Abstract
Background
Statin may confer anticancer effect. However, the association between statin and risk of hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV) or hepatitis C (HCV) virus infection remains inconsistent according to results of previous studies. A meta-analysis was performed to summarize current evidence.
Methods
Related follow-up studies were obtained by systematic search of PubMed, Cochrane’s Library, and Embase databases. A random-effect model was used to for the meta-analysis. Stratified analyses were performed to evaluate the influences of study characteristics on the outcome.
Results
Thirteen studies with 519,707 patients were included. Statin use was associated with reduced risk of HCC in these patients (risk ratio [RR]: 0.54, 95% CI: 0.44 to 0.66, p < 0.001; I2 = 86%). Stratified analyses showed that the association between statin use and reduced HCC risk was consistent in patients with HBV or HCV infection, in elder (≥ 50 years) or younger (< 50 years) patients, in males or females, in diabetic or non-diabetic, and in those with or without cirrhosis (all p < 0.05). Moreover, lipophilic statins was associated with a reduced HCC risk (RR: 0.52, p < 0.001), but not for hydrophilic statins (RR: 0.89, p = 0.21). The association was more remarkable in patients with highest statin accumulative dose compared to those with lowest accumulative dose (p = 0.002).
Conclusions
Satin use was independently associated with a reduced risk of HCC in patients with HBV or HCV infection.
Keywords: Statin, Hepatocellular carcinoma, Hepatitis B virus, Hepatitis C virus, Cirrhosis, Meta-analysis
Background
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers in digestive system, and approximately 500,000 cases of HCC are newly diagnosed annually worldwide [1]. Patients with HCC are of poor prognosis due to limited treatment options, and the median survival of these patients is less than 1 year [2–4]. Patients with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection are primarily the high-risk population for the development of HCC [2–4]. Although HBV suppression or HCV eradication has been increasingly applied, the incidence of HCC in patients with HBV or HCV infection remains high [5, 6]. Therefore, identification of novel chemoprevention agents for HCC remains of great clinical importance, particularly for high-risk population such as patients with HBV or HCV infection [7].
Statins, also known as 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors, are a category of cholesterol-lowering medications which have become the mainstays for the primary and secondary prevention of cardiovascular diseases [8]. Moreover, increasing evidence demonstrates the potential pleiotropy of statins, such as anti-inflammation, immunomodulation, pro-apoptosis, anti-proliferation, and anti-invasion, all of which have been implicated in carcinogenesis and metastasis [9, 10]. Therefore, statins have been suggested as anticancer agents [11]. A previous meta-analysis indicated that use of statin may be related with a 37% reduced risk of HCC incidence [12]. However, this meta-analysis included a patient population of heterogeneous spectrum of clinical statuses, which makes the interpretation of the results difficult [12]. Moreover, cross-sectional studies were included despite of follow-up studies, which may introduce additional biases [12]. Besides, previous studies evaluating the association between statin use and HCC risk in patients with HBV or HCV infection retrieved inconsistent results. Although most studies indicated that statin use was associated with a reduced risk of HCC in patients with HBV or HCV infection [13–22], some studies showed a nonsignificant association between statin use and HCC risk in these patients [23–25]. Therefore, we aimed to perform a meta-analysis of longitudinal follow-up studies to systematically evaluate the association between statin use and HCC risk in high-risk patients with HBV and HCV infection. Moreover, we explored the potential influences of study characteristics on this association, including virus type, age, gender, diabetic status of the patients, with or without cirrhosis, characteristics of statins, and accumulative dose of statins.
Methods
The MOOSE (Meta-analysis of Observational Studies in Epidemiology) [26] and Cochrane’s Handbook [27] guidelines were followed during the designing, performing, and reporting of the meta-analysis.
Literature search
Systematic search of electronic databases of PubMed, Cochrane’s Library, and Embase were performed to identify potentially relevant studies, via the following terms: (1) “statin” OR “3-hydroxy-3-methyl-glutaryl CoA reductase inhibitor” OR “CS-514” OR “statin” OR “simvastatin” OR “atorvastatin” OR “fluvastatin” OR “lovastatin” OR “rosuvastatin” OR “pravastatin” OR “pitavastatin”; and (2) “chronic hepatitis B” OR “chronic hepatitis C” OR “hepatitis B virus” OR “hepatitis C virus” OR “HBV” OR “HCV”. We used this extensive search strategy to avoid missing of potentially relevant studies. The search was limited to human studies, and no language restriction was applied. Besides, we also studied the reference lists of related original studies and review articles using a manual approach. The final literature search was conducted on September 15, 2019.
Study selection
The inclusion criteria were: (1) full-length articles reporting longitudinal follow-up studies, including randomized controlled trials (RCTs), cohort studies, and nested case-control studies; (2) enrolled at least 1000 adult patients with HBV or HCV infection and without HCC at baseline; (3) investigated the association between statin use and HCC risk during follow-up, with a minimal follow-up duration of 1 year; and (4) reported the relative risk for this association after adjustment of potential confounding factors. Review articles, preclinical studies, and studies irrelevant to the purpose of current meta-analysis were excluded.
