DOI] [Google Scholar]
- Pendlebury S. R.; Wang X.; Le Formal F.; Cornuz M.; Kafizas A.; Tilley S. D.; Grätzel M.; Durrant J. R. Ultrafast charge carrier recombination and trapping in hematite photoanodes under applied bias. J. Am. Chem. Soc. 2014, 136, 9854–9857. 10.1021/ja504473e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima A.; Zhang X.; Tryk D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. 10.1016/j.surfrep.2008.10.001. [DOI] [Google Scholar]
- Wang X.; Kafizas A.; Li X.; Moniz S. J. A.; Reardon P. J. T.; Tang J.; Parkin I. P.; Durrant J. R. Transient absorption spectroscopy of anatase and rutile: the impact of morphology and phase on photocatalytic activity. J. Phys. Chem. C 2015, 119, 10439–10447. 10.1021/acs.jpcc.5b01858. [DOI] [Google Scholar]
- Kafizas A.; Wang X.; Pendlebury S. R.; Barnes P.; Ling M.; Sotelo-Vazquez C.; Quesada-Cabrera R.; Li C.; Parkin I. P.; Durrant J. R. Where do photogenerated holes go in anatase: rutile TiO2 A transient absorption spectroscopy study of charge transfer and lifetime. J. Phys. Chem. A 2016, 120, 715–723. 10.1021/acs.jpca.5b11567. [DOI] [PubMed] [Google Scholar]
- Wang L.; McCleese C.; Kovalsky A.; Zhao Y.; Burda C. Femtosecond time-resolved transient absorption spectroscopy of CH3NH3PbI3 perovskite films: evidence for passivation effect of PbI2. J. Am. Chem. Soc. 2014, 136, 12205–12208. 10.1021/ja504632z. [DOI] [PubMed] [Google Scholar]
- Clarke T.; Ballantyne A.; Jamieson F.; Brabec C.; Nelson J.; Durrant J. Transient absorption spectroscopy of charge photogeneration yields and lifetimes in a low bandgap polymer/fullerenefilm. Chem. Commun. 2008, 89. 10.1039/B813815J. [DOI] [PubMed] [Google Scholar]
- Anderson L. J. E.; Mayer K. M.; Fraleigh R. D.; Yang Y.; Lee S.; Hafner J. H. Quantitative measurements of individual gold nanoparticle scattering cross sections. J. Phys. Chem. C 2010, 114, 11127–11132. 10.1021/jp1040663. [DOI] [Google Scholar]
- Wang L.; Wang H.-Y.; Gao B.-R.; Pan L.-Y.; Jiang Y.; Chen Q.-D.; Han W.; Sun H.-B. Transient absorption spectroscopic study on band-structure-type change in CdTe/CdS core-shell quantum dots. IEEE J. Quantum Electron. 2011, 47, 1177–1184. 10.1109/jqe.2011.2159853. [DOI] [Google Scholar]
- Peiró A. M.; Colombo C.; Doyle G.; Nelson J.; Mills A.; Durrant J. R. Photochemical reduction of oxygen adsorbed to nanocrystalline TiO2 films: A transient absorption and oxygen scavenging study of different TiO2 preparations. J. Phys. Chem. B 2006, 110, 23255–23263. 10.1021/jp064591c. [DOI] [PubMed] [Google Scholar]
- Devahasdin S.; Fan C. Jr.; Li K.; Chen D. H. TiO2 photocatalytic oxidation of nitric oxide: transient behavior and reaction kinetics. J. Photochem. Photobiol., A 2003, 156, 161–170. 10.1016/s1010-6030(03)00005-4. [DOI] [Google Scholar]
- Tang J.; Durrant J. R.; Klug D. R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. 10.1021/ja8034637. [DOI] [PubMed] [Google Scholar]
- Kundu S.; Kafizas A.; Hyett G.; Mills A.; Darr J. A.; Parkin I. P. An investigation into the effect of thickness of titanium dioxide and gold–silver nanoparticle titanium dioxide composite thin-films on photocatalytic activity and photo-induced oxygen production in a sacrificial system. J. Mater. Chem. 2011, 21, 6854–6863. 10.1039/c0jm03492d. [DOI] [Google Scholar]
- Cowan A. J.; Tang J.; Leng W.; Durrant J. R.; Klug D. R. Water splitting by nanocrystalline TiO2 in a complete photoelectrochemical cell exhibits efficiencies limited by charge recombination. J. Phys. Chem. C 2010, 114, 4208–4214. 10.1021/jp909993w. [DOI] [Google Scholar]
