Abstract
Background
Vector-borne diseases are a major public health concern and cause significant morbidity and mortality. Zika virus (ZIKV) is the etiologic agent of a massive outbreak in the Americas that originated in Brazil in 2015 and shows a strong association with congenital ZIKV syndrome in newborns. Cache Valley virus (CVV) is a bunyavirus that causes mild to severe illness in humans and ruminants. In this study, we investigated the vector competence of Virginia mosquitoes for ZIKV and CVV to explore their abilities to contribute to potential outbreaks.
Methods
To determine vector competence, mosquitoes were fed a blood meal comprised of defibrinated sheep blood and virus. The presence of midgut or salivary gland barriers to ZIKV infection were determined by intrathoracic inoculation vs oral infection. After 14-days post-exposure, individual mosquitoes were separated into bodies, legs and wings, and saliva expectorant. Virus presence was detected by plaque assay to determine midgut infection, dissemination, and transmission rates.
Results
Transmission rates for Ae. albopictus orally infected (24%) and intrathoracically inoculated (63%) with ZIKV was similar to Ae. aegypti (48% and 71%, respectively). Transmission rates of ZIKV in Ae. japonicus were low, and showed evidence of a midgut infection barrier demonstrated by low midgut infection and dissemination rates from oral infection (3%), but increased transmission rates after intrathoracic inoculation (19%). Aedes triseriatus was unable to transmit ZIKV following oral infection or intrathoracic inoculation. CVV transmission was dose-dependent where mosquitoes fed high titer (ht) virus blood meals developed higher rates of midgut infection, dissemination, and transmission compared to low titer (lt) virus blood meals. CVV was detected in the saliva of Ae. albopictus (ht: 68%, lt: 24%), Ae. triseriatus (ht: 52%, lt: 7%), Ae. japonicus (ht: 22%, lt: 0%) and Ae. aegypti (ht: 10%; lt: 7%). Culex pipiens and Cx. restuans were not competent for ZIKV or CVV.
Conclusions
This laboratory transmission study provided further understanding of potential ZIKV and CVV transmission cycles with Aedes mosquitoes from Virginia. The ability for these mosquitoes to transmit ZIKV and CVV make them a public health concern and suggest targeted control programs by mosquito and vector abatement districts.
Keywords: Zika virus, Cache Valley virus, Vector competence, Aedes japonicus, Aedes albopictus, Aedes triseriatus, Aedes aegypti, Culex pipiens, Culex restuans, Infection barriers
Background
Vector-borne pathogens are a major public health concern and cause significant morbidity and mortality globally. In recent years, vector-borne pathogens have appeared in new regions, even as endemic diseases have increased in incidence. Human travel and trade are often responsible for the introduction of invasive pathogens but ecological factors such as climate and presence of competent vectors will determine whether the pathogen becomes established. For example, since its introduction in 1999, West Nile virus (WNV) (family Flaviviridae, genus Flavivirus) is now the leading cause of vector-borne encephalitis in the USA [1]. Also impacting vector-borne disease emergence are invasive mosquitoes that may alter the transmission cycles of pathogens, whether native or introduced [2]. Aedes albopictus and Ae. japonicus are two of the most invasive mosquito species worldwide [3] and both have been known to function as competent vectors for several enzootic mosquito-borne viruses in the USA [4, 5].
Zika virus (ZIKV) (family Flaviviridae, genus Flavivirus) is an arthropod-borne virus (arbovirus) of humans and has been linked to congenital malformations and microcephaly in developing fetuses, and Guillain-Barré syndrome in adults [6]. Since its introduction to Brazil in 2015, ZIKV has spread into many new areas within the Americas [7]. ZIKV is transmitted primarily by urban and sylvatic Aedes mosquitoes, with Ae. aegypti serving as the main vector for human infection outside of Africa [8–10]. This emerging mosquito-borne virus has caused epidemics throughout Africa, Asia, the Pacific Islands and the Americas [11, 12]. Due to the lack of knowledge of ZIKV replication in North American mosquitoes, experimental vector competence studies are necessary to better understand the potential transmission of ZIKV by additional species. Recent studies have shown that some Aedes, Culex and Coquillettidia mosquitoes from temperate regions of North America were not competent for ZIKV [13, 14], but this is a small representation of the species and strain diversity of mosquitoes that are found in the USA.
