Abstract
The loss of insulin-producing β-cells is the central pathological event in type 1 and 2 diabetes, which has led to efforts to identify molecules to promote β-cell proliferation, protection, and imaging. However, the lack of β-cell specificity of these molecules jeopardizes their therapeutic potential. A general platform for selective release of small-molecule cargoes in β-cells over other islet cells ex vivo or other cell-types in an organismal context will be immensely valuable in advancing diabetes research and therapeutic development. Here, we leverage the unusually high Zn(II) concentration in β-cells to develop a Zn(II)-based prodrug system to selectively and tracelessly deliver bioactive small molecules and fluorophores to β-cells. The Zn(II)-targeting mechanism enriches the inactive cargo in β-cells as compared to other pancreatic cells; importantly, Zn(II)-mediated hydrolysis triggers cargo activation. This prodrug system, with modular components that allow for fine-tuning selectivity, should enable the safer and more effective targeting of β-cells.
Death and/or dysfunction of β-cells in type 1 and 2 diabetes critically reduces insulin levels, ultimately necessitating insulin injection.1,2 β-Cells reside in pancreatic islets, together with α-, δ-, ε-, and pancreatic polypeptide (PP)-cells. Multiple avenues in diabetes research and therapeutic development will benefit immensely from methods that selectively release small molecules into β-cells over other islet cells ex vivo or other cell-types in vivo. First, targeted release of small molecules that induce β-cell proliferation,3−9 even semispecifically, will be useful in several contexts. For example, the transplantation of β-cells derived from induced pluripotent stem cells is an emerging therapeutic avenue,10−12 but the yields of mature β-cells are low,12 requiring selective expansion of β-cells ex vivo that can be accomplished by the targeted release of β-cell mitogens.13 Targeted delivery of β-cell mitogens in vivo can also alleviate concerns about nontarget cell proliferation. Second, β-cell-specific release of imaging agents will also be useful in several contexts, including diagnostics. Islets are often transplanted in type 1 diabetes patients,14 and given the heterogeneous nature of the islets, a nondestructive imaging method for the precise and rapid quantification of β-cell mass in the islets before transplantation would be valuable. Furthermore, the availability of facile β-cell imaging methods would miniaturize small-molecule screening assays employing human islets, which are in scarce supply,13,15 and improve current in vivo β-cell imaging modalities that are only semispecific.16,17
Antibody–drug conjugates provide an attractive approach for cell-specific targeting, but the identification of specific β-cell surface markers is challenging. Cell-specific release of small molecules can be accomplished through a prodrug strategy, where an inactive analog of the parent compound is converted to the active agent only in the target cell by an enzyme. Here, we take advantage of an unusually high concentration of zinc ion (Zn(II)) in β-cells to report a β-cell-specific zinc-based prodrug system (ZnPD) that consists of an inactivated small-molecule cargo linked via a scaffold to a Zn(II)-binding ligand. Zn(II) catalyzes the hydrolysis of the bond between the inactive cargo and the scaffold, thereby releasing the active agent. By using this system, we demonstrate the selective release of multiple fluorophores and a β-cell mitogen in human β-cells across several cell types.
The development of ZnPD was motivated by several observations and design principles. First, β-cells have an unusually high Zn(II) concentration in insulin vesicles (up to ∼30 mM, ∼100 μM of which is “loosely bound”),18 which contrasts with the cytosolic Zn(II) concentration in most cells of ∼400 pM,19 the concentration in plasma and interstitial fluid of ∼1 nM, and the fact that free Zn(II) concentrations above 100 nM are toxic in cell culture.20 Indeed, fluorophores bearing Zn(II)-chelating groups have been used extensively over the years to selectively image β-cells.21−25 Second, Zn(II) can catalyze hydrolytic reactions,26,27 providing an opportunity to switch the inactive cargo to an active compound, akin to other prodrugs. Furthermore, when the cargo is released from the ZnPD, it can diffuse from the insulin granules to other parts within the β-cell where the cargo targets are likely to reside. For most cargoes (e.g., small-molecule inducers of β-cell proliferation), both the activation mechanism and the escape from insulin granules are critical—selective activation prevents proliferation in off-target tissues, whereas the exit from the granules ensures that the small molecule reaches its protein targets. Third, the hydrolytic mechanism allows traceless release of the cargo in its native form, without any modifications, allowing ZnPDs to be developed for small molecules that cannot tolerate modifications without a loss of activity. Fourth, several Zn(II) ligands exist with affinities ranging from pM to mM, allowing the precise fine-tuning of β-cell specificity.28 Finally, although the aqueous Zn(II) ion is not a potent Lewis acid, multiple tridentate coordinating ligands exist that can be placed proximally to the scissile bond of a ZnPD, thereby facilitating a high effective molarity of Zn(II) with an available coordination site and potent Lewis acidity.
