Abstract
More than 40 compounds have been formally licensed for clinical use as antiviral drugs, and half of these are used for the treatment of HIV infections. The others have been approved for the therapy of herpesvirus (HSV, VZV, CMV), hepadnavirus (HBV), hepacivirus (HCV) and myxovirus (influenza, RSV) infections. New compounds are in clinical development or under preclinical evaluation, and, again, half of these are targeting HIV infections. Yet, quite a number of important viral pathogens (i.e. HPV, HCV, hemorrhagic fever viruses) remain in need of effective and/or improved antiviral therapies.
I. Introduction
There are at present a forty some antiviral drugs that have been formally licensed for clinical in the treatment of viral infections.31 These are mainly used in the treatment of infections caused by human immunodeficiency virus (HIV), hepatitis B virus (HBV), herpesviruses [herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV)], orthomyxoviruses (influenza), paramyxoviruses [respiratory syncytial virus (RSV)], and hepaciviruses [hepatitis C virus (HCV)]. As these are the viruses that are most in demand of antiviral therapy, they have prompted the search for new antiviral strategies and drugs directed towards either the same molecular targets as the approved antiviral drugs or to other targets.
Most of the newly described antiviral compounds (that are currently in development) are targeted at HIV, HBV or HCV. Some are targeted at HSV, VZV or CMV, but, there are, in addition, many other important viral pathogens for which medical intervention, either prophylactic or therapeutic, is highly needed, and, these are, among the DNA viruses, the papillomaviruses [human papilloma virus (HPV)], adenoviruses, poxviruses (variola, vaccinia, monkeypox, …) and the herpesviruses Epstein–Barr (EBV) and human herpesvirus type 6 (HHV-6), and, among the RNA viruses, enteroviruses (i.e. Coxsackie B and Echo), coronaviruses [i.e. severe acute respiratory syndrome (SARS)-associated coronavirus], flaviviruses (i.e. Dengue, Yellow fever) and other RNA viruses associated with hemorrhagic fever [arenaviruses (i.e. Lassa fever), bunyaviruses (i.e. Rift Valley fever, Crimean-Congo fever) and filoviruses (i.e. Ebola and Marburg)].
Here I will describe, for each viral family, (i) which are the antiviral drugs that have been formally approved, (ii) which are the compounds that are under clinical development and thus may be considered as antiviral drug candidates, and (iii) which compounds are in the preclinical stage of development and still have a long route ahead before they could qualify as antiviral drugs (Table 1 , Figure 1 ). The virus families to be addressed are the following: parvo-, polyoma-, papilloma-, adeno-, herpes-, pox-, picorna-, flavi-, corona-, orthomyxo-, paramyxo-, arena-, bunya-, rhabdo-, filo-, reo-, and retroviruses.
Table 1.
The past, present and future of antiviral drugs (Part II: RNA viruses except retroviruses)
| Virus | Compound |
||
|---|---|---|---|
| Approved for medical use | In clinical development | In preclinical evaluation | |
| Picorna (Entero, Rhino) | – | Pleconaril | Pyrrolidinyl pentenoic acid (ethyl ester) |
| Ruprintrivir | Mycophenolic acid (MPA) mofetil | ||
| Interferon (inducers) | |||
| Alpha and Flavi (Yellow fever, Dengue, West Nile, …) | – | – | Triaryl pyrazoline Interferon (inducers) |
| Hepaci (HCV) | Pegylated interferon-α | BILN 2061 (Ciluprevir) | SCH 446211 (SCH6) |
| combined with ribavirin | VX-950 (Telaprevir) | -C-methylcytidine | |
| NM 283 (Valopicitabine) | -O-methylcytidine | ||
| Viramidine (Taribavirin) | -C-methyladenosine | ||
| SCH 503034 | 7-Deaza--C-methyladenosine | ||
| DEBIO-025 | -C-methylguanosine | ||
| -Deoxy--fluoro--C-methylcytidine | |||
| -Azidocytidine (R1479) | |||
| DKA compd 30 | |||
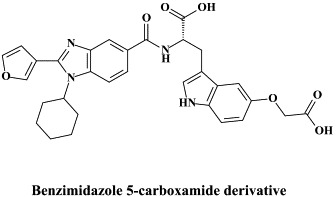
| Benzimidazole derivative | |||
| Benzimidazole 5-carboxamide derivative | |||
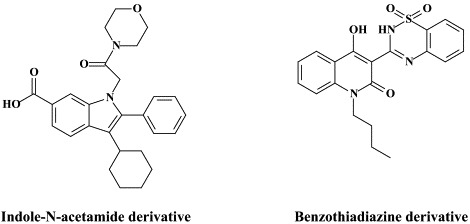
| Indole-N-acetamide derivative | |||
| Benzothiadiazine derivative | |||
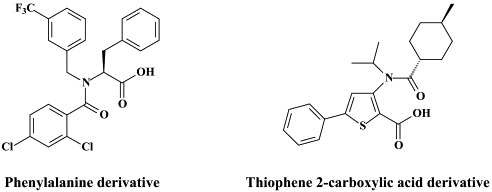
| Phenylalanine derivative | |||
| Thiophene 2-carboxylic acid derivative | |||
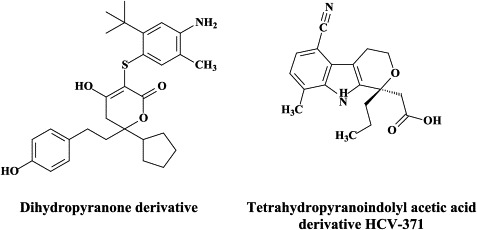
| Dihydropyranone derivative | |||
| Tetrahydropyranoindolyl acetic acid derivative HCV-371 | |||
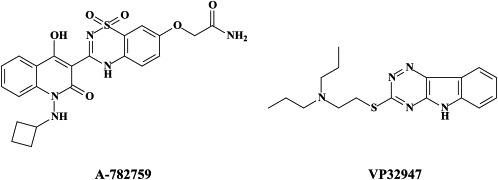
| N-1-Aza-4-hydroxyquinolone benzothiadiazine A-782759 | |||
| Pesti (BVDV) | VP32947 | ||
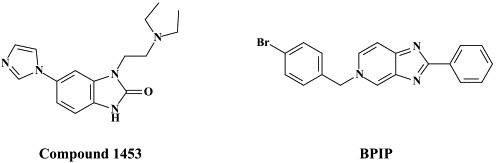
| Compound 1453 | |||
| BPIP | |||
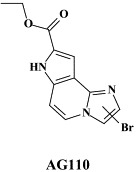
| AG110 | |||
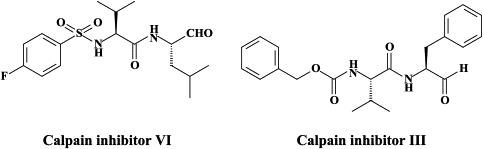
| Corona (SARS) | – | Pegylated interferon-α (“off label”) | Calpain inhibitors (III, VI) Niclosamide anilide |
| Phe–Phe dipeptide | |||
| TG-0205221 | |||
| Bananin | |||
| Valinomycin | |||
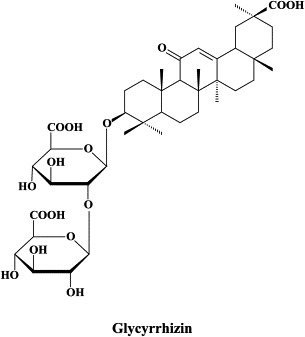
| Glycyrrhizin | |||
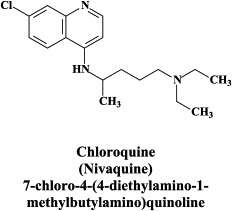
| Chloroquine | |||
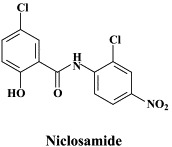
| Niclosamide | |||
| Nelfinavir | |||
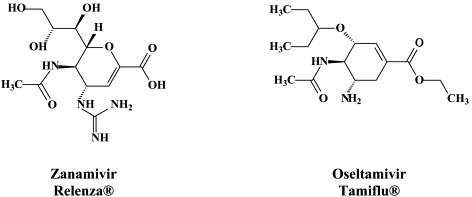
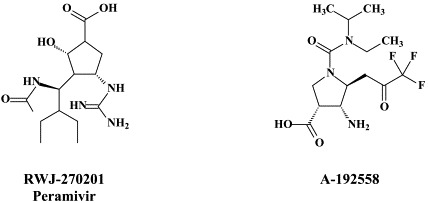
| Orthomyxo (Influenza) | Amantadine | – | RWJ-270201 |
| Rimantadine | A-192558 | ||
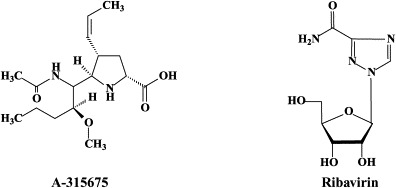
| Zanamivir | A-315675 | ||
| Oseltamivir | T-705 | ||
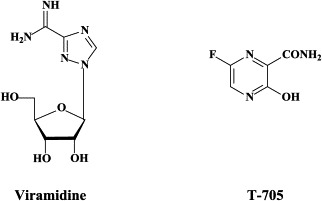
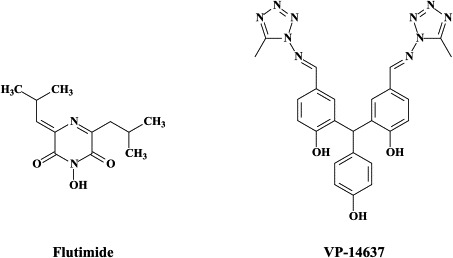
| Ribavirin (‘off label’) | Flutimide | ||
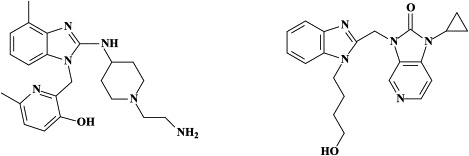
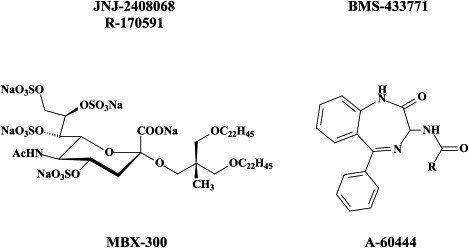
| Paramyxo (Parainfluenza, Measles, | Ribavirin (approved for RSV only) | – | VP-14637 JNJ-2408068 |
| Mumps, RSV, hMPV, …) | BMS-433771 | ||
| MBX-300 | |||
| A-60444 | |||
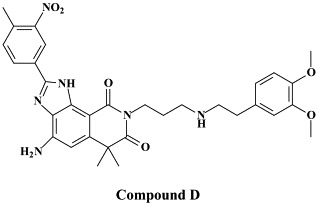
| Compound D | |||
| YM-53403 | |||
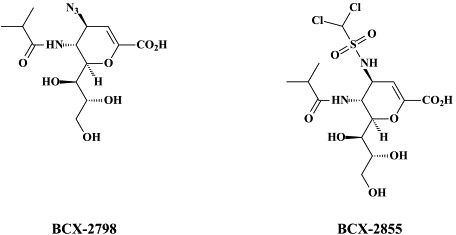
| BCX 2798 & BCX-2855 | |||
| Arena (Lassa, …) | Ribavirin (“off label”) | – | – |
| Bunya (Crimean–Congo, Rift | Ribavirin (“off label”) | – | – |
| Valley, …) | |||
| Rhabdo (Rabies) | – | – | – |
| Filo (Ebola, Marburg) | – | – | 3-Deazaneplancin A |
Figure 1.
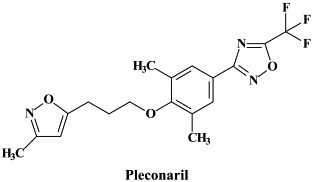
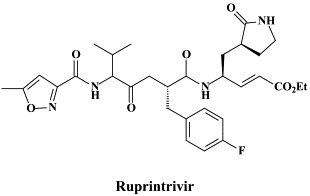
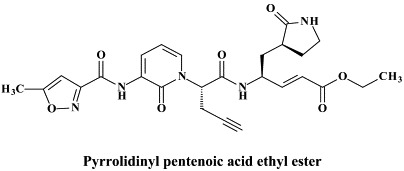
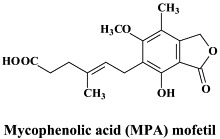
Structural formulae of antiviral compounds.
II. Picornaviruses (entero- and rhinoviruses)
Among the enteroviruses, polio and hepatitis A can be efficiently controlled by vaccination: for polio both a life attenuated and an inactivated (“killed”) virus vaccine, whereas for hepatitis A an inactivated virus vaccine is available. The other enteroviruses (Coxsackie A and B and echoviruses) and the rhinoviruses need to be approached by chemotherapeutic agents. No single antiviral drug has ever been licensed for clinical use against entero- or rhinovirus infections. The most extensively studied for its potential against enteroviruses has been pleconaril. This compound binds to a hydrophobic pocket beneath the “canyon floor” of the VP1 capsid protein of picornaviruses,93 thereby “freezing” the viral capsid and preventing its dissociation (uncoating) from the viral RNA genome. The clinical efficacy of pleconaril has been evaluated in experimentally induced enterovirus (Coxsackie A21) respiratory infections in adult volunteers145 and, on a compassionate basis, against potentially life-threatening enterovirus infections.142 Pleconaril has also been shown to reduce the duration and severity of picornavirus-associated viral respiratory illnesses in adolescents and adults.66., 67. Pleconaril would also shorten the course of enteroviral meningitis (as compared to placebo recipients), albeit only modestly in patients with more severe disease.37
For the prevention and/or treatment of rhinovirus infections (“common colds”) inhibitors of the human rhinovirus (HRV) 3C protease have been extensively investigated. Ruprintrivir is an irreversible 3C protease inhibitor,43 which, upon intranasal administration in human volunteers, appeared to be safe and well tolerated.75 In experimentally induced rhinovirus colds in healthy volunteers, ruprintrivir prophylaxis reduced the proportion of subjects with positive viral cultures but did not decrease the frequency of colds.68 Another, irreversible inhibitor of HRV 3C protease, here referred to as a pyrrolidinyl pentenoic acid ethyl ester [compound 344 or compound 1130] offers the advantage to be orally bioavailable: in healthy volunteers, single oral doses of this compound appeared to be safe and well tolerated, although the compound is currently not progressing toward clinical development.130
Despite extensive research efforts that have led to the discovery of many potent antiviral agents, no drug today has been approved for the treatment or prevention of rhinovirus-associated illnesses. There are several reasons to consider when trying to understand this situation.129 In the majority of individuals, rhinovirus-induced colds are mild and self-limiting. This alone dictates that potential drugs must be very safe and have a high risk/benefit ratio. The agent must have limited side effects and have no or low risk of resistance development and, furthermore, must be administered with low frequency (e.g. less than three times a day). To date no agent has been able to achieve these criteria and demonstrate appropriate clinical efficacy.129
In great need of antiviral treatment are the often severe complications of Coxsackie B virus infections, such as myocarditis which may lead to idiopathic dilated cardiomyopathy. In mice, Coxsackie B3 virus-induced myocarditis is inhibited by the immunosuppressive agent mycophenolic acid (MPA) mofetil.124 This beneficial outcome must apparently result from the immunosuppressive effect of MPA (through inhibition of IMP dehydrogenase and, hence, GTP supply), since MPA did not reduce the infectious virus titers in the myocard. A more pronounced inhibitory effect on Coxsackie B3 virus-induced myocarditis, accompanied by a marked reduction in the virus titers in the heart, was obtained with the interferon inducer poly(I)⋅poly(C) and poly(I)⋅poly(C12U) (also known as Ampligen), and to a lesser extent with (pegylated) interferon-α2b.125 Combination of an inhibitor of viral replication (such as Ampligen) with an immunosuppressant (such as MPA mofetil) could be an ideal treatment strategy for viral myocarditis, whether due to Coxsackie B or other viruses. How to implement such as treatment regimen in the clinical setting should be further addressed.