Data extracting and quality evaluation
Two authors indepdently performed database search, data extraction, and study quality assessment according to predefined criteria. If discrepancies occurred, they were solved by consensus between the two authors or discussion with the corresponding author. Data extracted included: (1) study information: name of first author, publication year, and study country; (2) study design characteristics; (3) patient characteristics: disease status, sample size, age, sex, prevalence of diabetes, and proportions of patients with cirrhosis at baseline; (4) definition of statin use; (5) follow-up durations; (6) strategy for HCC validation and number of HCC cases during follow-up; and (7) confounding factors adjusted. The Newcastle-Ottawa Scale was used as an instrument for study quality evaluation [28]. This scale ranges from 1 to 9 stars, and assesses study quality mainly regarding three domains, including study group selection, between-group comparability, and validation of the outcome of interest.
Statistical analyses
A risk ratio (RR) with corresponding 95% confidence interval (CI) was used as the main measure for the association between statin use and HCC risk in patients with HBV or HCV infection. Data of RRs and their corresponding stand errors (SEs) were calculated from 95% CIs or p values, and a logarithmical transformation was performed to stabilize variance and normalized the distribution [27]. The Cochrane’s Q test was performed to evaluate the heterogeneity, and the I2 statistic was also estimated [29]. An I2 > 50% indicates significant heterogeneity. We used a random-effect model for the meta-analysis of RR data because this model incorporates the potential heterogeneity among the included studies to calculate a more generalized result [27], By omitting one individual study at a time, we performed sensitivity analyses to test the robustness of the results [30]. We also performed stratified analyses to evaluate the influences of virus type, age, gender, diabetic status, with or without cirrhosis, lipophilic or hydrophilic statins, and accumulative dose of statin on the results. The potential publication bias was initially detected by visual inspection of the symmetry of funnel plots, then complemented with the Egger’s regression asymmetry test [31]. RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) software was used for the meta-analysis.
Results
Literature search
Figure 1 shows the literature search process. Briefly, 732 articles were obtained via initial search of the PubMed, Cochrane’s Library, and Embase databases, and 707 were excluded through screening of the titles and abstracts mainly because they were not relevant to the purpose of the meta-analysis. Subsequently, 25 records underwent full-text review. Of these, 12 were further excluded because four of them did not evaluate statin use as exposure, five did not report outcome of HCC risk, one did not provide available data for the multivariate adjusted association between statin use and HCC risk, and the remaining three were abstracts of already included studies. Finally, we included 13 studies in this meta-analysis [13–25].
Fig. 1.
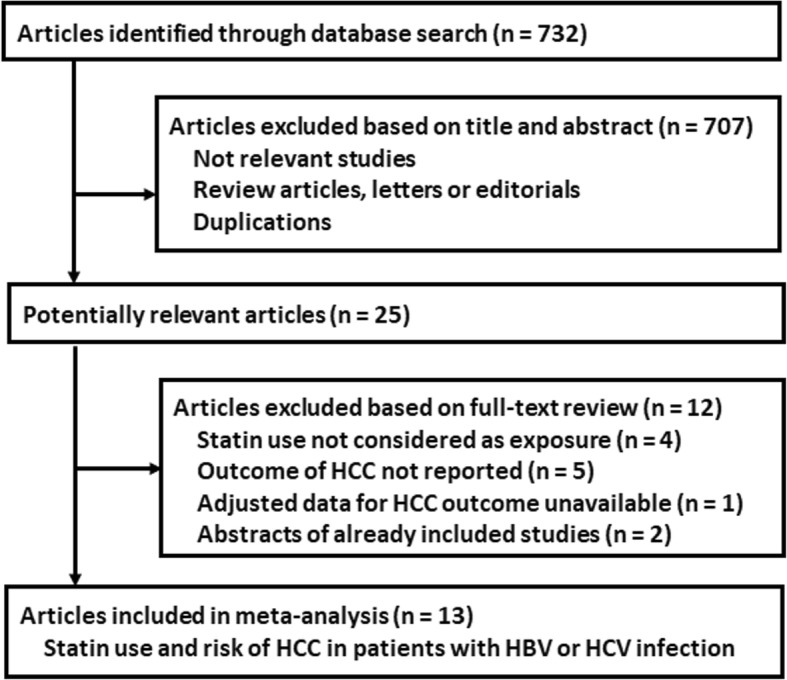
Flowchart of database search and study identification
Study characteristics and quality evaluation
The characteristics of the studies were presented in Table 1. All of them were observational studies, among which one was a prospective cohort study, another one was a nested case-control study, and the remaining 11 were retrospective cohort studies. Since two studies reported the association between statin use and HCC risk in patients with HBV and HCV infection separately, these datasets were included independently [22, 24]. Overall, 15 datasets from 13 studies, with 519,707 adult patients with HBV or HCV infection were included [13–25]. These studies were performed in China [13, 14, 16, 17, 21, 24], Korea [19, 20], the US [15, 18, 23, 25], and Sweden [22]. The mean age of the patients varied from 35 to 64 years, with percentiles of male ranging from 49 to 98%. Statin use was validated by prescription records in all studies and defined by accumulative statin dose of more than 28~30 cumulative defined daily dose (cDDD) in most studies [13–17, 20–23]. The follow-up duration varied from 2.5 to 10.7 years. The International Classification of Diseases (ICD) version 9 or 10 codes were used to validate HCC cases, and a total of 40,588 patients with HCC were included. Potential confounding factors including age, sex, diabetic status, comorbidities, and concurrent medications, were adjusted when presenting the outcome. The NOS scores of the included studies ranged from seven to nine, indicating generally good study quality.
Table 1.