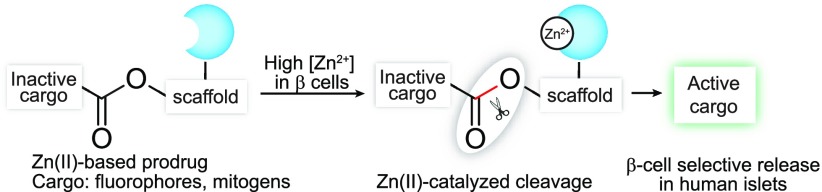
Before constructing ZnPDs, we first determined the rate of Zn(II)-mediated hydrolysis in β-cells compared to other islet cells. We employed a previously reported reaction-based probe, DA-ZP1, a PET-based zinc sensor that requires Zn(II)-triggered hydrolysis of a tethered acetate ester to turn-on fluorescence (Figure 1A) in a way that is selective for Zn(II) over other biologically relevant metal ions.27 DA-ZP1’s activity in β-cells has not been demonstrated. To confirm that the unmasking of DA-ZP1 fluorescence in cells was not catalyzed by an esterase and that proximally located dipicolyl ligands are necessary for hydrolytic cleavage, we tested the compound DA-FC, which lacks the Zn(II)-binding dipicolyl moieties. As expected, DA-FC did not fluoresce in the presence of Zn(II) (Figure 1B).27 To demonstrate that the high intracellular Zn(II) concentrations could release fluorescent cargo selectively in β-cells, we incubated DA-ZP1 in a β-cell line (INS-1E), an α-cell line (αTC1.6), a ductal cell line (PANC-1), and cells of other lineages (Figures 1C, D and S1A). After a 1 h incubation, the β-cells were highly fluorescent compared to the other cell types, with minimal background fluorescence at concentrations much greater than the Kd of DA-ZP1 (0.6 nM).26 β-Cells treated with the control compound DA-FC were not fluorescent. Finally, we monitored the kinetics of fluorophore release in β-cells at various concentrations of DA-ZP1 and observed that this release occurred within minutes, confirming the feasibility of our proposed ZnPD approach (Figure S1B and S1C). We further evaluated the intracellular localization of the released ZP1 (Zinpyr1) with nuclear and mitochondrial counterstaining and observed broad cellular distribution (Figure S2A). Finally, we confirmed the direct role of cellular Zn(II) by using a known chelator, N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN),27 that depletes Zn(II), preventing DA-ZP1 hydrolysis (Figure S2B). To better mimic human β-cells,29,30 we also tested DA-ZP1 for β-cell selectivity in human pancreatic islets. Here, we incubated DA-ZP1 in dissociated human islets, costaining for C-peptide (to identify β-cells). We observed a high level of C-peptide staining in DA-ZP1-positive cells (Figure 1E). Subsequent fluorescence-activated cell sorting (FACS) of the treated islets and immunohistochemical quantification of the number of cells positive for C-peptide demonstrated that ∼81% of the DA-ZP1-targeted cells were also positive for C-peptide (Figures 1F, G and S3).
Figure 1.
(A) Zn(II)-mediated unmasking of DA-ZP1 releases active fluorophore ZP1. (B) Structure of DA-FC and graph of Zn(II)-mediated fluorescence release of DA-ZP1 and DA-FC. (C) Selective unmasking of DA-ZP1 fluorescence in INS-1E cells compared to other cell types. (D) Representative images of DA-ZP1- or DA-FC-treated cells under the FITC channel (top) measuring ZP1 release, DAPI staining (middle), and the overlay (bottom). (E) Representative confocal images of dissociated human islets treated with DA-ZP1 followed by immunostaining for C-peptide. (F) Quantification of dispersed human islets treated with DA-ZP1 (n = 4). See also Figure S3. Human pancreatic donor information is available in Table S1. (G) Dispersed human islets were stained, and DA-ZP1+ and DA-ZP1– cells were collected after FACS (n = 4). Representative images show C-peptide (green) and glucagon (red) staining in unsorted, DA-ZP1+, DA-ZP1– cell populations. Nuclei stained with DAPI (blue).
