Abstract
High tumor mutation burden (TMB), which is associated with increased tumor immunogenicity, has been identified to predict improved response to immune checkpoint inhibitors (ICIs) therapy in non-small cell lung cancer (NSCLC). As host immunity is also significant to eliminate cancer cells, however, its clinical impact on cancer immunotherapy is still largely unknown. Here we explored the influence of age, which is an important characteristic to evaluate immune response of patients, on TMB-based predictive system for ICIs therapy in NSCLC. Our results showed that high TMB was capable of predicting better durable clinical benefit (DCB) in agelow group, while it was insignificant in agehigh group. Besides, the predictive power of TMB for progression-free survival (PFS) and overall survival (OS) was better in agelow group than in agehigh group. Our study illustrated that the predictive value of TMB for ICIs therapy was better in young patients than in elderly patients in NSCLC.
Keywords: Tumor mutation burden, TMB, Age, Immune checkpoint inhibitor, ICI, NSCLC, Immunosenescence
To the Editor,
Tumor mutation burden (TMB) is widely demonstrated to predict the efficacy of immune checkpoint inhibitors (ICIs) in diverse cancers, especially in non-small cell lung cancer (NSCLC) and melanoma [1, 2]. High TMB presents enriched clonal neoantigens and increased tumor immunogenicity, which can improve the response to cancer immunotherapy [3]. However, as host immunity is also significant to eliminate cancer cells, its clinical impact on cancer immunotherapy is still largely unknown. Immunosenescence, which refers to the decline of immune system with aging, may contribute to reduced tumor cell clearance efficiency in body, leading to increased cancer incidence in the elderly [4].
Based on these facts and evidence, we hypothesized that TMB could show better predictive value for cancer immunotherapy in young patients than in elderly patients in NSCLC. In order to test the hypothesis, published clinical data was identified through systematic literature search. Durable clinical benefit (DCB), progression-free survival (PFS) and overall survival (OS) were adopted as endpoints for assessment. Detailed methods were explained in Additional file 1.
We identified three NSCLC immunotherapy cohorts containing 665 patients [1, 5, 6]. Detailed characteristics of patients included were summarized in Additional file 2: Table S1.
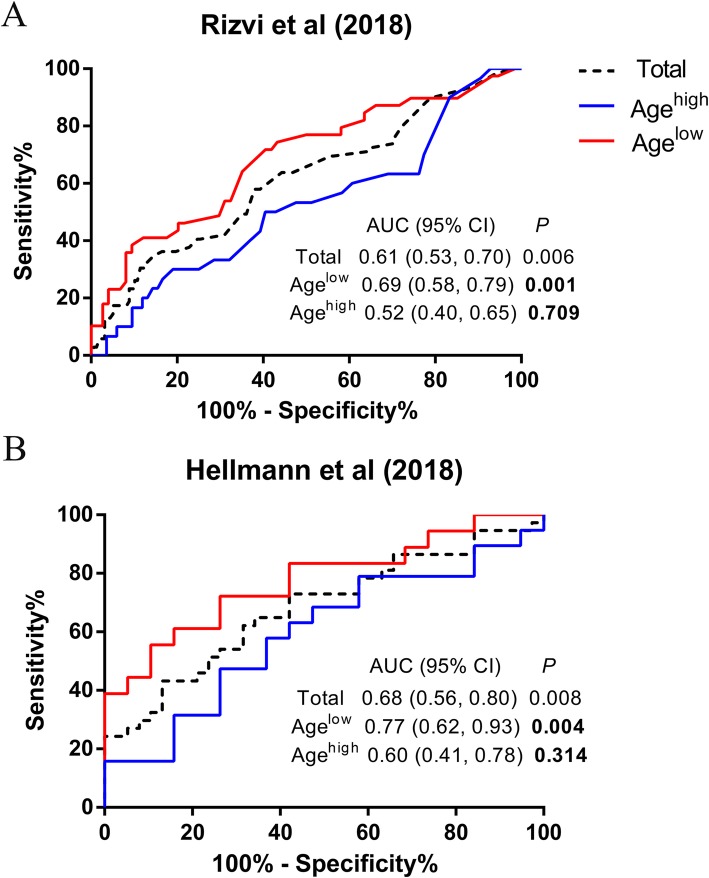
Firstly, as was shown in Fig. 1, high TMB was capable of predicting better DCB in agelow group. However, the predictive power was insignificant in agehigh group, indicating high TMB failed to forecast clinical benefit in the group.