Cache Valley virus (CVV) (family Peribunyaviridae, genus Orthobunyavirus) is a neuroinvasive arbovirus that is also spread by mosquitoes. Although CVV infection typically causes mild symptoms in humans, fever, meningitis, and encephalitis have been reported [15]. The symptoms of CVV infection are more severe in ruminants, such as sheep or cattle, and include stillbirths, congenital malformations, spontaneous abortions, and death [16]. CVV has a widespread distribution in North America and has been isolated from many species of mosquitoes including Ae. albopictus and Ae. japonicus [17–20]. The principal vector is unknown, but vector competence studies and field isolations have shown that Anopheles quadrimaculatus and An. punctipennis may play a significant role in the natural transmission cycle [21, 22]. Laboratory transmission studies have also shown that Cx. tarsalis, Ae. taeniorhynchus, Ae. sollicitans and Cq. perturbans are competent vectors of CVV [22–24].
Aedes aegypti and Ae. albopictus are the most important mosquito species responsible for virus transmission to humans in urban environments. Both species are competent vectors for ZIKV, dengue virus (DENV) and yellow fever virus (YFV) [25–27]. The Asian rock pool mosquito, Ae. japonicus, is a relatively new invasive species that can be found in subtropical and temperate regions of the USA. Although Ae. japonicus is not an aggressive human biter, blood meal analysis from field collected mosquitoes have shown high incidences of human blood consumption [28]. Laboratory transmission studies show that Ae. japonicus is a competent vector of WNV, La Crosse virus (LACV), Eastern equine encephalitis virus (EEE) and St. Louis encephalitis virus (SLEV) [29–32]. Aedes triseriatus, the principal vector of LACV, is found extensively throughout eastern USA and parts of Central America [33]. Under laboratory conditions, Ae. triseriatus is a competent vector for WNV, DENV, YFV, EEE and SLE [34]. WNV has been isolated from Culex pipiens and Cx. restuans and both species have been shown to be competent vectors of the virus [35, 36]. Laboratory transmission studies have found that Cx. pipiens is refractory to CVV and ZIKV infections [13, 23, 25, 37, 38].
Between 2015 and 2018, there were more than 5000 imported ZIKV cases in the USA, with over 100 cases in Virginia [39]. Within the continental USA, reports of local transmission by mosquito vectors have occurred in Florida and Texas [40–42]. There have been no human CVV cases reported in Virginia, but the virus has been isolated from field-collected Ae. japonicus within the state [20]. Although CVV has been detected in field mosquitoes, only a few transmission studies have been conducted to determine potential vectors for the virus. With the wide distribution of Ae. albopictus, Ae. japonicus, Ae. triseriatus, Cx. pipiens and Cx. restuans throughout Virginia [43], it is crucial to determine the vector competence of these local mosquito strains. In this study, we investigated the vector competence of Virginia mosquitoes common in urban and suburban environments for ZIKV and CVV to explore their abilities to contribute to potential outbreaks and help inform local mosquito control strategies.
Methods
Mosquito collection and rearing
All eggs were derived from female mosquitoes collected using gravid traps in forested areas around Blacksburg, VA. After laying eggs, adult mosquitoes were tested for arboviruses by Vero cell plaque assay to ensure the absence of virus in the F1 progeny. A laboratory strain of Ae. aegypti from Vero Beach, FL, was used as our reference vector species and was subsequently tested for CVV vector competence. Mosquitoes were reared in environmental chamber conditions set at 24 °C with 75% RH and 16L:8D photoperiod using methods by Jackson et al. [44] to ensure consistent adult size.
Cells and virus
African green monkey kidney (Vero) cells (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Corning, Corning, NY, USA) with 10% fetal bovine serum (FBS), 100 U/ml of penicillin and 100 μg/ml of streptomycin, and maintained at 37 °C with 5% CO2.