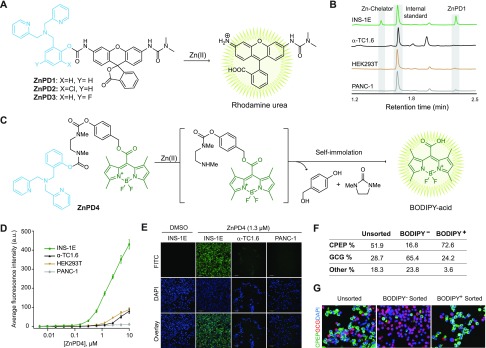
These findings prompted us to design our first ZnPD with a rhodamine urea (ZnPD1, Figure 2A) that has a reported pro-fluorophore system.31 We tested various linkers for connecting the cargo to the scaffold and found that a carbamate linkage (ZnPD1) was stable in buffers and serum-containing cell-culture media (Figure S4A). This stability could be fine-tuned by substituting electron-withdrawing groups at the ortho-/para-position of the scaffold, although ZnPD2 (chloro-substituted) and ZnPD3 (fluoro-substituted) were too labile, prematurely releasing their cargo in INS-1E media (Figure S4B and S4C). ZnPD1, lacking electron-withdrawing groups, released rhodamine urea in a Zn(II)-dependent fashion in PBS (Figure S4A), which was also confirmed by liquid chromatography–mass spectrometry (LC-MS) (Figure S4D). Removing the Zn(II)-binding ligand from ZnPD1 (ZnPD1ctrl, Figure S4E) prevented the release of rhodamine urea (Figure S4F), again confirming the proximity effect. We then demonstrated dose-dependent release of rhodamine urea in INS-1E cells at low micromolar concentrations (Figure S5). Unfortunately, the low fluorescence intensity of rhodamine urea prevented us from performing cell-selectivity studies through fluorescence, although we did confirm selective enrichment by extracting the compound from the cells followed by LC-MS analysis (Figure 2B).
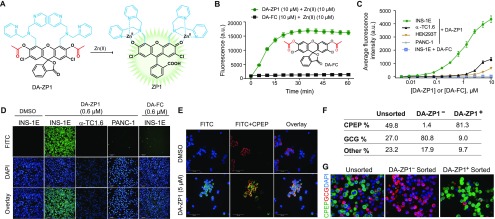
Figure 2.
(A) Structures of ZnPD1–3 and their Zn(II)-mediated unmasking of rhodamine urea fluorophore. (B) LC-MS chromatogram of ZnPD1-treated cell extracts. (C) Structures of ZnPD4 and Zn(II)-catalyzed release of BODIPY fluorophore via cascading self-immolation. (D) Selective unmasking of the ZnPD4 fluorophore in INS-1E cells compared to other cells. (E) Representative images of ZnPD4-treated cells under FITC channel (top), DAPI staining (middle), and the overlay (bottom). (F) Quantification of dispersed human islets treated with ZnPD4 (n = 3). See also Figure S7. Human pancreatic donor information is available in Table S1. (G) Dispersed human islets were stained, and BODIPY+ and BODIPY– cells were collected after FACS (n = 3). Representative images show C-peptide (green) and glucagon (red) staining in unsorted, BODIPY+, BODIPY– cell populations. Nuclei stained with DAPI (blue).
To demonstrate that this prodrug system is generalizable to other cargo types and hydrolyzable bonds, we used a boron dipyrromethene (BODIPY)-based caged probe,32 where the meso-carboxyl moiety of BODIPY was caged with a self-immolative linker (ZnPD4, Figure 2C).33−35 Upon Zn(II) binding, the carbamate linkage undergoes hydrolysis and triggers self-immolation, yielding the native fluorophore (Figure S6A). With ZnPD4, we observed selective fluorescence emission in only INS-1E cells but no other cell lines (Figure 2D and E), with dose and kinetic studies showing the fast turn-on of BODIPY fluorescence in INS-1E cells (Figure S6B and S6C). After the successful, selective release of BODIPY in INS-1E cells, we used ZnPD4 to capture β-cells from dissociated human islets (Figures 2F, G and S7) and observed an enrichment of 73% in β-cell population.