Fig. 1.
ROC curve analysis of the association between TMB and DCB in young and elderly patients in NSCLC. ROC curves of (a) Rizvi cohort, (b) Hellmann cohort. ROC: receiver operator characteristic; TMB: tumor mutation burden; DCB: durable clinical benefit; NSCLC: non-small cell lung cancer; AUC: area under curve; CI: confidence interval
Secondly, it was found that in agelow group, high TMB dramatically illustrated improved PFS (Rizvi cohort: Hazard ratio [HR] 0.55, 95% confidence interval [CI] 0.35, 0.80, P = 0.003, Fig. 2a; Hellman cohort: HR 0.26, 95% CI 0.08, 0.45, P < 0.001, Fig. 2c). The results were still significant in multivariate analysis (Rizvi cohort: Adjusted HR 0.54, 95% CI 0.36, 0.82, P = 0.004; Hellman cohort: Adjusted HR 0.23, 95% CI 0.09, 0.55, P = 0.001). However, there was no correlation between PFS and TMB level in agehigh group (Rizvi cohort: HR 1.03, 95% CI 0.70, 1.51, P = 0.898, Fig. 2b; Hellman cohort: HR 0.71, 95% CI 0.32, 1.55, P = 0.388, Fig. 2d). In the adjusted model, the conclusion was unchanged (Rizvi cohort: Adjusted HR 1.10, 95% CI 0.71, 1,71, P = 0.677; Hellman cohort: Adjusted HR 0.60, 95% CI 0.24, 1.50, P = 0.275). Then, the result of meta-analysis further illustrated that predictive power of TMB was more significant in agelow group than in agehigh group (Heterogeneity between two groups: P = 0.007, Fig. 3). In addition, in order to exclude whether the specific cutoff of TMB had an effect on the result, TMB at the highest quarter was adopted as another cutpoint. As was shown in Additional file 2: Figure S1, high TMB still showed better predictive power of PFS in agelow group rather than in agehigh group (Heterogeneity between two groups: P = 0.012).
Fig. 2.
Kaplan–Meier curves and HR analysis of the association between TMB and PFS in young and elderly patients in NSCLC. Kaplan–Meier curves of (a) Agelow group and (b) Agehigh group in Rizvi cohort, (c) Agelow group and (d) Agehigh group in Hellmann cohort. HR: hazard ratio; TMB: tumor mutation burden; PFS: progression-free survival; NSCLC: non-small cell lung cancer; CI: confidence interval
Fig. 3.
Forest plot of the association between TMB and PFS in young and elderly patients in NSCLC. TMB: tumor mutation burden; PFS: progression-free survival; NSCLC: non-small cell lung cancer; HR: hazard ratio; CI: confidence interval
Moreover, the predictive value of TMB for OS of immunotherapy was evaluated in the two age groups. When using median TMB as cutoff, though both agelow (HR 0.72, 95% CI 0.46, 1.07, P = 0.112, Additional file 2: Figure S2A) and agehigh (HR 1.03, 95% CI 0.72, 1.47, P = 0.881, Additional file 2: Figure S2B) groups showed insignificant predictive value, the former presented a better tendency. When adopting TMB at the highest quarter as cutpoint, high TMB illustrated meaningful predictive power in agelow group (HR 0.43, 95% CI 0.30, 0.76, P = 0.007, Additional file 2: Figure S2C), while it was still insignificant in agehigh group (HR 0.80, 95% CI 0.53, 1.19, P = 0.282, Additional file 2: Figure S2D). In multivariate analysis, the conclusion was unchanged (Agelow group: Adjusted HR 0.43, 95% CI 0.24, 0.75, P = 0.003; Agehigh group: Adjusted HR 0.82, 95% CI 0.54, 1.25, P = 0.354).
In the present study, we found that TMB could present better predictive value on the response to cancer immunotherapy in young patients than in elderly patients in NSCLC. High TMB is associated with enhanced tumor immunogenicity [3], which is an important factor in determining efficacy of cancer immunotherapy [7]. However, in addition to sufficient antigen presentation, a dynamic host immunity may also be necessary for eliminating cancer cells during ICIs therapy. In the process of aging, immune cells are gradually reduced in quantity and becoming defective [4]. Interestingly, PD-1/PD-L1 blockade could not completely restore exhausted T-cell responses in aged mice [8], suggesting the significance of basic immunity of host to confront tumor. To note, age itself may not be an appropriate marker to predict cancer immunotherapy response due to its complex influence on body besides host immunity [9].