To investigate whether RNA interference (RNAi) can protect against Coxsackie virus B3 infection, several Coxsackievirus B3-specific small interfering RNAs (siRNAs) targeting distinct regions of the viral genome were evaluated, the most effective one (in inhibiting virus replication) being that targeting the viral protease 2A.200 A primordial requirement for being effective was a perfect sequence match in the central region of the target.200 As shown for poliovirus,54 the virus may readily escape from RNA interference (RNAi) through unique point mutations (i.e. resulting in G:U mismatches) within the targeted regions; however, the emergence of resistant virus could be prevented by using a pool of siRNAs to simultaneously target multiple sites in the viral genome.54
III. Alpha- and flaviviruses (yellow fever, dengue, west nile, …)
No antivirals are currently available for the treatment of alpha- or flavivirus infections (although there is a live virus vaccine routinely used for the prophylaxis of Yellow fever), and the prospects for an effective therapy of flavivirus infections do not seem encouraging.96 Antiviral compounds such as ribavirin have only weak activity against flaviviruses. Greater hope may be vested in interferon and interferon inducers. Based on infection of hamsters with the murine Modoc virus, an experimental flavivirus encephalitis model has been developed, which is reminiscent of Japanese encephalitis virus infection in humans.97 In a related model with Modoc virus in SCID mice, both interferon-α2b (whether pegylated or not) and interferon inducers [poly(I)⋅poly(C) and Ampligen) were shown to significantly delay virus-induced morbidity (paralysis) and mortality (due to progressive encephalitis).98 Ribavirin did not provide any beneficial effect in this model, whether given alone or in combination with interferon.
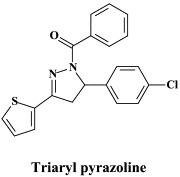
Recently, new inhibitors of flavivirus infection have been identified through high-throughput screening of a compound library. In particular, triaryl pyrazoline {[5-(4-chloro-phenyl)-3-thiophen-2-yl-4,5-dihydro-pyrazol-1-yl]-phenyl-methanone} was found to inhibit the in vitro replication of a number of flaviviruses (i.e. West Nile, Dengue, Yellow fever, St. Louis encephalitis) as well as some other viruses such as Western equine encephalitis virus (an alphavirus), mouse hepatitis virus (a coronavirus) and vesicular stomatitis virus (a rhabdovirus).134 The triaryl pyrazoline would be specifically targeted at viral RNA synthesis.134
RNA interference (RNAi) as demonstrated for hepadna-, picorna-, hepaci-, corona-, myxo- and retroviruses may also be further explored as an antiviral approach to control alpha- and flaviviruses, for example, at the level of their vector mosquitoes. RNA interference acts as a natural antiviral response to infection of Anopheles gambiae by the alphavirus O'nyong-O'nyong82 and for two mosquito-borne viruses, Semliki Forest virus (SFV) and Dengue virus (serotype 1), it has been shown that their replication in mosquito cells could be specifically blocked by si (double-stranded) RNA.13 This indicates that the use of specific siRNAs may inhibit virus replication in the insect host and thus prevent disease transmission.
IV. Hepaciviruses (HCV)
Current, approved therapy for chronic hepatitis C consists of pegylated interferon-α2 (180 μg, parenterally, once weekly) combined with ribavirin (1000 or 1200 orally, daily).49., 112. This treatment regimen is associated with a sustained viral response in at least 50% of the patients infected with HCV genotype 1, and of 80% in patients infected with another genotype (2, 3 or 4) of HCV. Duration of treatment is 48 weeks (or longer) for patients infected with HCV genotype 1, but may be reduced to 24 weeks for patients infected with another genotype.
Although interferon is generally acting as an immunomodulatory agent (i.e. in the treatment of hepatitis B) and ribavirin is an antiviral agent, when the two agents are used in combination against hepatitis C, they appear to act the other way around.31 Interferon appears to be targeted at the phosphoprotein encoded by the non-structural NS5A gene of the HCV genome.166 thus achieving its antiviral effect, whereas ribavirin, akin to MPA, primarily acts as an inhibitor of IMP dehydrogenase, thus reducing the biosynthesis of GTP. Ribavirin has recently been shown to modulate T-cell reactivity to HCV, i.e., by suppressing IL-10 production.140
The combination of peginterferon α-2a with ribavirin has also been advocated for the therapy of HCV infection in patients with HIV coinfection.178 However, it should not be forgotten that, should these patients be treated (for their HIV infection) with azidothymidine (zidovudine, ZDV), the latter may be antagonized by ribavirin.180 Therefore it was re-assuring to note that ribavirin (at 800 mg/day) administered in combination with peginterferon α-2a did not significantly affect the intracellular phosphorylation or plasma pharmacokinetics of ZDV (or other pyrimidine dideoxynucleosides such as 3TC or d4T) in HIV/HCV-co-infected patients.141
In addition to peginterferon α-2a and peginterferon α-2b, other interferons are in clinical development, e.g. albuferon-α (IFN-α-2b fused to human serum albumin) which allows dosing at intervals of 2–4 weeks compared with one week for the peginterferons.30 Consensus interferon (i.e. alfacon-1), when combined with ribavirin, has been shown to achieve a higher sustained response rate in naïve patients with chronic hepatitis C as compared to standard IFN-α and ribavirin.30 Recently, a novel IFN-α variant (GEA 007.1) has been described which would have a better inhibitory activity than the standard IFN-α2b in the HCV replicon system, due to a more potent activation of the JAK-STAT signaling pathway.48
In the combination with peginterferon, ribavirin may be advantageously replaced by viramidine (taribavirin), its amidine analogue (which is converted, mainly in hepatocytes, by adenosine deaminase, to ribavirin), as the latter has a reduced uptake by, and, therefore, lesser toxicity for red blood cells, as compared to ribavirin.186 Viramidine would give less anemia as compared with ribavirin. Phase III studies with viramidine combined with peginterferon, as compared to ribavirin combined with peginterferon, are eagerly awaited to assess which one to choose, ribavirin or viramidine.
Taking into account the duration of the combined interferon plus ribavirin treatment, the therewith associated side effects and costs, and the partial responses observed with this treatment regimen, fierce attempts have been made, rightfully, to develop more selective anti-HCV agents, targeted at specific viral proteins such as the NS3.4A serine protease and RNA helicase, and the NS5B RNA replicase (RNA-dependent RNA polymerase). Also the HCV p7 protein, which forms an ion channel and can be blocked by long-alkyl-chain iminosugar derivatives, has been considered as a potential target for antiviral therapy.131
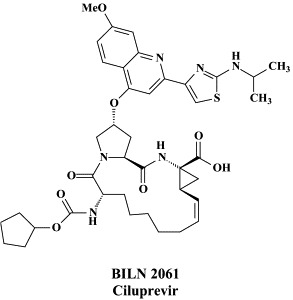
Proof-of-principle that compounds targeted at the NS3.4A protease could reduce plasma concentrations of HCV RNA has already been delivered with BILN 2061 administered orally for no longer than 2 days in patients infected with HCV genotype 1.89 BILN 2061 (Ciluprevir), for which an efficient large-scale synthetic procedure has been described recently,196 was able to reduce HCV RNA levels by 2–3 in patients infected with HCV genotype 1, after 2 days of treatment,72 but in patients infected with HCV genotypes 2 or 3, it proved less effective, apparently due to a lower affinity of BILN 2061 for the HCV protease of genotypes 2 and 3, as compared to genotype 1.138 Replacement of five residues at positions 78, 79, 80, 122, and 132 could account for most of the reduced sensitivity of genotype 2b protease,169 while replacement of residue 168 alone,169 or in combination with substitution of residues at positions 123 and 132,177 could account for the reduced sensitivity of genotype 3a. Apparently, the rigidity of BILN 2061, while conferring greater potency against genotype 1, rendered it more sensitive to variations near its binding site at the NS3.4A protease.177 Despite the robust antiviral response observed with BILN 2061 in genotype 1 HCV-infected individuals, further clinical development of this compound was halted because of (cardio)toxicity issues in animals.
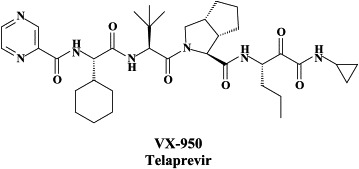
Another NS3.4A protease inhibitor, which differs in its in vitro resistance profile from BILN 2061, is VX-950 (telaprivir).102 While substitution of Ala156 with either valine (A156V) or threonine (A156T) led to cross-resistance between BILN 2061 and VX-950 [but also reduced fitness (or replication capacity) in a transient replicon cell system,103 the major BILN 2061-resistant mutants (D168V and D168A) were fully susceptible to VX-950, and, vice versa, the dominant VX-950-resistant mutant (A156S) remained sensitive to BILN 2061.102., 103. Thus, VX-950 and BILN 2061 must elicit resistance to HCV protease (NS3.4A) by different mechanisms.103
VX-950 (750 mg every 8 hours) was found to achieve, at the end of a 14 day-treatment, a main reduction of HCV RNA of 4.4 . Out of all patients receiving VX-950, 26/28 showed a >3 log decline of HCV RNA.137 [In some patients dosed with VX-950, the virus became undetectable at day 14 of dosing.] The overall preclinical profile of VX-950 supports its candidacy as a novel oral therapy against hepatitis C.132 The combination of VX-950 and interferon-α was found to be additive to synergistic in reducing HCV RNA in replicon cells, and this combination also suppressed the emergence of in vitro resistance mutations against VX-950 in replicon cells.104 Following promising results with VX-950 in phase I clinical trials, the compound has now progressed to phase II trials where it is evaluated in combination with peginterferon-α with or without ribavirin.
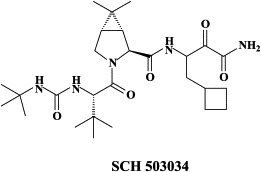
In addition to VX-950, other, 7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid-based macrocyclic inhibitors of HCV NS3.4A protease20 as well as SCH 503034, a mechanism-based inhibitor of HCV NS3.4A protease,109., 179. are in preclinical development. In fact, the latter was found to act synergistically with α-interferon in suppressing HCV replicon synthesis.109 SCH 503034 [compound 70 in Venkatraman et al.179] demonstrated good oral bioavailability in rats and dogs179 and has been advanced to clinical trials in humans for the treatment of HCV infections. SCH 503034 at the highest dose used 400 mg three times daily for 14 days achieved a 1.5 decline in viremia201 SCH 503034 is now being evaluated in combination with pegylated interferon-α, with or without ribavirin in ongoing 48-week phase II studies.
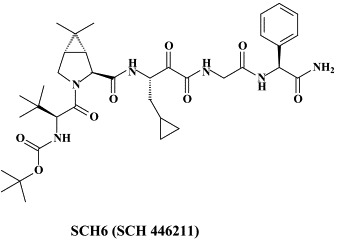
The HCV protease inhibitor SCH 503034 selected for a number of mutations, i.e. T54A, V170A, A156S and A156T; the A156T mutation conferred the highest level of resistance to SCH 503034 but also led to the greatest reduction in fitness.176 The A156T mutation also conferred high level resistance to SCH6 (SCH 446211), a novel ketoamide (structurally related to SCH 503034) inhibitor of the NS3.4A protease.198 A novel mutation, R109K, was identified which conferred moderate resistance only to SCH6. Unlike R109K which had minimal impact on NS3.4A enzymatic activity, A156T significantly reduced the enzymatic activity, polyprotein processing and replication fitness.198 However, three separate second-site mutations, P89L, Q86R and G162R were capable of partially reversing A156T-associated fitness without significantly reducing resistance to the protease inhibitor.198
In addition to the NS3.4A protease, the NS5B RNA replicase has also been perceived as an attractive target for the development of HCV inhibitors. Highly potent and selective antiviral agents (i.e. VP32947) [N-propyl-N-[2-(2H-1,2,4-triazino[5,6-b]indol-3-ylthio)ethyl]-1-propanamine]5 -and compound 1453 [1-[2-diethylamino)ethyl]-6-(1H-imidazol-1-yl)-1,3-dihydro-2H-benzimidazol-2-one]165 targeted at the viral RNA replicase have been described to inhibit the replication of bovine viral diarrhoea virus (BVDV), a pestivirus which could be considered as a surrogate virus for HCV5., 165. We have recently described two novel series of compounds [prototypes: 5-[(4-bromophenyl)methyl]-2-phenyl-5H-imidazo[4,5-c]pyridine (BPIP)]126 and ethyl-2-methylimidazo[1,2-a]pyrrolo[2,3-c]pyridin-8-carboxylate AG110,128 which act as “non-nucleoside” RNA replicase inhibitors (NNRRIs) and effect a highly potent and selective inhibition of the replication of BVDV.126., 128. From the BPIP class of compounds,135 new congeners have been derived that act equally efficiently against HCV replication.136 In future treatment strategies for HCV infections, these NNRRIs may likely to be combined with “nucleoside” RNA replicase inhibitors (NRRIs), in analogy with the strategy followed for the treatment of HIV infections, where NRTIs are combined with NNRTIs (see infra).
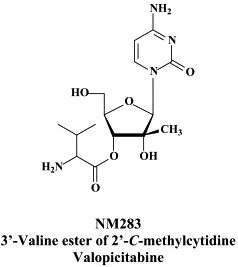
The sole NRRI which has already proceeded to phase I/II clinical trials for the therapy of hepatitis C is the 3′-O-valine ester of 2′-C-methylcytidine (NM-283, valopicitabine)I, which can be administered by the oral route2., 33. and shows enhanced antiviral efficacy if combined with peginterferon (valopicitabine is currently in phase II combination trials with pegylated interferon-α). It would be wise not to add ribavirin to this combination, as it has been shown that ribavirin antagonizes the in vitro anti-HCV activity of 2′-C-methylcytidine, the active compound of valopicitabine.28
HCV replicons that are resistant to 2′-C-methylcytidine (and 2′-C-methylpurine nucleosides) can be readily isolated in vitro, and resistance is the result of the S282T mutation in the NS5B gene. As a consequence of the S282T mutation, 2′-C-methyl CTP is no longer incorporated during the initiation step of RNA synthesis. In addition, the presence of the S282T mutation also compromises incorporation of the natural nucleotides, which may translate in decreased viral fitness.45
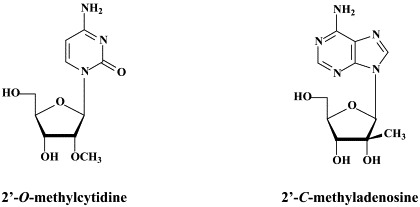
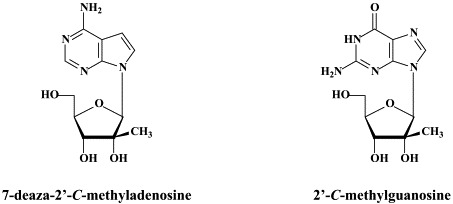
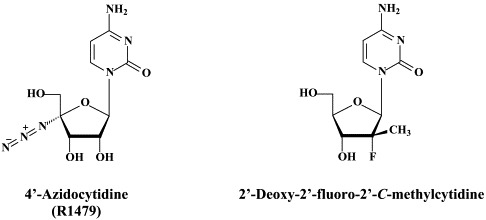
In addition to 2′-C-methylcytidine, several other ribonucleoside analogues (NRRIs) have been reported to inhibit HCV replication (as reviewed by):174 2′-O-methylcytidine,14, 2′-C-methyladenosine,14., 171. 7-deaza-2′-C-methyladenosine,122 2′-C-methylguanosine,46., 115. 2′-deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130),159 and 4′-azidocytidine (R1479).86
PSI-6130 is a specific inhibitor of HCV.159 It has no anti-BVDV activity. The fact that PSI-6130 shows reduced activity against the RdRp S282T mutant, which is resistant to 2′-C-methylated nucleosides suggests that PSI-6130 is an inhibitor of HCV RNA synthesis.159 In contrast, R1479 (4′-azidocytidine) did not show cross-resistance with 2′-C-methylcytidine.91 Thus, the S282T mutation86 did not confer resistance to R1479. In vitro studies mapped resistance to R1479 to amino acid substitutions S96T and S96T/N142T of the NS5B polymerase.91
All the aforementioned nucleoside analogues are, in principle, non-obligate chain-terminating nucleoside analogues. It would now seem interesting to prepare and evaluate the corresponding obligatory chain-terminating 3′-deoxyribonucleoside analogues for their anti-HCV activity. This was recently done for 2′-C-methylcytidine and 2′-C-methyluridine, which were modified at the C-3′ position.133 The newly synthesized compounds were quoted as antivirally inactive, but HCV did not figure among the viruses that were evaluated for their susceptibility to the compounds.