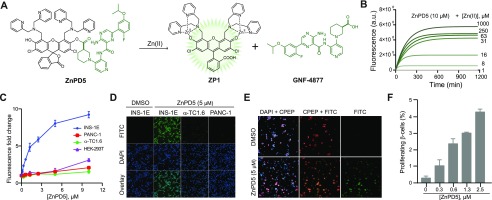
We next designed ZnPD5 (Figure 3A) based on DA-ZP1 for simultaneous release of fluorescence and a small molecule (GNF-4877) that promotes pancreatic β-cell proliferation.36,37 We could follow the Zn(II)-triggered hydrolysis of ZnPD5 through a steep rise in fluorescence intensity (Figures 3A, 3B and S8A) followed by saturation, indicating reaction completion that was also confirmed using LC-MS (Figure S8B). Additionally, ZnPD5 was stable in cell culture media and hydrolyzed only in the presence of added Zn(II) (Figure S8C). More importantly, we observed selective fluorescence emission from INS-1E cells when incubated with ZnPD5 (Figures 3C, D and S8D). The direct role of Zn(II) for ZnPD5 was confirmed by using the metal chelator TPEN, whereby INS1E cells preincubated with TPEN failed to hydrolyze ZnPD5 (Figure S8E). Next, we tested ZnPD5 in human islets and observed fluorescence release in β-cells (Figures 3E and S9A, B) as well as dose-dependent induction of proliferation (Figures 3F, S9C and D). The degree of proliferation induced by ZnPD5 is similar to that of GNF-4877 suggesting efficacious release of the cargo (i.e., GNF-4877).
Figure 3.
(A) Structures of ZnPD5 and its Zn(II)-catalyzed release of GNF-4877. (B) Reaction kinetics of ZnPD5 with different concentrations of Zn(II) as monitored by fluorescence spectroscopy. (C) Fold change in fluorescence in INS1E cells versus other cells. (D) Representative images of ZnPD5-treated cells under the FITC channel (top), DAPI staining (middle), and the overlay (bottom). (E) Representative fluorescence images of intact human islets showing β-cell selective hydrolysis of ZnPD5 under the FITC channel (green) and C-peptide (red). Intact islet cells were treated with either DMSO (top) or 150 nM of ZnPD5 (bottom). (F) Dose-dependent induction of β-cell proliferation by ZnPD5 in human islets.
We report the first examples of a rationally designed zinc-based prodrug system for selective and traceless cargo release in β-cells. These ZnPDs are nonenzymatic, cell-permeable, and modular in nature, with a specificity that can be fine-tuned. Furthermore, this approach can deliver various small molecules, including β-cell mitogens. Using controls lacking zinc-binding moieties (e.g., ZnPD1ctrl and DA-FC), we show that cargo release is not due to cellular esterases and confirm the potency of Zn(II) for cleaving various stable bonds (e.g., carbamates) in β-cells. Importantly, this prodrug system uses an inactive analog of the cargo; zinc hydrolysis is required to release the active cargo. Thus, this system should reach a level of selectivity that cannot be achieved by uncleavable systems, which rely on a relative enrichment in the β-cell population.24 We envision that these studies will propel the development of β-cell targeting approaches for imaging and therapeutic development.
Acknowledgments
We thank Dr. F. Wang (MIT) and also are thankful for support from the Burroughs Wellcome Fund (Career Award at the Scientific Interface) and NIH (UC4DK116255, R01 DK113597, RO1 DK067536, and GM065519). Human pancreatic islets were provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope (UC4DK098085) and the JDRF-funded IIDP Islet Award Initiative. This work is dedicated to Professor Laura L. Kiessling on the occasion of her 60th birthday
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c00099.
Experimental details and synthesis methods, and human islet donor information (PDF)
Author Contributions
▲ M.L., B.M., and D.M. contributed equally to this work
The authors declare the following competing financial interest(s): Broad Institute has filed PCT/US2018/028660 that claims inventions related to targeted delivery to beta cells.