The Asian lineage of ZIKV, PRVABC59 (GenBank: KU501215) and CVV strain 4B (GenBank: KX583998) was used in this study. PRVABC59 was isolated from the serum sample of a patient traveling from Puerto Rico in 2015. CVV4B was isolated from field-caught Ae. japonicus during a 2015 field study in Blacksburg, VA. Both viruses were maintained through passage on Vero cells and stored at − 80 °C. Infected blood meals consisted of 1 ml of virus mixed with 9 ml of defribinated sheep blood (Colorado Serum Company, Denver, CO, USA).
Mosquito infection
For oral infection, 1-week-old female mosquitoes were starved 24 h before blood-feeding and provided cotton balls soaked with deionized water. Approximately 40–50 female mosquitoes were placed into 1-gallon cages covered with a mesh screen top. The mosquitoes were offered infected blood meals contained in a glass water-jacketed membrane feeder attached to a circulating 37 °C water bath. Pig intestine sausage casing was used as the membrane. After a 2-h feeding period, fully engorged females were anesthetized on ice and transferred to a new 1-liter cage. A 0.5 ml sample of the infected blood was removed after the feeding period and stored at − 80 °C for later virus titer. Parenteral infection was done by intrathoracic inoculation of week-old females that had never taken a blood meal with 0.2 µl of virus [45]. Table 1 shows the titers of virus infected blood meals and virus inoculum. Infected mosquitoes were maintained at 24 °C with 75% RH and 16L: 8D photoperiod and provided 10% sucrose solution for sustenance.
Table 1.
ZIKV and CVV blood meal titers
| Species | CVV high blood meal titer (pfu/ml) | CVV low blood meal titer (pfu/ml) | ZIKV blood meal titer (pfu/ml) | ZIKV intrathoracic inoculation titer (pfu/ml) |
|---|---|---|---|---|
| Aedes albopictus | 5.25 × 106 | 2.90 × 103 | 3.00 × 106 | 5.25 × 104 |
| Aedes aegypti | 1.98 × 107 | 6.28 × 103 | 6.50 × 106 | 5.25 × 104 |
| Aedes japonicus | 1.99 × 106 | 4.60 × 103 | 3.72 × 107 | 7.50 × 104 |
| Aedes triseriatus | 2.99 × 106 | 1.40 × 103 | 4.50 × 107 | 1.80 × 105 |
Saliva extraction
After 14-DPE, female mosquitoes were removed from cages and immobilized by chilling on ice. Saliva was extracted by inserting the proboscis into a capillary tube filled with a 1:1 mixture of 10% sucrose and fetal bovine serum (FBS) [46]. The mosquitoes were given 30 min to feed and salivate. The saliva, legs and wings and body were placed into separate microcentrifuge tubes with DMEM and stored at − 80 °C until virus testing.
Virus detection
Mosquito bodies, leg and wing and saliva samples were homogenized with metal pellets in 2 ml of Vero media on a vortex mixer and then clarified by centrifugation at 1500× g for two min. Supernatants were tested for infection using Vero cell plaque assay following the methods of Barker et al. [47]. If virus was recovered from the body but not the legs and wings, the mosquito was classified as having a non-disseminated infection; if virus was detected in the wings and legs, the mosquito was classified as having a disseminated infection; mosquitoes with virus in saliva were classified as transmitting. Infection rate was determined as the percentage of the orally infected mosquitoes positive for virus in the body. Dissemination rate was determined as the percentage of orally infected mosquitoes that were positive for virus in the legs and wings, regardless of infection status. Transmission rate was determined as the percentage of orally infected mosquitoes that had virus in the salivary expectorant, regardless of infection status. Infectious blood meals were thawed at room temperature, diluted with a series of 10-fold serial dilutions and tested for virus concentration using plaque assay.
Statistical analysis
A Chi-square test was used to compare mean infection, dissemination and transmission rates among mosquito species followed by Fisher’s exact tests for pairwise comparisons [48]. GraphPad Prism 6.0 (La Jolla, CA, USA) was used for all statistical analysis. All statistical analyses were carried out at a significance level of α = 0.05.