- Raffi M.; Mehrwan S.; Bhatti T. M.; Akhter J. I.; Hameed A.; Yawar W.; ul Hasan M. M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. 10.1007/s13213-010-0015-6. [DOI] [Google Scholar]
- Mattioli G.; Alippi P.; Filippone F.; Caminiti R.; Amore Bonapasta A. Deep versus Shallow Behavior of Intrinsic Defects in Rutile and Anatase TiO2 Polymorphs. J. Phys. Chem. C 2010, 114, 21694–21704. 10.1021/jp1041316. [DOI] [Google Scholar]
- Deák P.; Aradi B.; Frauenheim T. Polaronic effects in TiO2 calculated by the HSE06 hybrid functional: Dopant passivation by carrier self-trapping. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 83, 155207. 10.1103/PhysRevB.83.155207. [DOI] [Google Scholar]
- Morgan B. J.; Watson G. W. Polaronic trapping of electrons and holes by native defects in anatase TiO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2009, 80, 233102. 10.1103/PhysRevB.80.233102. [DOI] [Google Scholar]
- Oba F.; Nishitani S. R.; Isotani S.; Adachi H.; Tanaka I. Energetics of native defects in ZnO. J. Appl. Physiol. 2001, 90, 824–828. 10.1063/1.1380994. [DOI] [Google Scholar]
- Oba F.; Togo A.; Tanaka I.; Paier J.; Kresse G. Defect energetics in ZnO: A hybrid Hartree-Fock density functional study. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 77, 245202. 10.1103/PhysRevB.77.245202. [DOI] [Google Scholar]
- Janotti A.; Van de Walle C. G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. 10.1103/PhysRevB.76.165202. [DOI] [Google Scholar]
- Ágoston P.; Albe K.; Nieminen R. M.; Puska M. J. Intrinsic n-Type Behavior in Transparent Conducting Oxides: A Comparative Hybrid-Functional Study of In2O3, SnO2 and ZnO. Phys. Rev. Lett. 2009, 103, 245501. 10.1103/PhysRevLett.103.245501. [DOI] [PubMed] [Google Scholar]
- Scanlon D. O.; Watson G. W. On the possibility of p-type SnO2. J. Mater. Chem. 2012, 22, 25236–25245. 10.1039/C2JM34352E. [DOI] [Google Scholar]
- Kılıç Ç.; Zunger A. Origins of Coexistence of Conductivity and Transparency in SnO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2002, 88, 095501. 10.1103/PhysRevLett.88.095501. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Janotti A.; Scheffler M.; Van de Walle C. G. Sources of Electrical Conductivity in SnO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2008, 101, 055502. 10.1103/PhysRevLett.101.055502. [DOI] [PubMed] [Google Scholar]
- Scanlon D. O. Defect engineering of BaSnO3 for high-performance transparent conducting oxide applications. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 87, 161201. 10.1103/PhysRevB.87.161201. [DOI] [Google Scholar]
- Sallis S.; Scanlon D. O.; Chae S. C.; Quackenbush N. F.; Fischer D. A.; Woicik J. C.; Guo J.-H.; Cheong S. W.; Piper L. F. J. La-doped BaSnO3—Degenerate perovskite transparent conducting oxide: Evidence from synchrotron x-ray spectroscopy. Appl. Phys. Lett. 2013, 103, 042105. 10.1063/1.4816511. [DOI] [Google Scholar]
- Deák P.; Aradi B.; Frauenheim T. Quantitative theory of the oxygen vacancy and carrier self-trapping in bulk TiO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 86, 195206. 10.1103/PhysRevB.86.195206. [DOI] [Google Scholar]
- Deák P.; Aradi B.; Frauenheim T. Oxygen deficiency in TiO2: Similarities and differences between the Ti self-interstitial and the O vacancy in bulk rutile and anatase. Phys. Rev. B: Condens. Matter Mater. Phys. 2015, 92, 045204. 10.1103/PhysRevB.92.045204. [DOI] [Google Scholar]
- Quesada-Gonzalez M.; Williamson B. A. D.; Sotelo-Vazquez C.; Kafizas A.; Boscher N. D.; Quesada-Cabrera R.; Scanlon D. O.; Carmalt C. J.; Parkin I. P. Deeper Understanding of Interstitial Boron-Doped Anatase Thin Films as A Multifunctional Layer Through Theory and Experiment. J. Phys. Chem. C 2018, 122, 714–726. 10.1021/acs.jpcc.7b11142. [DOI] [Google Scholar]