Supplementary Material
References
- Butler A. E.; Janson J.; Bonner-Weir S.; Ritzel R.; Rizza R. A.; Butler P. C. beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The Stunned beta Cell: A Brief History. Cell Metab. 2010, 11, 349–352. 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Tuttle R. L.; Gill N. S.; Pugh W.; Lee J. P.; Koeberlein B.; Furth E. E.; Polonsky K. S.; Naji A.; Birnbaum M. J. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKB alpha. Nat. Med. 2001, 7, 1133–1137. 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- Shen W.; Tremblay M. S.; Deshmukh V. A.; Wang W.; Filippi C. M.; Harb G.; Zhang Y. Q.; Kamireddy A.; Baaten J. E.; Jin Q.; Wu T.; Swoboda J. G.; Cho C. Y.; Li J.; Laffitte B. A.; McNamara P.; Glynne R.; Wu X.; Herman A. E.; Schultz P. G. Small-Molecule Inducer of beta Cell Proliferation Identified by High-Throughput Screening. J. Am. Chem. Soc. 2013, 135, 1669–1672. 10.1021/ja309304m. [DOI] [PubMed] [Google Scholar]
- Vetere A.; Wagner B. K. Chemical methods to induce Beta-cell proliferation. Int. J. Endocrinol. 2012, 2012, 925143. 10.1155/2012/925143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes J. P.; Ryu J. H.; Lam K.; Carolan P. J.; Utz K.; Hollister-Lock J.; Arvanites A. C.; Rubin L. L.; Weir G.; Melton D. A. Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 3915–3920. 10.1073/pnas.1201149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O.; Adams B. A.; Yoo D.; Ellis G. C.; Gut P.; Anderson R. M.; German M. S.; Stainier D. Y. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell Metab. 2012, 15, 885–894. 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ouaamari A.; Dirice E.; Gedeon N.; Hu J.; Zhou J. Y.; Shirakawa J.; Hou L.; Goodman J.; Karampelias C.; Qiang G.; Boucher J.; Martinez R.; Gritsenko M. A.; De Jesus D. F.; Kahraman S.; Bhatt S.; Smith R. D.; Beer H. D.; Jungtrakoon P.; Gong Y.; Goldfine A. B.; Liew C. W.; Doria A.; Andersson O.; Qian W. J.; Remold-O’Donnell E.; Kulkarni R. N. SerpinB1 Promotes Pancreatic beta Cell Proliferation. Cell Metab. 2016, 23, 194–205. 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetere A.; Choudhary A.; Burns S. M.; Wagner B. K. Targeting the pancreatic beta-cell to treat diabetes. Nat. Rev. Drug Discovery 2014, 13, 278–289. 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- Millman J. R.; Xie C.; Van Dervort A.; Gurtler M.; Pagliuca F. W.; Melton D. A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F. W.; Melton D. A. How to make a functional beta-cell. Development 2013, 140, 2472–2483. 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F. W.; Millman J. R.; Gurtler M.; Segel M.; Van Dervort A.; Ryu J. H.; Peterson Q. P.; Greiner D.; Melton D. A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Alvarez-Perez J. C.; Felsenfeld D. P.; Liu H. T.; Sivendran S.; Bender A.; Kumar A.; Sanchez R.; Scott D. K.; Garcia-Ocana A.; Stewart A. F. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftanel M. A.; Harlan D. M. Pancreatic islet transplantation. PLoS Med. 2004, 1, e58 10.1371/journal.pmed.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser R. E.; Gannon M. An assay for small scale screening of candidate beta cell proliferative factors using intact islets. BioTechniques 2013, 55, 310–312. 10.2144/000114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner T.; Thurber G.; Gaglia J.; Vinegoni C.; Liew C. W.; Upadhyay R.; Kohler R. H.; Li L.; Kulkarni R. N.; Benoist C.; Mathis D.; Weissleder R. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 12815–12820. 10.1073/pnas.1109859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.; Bonner-Weir S.; Weissleder R. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes 2001, 50, 2231–2236. 10.2337/diabetes.50.10.2231. [DOI] [PubMed] [Google Scholar]
- Li Y. V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–89. 10.1007/s12020-013-0032-x. [DOI] [PubMed] [Google Scholar]