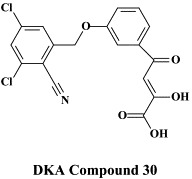
A new class of HCV NS5B RNA replicase inhibitors is represented by the -diketo acids (DKAs),162., 163. one of the more active DKAs being DKA compound 30. These compounds are reminiscent of the DKA type of HIV integrase inhibitors (see infra) and are assumed to inhibit the HCV polymerate activity via chelation of the active site Mg++ ions. In a certain sense they may be considered as “pyrophosphate mimics”, thus acting as product-like inhibitors of the polymerase reaction.174
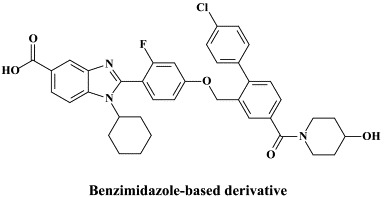
In recent years, a pleiade of NNRRIs has been described to act in a very similar (“allosteric”) fashion with HCV NS5B RNA replicase as NNRTIs do with respect to the HIV reverse transcripase: benzimidazole-based derivatives172 [i.e., benzimidazole 5-carboxamide derivatives,7., 88. indole-N-acetamide derivatives,39., 61. benzo-1,2,4-thiadiazine derivatives,38., 173. phenylalanine derivatives,183 thiophene 2-carboxylic acid derivatives,8., 17. dihydropyranone derivatives,107 the tetrahydropyranoindolyl acetic acid derivative HCV-371 (R-enantiomer of the racemic mixture HCV-570)73., 74. and the N-1-aza-4-hydroxyquinolone benzothiadiazine A-782759.117 The allosteric binding site for some of these compounds have already been identified by crystallographic studies.8., 17., 39., 183. It corresponds to a narrow cleft on the protein's surface in the “thumb” domain, about 30–35 Å from the enzyme's catalytic center.17., 183.
Curiously, most of the NNRRIs that are active against the HCV NS5B polymerase contain, besides a large hydrophobic region, a carboxylic acid group (or a similar motif) that allows hydrogen bonding with main chain amide nitrogen atoms (i.e. Ser 476 and Tyr 477, as demonstrated for the phenylalanine derivative).183 For the indole-N-acetamide derivatives, it has been suggested that they may displace part of the fingertip loop anchoring the fingers domain to the thumb domain.39 Using the thiophene 2-carboxylate type of inhibitors, it has been reported that these inhibitors can only be soaked in crystal form I (which adopts a “closed” conformation that is believed to be the active form, and not in form II (which adopts an “open” conformation, and is thus in the inactive form.8 Resistant mutations that emerged with the benzimidazole 5-carboxamide and related compounds were found at three amino acid positions in the thumb domain: Pro495 (P495S/L/A/T), Pro496 (P496S/A) and Val499 (V499A); mutation at each of these positions conferred different levels of resistance (in decreasing order P495S/L/A/T > P496S/A > 499A.88
Mutations conferring resistance to both the HCV NS5B RNA replicase (i.e. H95Q, N411S, M414L, M414T or Y448H) and NS3 protease (i.e. A156V or D168V) have been identified).117 These mutations conferred high levels of resistance to A-782759 and BILN 2061, respectively. However, the A-782759-resistant mutants remained susceptible to the NRRIs and other classes of NNRRIs, as well as interferon. In addition, the dually (A-782759- and BILN 2061-) resistant mutants displayed significantly reduced replicative ability as compared to the wild-type. These findings support a rationale for drug combinations in the therapy of HCV infections.117
As recently reviewed,30 several other approaches, including ribozymes, antisense oligonucleotides, and RNA interference (RNAi) based on short interfering (si)RNAs could be envisaged to target the HCV genome. ISIS 14803, a 20-base antisense oligonucleotide complementary to the internal ribosome entry site (IRES), in a phase I clinical trial gave transient HCV reduction (1.2–1.7 ) in 3/28 patients but ALT (alanyl transaminase) flares up to 10-fold in 5/28 patients.113
Short interfering (si)RNAs, aimed at posttranscriptional gene silencing, may be considered an attractive approach to curtail HCV infections.87 The siRNA approach has proven to be efficacious in vivo, in suppressing (SARS) coronavirus infections100 and influenza A virus infections52., 175. in monkeys100 and mice,52., 175. respectively.
From the screening of various marketed compounds, arsenic trioxide (As2O3) emerged as a potent HCV inhibitor.77 Similarly, sodium stibogluconate (SSG), along with several other antimonial compounds, including Sb2O3 and SbCl3 were found to exert potent anti-HCV activity at concentrations that did not affect cell viability.78 When SSG was combined with interferon-α, these two drugs acted synergistically to suppress HCV replication.78
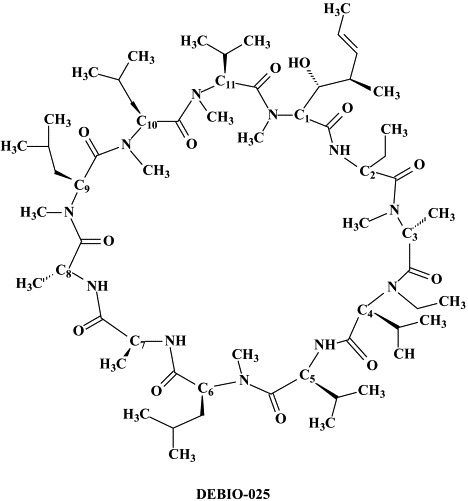
Various cellular targets may also be envisaged to interfere with HCV infections. In this sense, α-glucosidase inhibitors such as deoxynojirimycin have been shown to disturb the morphogenesis of HCV-like particles and may eventually be useful to fight HCV infections as part of drug combination protocols.18 Another cellular target for chemotherapeutic intervention is cyclophilin B (CyP B) which is critical for the efficient replication of the HCV genome. CyP B is a functional regulator of the HCV RNA polymerase.185 Cyclosporin A which interacts with CyP B inhibits HCV replication in vitro.185 We have recently demonstrated that the non-immunosuppressive cyclosporin DEBIO-025 is even 10-fold more potent an inhibitor of HCV replication.127 DEBIO-025 proved also additive to slightly synergistic when combined with interferon-α2a, and at concentrations of 0.5 and 1 μg/ml was able to clear the cells from their HCV replicon within three to four passages, whereas treatment with cyclosporin A at the same concentrations for seven consecutive passages did not result in clearance of the HCV replicon.127 DEBIO-025 may form an attractive new option for the therapy of HCV infections, particularly in HCV/HIV co-infected patients,127 and the clinical studies with DEBIO-025 have been recently initiated.
V. Coronaviruses (SARS)
As for HCV, there are several proteins encoded by the SARS coronavirus which could be considered as targets for chemotherapeutic intervention: i.e. the spike (S) protein, the 3C-like main protease, the NTPase/helicase, the RNA-dependent RNA polymerase (RNA replicase), and, possibly, other viral (or cellular) protein-mediated processes.157 The SARS coronavirus S protein mediates infection of permissive cells through interaction with its receptor, the angiotensin-converting enzyme 2 (ACE 2),101 and monoclonal antibody to the S1 domain was found to neutralize the virus by blocking its association with the receptor ACE 2.161
Also the fusion of the SARS coronavirus with the cell could be considered an attractive target. To the extent that this fusion process bears resemblance to the fusogenic mechanism of HIV, i.e. with regard to heptad repeat interactions and six-helix bundle formation, it might be feasible to develop SARS coronavirus inhibitors, analogous to the HIV fusion inhibitor enfuvirtide.105
Following receptor binding and induced conformational changes in the spike glycoprotein, a third step would be involved in the viral entry process, namely cathepsin L proteolysis within endosomes.154 The cathepsin-L-specific inhibitor, MDL 28170 [also known as calpain inhibitor III, or Z-Val-Phe(CHO)], at the same time inhibited cathepsin-L activity and S protein-mediated infection (at an IC50 of 2.5 nM and 0.1 μM, respectively). In addition to calpain inhibitor III, some other calpain inhibitors have been described as inhibitors of SARS coronavirus replication, the most selective (selectivity index >100) being calpain inhibitor VI [4-fluorophenylsulfonyl-Val-Leu(CHO)].6
The crystal structure of the SARS coronavirus protease has been revealed.94., 194. This offers a solid basis for the rational drug design of SARS protease inhibitors. For other potential targets such as the NTPase/helicase and the RNA replicase (RNA-dependent RNA polymerase) such structural basis still has to be delineated.
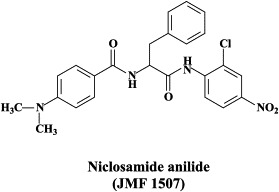
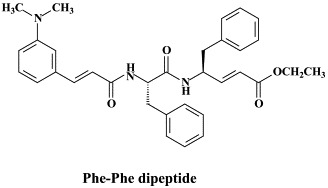
Of a number of peptidomimetic compounds (aziridinyl peptides, keto-glutamine analogues, chymotrypsin-like protease inhibitors and peptide anilides) that have been reported as inhibitors of the SARS coronavirus main protease, the niclosamide anilide, with a (), proved to the most potent (competitive) inhibitor.146 There are only a few cases where the 3C-like protease inhibitors were shown to inhibit both the SARS coronavirus protease activity and virus replication in cell culture. For example, the Phe–Phe dipeptide inhibitor was found to inhibit the 3C-like protease at an IC50 of 1 μM and inhibited virus replication in Vero cells at an EC50 of 0.18 μM, while not being toxic to the host cells at a concentration of 200 μM (selectivity index: >1000).147
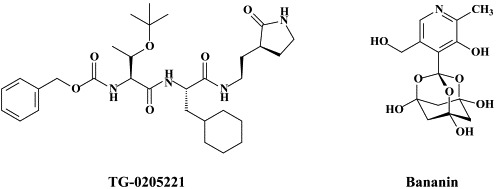
Recently, TG-0205221 was described as a potent SARS coronavirus 3C-like protease inhibitor (), which reduced virus production in cell culture by 4.7 at a concentration of 5 μM.195 An octapeptide, specifically designed for the SARS coronavirus main protease, namely AVLQSGFR, was reported to inhibit SARS coronavirus replication in Vero cells at an EC50 of 0.027 μg/ml, while not being cytotoxic at 100 μg/ml, thus establishing a selectivity index of >3700.51 Whether this highly selective antiviral effect was actually mediated by an inhibition of the SARS coronavirus main protease was not ascertained in this study.51
The SARS coronavirus NTPase/helicase has been considered a potential target for the development of anti-SARS agents.167. Bananin and three of its derivatives (iodobananin, vanillinbananin and eubananin) were shown to inhibit both the ATPase and helicase activity of the SARS coronavirus NTPase/helicase, with IC50 values (for the ATPase activity) in the range of 0.5–3 μM).168 Bananin was also found to inhibit SARS-CoV replication in fetal rhesus kidney (FRhK-4) cells at an EC50 of less than 10 μM and a CC50 of over 300 μM, thus exhibiting a selectivity index of over 30).168 Whether the antiviral effect obtained in cell culture was causally linked to inhibition of the NTPase/helicase was not ascertained.
The SARS coronavirus RNA-dependent RNA polymerase (RdRp), because of its pivotal role in viral replication, represents another potential target for anti-SARS therapy. This enzyme does not contain a hydrophobic pocket for non-nucleoside inhibitors similar to those that have proven effective against the HCV polymerase or HIV-1 reverse transcriptase.192 In fact, non-nucleoside HIV-1 reverse transcriptase inhibitors were shown to have no evident inhibitory effect on SARS coronavirus RdRp activity).21 At present, few, if any, nucleoside analogues have been recognized as specific inhibitors of the SARS coronavirus RdRp. There is N4-hydroxycytidine, which has been accredited with both anti-HCV and anti-SARS coronavirus effects. Against SARS coronavirus it proved active at an EC50 of 10 μM (selectivity index ⩾10)6 Whether this antiviral effect was mediated by an inhibition of the viral RdRp was not ascertained, however.
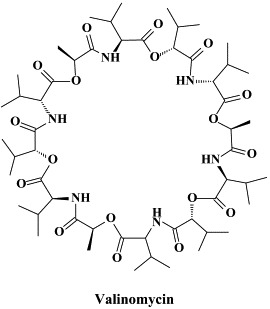
A wide variety of “old” and “new” compounds have been reported to inhibit the in vitro replication of the SARS coronavirus at relatively high concentration ().31 There is no shortage of small molecules that inhibit the replication of the SARS virus within the 1–10 μM (or higher) concentration range,188 but whether any of these molecules would be able to prevent or suppress SARS in vivo, remains to be determined. Typical examples of such miscellaneous compounds, often with an ill-defined mode of action but selectivity indexes up to 100, that have been reported to inhibit SARS coronavirus replication are valinomycin,188 glycyrrhizin,25 chloroquine,83 niclosamide189 and nelfinavir.193
Short interfering (si) RNAs have been developed that target the replicase70 and spike (S)202 genes of the SARS coronavirus genome, thereby silencing their expression in cell culture. Potent siRNA inhibitors of SARS coronavirus in vitro [i.e. the siRNA duplexes siSC2 (forward sequence: 5′-GCUCCUAAUUACACUCAACdtdt-3′) and siSC5 (forward sequence: 5′-GGAUGAGGAAGGCAAUUUAdtdt-3′), targeting the SARS coronavirus genome at S protein- and non-structural protein-12-coding regions, respectively] were further evaluated for their efficacy in a rhesus macaque SARS model),100 and found to provide relief from SARS coronavirus infection-induced fever, diminish SARS-CoV levels and reduce acute diffuse alveolar damage. Further studies with siRNA in prophylactic and therapeutic regimens against SARS coronavirus seem warranted.