Characteristics of the included follow-up studies
| Study | Country | Design | Patient characteristics | Sample size | Mean age years |
Male % |
Diabetes % |
Cirrhosis % |
Validation of statin use | Follow-up durations years |
HCC validation | HCC cases | Variables adjusted | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tsan 2012 [13] | China | RC | Adult patients with a first-time diagnosis of HBV infection | 33413 | 35.6 | 58.2 | 26.4 | 10.7 | Records of prescription for statins ≥28 cDDD | 9.8 | ICD-9 codes | 1021 | Age, sex, income, urbanization, diabetes, and liver cirrhosis | 9 (4, 2, 3) |
| Tsan 2013 [14] | China | RC | Adult patients with a first-time diagnosis of HCV infection | 260864 | 50.4 | 49.2 | 27.6 | 18.4 | Records of prescription for statins ≥28 cDDD | 10.7 | ICD-9 codes | 27883 | Age, sex, income, urbanization, diabetes, and liver cirrhosis | 9 (4, 2, 3) |
| Butt 2015 [15] | the US | RC | Adult patients with HCV infection | 7248 | 52.4 | 95.5 | 13.1 | 0 | Records of prescription for statins ≥28 cDDD | 10 | ICD-9 codes | 142 | Age, race, sex, development of cirrhosis, HCV RNA, BMI, dyslipidemia, diabetes, and alcohol abuse | 8 (3, 2, 3) |
| Hsiang 2015 [17] | China | RC | Adult patients with HBV infection | 53513 | 58.9 | 66.8 | 45.4 | 3.1 | Records of prescription for statins ≥28 cDDD | 4.6 | ICD-9 codes | 6883 | Age, sex, cirrhosis and complications, and diabetes | 8 (3, 2, 3) |
| Chen 2015 [16] | China | RC | Adult patients with HBV infection | 71847 | 41.5 | 57.8 | NR | NR | Records of prescription for statins ≥28 cDDD | 9 | ICD-9 codes | 1735 | Age, sex. CCI index, and using of other medications | 7 (2, 3, 2) |
| Mohanty 2016 [18] | the US | RC | Adult patients with HCV related compensated cirrhosis | 40512 | 56 | 98 | 34.1 | 100 | Records of prescription for statins ≥2 fills | 2.5 | ICD-9 codes | 173 | Age, race, sex, BMI, dyslipidemia, diabetes, and MELD Score | 7 (2, 3, 2) |
| Simon 2016 [23] | the US | RC | Adult patients with HCV infection without cirrhosis | 9135 | 52.9 | 95.7 | 17.1 | 0 | Records of prescription for statins ≥28 cDDD | 7.4 | ICD-9 codes | 239 | Age, sex, race, smoking, alcohol abuse, BMI, diabetes, and concurrent medications | 8 (3, 2, 3) |
| Chang 2017 [24] | China | RC | Adult patients with HBV or HCV related cirrhosis | 1350 | 57 | 73 | 74.5 | 100 | Records of prescription for statins ≥28 cDDD | 5.5 | ICD-9 codes | 111 | Age, sex, CCI index, diabetes, and concurrent medications | 7 (2, 3, 2) |