- Navas J.; Sánchez-Coronilla A.; Aguilar T.; Hernández N. C.; de los Santos D. M.; Sánchez-Márquez J.; Zorrilla D.; Fernández-Lorenzo C.; Alcántara R.; Martín-Calleja J. Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835–3845. 10.1039/c3cp54273d. [DOI] [PubMed] [Google Scholar]
- Pongwan P.; Wetchakun K.; Phanichphant S.; Wetchakun N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 2016, 42, 2815–2830. 10.1007/s11164-015-2179-y. [DOI] [Google Scholar]
- Yoong L. S.; Chong F. K.; Dutta B. K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. 10.1016/j.energy.2009.07.024. [DOI] [Google Scholar]
- Shannon R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A: Found. Crystallogr. 1976, 32, 751–767. 10.1107/s0567739476001551. [DOI] [Google Scholar]
- Mathew S.; Ganguly P.; Rhatigan S.; Kumaravel V.; Byrne C.; Hinder S.; Bartlett J.; Nolan M.; Pillai S. Cu-doped TiO2: visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. 10.3390/app8112067. [DOI] [Google Scholar]
- Kernazhitsky L.; Shymanovska V.; Gavrilko T.; Naumov V.; Fedorenko L.; Kshnyakin V.; Baran J. Room temperature photoluminescence of anatase and rutile TiO2 powders. J. Lumin. 2014, 146, 199–204. 10.1016/j.jlumin.2013.09.068. [DOI] [Google Scholar]
- Varley J. B.; Janotti A.; Van de Walle C. G. Mechanism of visible-light photocatalysis in nitrogen-doped TiO2. Adv. Mater. 2011, 23, 2343–2347. 10.1002/adma.201003603. [DOI] [PubMed] [Google Scholar]
- Santara B.; Giri P. K.; Imakita K.; Fujii M. Evidence for Ti interstitial induced extended visible absorption and near infrared photoluminescence from undoped TiO2 nanoribbons: an in situ photoluminescence study. J. Phys. Chem. C 2013, 117, 23402–23411. 10.1021/jp408249q. [DOI] [Google Scholar]
- Wang X.; Feng Z.; Shi J.; Jia G.; Shen S.; Zhou J.; Li C. Trap states and carrier dynamics of TiO2 studied by photoluminescence spectroscopy under weak excitation condition. Phys. Chem. Chem. Phys. 2010, 12, 7083–7090. 10.1039/b925277k. [DOI] [PubMed] [Google Scholar]
- Abazović N. D.; Čomor M. I.; Dramićanin M. D.; Jovanović D. J.; Ahrenkiel S. P.; Nedeljković J. M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B 2006, 110, 25366–25370. 10.1021/jp064454f. [DOI] [PubMed] [Google Scholar]
- Stefan M.; Pana O.; Leostean C.; Bele C.; Silipas D.; Senila M.; Gautron E. Synthesis and characterization of Fe3O4–TiO2 core-shell nanoparticles. J. Appl. Phys. 2014, 116, 114312. 10.1063/1.4896070. [DOI] [Google Scholar]
- Kernazhitsky L.; Shymanovska V.; Gavrilko T.; Naumov V.; Fedorenko L.; Kshnyakin V. Photoluminescence and Optical Absorption of Pure Nanocrystalline TiO2 Anatase and Rutile at Room Temperature. J. Nano- Electron. Phys. 2013, 5, 03047-1. [Google Scholar]
- Mathew S.; kumar Prasad A.; Benoy T.; Rakesh P. P.; Hari M.; Libish T. M.; Radhakrishnan P.; Nampoori V. P. N.; Vallabhan C. P. G. UV-visible photoluminescence of TiO2 nanoparticles prepared by hydrothermal method. J. Fluoresc. 2012, 22, 1563–1569. 10.1007/s10895-012-1096-3. [DOI] [PubMed] [Google Scholar]
- Lyons J. L.; Alkauskas A.; Janotti A.; Van de Walle C. G. First-principles theory of acceptors in nitride semiconductors. Phys. Status Solidi B 2015, 252, 900–908. 10.1002/pssb.201552062. [DOI] [Google Scholar]
- Frodason Y.; Johansen K.; Bjørheim T.; Svensson B.; Alkauskas A. Zn vacancy as a polaronic hole trap in ZnO. Phys. Rev. B 2017, 95, 094105. 10.1103/physrevb.95.094105. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.