Shortly after SARS coronavirus was identified as the causative agent of SARS, interferons were shown to inhibit the replication of SARS coronavirus in cell culture in vitro, interferon-β being more potent than either interferon-α or -γ.26 These observations were subsequently confirmed and extended in several other studies.71., 143., 158. Interferon-β, in conjunction with interferon-γ, was found to synergistically inhibit the replication of SARS coronavirus in Vero cells.143 Being a prophylactic rather than therapeutic agent, interferon(s) may have their highest utility in the prophylaxis or early post-exposure management of SARS. Pegylated interferon-α has been shown to reduce viral replication and excretion, viral antigen expression by type 1 pneumocytes and the attendant pulmonary damage in cynomolgous macaques that were infected experimentally with SARS coronavirus.58 Pegylated interferon-α is commercially available for the treatment of hepatitis C (where it is generally used in combination with ribavirin) and hepatitis B. Pegylated interferon-α as well as the other commercially available interferons (interferon-β, alfacon-1, etc.) could be considered for prevention and/or early post-exposure treatment of SARS should it re-emerge.
VI. Orthomyxoviruses (influenza A, B)
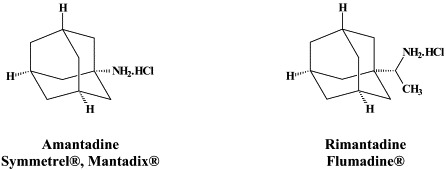
For many years, amantadine and rimantadine have been used for the prophylaxis and therapy of influenza A virus infections, but they never gained wide acceptance, primarily because of the risk of rapid emergence of drug-resistant virus mutants. These compounds interact specifically with the matrix M2 protein, which through its function as an hydrogen ion (H+) channel, helps in the decapsidation (“uncoating”) of the influenza A virus particles. Influenza A (H1N1) viruses harboring amantadine-resistance mutations are as virulent as wild-type virus strains1 and can be readily transmitted during antiviral pressure in the clinical setting.
In recent years, the neuraminidase inhibitors zanamivir181 and oseltamivir84 have become available for the therapy and/or prophylaxis of influenza A and B virus infections. Influenza has adopted a unique replication strategy by using one of its surface glycoproteins, hemagglutinin (H), to bind to the target cell receptor [which contains a terminal sialic acid (N-acetylneuraminic acid, NANA)], and another surface glycoprotein neuraminidase (N), to cleave off the terminal sialic acid, thus allowing the virus particles to leave the cells after the viral replicative cycle has been completed31 Neuraminidase inhibitors block the release of progeny virus particles from the virus-infected cells, thus preventing virus spread to other host cells.
When used therapeutically, neuraminidase inhibitors lead to a reduction in illness by 1–2 days, a reduction in virus transmission to household or healthcare contacts, a reduction in the frequency and severity of complications (such as sinusitis and bronchitis) and a diminished use of antibiotics.81 When used prophylactically, neuraminidase inhibitors significantly reduced the number of new influenza cases.69 Although resistance of human influenza viruses to neuraminidase inhibitors can develop,85 there is no evidence of naturally occurring resistance to either zanamivir or oseltamivir.114., 118. Zanamivir and oseltamivir should be effective against both influenza A and B, and, among influenza A, the prevailing variants H1N1, H3N2 and, also, the avian “flu” H5N1. Zanamivir, which must be taken through (oral) inhalation, and, in particular, oseltamivir, which can be more conveniently administered as oral capsules, should be stockpiled to affront a potential influenza pandemic in the future.123., 184. With the increasing threat of the avian “flu” (H5N1), the need for a sufficient supply of neuraminidase inhibitors, such as oseltamivir and zanamivir, has become extremely urgent. In comparison with the neuraminidase inhibitors, the existing influenza vaccines are likely to be of limited value against newly emerging influenza virus strains.
Following zanamivir and oseltamivir, similar structure-based neuraminidase inhibitors have been developed, such as the cyclopentane derivative RWJ-270201 (peramivir)152., 155. and the pyrrolidines A-192558182 and A-315675.36., 60. These novel neuraminidase inhibitors may themselves be considered as potential drug candidates, and, while being amenable to further optimization,110 lead to the development of yet newer compounds with improved activity, bioavailability and/or resistance profiles.
In fact, both peramivir (RWJ-270201) and A-315675 proved effective against a panel of five zanamivir-resistant and six oseltamivir-resistant A and B influenza virus strains.116 Oseltamivir resistance in clinical isolates of human influenza A has been associated with mutations at positions 119, 198, 274, 292 or 294 of the neuraminidase. Recently, resistance of avian influenza A H5N1 against oseltamivir was shown to be caused by the H274Y mutation.34., 92. The H274Y variant still appeared sensitive to zanamivir.92 Prominent among the other neuraminidase inhibitor-resistant influenza A (H3N2) virus mutations (not yet demonstrated for H5N1) are E119V and R292K: whereas the R292K mutation was associated with compromised virus growth and transmissibility, the growth and transmissibility of the E119V variant were comparable to those of wild-type virus.197
The emergence of antiviral drug resistance during oseltamivir treatment and its association with clinical failure in immunocompromised hosts79 and influenza A H5N1-infected patients34 has highlighted the need for alternative therapies and the use of antiviral combinations.63 The concept of using two or more antivirals to enhance the antiviral effects and perhaps reduce resistance emergence is decades old.62., 64., 90.
Are there other antiviral agents, besides amantadine, rimantadine and the neuraminidase inhibitors, which may be considered for their potential, in the prevention and/or therapy, of influenza A virus infections, including avian influenza (H5N1)? Ribavirin has since long been recognized as a broad-spectrum antiviral agent, with particular activity against both ortho- and paramyxoviruses.151 While earlier observations point to the lack of oral ribavirin (at 1 g daily) in naturally occurring influenza A (H1N1) virus infection,156 intravenous ribavirin (at 5 mg/kg/hour for 8 hours, followed by 1.5 mg/kg/hour for 2 to 6 days) may be worth exploring as a therapeutic strategy for serious influenza and parainfluenza virus infections.65
Recently, viramidine, the carboxamidine analogue of ribavirin was shown to have similar efficacy as ribavirin against influenza virus infections, and considering the lesser toxicity, viramidine may warrant further evaluation as a possible therapy for influenza, including H5N1.153 Yet, other recently described compounds with specific activity against influenza A, B and C viruses are T-705 (6-fluoro-3-hydroxy-2-pyrazine carboxamide) and the 2,6-diketopiperazine flutimide, which would target the viral polymerase50 and cap-dependent endonuclease,170 respectively. In fact, the polymerase (PA, PB1, PB2) complex contributes to the high virulence of avian influenza H5N1, and has been considered an attractive target for novel anti-influenza virus drug development.144
Other approaches to curtail a potential influenza virus epidemic include carbohydrate-binding molecules, such as the defensing retrocyclin 2, which inhibit viral fusion and entry by cross-linking membrane glycoproteins, and which have been shown to inhibit influenza A virus infection,95 as well as T- and M-tropic HIV-1 virus infections.29 Furthermore, as has been shown for several other virus infections, i.e. HCV infections87 and (SARS) coronavirus infections,70., 100., 202. short interfering RNAs (siRNAs) specific for conserved influenza virus genes may also be expected, and have in fact been demonstrated, to protect animals against highly pathogenic avian influenza A viruses.52., 175.
VII. Paramyxoviruses (parainfluenza, measles, mumps, RSV, hMPV, Nipah, …)
Of the paramyxoviruses, parainfluenza (types 1–5) has received little attention from either a preventative or curative viewpoint; mumps and measles, like the rubellivirus rubella, are now considered to be sufficiently contained by vaccination (although, despite existence of a vaccine, over half a million of deaths per year result from measles virus, which has prompted the search for fusion inhibitors that block measles virus entry),164 which makes RSV (respiratory syncytial virus) and hMPV (human metapneumovirus) as well as Nipah, the paramyxoviruses with the greatest need for antiviral therapy.
For RSV the only approved antiviral therapy is aerosol administration of ribavirin. In practice, however, ribavirin is rarely used owing to the technical burden of delivery by aerosol under the given circumstances (RSV bronchopneumonitis in young infants). Recently, intravenous ribavirin (together with oral corticosteroids) has proved to be a safe and cost-effective treatment for RSV infection after lung transplantation: RSV represents a risk factor for bronchiolitis obliterans syndrome, the major limiting factor for long-term survival after lung transplantation.55
Given the high incidence of RSV infections (which are often diagnosed as influenza), there is a high (an as yet unmet) medical need for an appropriate therapy (and prophylaxis) of RSV infections; The same holds for hMPV infections, which usually occur during the same (winter) season as RSV, mainly in young children, elderly people and immunocompromised individuals. Ribavirin certainly holds promise for the treatment of hMPV infections, as has recently been demonstrated in the mouse model for hMPV.59
As has been mentioned above for HCV,87 short interfering RNAs (siRNAs) may also be applicable, if properly designed and administered (intranasally) in the treatment of respiratory virus infections:9 using the RNA interference (RNAi) approach, Bitko et al.9 demonstrated that respiratory syncytial virus and parainfluenza virus infections, both individual and joint, could be specifically prevented and inhibited by siRNAs instilled intranasally in the mouse, with or without transfection reagents. In the case of measles, however, the RNAi approach may either prevent or enhance viral transcription, depending on the target mRNA [nucleocapsid (N), phosphoprotein (P) and polymerase (L) mRNA versus the matrix (M) mRNA].139 For avian metapneumovirus, one of the major causes of respiratory infections in poultry, siRNAs specific towards the phosphoprotein (P) led to marked inhibition of virus replication.119
Recently, a number of small molecules, i.e. VP-14637 and JNJ-2408068 (formerly known as R-170591), although structurally dissimilar, have been shown to fit into a small hydrophobic cavity in the inner core of the RSV fusion (F) protein, thereby interacting with the heptad repeats HR1 and HR2 domains, and to inhibit RSV fusion.4., 41., 42., 190. Although the therapeutic potential of these compounds in the treatment of RSV infections is presently unclear, there is no doubt that further exploration of the mechanism of interaction between these inhibitors and the F protein should facilitate the design of new RSV fusion inhibitors.42 In cotton rats, treatment by VP-14637 (or JNJ-2408068) small droplet aerosol for 60 min (or 15 min) significantly reduced mean lung virus titers.190., 191.
BMS-433771 was found to be a potent inhibitor of RSV replication in vitro:22 it exhibited excellent potency against multiple laboratory and clinical isolates of both A and B RSV with an average EC50 of 20 nM. BMS-433771 inhibits fusion the (viral and cellular) lipid membranes during both the early and virus entry stage and late-stage syncytium formation.22 BMS-433771 was shown to be orally active against RSV in BALB/c mice and cotton rats, even if administered as a single oral dose 1 hour prior to intranasal RSV inoculation.23 It could be considered the prototype of small-molecular-weight inhibitors that target the formation of the six helical coiled-coil bundles as a prelude to virus-cell fusion, not only of RSV but also HIV.24 In fact, starting from BMS-433771 new water-soluble (i.e. carboxylic acid-substituted benzimidazol-2-one derivatives have been synthesized with potent in vitro and in vivo activity against RSV.199
Peptides containing multiple copies of alternating HR1 and HR2 sequences of the terminal heptal repeats of the F-protein have been designed to inhibit F-protein-mediated fusion.120 MBX 300 or [2,2-bis(docosyloxymethyl)propyl-5-acetoamido-3,5-dideoxyl-4,7,8,9- tetra-O-(sodium-oxysulfonyl)-D-glycero-D-galacto-2-nonulopyranosid]-onate, on the other hand, inhibits RSV attachment to the cell by targeting the G-protein.40
RSV attachment/fusion inhibitors targeted at either the G- or F-protein have, despite their marked in vitro potency and in vivo efficacy in the cotton rat model, not made much progress in the clinical setting, in part because of their poor pharmacokinetic properties. Therefore, new RSV inhibitors acting at a target distinct from the attachment/fusion process have been searched for. One such target is the viral nucleocapsid (N) protein which appears to be the target for the 1,4-benzodiazepines (i.e. A-60444).15 The compound has entered phase II clinical trials (according to),150 but data have not yet been made available.
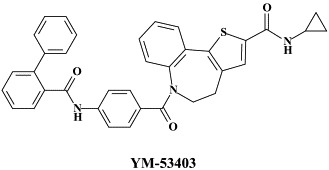
Another target protein worth pursuing for potential anti-RSV agents is the RNA-dependent RNA polymerase L-protein, which has been identified as the point of attack for 4-amino-8-(3-{[2-(3,4-dimethoxyphenyl)ethyl]amino}propyl)-6,6-dimethyl-2-(4-methyl-3-nitrophenyl)- 1H-imidazo[4,5-h]-isoquinoline-7,9()-dione (“Compound D”)106 and 6-{4-[(biphenyl-2-ylcarbonyl)amino]benzoyl}-N-cyclopropyl-5,6-dihydro-4H-thieno[3,2-d][1]benzazepine-2-carboxamide (YM-53403).160 Whether these compounds (“compound D” and YM-53403) have any potential in the therapy and/or prophylaxis of RSV infections remains to be further evaluated.
Although human parainfluenza viruses are important respiratory tract pathogens, especially in children, they have received little attention from either prophylactic (vaccine) or therapeutic viewpoint. Yet, they contain a unique target, the major surface glycoprotein hemagglutinin-neuraminidase (HN) that serves, at the same time, for cell attachment and virus spread. The HN inhibitors BCX 2798 and BCX 2855 were found to inhibit both functions, and to block infection with parainfluenza viruses both in vitro and in vivo 3 These compounds may limit parainfluenza virus infections in humans. Other compounds which may be further pursued for their activity against parainfluenza viruses, RSV, as well as influenza viruses, include flavonoids,187 uncinosides108 and polyoxotungstates,148 and diffaeoylquinic acid isolated from the ethnobotanical L-deflexicalyx.121
VIII. Arena-, bunya-, rhabdo- and filoviruses
Of the 23 arenaviruses known, five are associated with viral hemorrhagic fever: Lassa, Junin, Machupo, Guanarito and Sabia.19 Ribavirin has proven to be effective in the post-exposure prophylaxis and therapy of experimental arenavirus infections in animal models, and anecdotal reports suggest that it might also be effective in the treatment of arenavirus infections (i.e. Machupo and Sabia) in humans.19 The most convincing evidence for the (clinical) efficacy was obtained in the case of Lassa fever, where it was found to reduce the case-fatality rate, irrespective of the time point in the illness when treatment was started111
Of equal significance is that in a prospective, double-blind, placebo-controlled clinical trial, intravenous ribavirin therapy was shown to reduce mortality due to Hantaan infections [HFRS (Hemorrhagic Fever with Renal Syndrome)],76 which is not surprising as the (−)RNA bunyaviruses, and in particular, hantaviruses, are among the most sensitive RNA viruses to ribavirin in vitro.