| Kim 2017 [19] | Korea | NCC | Adult patients with HBV or HCV infection | 1374 | 52.5 | 81.4 | 100 | 8.9 | Records of prescription for statins | 5 | ICD-10 codes | 229 | Age, sex, diabetic duration, CCI, and concurrent medications | 7 (2, 3, 2) |
| Simon 2019 [22] | Sweden | PC | Adult patients with HBV or HCV infection | 16668 | 47.4 | 65.5 | 30.5 | 10 | Records of prescription for statins ≥30 cDDD | 8 | ICD-10 codes | 616 | Age, sex, cirrhosis and complications, diabetes, and concurrent medications | 8 (3, 2, 3) |
| Kaplan 2019 [25] | the US | RC | Adult patients with HCV infection | 5455 | 64 | 97.6 | 70.8 | 100 | Records of prescription for statins | 8 | ICD-10 codes | 133 | Age, sex, CCI, diabetes, comorbidities, and concurrent medications | 7 (2, 3, 2) |
| Goh 2019 [20] | Korea | RC | Adult patients with HBV infection | 7713 | 47.3 | 66.2 | 11.4 | 24.1 | Records of prescription for statins ≥28 cDDD | 9.2 | ICD-10 codes | 702 | Age, sex, cirrhosis, diabetes, hypertension, HBV DNA level, antiviral treatment, and antiplatelet therapy | 9 (4, 2, 3) |
| Lee 2019 [21] | China | RC | Adult patients with HBV infection | 10615 | 58.8 | 72.4 | 29 | 17.1 | Records of prescription for statins ≥28 cDDD | 5 | ICD-10 codes | 721 | Age, sex, cirrhosis, diabetes, hypertension, hyperlipidemia, and concurrent medications | 8 (3, 2, 3) |
HCC hepatocellular carcinoma, NOS the Newcastle-Ottawa Scale, RC retrospective cohort, PC prospective cohort, NCC nested case-control, HBV hepatitis B virus, HCV hepatitis C virus, cDDD cumulative defined daily dose, ICD International Classification of Diseases, BMI body mass index, CCI Charlson comorbidity index, MELD model for end-stage liver disease
Results of main meta-analysis
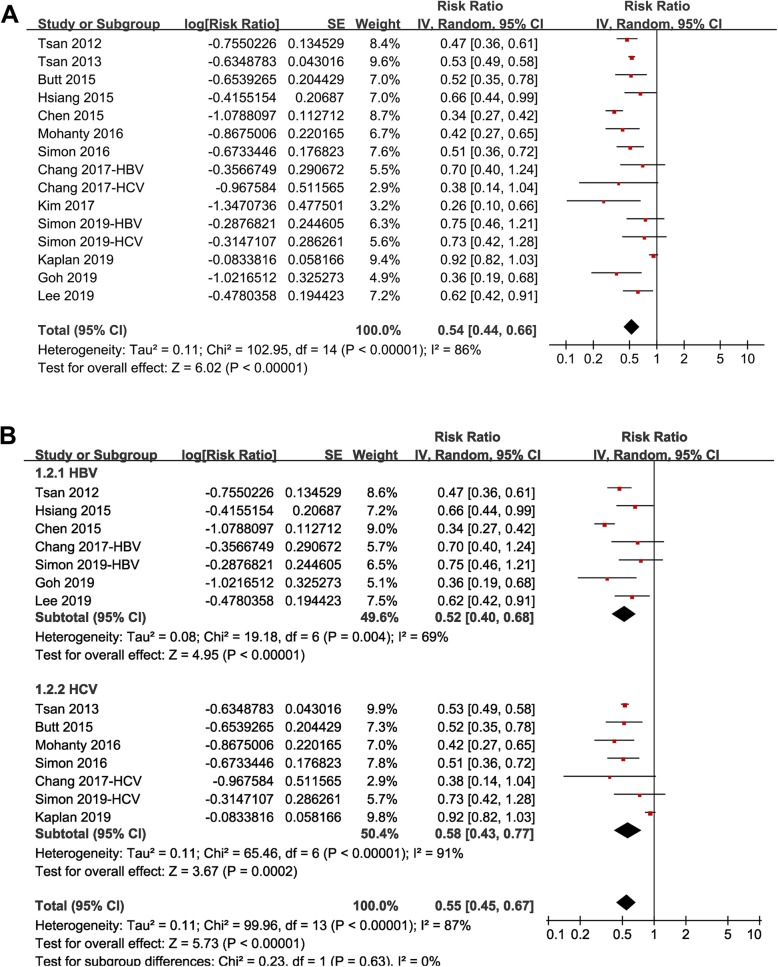
Pooled results of all included studies using a random-effect model showed that statin use was associated with a reduced risk of HCC in patients with HBV or HCV infection (RR: 0.54, 95% CI: 0.44 to 0.66, p < 0.001; Fig. 2a) with significant heterogeneity (p for Cochrane’s Q test < 0.001, I2 = 86%). Sensitivity analyses by omitting one datasets at a time did not significantly change the results (RR: 0.50 to 0.56, p all < 0.05).
Fig. 2.
Forest plots for the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection: a overall meta-analysis; and b stratified analyses according to type of virus infected