- Carpenter M. C.; Lo M. N.; Palmer A. E. Techniques for measuring cellular zinc. Arch. Biochem. Biophys. 2016, 611, 20–29. 10.1016/j.abb.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozym R. A.; Chimienti F.; Giblin L. J.; Gross G. W.; Korichneva I.; Li Y. A.; Libert S.; Maret W.; Parviz M.; Frederickson C. J.; Thompson R. B. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010, 235, 741–750. 10.1258/ebm.2010.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Chen S.; Bellomo E. A.; Tarasov A. I.; Kaut C.; Rutter G. A.; Li W. H. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR). Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 21063–21068. 10.1073/pnas.1109773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S. A novel method for the detection of viable human pancreatic beta cells by flow cytometry using fluorophores that selectively detect labile zinc, mitochondrial membrane potential and protein thiols. Cytometry, Part A 2008, 73A, 615–625. 10.1002/cyto.a.20560. [DOI] [PubMed] [Google Scholar]
- Gee K. R.; Zhou Z. L.; Qian W. J.; Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 2002, 124, 776–778. 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Horton T. M.; Allegretti P. A.; Lee S.; Moeller H. P.; Smith M.; Annes J. P. Zinc-Chelating Small Molecules Preferentially Accumulate and Function within Pancreatic beta Cells. Cell Chem. Biol. 2019, 26, 213–222. 10.1016/j.chembiol.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. A.; Hayes D.; Smith S. J.; Friedle S.; Lippard S. J. New strategy for quantifying biological zinc by a modified zinpyr fluorescence sensor. J. Am. Chem. Soc. 2008, 130, 15788–15789. 10.1021/ja807156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyan W.; Zhang D. Y.; Lippard S. J.; Radford R. J. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 143–148. 10.1073/pnas.1310583110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow M. L.; Radford R. J.; Chyan W.; Anderson C. T.; Zhang D. Y.; Loas A.; Tzounopoulos T.; Lippard S. J. Reaction-Based Probes for Imaging Mobile Zinc in Live Cells and Tissues. ACS Sens. 2016, 1, 32–39. 10.1021/acssensors.5b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que E. L.; Domaille D. W.; Chang C. J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- Kulkarni R. N.; Mizrachi E. B.; Ocana A. G.; Stewart A. F. Human beta-cell Proliferation and Intracellular Signaling: Driving in the Dark Without a Road Map. Diabetes 2012, 61, 2205–2213. 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E.; Kulkarni R. N.; Scott D. K.; Mauvais-Jarvis F.; Stewart A. F.; Garcia-Ocana A. Human beta-Cell Proliferation and Intracellular Signaling Part 2: Still Driving in the Dark Without a Road Map. Diabetes 2014, 63, 819–831. 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis L. D.; Chao T. Y.; Raines R. T. Fluorogenic label for biomolecular imaging. ACS Chem. Biol. 2006, 1, 252–260. 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; He X.; Su M.; Zhai W.; Zhang H.; Li C. A General Strategy Toward Highly Fluorogenic Bioprobes Emitting across the Visible Spectrum. J. Am. Chem. Soc. 2017, 139, 10157–10163. 10.1021/jacs.7b05920. [DOI] [PubMed] [Google Scholar]
- Blencowe C. A.; Russell A. T.; Greco F.; Hayes W.; Thornthwaite D. W. Self-immolative linkers in polymeric delivery systems. Polym. Chem. 2011, 2, 773–790. 10.1039/C0PY00324G. [DOI] [Google Scholar]
- Alouane A.; Labruere R.; Le Saux T.; Schmidt F.; Jullien L. Self-Immolative Spacers: Kinetic Aspects, Structure-Property Relationships, and Applications. Angew. Chem., Int. Ed. 2015, 54, 7492–7509. 10.1002/anie.201500088. [DOI] [PubMed] [Google Scholar]
- Roth M. E.; Green O.; Gnaim S.; Shabat D. Dendritic, Oligomeric, and Polymeric Self-Immolative Molecular Amplification. Chem. Rev. 2016, 116, 1309–1352. 10.1021/acs.chemrev.5b00372. [DOI] [PubMed] [Google Scholar]
- Shen W.; Taylor B.; Jin Q.; Nguyen-Tran V.; Meeusen S.; Zhang Y. Q.; Kamireddy A.; Swafford A.; Powers A. F.; Walker J.; Lamb J.; Bursalaya B.; DiDonato M.; Harb G.; Qiu M.; Filippi C. M.; Deaton L.; Turk C. N.; Suarez-Pinzon W. L.; Liu Y.; Hao X.; Mo T.; Yan S.; Li J.; Herman A. E.; Hering B. J.; Wu T.; Martin Seidel H.; McNamara P.; Glynne R.; Laffitte B. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat. Commun. 2015, 6, 8372. 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar S. S.; Reineke T. M. Theranostics: combining imaging and therapy. Bioconjugate Chem. 2011, 22, 1879–903. 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.