Of the bunyaviruses, one of the most feared (because it is highly infectious, easily transmitted between humans, and associated with a case-fatality rate of approximately 30%) is Crimean–Congo hemorrhagic fever virus.27 Bunyaviruses are sensitive to ribavirin, and this has also been demonstrated in experimental animal models.149 Also, interferon and interferon inducers have proved effective in the treatment of experimental bunyavirus infections, and, likewise, interferon-α should be considered for the treatment of arenavirus infections, as warranted by its efficacy in the therapy of Pichinde virus infection in hamsters.56
Of the rhabdoviruses, rabies, which is almost invariably fatal if no control measures are taken, can be contained by repeated injections of specific immunoglobulin and/or the inactivated (“killed”) rabies vaccine as soon as possible after the infection. For the filovirus infections Ebola and Marburg no vaccine is (yet) available. Specific immunoglobulin or interferon-α may only be of limited value in the treatment of filovirus infections, as indicated by experimental findings in rhesus macaques infected with Ebola (Zaire) virus.80
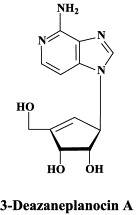
No antiviral drugs that are currently in clinical use, including ribavirin, provide meaningful protection against filoviruses in vivo.10 A possible therapeutic strategy may be based on the use of S-adenosylhomocysteine (SAH) hydrolase inhibitors.31 SAH hydrolase inhibitors, such as 3-deazaneplanocin A, interfere with S-adenosylmethionine (SAM)-dependent methylation reactions, particularly those involved in the “capping” of viral mRNA. Some viruses, such as the rhabdovirus VSV (vesicular stomatitis virus), heavily rely on mRNA capping and are particularly sensitive to inhibition by SAH hydrolase inhibitors, including 3-deazaneplanocin A.32
As, biochemically, filo- and rhabdoviruses are quite similar in their replication machinery, both requiring 5′-capping of their mRNAs, SAH hydrolase inhibitors such as 3-deazaneplanocin A may logically be expected to be effective in the treatment of Ebola virus infections. In fact, when administered as a single dose of 1 mg/kg, 3-deazaneplanocin A was found to protect mice against a lethal infection with Ebola virus (Zaire strain).11., 12. This protective effect was accompanied, and probably mediated, by the production of high concentrations of interferon in the Ebola virus-infected mice.12 It can be hypothesized that, by blocking the 5′-capping of the nascent (+)RNA viral strands (and, hence, their maturation towards mRNAs), 3-deazaneplanocin A stimulated the formation of double-stranded (±)RNA complexes, which have since long been known as excellent inducers of interferon.16
Like SAH hydrolase, IMP (inosine monophosphate) dehydrogenase is another cellular enzyme that may be envisaged as a target for antiviral agents. IMP dehydrogenase is a crucial enzyme involved in the biosynthesis of GTP, and, although ribavirin may act against distinct viruses by distinct mechanisms (i.e. IMP dehydrogenase inhibition, immunomodulatory effect, RNA capping interference, polymerase inhibition, lethal mutagenesis),57 the predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase.99
Recently, a new class of compounds, phosphorodiamidate morpholino oligomers (PMO), conjugated to arginine-rich cell-penetrating peptides (P-PMO) and designed to base pair with the translation start region of Ebolavirus VP35 positive-sense RNA, were reported to inhibit Ebolavirus replication and to protect mice against a lethal Ebolavirus infection.47 Similarly, siRNAs targeting Ebola virus (Zaire) polymerase (L) gene and delivered using SNALPs (stable nucleic acid–lipid particles) were found to completely protect guinea pigs against death when administered shortly after Ebola virus infection.53
IX. Reoviruses
Of the reoviruses, rotavirus, which is associated with viral gastrointestinal infections, is by far the most clinically important pathogen. Several attempts have been, and are still being, made to develop an effective vaccine for rotavirus infections. Current treatment for rotavirus diarrhoea is mainly based on the administration of fluids to prevent dehydration. There are no serious attempts to develop an antiviral drug for this disease, although it is noteworthy that the replication of reo- (and rota)viruses is exquisitely sensitive to SAH hydrolase inhibitors such as 3-deazaneplanocin A.32 Also, small interfering RNA (siRNA) corresponding to the VP4 gene has been shown to efficiently inhibit the synthesis of this protein, and to reduce the yield of viral progeny in virus-infected cells.35
X. Conclusion
About forty compounds are registered as antiviral drugs, at least half of which are used to treat HIV infections. An even greater number of compounds are under clinical or preclinical development, with again, as many targeting HIV as all the other viruses taken together. This implies that HIV, since its advent, has remained the main stay in antiviral drug development. Antiviral agents can, as guided by the anti-HIV agents as examples, be divided in roughly five categories:
-
(i)
nucleoside analogues,
-
(ii)
nucleotide analogues (or acyclic nucleoside phosphonates),
-
(iii)
non-nucleoside analogues,
-
(iv)
protease inhibitors, and
-
(v)
virus–cell fusion inhibitors.
Molecular targets are for (i) and (ii) the viral DNA polymerase (whether DNA-dependent as in the case of herpesviruses, or RNA-dependent as in the case of HIV or HBV); for (iii) RNA-dependent DNA polymerase (reverse transcriptase), associated with HIV, or RNA-dependent RNA polymerase (RNA replicase) associated with HCV; for (iv) the proteases associated with HIV and HCV; and for (v) the fusion process of HIV (and, potentially, other viruses such as the SARS coronavirus and RSV). Antiviral agents may also exert their antiviral effects through an interaction with cellular targets such as IMP dehydrogenase (ribavirin) and SAH hydrolase (3-deazaneplanocin A). The latter enzymes are essential for viral RNA synthesis (through the supply of GTP) and viral mRNA maturation (through 5′-capping), respectively. Finally, interferons (now generally provided in their pegylated form) may be advocated in the therapy of those viral infections (actually, HBV and HCV; prospectively, Coxsackie B, SARS, …) that, as yet, cannot be sufficiently curbed by other therapeutic measures.
References
- 1.Abed Y., Goyette N., Boivin G. Generation and characterization of recombinant influenza A (H1N1) viruses harboring amantadine resistance mutations. Antimicrob. Agents Chemother. 2005;49:556–559. doi: 10.1128/AAC.49.2.556-559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afdhal N., Godofsky E., Dienstag J. Final phase I/II trial results for NM283, a new polymerase inhibitor for hepatitis C: antiviral efficacy and tolerance in patients with HCV-1 infection, including previous interferon failures. Hepatology. 2004;40:726A. [Google Scholar]
- 3.Alymova I.V., Taylor G., Takimoto T., Lin T.H., Chand P., Babu Y.S., Li C., Xiong X., Portner A. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob. Agents Chemother. 2004;48:1495–1502. doi: 10.1128/AAC.48.5.1495-1502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andries K., Moeremans M., Gevers T., Willebrords R., Sommen C., Lacrampe J., Janssens F., Wyde P.R. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res. 2003;60:209–219. doi: 10.1016/j.antiviral.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Baginski S.G., Pevear D.C., Seipel M., Sun S.C., Benetatos C.A., Chunduru S.K., Rice C.M., Collett M.S. Mechanism of action of a pestivirus antiviral compound. Proc. Natl. Acad. Sci. USA. 2000;97:7981–7986. doi: 10.1073/pnas.140220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J., Sidwell R.W. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and β-D-N4-hydroxycytidine. Antiviral Chem. Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu P.L., Bos M., Bousquet Y., DeRoy P., Fazal G., Gauthier J., Gillard J., Goulet S., McKercher G., Poupart M.A., Valois S., Kukolj G. Non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase: discovery of benzimidazole 5-carboxylic amide derivatives with low-nanomolar potency. Bioorg. Med. Chem. Lett. 2004;14:967–971. doi: 10.1016/j.bmcl.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Biswal B.K., Cherney M.M., Wang M., Chan L., Yannopoulos C.G., Bilimoria D., Nicolas O., Bedard J., James M.N.G. Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors. J. Biol. Chem. 2005;280:18202–18210. doi: 10.1074/jbc.M413410200. [DOI] [PubMed] [Google Scholar]
- 9.Bitko V., Musiyenko A., Shulyayeva O., Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 10.Bray M. Defense against filoviruses used as biological weapons. Antiviral Res. 2003;57:53–60. doi: 10.1016/s0166-3542(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 11.Bray M., Driscoll J., Huggins J.W. Treatment of lethal Ebola virus infection in mice with a single dose of an S-adenosyl-L-homocysteine hydrolase inhibitor. Antiviral Res. 2000;45:135–147. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 12.Bray M., Raymond J.L., Geisbert T., Baker R.O. 3-Deazaneplanocin A induces massively increased interferon-alpha production in Ebola virus-infected mice. Antiviral Res. 2002;55:151–159. doi: 10.1016/s0166-3542(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 13.Caplen N.J., Zheng Z., Falgout B., Morgan R.A. Inhibition of viral gene expression and replication in mosquito cells by dsRNA-triggered RNA interference. Mol. Ther. 2002;6:243–251. doi: 10.1006/mthe.2002.0652. [DOI] [PubMed] [Google Scholar]
- 14.Carroll S.S., Tomassini J.E., Bosserman M., Getty K., Stahlhut M.W., Eldrup A.B., Bhat B., Hall D., Simcoe A.L., LaFemina R., Rutkowski C.A., Wolanski B., Yang Z., Migliaccio G., De Francesco R., Kuo L.C., MacCoss M., Olsen D.B. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 2003;278:11979–11984. doi: 10.1074/jbc.M210914200. [DOI] [PubMed] [Google Scholar]
- 15.Carter M.C., Alber D.G., Baxter R.C., Bithell S.K., Budworth J., Chubb A., Cockerill G.S., Dowdell V.C.L., Henderson E.A., Keegan S.J., Kelsey R.D., Lockyer M.J., Stables J.N., Wilson L.J., Powell K.L. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. J. Med. Chem. 2006;49:2311–2319. doi: 10.1021/jm051185t. [DOI] [PubMed] [Google Scholar]
- 16.Carter W.A., De Clercq E. Viral infection and host defense. Science. 1974;186:1172–1178. doi: 10.1126/science.186.4170.1172. [DOI] [PubMed] [Google Scholar]
- 17.Chan I., Das S.K., Reddy T.J., Poisson C., Prouix M., Pereira O., Courchesne M., Roy C., Wang W., Siddiqui A., Yannopoulos C.G., Nguyen-Ba N., Labrecque D., Bethell R., Hamel M., Courtemanche-Asselin P., L'Heureux L., David M., Nicolas O., Brunette S., Bilimoria D., Bedard J. Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 1: Sulfonamides. Bioorg. Med. Chem. Lett. 2004;14:793–796. doi: 10.1016/j.bmcl.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 18.Chapel C., Garcia C., Roingeard P., Zitzmann N., Dubuisson J., Dwek R.A., Trépo C., Zoulim F., Durante D. Antiviral effect of alpha-glucosidase inhibitors on viral morphogenesis and binding properties of hepatitis C virus-like particles. J. Gen. Virol. 2006;87:861–871. doi: 10.1099/vir.0.81503-0. [DOI] [PubMed] [Google Scholar]
- 19.Charrel R.N., de Lamballerie X. Arenaviruses other than Lassa virus. Antiviral Res. 2003;57:89–100. doi: 10.1016/s0166-3542(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen K.X., Njoroge F.G., Pichardo J., Prongay A., Butkiewicz N., Yao N., Madison V., Girijavallabhan V. Potent 7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid-based macrocyclic inhibitors of hepatitis C virus NS3 protease. J. Med. Chem. 2006;49:567–574. doi: 10.1021/jm050520a. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A., Zhang W., Xie Y., Jiang W., Arnold E., Sarafianos S.G., Ding J. Expression, purification, and characterization of SARS coronavirus RNA polymerase. Virology. 2005;335:165–176. doi: 10.1016/j.virol.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianci C., Yu K.-L., Combrink K., Sin N., Pearce B., Wang A., Civiello R., Voss S., Luo G., Kadow K., Genovesi E.V., Venables B., Gulgeze H., Trehan A., James J., Lamb L., Medina I., Roach J., Yang Z., Zadjura L., Colonno R., Clark J., Meanwell N., Krystal M. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob. Agents Chemother. 2004;48:413–422. doi: 10.1128/AAC.48.2.413-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cianci C., Genovesi E.V., Lamb L., Medina I., Yang Z., Zadjura L., Yang H., D'Arienzo C., Sin N., Yu K.L., Combrink K., Li Z., Colonno R., Meanwell N., Clark J., Krystal M. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob. Agents Chemother. 2004;48:2448–2454. doi: 10.1128/AAC.48.7.2448-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cianci C., Meanwell N., Krystal M.J. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. Antimicrob. Chemother. 2005;55:289–292. doi: 10.1093/jac/dkh558. [DOI] [PubMed] [Google Scholar]
- 25.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clement J.P. Hantavirus. Antiviral Res. 2003;57:121–127. doi: 10.1016/s0166-3542(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 28.Coelmont L., Paeshuyse J., Windisch M.P., De Clercq E., Bartenschlager R., Neyts J. Ribavirin antagonizes the in vitro anti-hepatitis C virus activity of 2′-C-methylcytidine, the active component of valopicitabine. Antimicrob. Agents Chemother. 2006;50:3444–3446. doi: 10.1128/AAC.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole A.M., Hong T., Boo L.M., Nguyen T., Zhao C., Bristol G., Zack J.A., Waring A.J., Yang O.O., Lehrer R.I. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornberg M., Manns M.P. Future trends in hepatitis C therapy. Future Virol. 2006;1:99–107. [Google Scholar]
- 31.De Clercq E. Antivirals and antiviral strategies. Nature Rev. Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Clercq E., Cools M., Balzarini J., Marquez V.E., Borcherding D.R., Borchardt R.T., Drach J.C., Kitaoka S., Konno T. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A, and their 5′-nor derivatives. Antimicrob. Agents Chemother. 1989;33:1291–1297. doi: 10.1128/aac.33.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Francesco R., Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 34.de Jong M.D., Tran T.T., Truong H.K., Vo M.H., Smith G.J., Nguyen V.C., Bach V.C., Phan T.Q., Do Q.H., Guan Y., Peiris J.S., Tran T.H., Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 35.Déctor M.A., Romero P., López S., Arias C.F. Rotavirus gene silencing by small interfering RNAs. EMBO Reports. 2002;3:1175–1180. doi: 10.1093/embo-reports/kvf234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeGoey D.A., Chen H.-J., Flosi W.J., Grampovnik D.J., Yeung C.M., Klein L.L., Kempf D.J. Enantioselective synthesis of antiinfluenza compound A-315675. J. Org. Chem. 2002;67:5445–5453. doi: 10.1021/jo0162890. [DOI] [PubMed] [Google Scholar]