There are several strengths in the study. Firstly, different studies including a large number of patients were analyzed, which increased the credibility of the results. Besides, both univariate and multivariate analyses were adopted, which improved the accuracy of the conclusion. However, there are quite a few limitations in the present study. First of all, different TMB testing methodologies and cutoffs were utilized, as there is still no uniform standard, which could lead to heterogeneity of the results. In addition, analysis in the study was conducted only in NSCLC, while related open access data in most cancers are still insufficient.
In conclusion, we revealed that the predictive power of TMB on ICIs therapy was better in young patients than in elderly patients in NSCLC. Therefore, more effective markers need to be identified to differentiate the efficacy of cancer immunotherapy in elderly patients in NSCLC. In addition, the combination of tumor immunogenicity and host immunity evaluation may better identify patient subgroups which are suitable for cancer immunotherapy.
Supplementary information
Additional file 1. Supplementary Methods
Additional file 2: Table S1. Clinical characteristics of included NSCLC cohorts treated with immune checkpoint inhibitors. Figure S1. Forest plot of the association between TMB (using the highest quarter as cutoff) and PFS in young and elderly patients in NSCLC. Figure S2. Kaplan–Meier curves and HR analysis of the association between TMB and OS in young and elderly patients in NSCLC. Kaplan–Meier curves of (A-B) using median TMB as cutoff and (C-D) using the highest quarter as cutoff.
Acknowledgements
Not applicable.
Abbreviations
- TMB
Tumor mutation burden
- ICIs
Immune checkpoint inhibitors
- NSCLC
Non-small cell lung cancer
- DCB
Durable clinical benefit
- PFS
Progression-free survival
- OS
Overall survival
- HR
Hazard ratio
- CI
Confidence interval
- ROC
Receiver operator characteristic
- AUC
Area under curve
Authors’ contributions
JH contributed to the conception and design of the work. YFW contributed to conception, design, data analysis and editing the manuscript. JMX and JWX contributed to design and data analysis. YQW, LW, and WL contributed to data acquisition and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by National Key R&D Program of China (No. 2017YFC0113500), Major Science and Technology Projects of Zhejiang Province (No. 2014C03032), Lung Cancer Diagnosis and Treatment Technology Research Center of Zhejiang Province (JBZX-202007), Key Disciplines of Traditional Chinese Medicine in Zhejiang Province (No. 2017-XK-A33).
Availability of data and materials
The datasets analyzed during the current study are available at cBioPortal (Samstein et al. (2019): https://www.cbioportal.org/study/summary?id=tmb_mskcc_2018 [1]; Rizvi et al. (2018): https://www.cbioportal.org/study/summary?id=nsclc_pd1_msk_2018 [5]) and ScienceDirect (Hellmann et al. (2018): 10.1016/j.ccell.2018.03.018 [6]).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40364-020-00188-2.
References
- 1.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Xu J, Du C, Wu Y, Xia D, Lv W, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol. 2019;9:1161. doi: 10.3389/fonc.2019.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging, immune senescence, and immunotherapy: a comprehensive review. Semin Oncol. 2018;45(4):187–200. doi: 10.1053/j.seminoncol.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung Cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell. 2018;33(5):843–852. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/ PD-L1 pathway. Aging Cell. 2010;9(5):785–798. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawelec G. Does patient age influence anti-cancer immunity? Semin Immunopathol. 2019;41(1):125–131. doi: 10.1007/s00281-018-0697-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Methods
Additional file 2: Table S1. Clinical characteristics of included NSCLC cohorts treated with immune checkpoint inhibitors. Figure S1. Forest plot of the association between TMB (using the highest quarter as cutoff) and PFS in young and elderly patients in NSCLC. Figure S2. Kaplan–Meier curves and HR analysis of the association between TMB and OS in young and elderly patients in NSCLC. Kaplan–Meier curves of (A-B) using median TMB as cutoff and (C-D) using the highest quarter as cutoff.
Data Availability Statement
The datasets analyzed during the current study are available at cBioPortal (Samstein et al. (2019): https://www.cbioportal.org/study/summary?id=tmb_mskcc_2018 [1]; Rizvi et al. (2018): https://www.cbioportal.org/study/summary?id=nsclc_pd1_msk_2018 [5]) and ScienceDirect (Hellmann et al. (2018): 10.1016/j.ccell.2018.03.018 [6]).