Results of stratified analyses
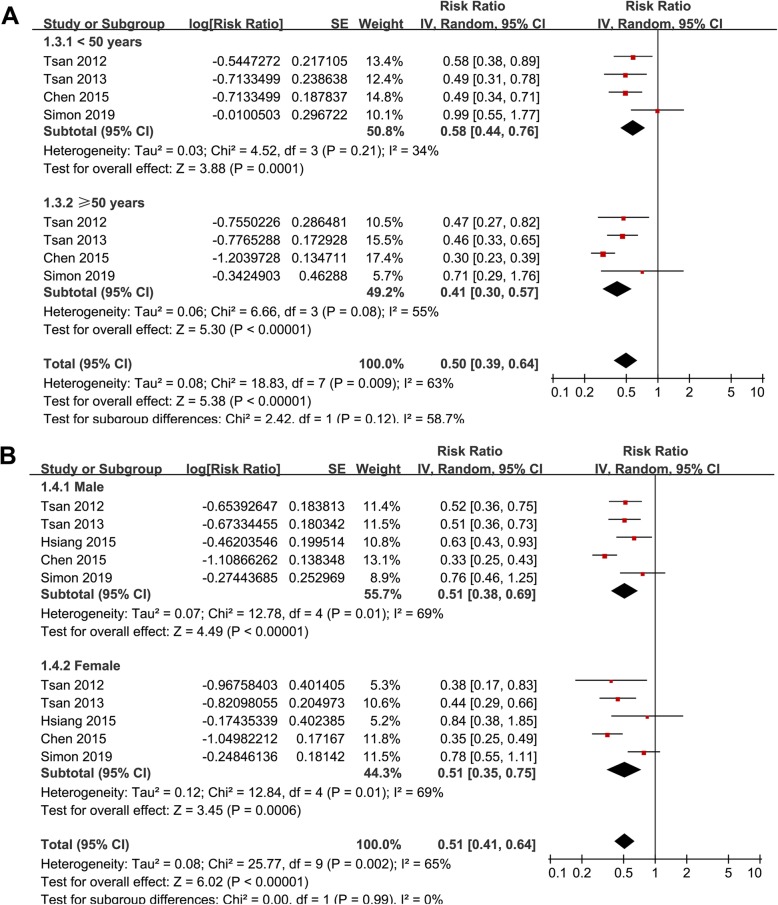
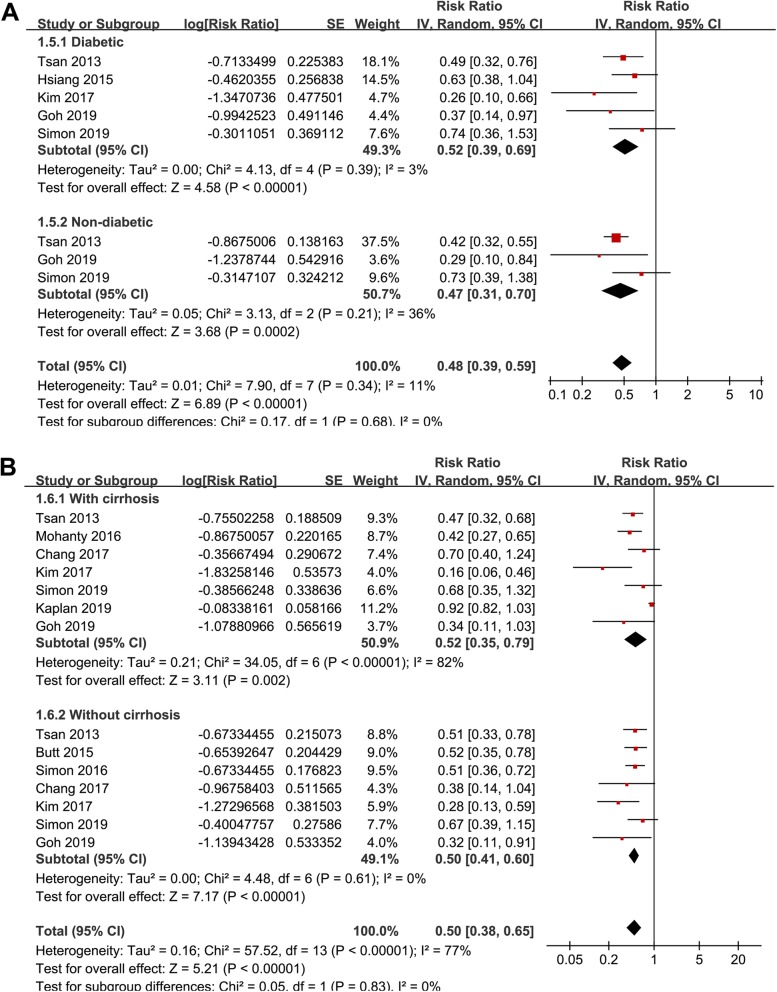
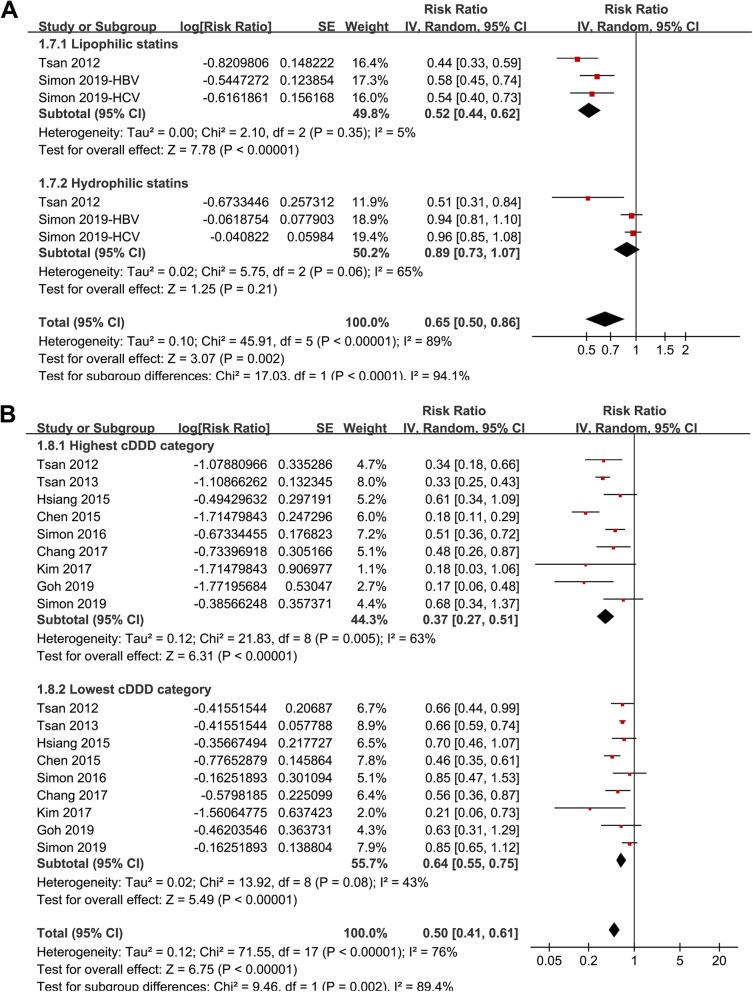
Stratified analyses showed that the association between statin use and reduced risk of HCC was consistent in patients with HBV (RR: 0.52, 95% CI: 0.40 to 0.68, p < 0.001) or HCV infection (RR: 0.58, 95% CI: 0.43 to 0.77, p < 0.001; Fig. 2b), in patients < 50 years (RR: 0.58, 95% CI: 0.44 to 0.76, p < 0.001) or ≥ 50 years (RR: 0.41, 95% CI: 0.30 to 0.57, p < 0.001; Fig. 3a), in males (RR: 0.51, 95% CI: 0.38 to 0.69, p < 0.001) or females (RR: 0.51, 95% CI: 0.35 to 0.75, p < 0.001; Fig. 3b), in diabetic (RR: 0.52, 95% CI: 0.39 to 0.69, p < 0.001) or non-diabetic patients (RR: 0.47, 95% CI: 0.31 to 0.70, p < 0.001; Fig. 4a), and in patients with (RR: 0.52, 95% CI: 0.35 to 0.79, p = 0.002) or without cirrhosis (RR: 0.50, 95% CI: 0.41 to 0.60, p < 0.001; Fig. 4b). The association between statin use and reduced risk of HCC were not significantly affected by the above patient characteristics (p for subgroup difference all > 0.05). However, stratified analyses with three datasets in each stratum showed that use of lipophilic statins was associated with reduced risk of HCC in patients with HBV or HCV infection (RR: 0.52, 95% CI: 0.44 to 0.62, p < 0.001), but not for hydrophilic statins (RR: 0.89, 95% CI: 0.73 to 1.07, p = 0.21; p for subgroup difference < 0.001; Fig. 5a). Nine studies reported the potential dose-response relationship between statin use and risk of HCC according to the cDDD of statins [13, 14, 16, 17, 19, 20, 22–24]. However, difference cut-off values for cDDD were used, which prevented a dose-response analysis in our meta-analysis. Subsequently, we performed stratified analyses comparing the association between statin use and HCC risk in patients with highest and lowest cDDD categories in each study. Results showed that the association between statin use and reduced risk of HCC was more remarkable in patients with highest cDDD category for statin prescription (RR: 0.37, 95% CI: 0.27 to 0.51, p < 0.001) compared to those with lowest cDDD category (RR: 0.64, 95% CI: 0.55 to 0.75, p < 0.001; p for subgroup difference = 0.002; Fig. 5b).
Fig. 3.
Stratified analyses for the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection; a stratified analyses according to patient age; and b stratified analyses according to patient sex
Fig. 4.