- 37.Desmond R.A., Accortt N.A., Talley L., Villano S.A., Soong S.-J., Whitley R.J. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob. Agents Chemother. 2006;50:2409–2414. doi: 10.1128/AAC.00227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhanak D., Duffy K.J., Johnston V.K., Lin-Goerke J., Darcy M., Shaw A.N., Gu B., Silverman C., Gates A.T., Nonnemacher M.R., Earnshaw D.L., Casper D.J., Kaura A., Baker A., Greenwood C., Gutshall L.L., Maley D., DelVecchio A., Macarron R., Hofmann G.A., Alnoah Z., Cheng H.Y., Chan G., Khandekar S., Keenan R.M., Sarisky R.T. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 2002;277:38322–38327. doi: 10.1074/jbc.M205566200. [DOI] [PubMed] [Google Scholar]
- 39.Di Marco S., Volpari C., Tomei L., Altamura S., Harpers S., Narjes F., Koch U., Rowley M., De Francesco R., Migliaccio G., Carfi A. Interdomain communication in hepatitis C virus polymerase abolished by small molecule inhibitors bound to a novel allosteric site. J. Biol. Chem. 2005;280:29765–29770. doi: 10.1074/jbc.M505423200. [DOI] [PubMed] [Google Scholar]
- 40.Douglas J.L. In search of a small-molecule inhibitor for respiratory syncytial virus. Expert Rev. Anti-Infect. Ther. 2004;2:625–639. doi: 10.1586/14787210.2.4.625. [DOI] [PubMed] [Google Scholar]
- 41.Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., Cai R., Swaminathan S., Cihlar T. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J. Virol. 2003;77:5054–5064. doi: 10.1128/JVI.77.9.5054-5064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., Cai R., Swaminathan S., Chen X., Cihlar T. Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncytial virus fusion by similar mechanisms. Antimicrob. Agents Chemother. 2005;49:2460–2466. doi: 10.1128/AAC.49.6.2460-2466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dragovich P.S., Prins T.J., Zhou R., Webber S.E., Marakovits J.T., Fuhrman S.A., Patick A.K., Matthews D.A., Lee C.A., Ford C.E., Burke B.J., Rejto P.A., Hendrickson T.F., Tuntland T., Brown E.L., Meador J.W., 3rd, Ferre R.A., Harr J.E., Kosa M.B., Worland S.T. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 1999;42:1213–1224. doi: 10.1021/jm9805384. [DOI] [PubMed] [Google Scholar]
- 44.Dragovich P.S., Prins T.J., Zhou R., Johnson T.O., Hua Y., Luu H.T., Sakta S.K., Brown E.L., Maldonado F.C., Tuntland T., Lee C.A., Fuhrman S.A., Zalman L.S., Patick A.K., Matthews D.A., Wu E.Y., Guo M., Borer B.C., Nayyar N.K., Moran T., Chen L., Rejto P.A., Rose P.W., Guzman M.C., Dovalsantos E.Z., Lee S., McGee K., Mohajeri M., Liese A., Tao J., Kosa M.B., Liu B., Batugo M.R., Gleeson J.P., Wu Z.P., Liu J., Meador J.W., 3rd, Ferre R.A. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 8. Pharmacological optimization of orally bioavailable 2-pyridone-containing peptidomimetics. J. Med. Chem. 2003;46:4572–4585. doi: 10.1021/jm030166l. [DOI] [PubMed] [Google Scholar]
- 45.Dutartre H., Bussetta C., Boretto J., Canard B. General catalytic deficiency of hepatitis C virus RNA polymerase with an S282T mutation and mutually exclusive resistance towards 2′-modified nucleotide analogues. Antimicrob. Agents Chemother. 2006;50:4161–4169. doi: 10.1128/AAC.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldrup A.B., Allerson C.R., Bennett C.F., Bera S., Bhat B., Bhat N., Bosserman M.R., Brooks J., Burlein C., Carroll S.S., Cook P.D., Getty K.L., MacCoss M., McMasters D.R., Olsen D.B., Prakash T.P., Prhavc M., Song Q., Tomassini J.E., Xia J. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 2004;47:2283–2295. doi: 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- 47.Enterlein S., Warfield K.L., Swenson D.L. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob. Agents Chemother. 2006;50:984–993. doi: 10.1128/AAC.50.3.984-993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escuret V., Martin A., Durantel D., Parent R., Hantz O., Trépo C., Menguy T., Bottius E., Dardy J., Maral J., Escary J.L., Zoulim F. Novel alpha interferon (IFN-α) variant with improved inhibitory activity against hepatitis C virus genotype 1 replication compared to IFN-α2b therapy in a subgenomic replicon system. Antimicrob. Agents Chemother. 2006;50:3984–3991. doi: 10.1128/AAC.00199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Goncales F.L., Jr, Haussinger D., Diago M., Carosi G., Dhumeaux D., Craxi A., Lin A., Hoffman J., Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 50.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan Y.-R., Huang H., Huang Y.-D., Rao C.M., Zhao Y., Liu J.S., Wu L., Wei D.Q. Synthesis and activity of an octapeptide inhibitor designed for SARS coronavirus main proteinase. Peptides. 2006;27:622–625. doi: 10.1016/j.peptides.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. USA. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geisbert T.W., Hensley L.E., Kagan E., Yu E.Z., Geisbert J.B., Daddario-DiCaprio K., Fritz E.A., Jahrling P.B., McClintock K., Phelps J.R., Lee A.C.H., Judge A., Jeffs L.B., MacLachlan I. Postexposure protection of guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J. Infect. Dis. 2006;193:1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gitlin L., Stone J.K., Andino R. Poliovirus escape from RNA interference: short interfering RNA-target recognition and implications for therapeutic approaches. J. Virol. 2005;79:1027–1035. doi: 10.1128/JVI.79.2.1027-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glanville A.R., Scott A.I.R., Morton J.M., Aboyoun C.L., Plit M.L., Carter I.W., Malouf M.A. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J. Heart Lung Transplant. 2005;24:2114–2119. doi: 10.1016/j.healun.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Gowen B.B., Barnard D.L., Smee D.F., Wong M.H., Pace A.M., Jung K.H., Winslow S.G., Bailey K.W., Blatt L.M., Sidwell R.W. Interferon alfacon-1 protects hamsters from lethal pichinde virus infection. Antimicrob. Agents Chemother. 2005;49:2378–2386. doi: 10.1128/AAC.49.6.2378-2386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nature Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamelin M.-E., Prince G.A., Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob. Agents Chemother. 2006;50:774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanessian S., Bayrakdarian M., Luo X. Total synthesis of A-315675: a potent inhibitor of influenza neuraminidase. J. Am. Chem. Soc. 2002;124:4716–4721. doi: 10.1021/ja0126226. [DOI] [PubMed] [Google Scholar]
- 61.Harper S., Pacini B., Avolio S., Di Fillippo M., Migliaccio G., Laufer R., De Francesco R., Rowley M., Narjes F. Development and preliminary optimization of indole-N-acetamide inhibitors of hepatitis C virus NS5B polymerase. J. Med. Chem. 2005;48:1314–1317. doi: 10.1021/jm049122i. [DOI] [PubMed] [Google Scholar]
- 62.Hayden F.G. Combinations of antiviral agents for treatment of influenza virus infections. J. Antimicrob. Chemother. 1986;18(Suppl. B):177–183. doi: 10.1093/jac/18.supplement_b.177. [DOI] [PubMed] [Google Scholar]
- 63.Hayden F.G. Antivirals for influenza: historical perspectives and lessons learned. Antiviral Res. 2006;71:372–378. doi: 10.1016/j.antiviral.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Hayden F.G., Douglas R.G.J., Simons R. Enhancement of activity against influenza viruses by combinations of antiviral agents. Antimicrob. Agents Chemother. 1980;18:536–541. doi: 10.1128/aac.18.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayden F.G., Sable C.A., Connor J.D., Lane J. Intravenous ribavirin by constant infusion for serious influenza and parainfluenzavirus infection. Antiviral Ther. 1996;1:51–56. [PubMed] [Google Scholar]
- 66.Hayden F.G., Coats T., Kim K., Hassman H.A., Blatter M.M., Zhang B., Liu S. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: efficacy and tolerability in phase II clinical trials. Antiviral Ther. 2002;7:53–65. [PubMed] [Google Scholar]
- 67.Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A., Liu S., Hudson S., Pevear D.C., Collett M., McKinlay M. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003;36:1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayden F.G., Turner R.B., Gwaltney J.M., Chi-Burris K., Gersten M., Hsyu P., Patick A.K., Smith G.J., 3rd, Zalman L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayden F.G., Belshe R., Villanueva C., Lanno R., Hughes C., Small I., Dutkowski R., Ward P., Carr J. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J. Infect. Dis. 2004;189:440–449. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- 70.He M.-L., Zheng B., Peng Y., Peiris J.S., Poon L.L., Yuen K.Y., Lin M.C., Kung H.F., Guan Y. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290:2665–2666. doi: 10.1001/jama.290.20.2665. [DOI] [PubMed] [Google Scholar]
- 71.Hensley L.E., Fritz E.A., Jahrling P.B., Karp C.L., Huggins J.W., Geisberg T.W. Interferon-beta 1a and SARS coronavirus replication. Emerging Infect. Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinrichsen H., Benhamou Y., Wedemeyer H., Reiser M., Sentjens R.E., Calleja J.L., Forns X., Erhardt A., Cronlein J., Chaves R.L., Yong C.L., Nehmiz G., Steinmann G.G. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology. 2004;127:1347–1355. doi: 10.1053/j.gastro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Howe A.Y.M., Bloom J., Baldick C.J., Benetatos C.A., Cheng H., Christensen J.S., Chunduru S.K., Coburn G.A., Feld B., Gopalsamy A., Gorczyca W.P., Herrmann S., Johann S., Jiang X., Kimberland M.L., Krisnamurthy G., Olson M., Orlowski M., Swanberg S., Thompson I., Thorn M., Del Vecchio A., Young D.C., van Zeijl M., Ellingboe J.W., Upeslacis J., Collett M., Mansour T.S., O'Connell J.F. Novel nonnucleoside inhibitor of hepatitis C virus RNA-dependent RNA polymerase. Antimicrob. Agents Chemother. 2004;48:4813–4821. doi: 10.1128/AAC.48.12.4813-4821.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howe A.Y.M., Cheng H., Thompson I., Chunduru S.K., Herrmann S., O'Connell J., Agarwal A., Chopra R., Del Vecchio A.M. Molecular mechanisms of a thumb domain hepatitis C virus nonnucleoside RNA-dependent RNA polymerase inhibitor. Antimicrob. Agents Chemother. 2006;50:4103–4113. doi: 10.1128/AAC.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsyu P.-H., Pithavala Y.K., Gersten M., Penning C.A., Kerr B.M. Pharmacokinetics and safety of an antirhinoviral agent, ruprintrivir, in healthy volunteers. Antimicrob. Agents Chemother. 2002;46:392–397. doi: 10.1128/AAC.46.2.392-397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huggins J.W., Hsiang C.M., Cosgriff T.M., Guang M.Y., Smith J.I., Wu Z.O., LeDuc J.W., Zheng Z.M., Meegan J.M., Wang Q.N. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J. Infect. Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 77.Hwang D.R., Tsai Y.C., Lee J.C., Huang K.K., Lin R.K., Ho C.H., Chiou J.M., Lin Y.T., Hsu J.T., Yeh C.T. Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrob. Agents Chemother. 2004;48:2876–2882. doi: 10.1128/AAC.48.8.2876-2882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hwang D.R., Lin R.-K., Leu G.-Z., Lin T.-Y., Lien T.-W., Yu M.-C., Yeh C.-T., Hsu J.T.-A. Inhibition of hepatitis C virus replication by antimonial compounds. Antimicrob. Agents Chemother. 2005;49:4197–4202. doi: 10.1128/AAC.49.10.4197-4202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ison M.G., Gubareva L.V., Atmar R.L., Treanor J., Hayden F.G. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J. Infect. Dis. 2006;193:760–764. doi: 10.1086/500465. [DOI] [PubMed] [Google Scholar]
- 80.Jahrling P.B., Geisbert T.W., Geisbert J.B., Swearengen J.R., Bray M., Jaax N.K., Huggins J.W., LeDuc J.W., Peters C.J. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 1999;179(Suppl.):S224–S234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 81.Kaiser L., Wat C., Mills T., Mahoney P., Ward P., Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 82.Keene K.M., Foy B.D., Sanchez-Vargas I., Beaty B.J., Blair C.D., Olson K.E. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim C.U., Lew W., Williams M.A., Liu H., Zhang L., Swaminathan S., Bischofberger N., Chen M.S., Mendel D.B., Tai C.Y., Laver W.G., Stevens R.C. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 85.Kiso M., Mitamura K., Sakai-Tagawa Y., Shiraishi K., Kawakami C., Kimura K., Hayden F.G., Sugaya N., Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 86.Klumpp K., Léveque V., Le Pogam S., Ma H., Jiang W.-R., Kang H., Granycome C., Singer M., Laxton C., Hang J.Q., Sarma K., Smith D.B., Heindl D., Hobbs C.J., Merrett J.H., Symons J., Cammack N., Martin J.A., Devos R., Najera I. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J. Biol. Chem. 2006;281:3793–3799. doi: 10.1074/jbc.M510195200. [DOI] [PubMed] [Google Scholar]
- 87.Kronke J., Kittler R., Buchholz F., Windisch M.P., Pietschmann T., Bartenschlager R., Frese M. Alternative approaches for efficient inhibition of hepatitis C virus RNA replication by small interfering RNAs. J. Virol. 2004;78:3436–3446. doi: 10.1128/JVI.78.7.3436-3446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kukolj G., McGibbon G.A., McKercher G., Marquis M., Lefebvre S., Thauvette L., Gauthier J., Goulet S., Poupart M.A., Beaulieu P.L. Binding site characterization and resistance to a class of non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase. J. Biol. Chem. 2005;280:39260–39267. doi: 10.1074/jbc.M506407200. [DOI] [PubMed] [Google Scholar]
- 89.Lamarre D., Anderson P.C., Bailey M., Beaulieu P., Bolger G., Bonneau P., Bos M., Cameron D.R., Cartier M., Cordingley M.G., Faucher A.M., Goudreau N., Kawai S.H., Kukolj G., Lagace L., LaPlante S.R., Narjes H., Poupart M.A., Rancourt J., Sentjens R.E., George R.St., Simoneau B., Steinmann G., Thibeault D., Tsantrizos Y.S., Weldon S.M., Yong C.L., Llinas-Brunet M. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature. 2003;426:186–189. doi: 10.1038/nature02099. [DOI] [PubMed] [Google Scholar]
- 90.Lavrov S.V., Eremkina E.I., Orlova T.G., Galegov G.A., Soloviev V.D., Zhdanov V.M. Combined inhibition of influenza virus reproduction in cell culture using interferon and amantadine. Nature. 1968;217:856–857. doi: 10.1038/217856a0. [DOI] [PubMed] [Google Scholar]
- 91.Le Pogam S., Jiang W.-R., Leveque V., Rajyaguru S., Ma H., Kang H., Jiang S., Singer M., Ali S., Klumpp K., Smith D., Symons J., Cammack N., Nájera I. In vitro selected Con1 subgenomic replicons resistant to 2′-C-methyl-cytidine or to R1479 show lack of cross resistance. Virology. 2006;351:349–359. doi: 10.1016/j.virol.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 92.Le Q.M., Kiso M., Someya K., Sakai Y.T., Nguyen T.H., Nguyen K.H., Pham N.D., Ngyen H.H., Yamada S., Muramoto Y., Horimoto T., Takada A., Goto H., Suzuki T., Suzuki Y., Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 93.Ledford R.M., Patel N.R., Demenczuk T.M., Watanyar A., Herbertz T., Collett M.S., Pevear D.C. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 2004;78:3663–3674. doi: 10.1128/JVI.78.7.3663-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee T.-W., Cherney M.M., Huitema C., Liu J., James K.E., Powes J.C., Eltis L.D., James M.N. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005;353:1137–1151. doi: 10.1016/j.jmb.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leikina E., Delanoe-Ayari H., Melikov K., Cho M.S., Chen A., Waring A.J., Wang W., Xie Y., Loo J.A., Lehrer R.I., Chernomordik L.V. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nature Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- 96.Leyssen P., Charlier N., Paeshuyse J., De Clercq E., Neyts J. Prospects for antiviral therapy. Adv. Virus Res. 2003;61:511–553. doi: 10.1016/s0065-3527(03)61014-6. [DOI] [PubMed] [Google Scholar]