Results
Vector competence for ZIKV following oral infection
There was a significant difference among infection (χ2 = 58.73, df = 5, P < 0.05), dissemination (χ2 = 71.21, df = 5, P < 0.05) and transmission (χ2 = 60.17, df = 5, P < 0.05) rates for Ae. aegypti, Ae. albopictus, Ae. japonicus and Ae. triseriatus after oral infection with ZIKV (Fig. 1). Rates for infection, dissemination and transmission were highest for Ae. aegypti (68%, 60% and 48%, respectively) and Ae. albopictus (49%, 41% and 24%, respectively). Aedes japonicus rates of infection, dissemination and transmission (20%, 9% and 3%) were significantly lower than Ae. aegypti (Fisher’s exact test, P < 0.0001, OR: 0.9257, 95% CI: 0.0326–0.2629; P < 0.0001, OR: 0.05970, 95% CI: 0.0187–0.1899; P < 0.0001, OR: 0.03052, 95% CI: 0.0061–0.1527) and Ae. albopictus (Fisher’s exact test, P < 0.0006, OR: 0.2077, 95% CI: 0.0849–0.5076; P = 0.0002, OR: 0.1313, 95% CI: 0.0454–0.3800; P = 0.0008, OR: 0.08764, 95% CI: 0.0178–0.4314) (Fig. 1). Although 25% of Ae. triseriatus became infected after imbibing an infectious blood meal, there was no dissemination or transmission of the virus. Neither Cx. pipiens nor Cx. restuans were infected after oral exposure to ZIKV (Table 2).
Fig. 1.
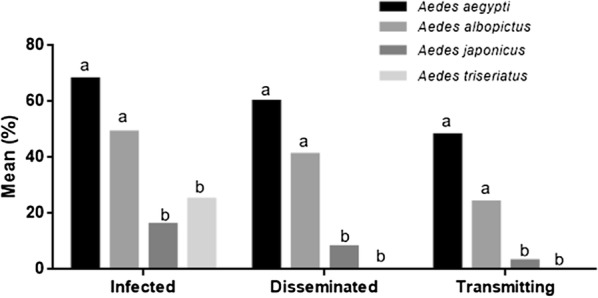
Vector competence for ZIKV PRVABC59. Aedes albopictus (n = 37), Ae. triseriatus (n = 28), Ae. japonicus (n = 73) and Ae. aegypti (n = 25) were provided infectious blood meals with an average titer of 2.57 × 107 pfu/ml (range = 5.75 × 106 to 7.5 × 107 pfu/ml). After 14 days, mosquitoes were dissected and the number infected (% mosquitoes with virus in the body), disseminated (% mosquitoes with virus in legs and wings, independent of infection status) and transmitting (% mosquitoes with virus in saliva expectorant, independent of infection status) were determined by Vero cell plaque assay. Different letters denote significance by two-tailed Fischer’s exact test and presented as mean % infected, disseminated and transmitting, α = 0.05
Table 2.
Vector competence of ZIKV and CVV in Culex pipiens and Cx. restuans
| Mosquito species | Sample size (n) | Mean titer (pfu/ml) | Mean non-disseminated infection (%) | Mean disseminated infection (%) | Mean transmitting (%) |
|---|---|---|---|---|---|
| ZIKV | |||||
| Cx. pipiens | 30 | 3.00 × 107 | 0 | 0 | 0 |
| Cx. restuans | 28 | 5.25 × 106 | 0 | 0 | 0 |
| CVV | |||||
| Cx. pipiens | 67 | 1.12 × 108 | 1 | 1 | 0 |
| Cx. restuans | 30 | 7.70 × 107 | 0 | 0 | 0 |
Transmission of ZIKV following parenteral infection
Parenteral infection by intrathoracic inoculation resulted in significantly higher rates of transmission compared to oral infection in Ae. albopictus (63% parenteral vs 24% oral) (Fisher’s exact test, P = 0.0080, OR: 0.1875, 95% CI: 0.0566–0.6209) and Ae. japonicus (19% parenteral vs 3% oral) (Fisher’s exact test, P = 0.0212, OR: 0.1197, 95% CI: 0.0202–0.7088) (Table 3). Mode of infection had no effect on transmission by Ae. aegypti (71% parenteral vs 48% oral) (Fisher’s exact test, P = 0.1395, OR: 0.3693, 95% CI: 0.1079–1.2630). No virus was detected in the saliva of Ae. triseriatus from either orally or parenterally infected groups (Table 3).