Stratified analyses for the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection; a stratified analyses according to diabetic status of patient; and b stratified analyses according to with or without cirrhosis
Fig. 5.
Stratified analyses for the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection; a stratified analyses according to the properties of statins (lipophilic or hydrophilic); and b stratified analyses according to the accumulative dosages of statins
Publication bias
The funnel plots for the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection were shown in Fig. 6. These plots were symmetry on visual inspection, suggesting low risk of publication bias. Results of Egger’s regression test also suggested low possibility of publication bias (p = 0.188).
Fig. 6.
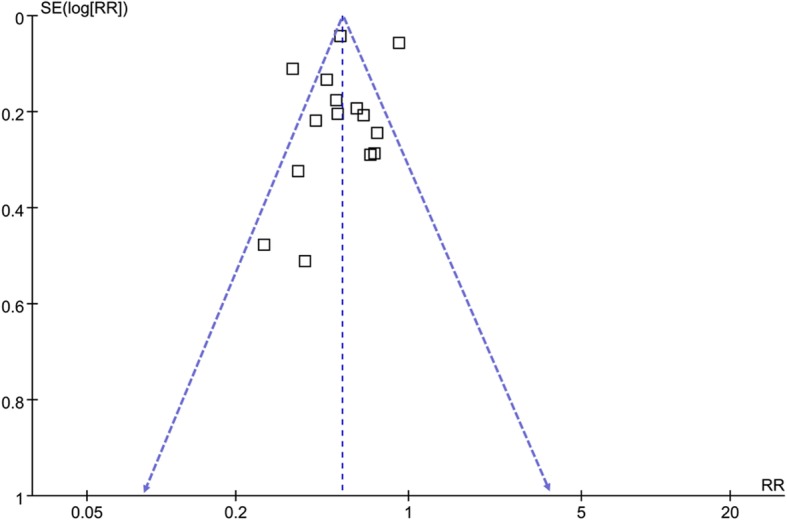
Funnel plots for the publication bias underlying the meta-analysis of the association between statin use and HCC risk in patients with HBV or HCV infection
Discussion
By summarizing the current evidence from epidemiological studies, our meta-analysis showed that statin use was indepdently associated with reduced risk of HCC in patients with HBV or HCV infection. Subsequently stratified analyses showed that the association between statin use and reduced risk of HCC in these patients were consistent in patients with HBV and HCV infection, in elder (≥ 50 years) and younger (< 50 years) patients, in males and females, in diabetic and non-diabetic, and in those with and without cirrhosis. Moreover, exploring stratified analyses showed that use of lipophilic statins was associated with reduced risk of HCC in patients with HBV or HCV infection, but not for hydrophilic statins. Besides, the association between statin use and reduced risk of HCC in these patients was more remarkable in patients with highest accumulative dose of statin prescription compared to those with lowest accumulative dose category. Taken together, these results demonstrated that statin use was indepdently associated with a reduced risk of HCC in patients with HBV or HCV infection, which may be primarily driven by studies with lipophilic statins and probably via a dose-dependent manner. Although large-scale prospective cohort studies and RCTs are needed to validate these findings, results of this meta-analysis highlight the potential role of statins as chemoprevention agents for HCC in patients with HBV or HCV infection.