- 97.Leyssen P., Croes R., Rau P., Heiland S., Verbeken E., Sciot R., Paeshuyse J., Charlier N., De Clercq E., Meyding-Lamadé U., Neyts J. Acute encephalitis, a poliomyelitis-like syndrome and neurological sequelae in a hamster model for flavivirus infections. Brain Pathol. 2003;13:279–290. doi: 10.1111/j.1750-3639.2003.tb00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leyssen P., Drosten C., Paning M., Charlier N., Paeshuyse J., De Clercq E., Neyts J. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob. Agents Chemother. 2003;47:777–782. doi: 10.1128/AAC.47.2.777-782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leyssen P., Balzarini J., De Clercq E., Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 2005;79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li B.-j., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., Xu J., Liu Y., Zheng B.J., Woodle M.C., Zhong N., Lu P.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nature Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin C., Lin K., Luong Y.-P., Rao B.G., Wei Y.Y., Brennan D.L., Fulghum J.R., Hsiao H.M., Ma S., Maxwell J.P., Cottrell K.M., Perni R.B., Gates C.A., Kwong A.D. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 2004;279:17508–17514. doi: 10.1074/jbc.M313020200. [DOI] [PubMed] [Google Scholar]
- 103.Lin C., Gates C.A., Rao B.G., Brennan D.L., Fulghum J.R., Luong Y.P., Frantz J.D., Lin K., Ma S., Wei Y.Y., Perni R.B., Kwong A.D. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J. Biol. Chem. 2005;280:36784–36791. doi: 10.1074/jbc.M506462200. [DOI] [PubMed] [Google Scholar]
- 104.Lin K., Perni R.B., Kwong A.D., Lin C. VX-950, a novel hepatitis C virus (HCV) NS3-4A protease inhibitor, exhibits potent antiviral activities in HCV replicon cells. Antimicrob. Agents Chemother. 2006;50:1813–1822. doi: 10.1128/AAC.50.5.1813-1822.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., Xiong H., Farmar J., Debnath A.K., Tien P., Jiang S. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liuzzi M., Mason S.W., Carter M., Lawetz C., McCollum R.S., Dansereau N., Bolger G., Lapeyre N., Gaudette Y., Lagace L., Massariol M.J., Do F., Whitehead P., Lamarre L., Scouten E., Bordeleau J., Landry S., Rancourt J., Fazal G., Simoneau B. Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase. J. Virol. 2005;79:13105–13115. doi: 10.1128/JVI.79.20.13105-13115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Love R.A., Parge H.E., Yu X., Hickey M.J., Diehl W., Gao J., Wriggers H., Ekker A., Wang L., Thomson J.A., Dragovich P.S., Fuhrman S.A. Crystallographic identification of a noncompetitive inhibitor binding site on the hepatitis C virus NS5B RNA polymerase enzyme. J. Virol. 2003;77:7575–7581. doi: 10.1128/JVI.77.13.7575-7581.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma L.-Y., Ma S.-C., Wei F., Lin R.C., But P.P., Lee S.H., Lee S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003;51:1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- 109.Malcolm B.A., Liu R., Lahser F., Agrawal S., Belanger B., Butkiewicz N., Chase R., Gheyas F., Hart A., Hesk D., Ingravallo P., Jiang C., Kong R., Lu J., Pichardo J., Prongay A., Skelton A., Tong X., Venkatraman S., Xia E., Girijavallabhan V., Njoroge F.G. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob. Agents Chemother. 2006;50:1013–1020. doi: 10.1128/AAC.50.3.1013-1020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maring C.J., Stoll V.S., Zhao C., Sun M., Krueger A.C., Stewart K.D., Madigan D.L., Kati W.M., Xu Y., Carrick R.J., Montgomery D.A., Kempf-Grote A., Marsh K.C., Molla A., Steffy K.R., Sham H.L., Laver W.G., Gu Y.G., Kempf D.J., Kohlbrenner W.E. Structure-based characterization and optimization of novel hydrophobic binding interactions in a series of pyrrolidine influenza neuraminidase inhibitors. J. Med. Chem. 2005;48:3980–3990. doi: 10.1021/jm049276y. [DOI] [PubMed] [Google Scholar]
- 111.McCormick J.B., King I.J., Webb P.A., Scribner C.L., Craven R.B., Johnson K.M., Elliott L.H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 112.McHutchison J.G., Manns M., Patel K., Poynard T., Lindsay K.L., Trépo C., Dienstag J., Lee W.M., Mak C., Garaud J.J., Albrecht J.K. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 113.McHutchison J.G., Patel K., Pockros P., Nyberg L., Pianko S., Yu R.Z., Dorr F.A., Kwoh T.J. A phase I trial of an antisense inhibitor of hepatitis C virus (ISIS 14803), administered to chronic hepatitis C patients. J. Hepatol. 2006;44:88–96. doi: 10.1016/j.jhep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 114.McKimm-Breschkin J., Trivedi T., Hampson A., Hay A., Klimov A., Tashiro M., Hayden F., Zambon M. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob. Agents Chemother. 2003;47:2264–2272. doi: 10.1128/AAC.47.7.2264-2272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Migliaccio G., Tomassini J.E., Carroll S.S., Tomei L., Altamura S., Bhat B., Bartholomew L., Bosserman M.R., Ceccacci A., Colwell L.F., Cortese R., De Francesco R., Eldrup A.B., Getty K.L., Hou X.S., LaFemina R.L., Ludmerer S.W., MacCoss M., McMasters D.R., Stahlhut M.W., Olsen D.B., Hazuda D.J., Flores O.A. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 2003;278:49164–49170. doi: 10.1074/jbc.M305041200. [DOI] [PubMed] [Google Scholar]
- 116.Mishin V.P., Hayden F.G., Gubareva L.V. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents Chemother. 2005;49:4515–4520. doi: 10.1128/AAC.49.11.4515-4520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mo H., Lu L., Pilot-Matias T., Pithawalla R., Mondal R., Masse S., Dekhtyar T., Ng T., Koev G., Stoll V., Stewart K.D., Pratt J., Donner P., Rockway T., Maring C., Molla A. Mutations conferring resistance to a hepatitis C virus (HCV) RNA-dependent RNA polymerase inhibitor alone or in combination with an HCV serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 2005;49:4305–4314. doi: 10.1128/AAC.49.10.4305-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monto A.S., McKimm-Breschkin J.L., Macken C., Hampson A.W., Hay A., Klimov A., Tashiro M., Webster R.G., Aymard M., Hayden F.G., Zambon M. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob. Agents Chemother. 2006;50:2395–2402. doi: 10.1128/AAC.01339-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munir S., Kaur K., Kapur V. Avian metapneumovirus phosphoprotein targeted RNA interference silences the expression of viral proteins and inhibits virus replication. Antiviral Res. 2006;69:46–51. doi: 10.1016/j.antiviral.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni L., Zhao L., Qian Y., Zhu J., Jin Z., Chen Y.W., Tien P., Gao G.F. Design and characterization of human respiratory syncytial virus entry inhibitors. Antiviral Ther. 2005;10:833–840. [PubMed] [Google Scholar]
- 121.Ojwang J.O., Wang Y.-H., Wyde P.R., Fischer N.H., Schuehly W., Appleman J.R., Hinds S., Shimasaki C.D. A novel inhibitor of respiratory syncytial virus isolated from ethnobotanicals. Antiviral Res. 2005;68:163–172. doi: 10.1016/j.antiviral.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Olsen D.B., Eldrup A.B., Bartholomew L., Bhat B., Bosserman M.R., Ceccacci A., Colwell L.F., Fay J.F., Flores O.A., Getty K.L., Grobler J.A., LaFemina R.L., Markel E.J., Migliaccio G., Prhavc M., Stahlhut M.W., Tomassini J.E., MacCoss M., Hazuda D.J., Carroll S.S. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 2004;48:3944–3953. doi: 10.1128/AAC.48.10.3944-3953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oxford J. Oseltamivir in the management of influenza. Expert Opin. Pharmacother. 2005;6:2493–2500. doi: 10.1517/14656566.6.14.2493. [DOI] [PubMed] [Google Scholar]
- 124.Padalko E., Verbeken E., Matthys P., Aerts J.L., De Clercq E., Neyts J. Mycophenolate mofetil inhibits the development of Coxsackie B3-induced myocarditis in mice. BMC Microbiol. 2003;3:25. doi: 10.1186/1471-2180-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Padalko E., Nuyens D., De Palma A., Verbeken E., Aerts J.L., De Clercq E., Carmeliet P., Neyts J. The interferon inducer ampligen [poly(I)-poly(C12U)] markedly protects mice against Coxsackie B3 virus-induced myocarditis. Antimicrob. Agents Chemother. 2004;48:267–274. doi: 10.1128/AAC.48.1.267-274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paeshuyse J., Leyssen P., Mabery E., Boddeker N., Vrancken R., Froeyen M., Ansari I.H., Dutartre H., Rozenski J., Gil L.H., Letellier C., Lanford R., Canard B., Koenen F., Kerkhofs P., Donis R.O., Herdewijn P., Watson J., De Clercq E., Puerstinger G., Neyts J. A novel, highly selective inhibitor of pestivirus replication that targets the viral RNA-dependent RNA polymerase. J. Virol. 2005;80:149–160. doi: 10.1128/JVI.80.1.149-160.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paeshuyse J., Kaul A., De Clercq E., Rosenwirth B., Dumont J.-M., Scalfaro P., Bartenschlager R., Neyts J. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;43:761–770. doi: 10.1002/hep.21102. [DOI] [PubMed] [Google Scholar]
- 128.J. Paeshuyse, J.-M. Chezal, M. Froeyen, P. Leyssen, H. Dutartre, R. Vrancken, B. Canard, C. Letellier, T. Li, H. Mittendorfer, F. Koenen, P. Kerhofs, E. De Clercq, P. Herdewijn, G. Puerstinger, A. Gueiffier, O. Chavignon, J.-C. Teulade, J. Neyts, The imidazopyrrolopyridine analogue, AG110 is a novel, highly selective inhibitor of pestiviruses that targets the viral RNA-dependent RNA polymerase at a hot spot for inhibition of viral replication, 2007, submitted for publication [DOI] [PMC free article] [PubMed]
- 129.Patick A.K. Rhinovirus chemotherapy. Antiviral Res. 2006;71:391–396. doi: 10.1016/j.antiviral.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patick A.K., Brothers M.A., Maldonado F., Binford S., Madonado O., Fuhrman S., Petersen A., Smith G.J., 3rd, Zalman L.S., Burns-Naas L.A., Tran J.Q. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005;49:2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pavlovic D., Neville D.C.A., Argaud O., Blumberg B., Dwek R.A., Fischer W.B., Zitzmann N. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc. Natl. Acad. Sci. USA. 2003;100:6104–6108. doi: 10.1073/pnas.1031527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perni R.B., Almquist S.J., Byrn R.A., Chandorkar G., Chaturvedi P.R., Courtney L.F., Decker C.J., Dinehart K., Gates C.A., Harbeson S.L., Heiser A., Kalkeri G., Kolaczkowski E., Lin K., Luong Y.P., Rao B.G., Taylor W.P., Thomson J.A., Tung R.D., Wei Y., Kwong A.D., Lin C. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob. Agents Chemother. 2006;50:899–909. doi: 10.1128/AAC.50.3.899-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pierra C., Amador A., Baradoux E., Storer R., Gosselin G. Synthesis of 2′-C-methylcytidine and 2′-C-methyluridine derivatives modified in the 3′-position as potential antiviral agents. Collect. Czech. Chem. Commun. 2006;71:991–1010. [Google Scholar]
- 134.Puig-Basagoiti F., Tilgner M., Forshey B.M., Philpott S.M., Espina N.G., Wentworth D.E., Goebel S.J., Masters P.S., Falgout B., Ren P., Ferguson D.M., Shi P.Y. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob. Agents Chemother. 2006;50:1320–1329. doi: 10.1128/AAC.50.4.1320-1329.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pürstinger G., Paeshuyse J., Herdewijn P., Rozenski J., De Clercq E., Neyts J. Substituted 5-benzyl-2-phenyl-5H-imidazo[4,5-c]pyridines: a new class of pestivirus inhibitors. Bioorg. Med. Chem. Lett. 2006;16:5345–5349. doi: 10.1016/j.bmcl.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 136.Pürstinger G., Paeshuyse J., De Clercq E., Neyts J. Antiviral 2,5-disubstituted imidazo[4,5-c]pyridines: from anti-pestivirus to anti-hepatitis C virus activity. Bioorg. Med. Chem. Lett. 2007;17:390–393. doi: 10.1016/j.bmcl.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 137.Reesink H.W., Zeuzem S., Weegink C.J., Forestier N., van Vliet A., van de Wetering de Rooij J. Final results of a phase 1b, multiple-dose study of VX-950, a hepatitis C virus protease inhibitor. Hepatology. 2005;42(Suppl. 1):234A. [Google Scholar]
- 138.Reiser M., Hinrichsen H., Benhamou Y., Reesink H.W., Wedemeyer H., Avendano C., Riba N., Yong C.L., Nehmiz G., Steinmann G.G. Antiviral efficacy of NS3-serine protease inhibitor BILN-2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology. 2005;41:832–835. doi: 10.1002/hep.20612. [DOI] [PubMed] [Google Scholar]
- 139.Reuter T., Weissbrich B., Schneider-Schaulies S., Schneider-Schaulies J. RNA interference with measles virus N, P, and L mRNAs efficiently prevents and with matrix protein mRNA enhances viral transcription. J. Virol. 2006;80:5951–5957. doi: 10.1128/JVI.02453-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.E.I. Rigopoulou, W.G.H. Abbott, R. Williams, N.V. Naoumov, Direct evidence for immunomodulatory properties on T-cell reactivity to hepatitis C virus, Antiviral Res. (2007), in press [DOI] [PubMed]
- 141.Rodriguez-Torres M., Torriani F.J., Soriano V., Borucki M.J., Lissen E., Sulkowski M., Dieterich D., Wang K., Gries J.M., Hoggard P.G., Back D. Effect of ribavirin on intracellular and plasma pharmacokinetics of nucleoside reverse transcriptase inhibitors in patients with human immunodeficiency virus-hepatitis C virus coinfection: results of a randomized clinical study. Antimicrob. Agents Chemother. 2005;49:3997–4008. doi: 10.1128/AAC.49.10.3997-4008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rotbart H.A., Webster A.D. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 2001;32:228–235. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 143.Sainz B., Jr, Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329:11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salomon R., Franks J., Govorkova E.A., Ilyushina N.A., Yen H.L., Hulse-Post D.J., Humberd J., Trichet M., Rehg J.E., Webby R.J., Webster R.G., Hoffmann E. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 2006;203:689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiff G.M., Sherwood J.R. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J. Infect. Dis. 2002;181:20–26. doi: 10.1086/315176. [DOI] [PubMed] [Google Scholar]
- 146.Shie J.-J., Fang J.-M., Kuo C.-J., Kuo T.H., Liang P.H., Huang H.J., Yang W.B., Lin C.H., Chen J.L., Wu Y.T., Wong C.H. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J. Med. Chem. 2005;48:4469–4473. doi: 10.1021/jm050184y. [DOI] [PubMed] [Google Scholar]