Table 3.
Transmission rate of Aedes japonicus and Ae. triseriatus intrathoracically inoculated with ZIKV
| Mosquito species | Infection method | Sample size (n) | Virus titer (pfu/ml) | Transmission (%) |
|---|---|---|---|---|
| Ae. albopictus | Intrathoracic | 19 | 5.25 × 104 | 63a |
| Oral | 37 | 3.00 × 106 | 24b | |
| Ae. aegypti | Intrathoracic | 21 | 5.25 × 104 | 71a |
| Oral | 25 | 6.50 × 106 | 48a | |
| Ae. japonicus | Intrathoracic | 21 | 7.50 × 104 | 19a |
| Oral | 73 | 3.72 × 107 | 3b | |
| Ae. triseriatus | Intrathoracic | 23 | 1.80 × 105 | 0a |
| Oral | 28 | 4.50 × 107 | 0a |
Notes: Differing letters denote significance of transmission rates of the same species after oral or intrathoracic infection by two-tailed Fischer’s exact test, α = 0.05
Vector competence to CVV
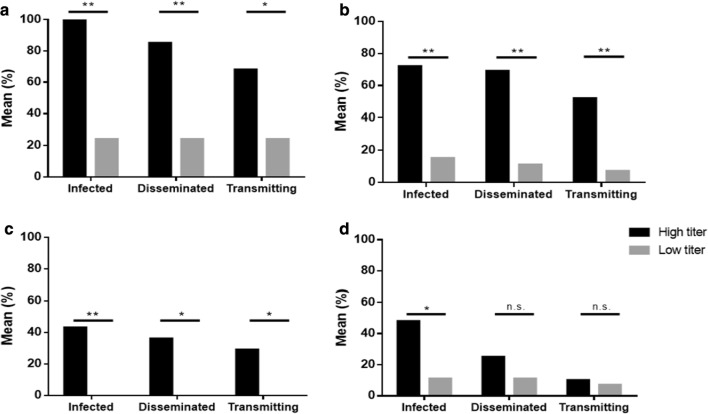
When fed a high titer (ht) virus blood meal, Ae. albopictus and Ae. triseriatus showed significantly higher rates of infection (χ2 = 127.5, df = 5, P < 0.0001), dissemination (χ2 = 107.8, df = 5, P < 0.0001) and transmission (χ2 = 88.08, df = 5, P < 0.0001) than Ae. japonicus or Ae. aegypti (Table 4). However, when fed low titer (lt) blood meals, there were no differences among rates for any of the species (χ2 = 5.61, df = 3, P > 0.05) (Table 4). Aedes albopictus was the most susceptible to CVV oral infection (ht: 100%, lt: 24%) and had the highest rate of dissemination (ht: 85%, lt: 24%) and transmission (ht: 68%, lt: 24%). Aedes triseriatus was also susceptible to CVV infection (ht: 72%, lt: 15%), dissemination (ht: 69%, lt: 11%) and transmission (ht: 52%, lt: 7%). None of the Ae. japonicus fed a low titer blood meal developed midgut infections. For Ae. aegypti¸ CVV was able to establish midgut infections (ht: 48%, lt: 11%), cause a disseminated infection (ht: 25%, lt: 11%) and transmit virus (ht: 10%; lt:7%). Figure 2 shows significant differences in infection, dissemination and transmission between high and low titer blood meals for Ae. albopictus, Ae. triseriatus and Ae. japonicus. Virus titer resulted in significant differences in infection for Ae. aegypti but not dissemination or transmission rates. Neither of the Culex species was able to transmit CVV. No Cx. restuans became infected and only one Cx. pipiens was positive for infection and dissemination (Table 2).