To the best of our knowledge, our study is the first meta-analysis focusing on the association between statin use and HCC risk in patients with HBV or HCV infection. The strengths of our study included follows. Firstly, this meta-analysis included only longitudinal follow-up studies, which could therefore establish a sequential association between statin use and reduced risk of HCC in patients with HBV or HCV infection. Secondly, we only studies with adequate adjustment of confounding factors, which therefore may suggest an independently association between statin use and reduced risk of HCC in these patients. Thirdly, we used sensitivity analysis to confirm the robustness of the finding, which was not primarily driven by either of the included study. Finally, multiple stratified analyses were performed to evaluate the stability of the results, which showed that association between statin use and reduced risk of HCC in these patients were consistent and not affected by hepatitis virus type, patient age, sex, diabetic status, and with and without cirrhosis. These results supported the hypothesis that statins may be applied as a chemoprevention agent against the development of HCC in high-risk patients with HBV or HCV infection. Since no RCTs have been published in this field, our results highlighted the need of large-scale RCTs to validate the potential chemoprevention role of statins for HCC.
Results of our stratified analyses showed that use of lipophilic statins was associated with reduced risk of HCC in patients with HBV or HCV infection, but not for hydrophilic statins. However, only three datasets were available for each stratum of the stratified analyses, and the results were mainly driven by one study [22]. Therefore, the results should be interpreted cautiously. Interestingly, previous studies did show that lipophilic statins seem to confer more remarkable anticancer efficacy than hydrophilic statins in some cancers, such as in gynecological cancers expressing high levels of HMG-CoA reductase [32]. The mechanisms for the potential different anticancer efficacies between lipophilic and hydrophilic statins remain to be determined. In addition, we found that the association between statin use and reduced risk of HCC was more remarkable in patients with highest accumulative dose of statin prescription compared to those with lowest accumulative dose category, suggesting a possible dose-dependent manner under the association. However, since the included studies applied cDDD with various cut-off values for categorization of statin dose, large scale studies are warranted to validate the dose-dependent association between statin use and reduced HCC risk in patients with HBV or HCV infection.
The potential molecular mechanisms underlying the chemoprevention effects of statins for HCC may be multiple. An early experimental study showed that combinatorial treatment with statin and protein kinase C-beta inhibitor displayed enhanced anti-tumor efficacy in cultured HCC cells and in a mouse model of HCC [33]. Subsequent studies showed that inhibition of HMG-CoA reductase by atorvastatin blocks both MYC phosphorylation and activation, suppressing tumor initiation and growth in vivo in a transgenic model of MYC-induced HCC as well as in human HCC-derived cell lines [34]. Moreover, in mouse and human HCC cell lines, treatment with fluvastatin, simvastatin, atorvastatin, rosuvastatin or lovastatin are all associated with induced cellular apoptosis in a p53 dependent manner [35]. Modulation other molecular pathways, such as inhibition of signal transducer and activator of transcription 3/SKP2 axis [36], inhibition of SRC/FAK cue [37], and activation of AMPK et al. [38] have also been involved in the potential anti-HCC effects of statins. The key mechanisms underlying the potential anti-HCC efficacy of statins in patients with HBV or HCV infection deserve further investigations.
Our study has limitations Firstly, significant heterogeneity was found for the meta-analysis. Although stratified analyses were performed to evaluate the patient and statin prescription characteristics on the outcome, we could not exclude some other study characteristics that may also contribute to the heterogeneity, such as concurrent medications including antiviral agents [39] and metformin [40]. Both have been indicated to confer anticancer effects. Moreover, due to the limited studies, results of some stratified analyses should be interpreted very cautiously, such as the findings that lipophilic statins and hydrophilic statins may be associated with HCC risk differently. This finding was mainly driven by one include study [22] as previously discussed. In addition, although we included studies with adjusted data, residual factors may remain existing which may confound the association, such as chronic alcoholism [41] and metabolic liver diseases [42]. Finally, a causative association between statin use and reduced HCC risk in patients with HBV or HCV infection could not be derived based on our finding, since this study was a meta-analysis of observational studies. Our finding should be considered as hypothesis-generating. Effect of additional statin therapy on HCC incidence in patients with HBV or HCV infection should be validated in large-scale RCTs.
Conclusions
In conclusion, results of meta-analysis demonstrated that statin use was indepdently associated with a reduced risk of HCC in patients with HBV or HCV infection, which may be primarily driven by studies with lipophilic statins and probably via a dose-dependent manner. Satins may be potential chemoprevention agents for HCC in patients with HBV or HCV infection.
Acknowledgments
Not applied.
Abbreviations
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C
- RR
Risk ratio
- HMG-CoA
3-hydroxy-3-methylglutaryl CoA
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- RCT
Randomized controlled trial
- CI
Confidence intervals
- SE
Stand error
Authors’ contributions
XL conceived and designed the study. LS and LL1 selected the studies and collected the data. XL, LS, YH, YC and LL2 analyzed data. All authors interpreted the results. XL, LS, and LL1 drafted and revised the paper. All authors revised the draft paper. All authors read and approved the final version of the manuscript.
Funding
No funding was received for this study.
Availability of data and materials
All relevant data for this study are presented in tables, figures and supplementary materials.