- 147.Shie J.-J., Fang J.-M., Kuo T.-H., Kuo C.J., Liang P.H., Huang H.J., Wu Y.T., Jan J.T., Cheng Y.S., Wong C.H. Inhibition of the severe acute respiratory syndrome 3CL protease by peptidomimetic alpha,beta-unsaturated esters. Bioorg. Med. Chem. 2005;13:5240–5252. doi: 10.1016/j.bmc.2005.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shigeta S., Mori S., Kodama E., Kodama J., Takahashi K., Yamase T. Broad spectrum anti-RNA virus activities of titanium and vanadium substituted polyoxotungstates. Antiviral Res. 2003;58:265–271. doi: 10.1016/s0166-3542(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 149.Sidwell R.W., Smee D.F. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 150.Sidwell R.W., Barnard D.L. Respiratory syncytial virus infections: recent prospects for control. Antiviral Res. 2006;71:379–390. doi: 10.1016/j.antiviral.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 151.Sidwell R.W., Huffman J.H., Khare G.P., Allen L.B., Witkowski J.T., Robins R.K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 152.Sidwell R.W., Smee D.F., Huffman J.H., Barnard D.L., Bailey K.W., Morrey J.D., Babu Y.S. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201. Antimicrob. Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sidwell R.W., Bailey K.W., Wong M.H., Barnard D.L., Smee D.F. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antiviral Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 154.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smee D.F., Huffman J.H., Morrison A.C., Barnard D.L., Sidwell R.W. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob. Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith C.B., Charette R.P., Fox J.P., Cooney M.K., Hall C.E. Lack of effect of oral ribavirin in naturally occurring influenza A virus (H1N1) infection. J. Infect. Dis. 1980;141:548–554. doi: 10.1093/infdis/141.5.548. [DOI] [PubMed] [Google Scholar]
- 157.Stadler K., Masignani V., Eickmann M., Becker S., Abrignani S., Klenk H.D., Rappuoli R. SARS—beginning to understand a new virus. Nature Rev. Microbiol. 2003;1:209–218. doi: 10.1038/nrmicro775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ströher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J. Infect. Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stuyver L.J., McBrayer T.R., Tharnish P.M., Clark J., Hollecker L., Lostia S., Nachman T., Grier J., Bennett M.A., Xie M.-Y., Schinazi R.F., Morrey J.D., Julander J.J., Furman P.A., Otto M.J. Inhibition of hepatitis C replicon RNA synthesis by β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antiviral Chem. Chemother. 2006;17:79–87. doi: 10.1177/095632020601700203. [DOI] [PubMed] [Google Scholar]
- 160.Sudo K., Miyazaki Y., Kojima N., Kobayashi M., Suzuki H., Shintani M., Shimizu Y. YM-53403, a unique anti-respiratory syncytial virus agent with a novel mechanism of action. Antiviral Res. 2005;65:125–131. doi: 10.1016/j.antiviral.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 161.Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Summa V., Petrocchi A., Pace P., Matassa V.G., De Francesco R., Altamura S., Tomei L., Koch U., Neuner P. Discovery of alpha,gamma-diketo acids as potent selective and reversible inhibitors of hepatitis C virus NS5b RNA-dependent RNA polymerase. J. Med. Chem. 2004;47:14–17. doi: 10.1021/jm0342109. [DOI] [PubMed] [Google Scholar]
- 163.Summa V., Petrocchi A., Matassa V.G., Taliani M., Laufer R., De Francesco R., Altamura S., Pace P. HCV NS5b RNA-dependent RNA polymerase inhibitors: from alpha,gamma-diketoacids to 4,5-dihydroxypyrimidine- or 3-methyl-5-hydroxypyrimi-dinonecarboxylic acids. Design and synthesis. J. Med. Chem. 2004;47:5336–5339. doi: 10.1021/jm0494669. [DOI] [PubMed] [Google Scholar]
- 164.Sun A., Prussia A., Zhan W., Murray E.E., Doyle J., Cheng L.-T., Yoon J.-J., Radchenko E.V., Palyulin V.A., Compans R.W., Liotta D.C., Plemper R.K., Snyder J.P. Nonpeptide inhibitors of measles virus entry. J. Med. Chem. 2006;49:5080–5092. doi: 10.1021/jm0602559. [DOI] [PubMed] [Google Scholar]
- 165.Sun J.H., Lemm J.A., O'Boyle D.R., 2nd, Racela J., Colonno R., Gao M. Specific inhibition of bovine viral diarrhea virus replicase. J. Virol. 2003;77:6753–6760. doi: 10.1128/JVI.77.12.6753-6760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tan S.L., Pause A., Shi Y., Sonenberg N. Hepatitis C therapeutics: current status and emerging strategies. Nature Rev. Drug Discovery. 2002;1:867–881. doi: 10.1038/nrd937. [DOI] [PubMed] [Google Scholar]
- 167.Tanner J.A., Watt R.M., Chai Y.B., Lu L.Y., Lin M.C., Peiris J.S., Poon L.L., Kung H.F., Huang J.D. The severe acute respiratory syndrome (SARS) coronavirus NTPase/helicase belongs to a distinct class of 5′ to 3′ viral helicases. J. Biol. Chem. 2003;278:39578–39582. doi: 10.1074/jbc.C300328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tanner J.A., Zheng B.-J., Zhou J., Watt R.M., Jiang J.Q., Wong K.L., Lin Y.P., Lu L.Y., He M.L., Kung H.F., Kesel A.J., Huang J.D. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem. Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thibeault D., Bousquet C., Gingras R., Lagacé L., Maurice R., White P.W., Lamarre D. Sensitivity of NS3 serine proteases from hepatitis C virus genotypes 2 and 3 to the inhibitor BILN 2061. J. Virol. 2004;78:7352–7359. doi: 10.1128/JVI.78.14.7352-7359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tomassini J.E., Davies M.E., Hastings J.C., Lingham R., Mojena M., Raghoobar S.L., Singh S.B., Tkacz J.S., Goetz M.A. A novel antiviral agent which inhibits the endonuclease of influenza viruses. Antimicrob. Agents Chemother. 1996;40:1189–1193. doi: 10.1128/aac.40.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tomassini J.E., Getty K., Stahlhut M.W., Shim S., Bhat B., Eldrup A.B., Prakash T.P., Carroll S.S., Flores O., MacCoss M., McMasters D.R., Migliaccio G., Olsen D.B. Inhibitory effect of 2′-substituted nucleosides on hepatitis C virus replication correlates with metabolic properties in replicon cells. Antimicrob. Agents Chemother. 2005;49:2050–2058. doi: 10.1128/AAC.49.5.2050-2058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tomei L., Altamura S., Bartholomew L., Biroccio A., Ceccacci A., Pacini L., Narjes F., Gennari N., Bisbocci M., Incitti I., Orsatti L., Harper S., Stansfield I., Rowley M., De Francesco R., Migliaccio G. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 2003;77:13225–13231. doi: 10.1128/JVI.77.24.13225-13231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tomei L., Altamura S., Bartholomew L., Bisbocci M., Bailey C., Bosserman M., Cellucci A., Forte E., Incitti I., Orsatti L., Koch U., De Francesco R., Olsen D.B., Carroll S.S., Migliaccio G. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 2004;78:938–946. doi: 10.1128/JVI.78.2.938-946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tomei L., Altamura S., Paonessa G., De Francesco R., Migliaccio G. HCV antiviral resistance: the impact of in vitro studies on the development of antiviral agents targeting the viral NS5B polymerase. Antiviral Chem. Chemother. 2005;16:225–245. doi: 10.1177/095632020501600403. [DOI] [PubMed] [Google Scholar]
- 175.Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tong X., Chase R., Skelton A., Chen T., Wright-Minogue J., Malcolm B.A. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antiviral Res. 2006;70:28–38. doi: 10.1016/j.antiviral.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 177.Tong X., Guo Z., Wright-Minogue J., Xia E., Prongay A., Madison V., Qiu P., Venkatraman S., Velazquez F., Njoroge F.G., Malcolm B.A. Impact of naturally occurring variants of HCV protease on the binding of different classes of protease inhibitors. Biochemistry. 2006;45:1353–1361. doi: 10.1021/bi051565g. [DOI] [PubMed] [Google Scholar]
- 178.Torriani F.J., Rodriguez-Torres M., Rockstroh J.K., Lissen E., Gonzalez-Garcia J., Lazzarin A., Carosi G., Sasadeusz J., Katlama C., Montaner J., Sette H., Jr, Passe S., De Pamphilis J., Duff F., Schrenk U.M., Dieterich D.T. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 179.Venkatraman S., Bogen S.L., Arasappan A., Bennett F., Chen K., Jao E., Liu Y.-T., Lovey R., Hendrata S., Huang Y., Pan W., Parekh T., Pinto P., Popov V., Pike R., Ruan S., Santhanam B., Vibulbhan B., Wu W., Yang W., Kong J., Liang X., Wong J., Liu R., Butkiewicz N., Chase R., Hart A., Agrawal S., Ingravallo P., Pichardo J., Kong R., Baroudy B., Malcolm B., Guo Z., Prongay A., Madison V., Broske L., Cui X., Cheng K.-C., Hsieh Y., Brisson J.-M., Prelusky D., Korfmacher W., White R., Bogdanowich-Knipp S., Pavlovsky A., Bradley P., Saksena A.K., Ganguly A., Piwinski J., Girijavallabhan V., Njoroge F.G. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[31.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: a potential therapeutic agent for the treatment of hepatitis C infection. J. Med. Chem. 2006;49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 180.Vogt M.W., Hartshorn K.L., Furman P.A., Chou T.C., Fyfe J.A., Coleman L.A., Crumpacker C., Schooley R.T., Hirsch M.S. Ribavirin antagonizes the effect of azidothymidine on HIV replication. Science. 1987;235:1376–1379. doi: 10.1126/science.2435003. [DOI] [PubMed] [Google Scholar]
- 181.von Itzstein M., Wu W.Y., Kok G.B., Pegg M.S., Dyason J.C., Jin B., Van Phan T., Smythe M.L., White H.F., Oliver S.W. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 182.Wang G.T., Chen Y., Wang S., Gentles R., Sowin T., Kati W., Muchmore S., Giranda V., Stewart K., Sham H., Kempf D., Laver W.G. Design, synthesis, and structural analysis of influenza neuraminidase inhibitors containing pyrrolidine cores. J. Med. Chem. 2001;44:1192–1201. doi: 10.1021/jm000468c. [DOI] [PubMed] [Google Scholar]
- 183.Wang M., Ng K.K., Cherney M.M., Yannopoulos C.G., Bedard J., Morin N., Nguyen-Ba N., Alaoui-Ismaili M.H., Bethell R.C., James M.N. Non-nucleoside analogue inhibitors bind to an allosteric site on HCV NS5B polymerase. Crystal structures and mechanism of inhibition. J. Biol. Chem. 2003;278:9489–9495. doi: 10.1074/jbc.M209397200. [DOI] [PubMed] [Google Scholar]
- 184.Ward P., Small I., Smith J., Suter P., Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J. Antimicrob. Chemother. 2005;55(Suppl. S1):i5–i21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- 185.Watashi K., Ishii N., Hijikata M., Inoue D., Murata T., Miyanari Y., Shimotohno K. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol. Cell. 2005;19:111–122. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 186.Watson J. Prospects for hepatitis C virus therapeutics: levovirin and viramidine as improved derivatives of ribavirin. Curr. Opin. Investig. Drugs. 2002;3:680–683. [PubMed] [Google Scholar]
- 187.Wei F., Ma S.-C., Ma L.-Y., But P.P.-H., Lin R.-C., Khan I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J. Nat. Prod. 2004;67:650–653. doi: 10.1021/np030470h. [DOI] [PubMed] [Google Scholar]
- 188.Wu C.-Y., Jan J.-T., Ma S.-H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu C.-Y., Jan J.-T., Chen C.-M., Hsieh H.P., Hwang D.R., Liu H.W., Liu C.Y., Huang H.W., Chen S.C., Hong C.F., Lin R.K., Chao Y.S., Hsu J.T. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wyde P.R., Chetty S.N., Timmerman P., Gilbert B.E., Andries K. Short duration aerosols of JNJ 2408068 (R170591) administered prophylactically or therapeutically protect cotton rats from experimental respiratory syncytial virus infection. Antiviral Res. 2003;60:221–231. doi: 10.1016/j.antiviral.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 191.Wyde P.R., Laquerre S., Chetty S.N., Gilbert B.E., Nitz T.J., Pevear D.C. Antiviral efficacy of VP4637 against respiratory syncytial virus in vitro and in cotton rats following delivery by small droplet aerosol. Antiviral Res. 2005;68:18–26. doi: 10.1016/j.antiviral.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 192.Xu X., Liu Y., Weiss S., Arnold E., Sarafianos S.G., Ding J. Molecular model of SARS coronavirus polymerase: implications for biochemical functions and drug design. Nucleic Acids Res. 2003;31:7117–7130. doi: 10.1093/nar/gkg916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A., Tamamura H., Fujii N., Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H., Gao G.F., Anand K., Bartlam M., Hilgenfeld R., Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang S., Chen S.J., Hsu M.F., Wu J.D., Tseng C.T., Liu Y.F., Chen H.C., Kuo C.W., Wu C.S., Chang L.W., Chen W.C., Liao S.Y., Chang T.Y., Hung H.H., Shr H.L., Liu C.Y., Huang Y.A., Chang L.Y., Hsu J.C., Peters C.J., Wang A.H., Hsu M.C. Synthesis, crystal structure, structure-activity relationships, and antiviral activity of a potent SARS coronavirus 3CL protease inhibitor. J. Med. Chem. 2006;49:4971–4980. doi: 10.1021/jm0603926. [DOI] [PubMed] [Google Scholar]
- 196.Yee N.K., Farina V., Houpis I.N., Haddad N., Frutos R.P., Gallou F., Wang X.-J., Wei X., Simpson R.D., Feng X., Fuchs V., Xu Y., Tan J., Zhang L., Xu J., Smith-Keenan L.L., Vitous J., Ridges M.D., Spinelli E.M., Johnson M., Donsbach K., Nicola T., Brenner M., Winter E., Kreye P., Samstag W. Efficient large-scale synthesis of BILN 2061, a potent HCV protease inhibitor, by a convergent approach based on ring-closing metathesis. J. Org. Chem. 2006;71:7133–7145. doi: 10.1021/jo060285j. [DOI] [PubMed] [Google Scholar]
- 197.Yen H.-L., Herlocher L.M., Hofmann E., Matrosovich M.N., Monto A.S., Webster R.G., Govorkova E.A. Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility. Antimicrob. Agents Chemother. 2005;49:4075–4084. doi: 10.1128/AAC.49.10.4075-4084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yi M., Tong X., Skelton A., Chase R., Chen T., Prongay A., Bogen S.L., Saksena A.K., Njoroge F.G., Veselenak R.L., Pyles R.B., Bourne N., Malcolm B.A., Lemon S.M. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor. J. Biol. Chem. 2006;281:8205–8215. doi: 10.1074/jbc.M510246200. [DOI] [PubMed] [Google Scholar]
- 199.Yu K.-L., Wang X.A., Civiello R.L., Trehan A.K., Pearce B.C., Yin Z., Combrink K.D., Gulgeze H.B., Zhang Y., Kadow K.F., Cianci C.W., Clarke J., Genovesi E.V., Medina I., Lamb L., Wyde P.R., Krystal M., Meanwell N.A. Respiratory syncytial virus fusion inhibitors. Part 3: Water-soluble benzimidazol-2-one derivatives with antiviral activity in vivo. Bioorg. Med. Chem. Lett. 2006;16:1115–1122. doi: 10.1016/j.bmcl.2005.11.109. [DOI] [PubMed] [Google Scholar]
- 200.Yuan J., Cheung P.K.M., Zhang H.M., Chau D., Yang D. Inhibition of Coxsackievirus B3 replication by small interfering RNAs requires perfect sequence match in the central region of the viral positive strand. J. Virol. 2005;79:2151–2159. doi: 10.1128/JVI.79.4.2151-2159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zeuzem S., Sarrazin C., Rouzier R., Tarral A., Brion N., Forestier N. Antiviral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype-1 (HCV-1) patients refractory to pegylated interferon (PEG-IFN-α) Hepatology. 2005;42(Suppl. 1):233A. [Google Scholar]
- 202.Zhang Y., Li T., Fu L., Yu C., Li Y., Xu X., Wang Y., Ning H., Zhang S., Chen W., Babiuk L.A., Chang Z. Silencing SARS-CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