Ethics approval and consent to participate
Not applicable. The authors declare that no patient data (details, images or videos relating to individual participants) are included in this article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Mak LY, Cruz-Ramon V, Chinchilla-Lopez P, Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Bailey HH, et al. Global epidemiology, prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262–279. doi: 10.1200/EDBK_200939. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491 e471. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo M, Lleo A. The impact of antiviral therapy on hepatocellular carcinoma epidemiology. Hepat Oncol. 2018;5(1):HEP03. doi: 10.2217/hep-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roderburg C, Tacke F, Trautwein C. Antiviral therapy in patients with viral hepatitis and hepatocellular carcinoma: indications and prognosis. Visc Med. 2016;32(2):121–126. doi: 10.1159/000444990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2019;20(1):78-89. 10.1111/apt.15573. [DOI] [PubMed]
- 8.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316(19):2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 9.Fatehi Hassanabad A. Current perspectives on statins as potential anti-cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Transl Lung Cancer Res. 2019;8(5):692–699. doi: 10.21037/tlcr.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altwairgi AK. Statins are potential anticancerous agents (review) Oncol Rep. 2015;33(3):1019–1039. doi: 10.3892/or.2015.3741. [DOI] [PubMed] [Google Scholar]
- 11.Mei Z, Liang M, Li L, Zhang Y, Wang Q, Yang W. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer. 2017;140(5):1068–1081. doi: 10.1002/ijc.30526. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 14.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31(12):1514–1521. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, Chung RT, Rogal SS. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology. 2015;62(2):365–374. doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 16.Chen CI, Kuan CF, Fang YA, Liu SH, Liu JC, Wu LL, Chang CJ, Yang HC, Hwang J, Miser JS, et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore) 2015;94(6):e462. doi: 10.1097/MD.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiang JC, Wong GL, Tse YK, Wong VW, Yip TC, Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: a propensity score landmark analysis. J Hepatol. 2015;63(5):1190–1197. doi: 10.1016/j.jhep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of Decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2016;150(2):430–440 e431. doi: 10.1053/j.gastro.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim G, Jang SY, Han E, Lee YH, Park SY, Nam CM, Kang ES. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer. 2017;140(4):798–806. doi: 10.1002/ijc.30506. [DOI] [PubMed] [Google Scholar]
- 20.Goh MJ, Sinn DH, Kim S, Woo SY, Cho H, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Statin Use and the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. Hepatology. 2019. 10.1002/hep.30973. [DOI] [PubMed]
- 21.Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, Wu CY. Association of Daily Aspirin Therapy with Risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med. 2019;179(5):633–640. doi: 10.1001/jamainternmed.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, Chung RT, Ludvigsson JF. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a Nationwide Swedish population. Ann Intern Med. 2019;171(5):318–327. doi: 10.7326/M18-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology. 2016;64(1):47–57. doi: 10.1002/hep.28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study. Hepatology. 2017;66(3):896–907. doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan DE, Serper MA, Mehta R, Fox R, John B, Aytaman A, Baytarian M, Hunt K, Albrecht J, Njei B, et al. Effects of hypercholesterolemia and statin exposure on survival in a large National Cohort of patients with cirrhosis. Gastroenterology. 2019;156(6):1693–1706 e1612. doi: 10.1053/j.gastro.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab. 2011; www.cochranehandbook.org.
- 28.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010. [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, Smalley S, Sadarangani A, Chen-Lin K, Oliva B, Branes J, Carvajal J, Gejman R, Owen GI, Cuello M. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J Cell Mol Med. 2010;14(5):1180–1193. doi: 10.1111/j.1582-4934.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim W, Yoon JH, Kim JR, Jang IJ, Bang YJ, Kim YJ, Lee HS. Synergistic anti-tumor efficacy of lovastatin and protein kinase C-beta inhibitor in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2009;64(3):497–507. doi: 10.1007/s00280-008-0897-1. [DOI] [PubMed] [Google Scholar]
- 34.Cao Z, Fan-Minogue H, Bellovin DI, Yevtodiyenko A, Arzeno J, Yang Q, Gambhir SS, Felsher DW. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71(6):2286–2297. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kah J, Wustenberg A, Keller AD, Sirma H, Montalbano R, Ocker M, Volz T, Dandri M, Tiegs G, Sass G. Selective induction of apoptosis by HMG-CoA reductase inhibitors in hepatoma cells and dependence on p53 expression. Oncol Rep. 2012;28(3):1077–1083. doi: 10.3892/or.2012.1860. [DOI] [PubMed] [Google Scholar]
- 36.Wang ST, Ho HJ, Lin JT, Shieh JJ, Wu CY. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017;8(2):e2626. doi: 10.1038/cddis.2016.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Sayed I, Helmy MW, El-Abhar HS. Inhibition of SRC/FAK cue: a novel pathway for the synergistic effect of rosuvastatin on the anti-cancer effect of dasatinib in hepatocellular carcinoma. Life Sci. 2018;213:248–257. doi: 10.1016/j.lfs.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Ridruejo E, Romero-Caimi G, Obregon MJ, Kleiman de Pisarev D, Alvarez L. Potential molecular targets of statins in the prevention of Hepatocarcinogenesis. Ann Hepatol. 2018;17(3):490–500. doi: 10.5604/01.3001.0011.7394. [DOI] [PubMed] [Google Scholar]
- 39.Bang CS, Song IH. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis. BMC Gastroenterol. 2017;17(1):46. doi: 10.1186/s12876-017-0606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48(1):78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 41.Kuper H, Ye W, Broome U, Romelsjo A, Mucci LA, Ekbom A, Adami HO, Trichopoulos D, Nyren O. The risk of liver and bile duct cancer in patients with chronic viral hepatitis, alcoholism, or cirrhosis. Hepatology. 2001;34(4 Pt 1):714–718. doi: 10.1053/jhep.2001.28233. [DOI] [PubMed] [Google Scholar]
- 42.Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol. 2015;30(1):14–20. doi: 10.1111/jgh.12700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data for this study are presented in tables, figures and supplementary materials.