Table 4.
Vector competence for CVV 4B by Aedes albopictus, Ae. triseriatus, Ae. japonicus and Ae. aegypti presented as mean % infected, disseminated and transmitting
| Species | Sample size (n) | Mean blood meal titer (pfu/ml) | % infected | % disseminated | % transmitting |
|---|---|---|---|---|---|
| High titer | |||||
| Ae. albopictus | 34 | 5.25 × 106 | 100a | 85a | 68a |
| Ae. triseriatus | 29 | 2.99 × 106 | 72a | 69a | 52a |
| Ae. japonicus | 74 | 1.99 × 106 | 41b | 38b | 28b |
| Ae. aegypti | 52 | 1.98 × 107 | 48a,b | 25b | 10b |
| Low titer | |||||
| Ae. albopictus | 21 | 2.90 × 103 | 24a | 24a | 24a |
| Ae. triseriatus | 55 | 1.40 × 103 | 15a | 11a | 7a |
| Ae. japonicus | 21 | 4.60 × 103 | 0a | 0a | 0a |
| Ae. aegypti | 44 | 6.28 × 103 | 11a | 11a | 7a |
Notes: Different letters denote significance between different species within respective categories of high or low titer blood meals by two-tailed Fischer’s exact test, α = 0.05
Fig. 2.
Vector competence for CVV with high versus low titer blood meals. Mosquitoes were provided low titer (lt) (1.2 × 103 to 4.6 × 103 pfu/ml) or high titer (ht) (1.6 × 105 to 5.5 × 107 pfu/ml) infectious blood meals. After 14 days, the mosquitoes were dissected and the number infected (% mosquitoes with virus in the body), disseminated (% mosquitoes with virus in legs and wings, independent of infections status) and transmitting (% mosquitoes with virus in saliva expectorant, independent of infection statues) were determined using Vero cell plaque assay. aAedes albopictus (lt: n = 21; ht: n = 34). bAedes triseriatus (lt: n = 55; ht: n = 29). cAedes japonicus (lt: n = 21; ht: n = 74). dAedes aegypti (lt: n = 44; ht: n = 52). Data are presented as mean % infected, disseminated and transmitting. *P < 0.01, **P < 0.001 and ns, not significant by two-tailed Fischer’s exact test
Discussion
Assessing the vector competence of local mosquitoes for imported and emerging viruses is critical for public health officials to anticipate patterns of arbovirus transmission, determine the relative roles of the different species for virus amplification and spread, and to select appropriate control responses. This study aimed to determine the risk of local ZIKV transmission and the emergence potential of CVV in Virginia by evaluating the vector competence of the most common mosquito species found in urban and suburban habitats.
A meta-analysis by McKenzie et al. [49] suggested that the vector competence of Ae. albopictus for Zika virus varied among geographically disparate populations. We found that the vector competence of a Virginia strain of Ae. albopictus was equivalent to that of a Florida strain of Ae. aegypti. Culex mosquitoes were found to be refractory to ZIKV infection. Other studies have also observed similar results, suggesting that it is unlikely this group plays a role in ZIKV transmission [13, 37, 50, 51]. We also found that Ae. japonicus from Virginia was capable of transmitting ZIKV, but at a much lower rate compared to Ae. aegypti and Ae. albopictus. A study by Aliota et al. [13] showed that laboratory strains of Ae. triseriatus were able to become infected with ZIKV PRVABC59, the same strain that we used, but no dissemination or transmission resulted. Our study showed similar results working with an F1 generation of field-caught Ae. triseriatus where only midgut infections resulted from oral exposure.
Upon ingesting an infectious blood meal, the virus must surmount several tissue barriers associated with the midgut and salivary glands [52]. We assessed the presence of tissue barriers by intrathoracic inoculation of ZIKV. Injecting virus directly into the hemolymph bypasses the midgut and permits the virus to reach and infect the salivary glands. We detected infectious virus in salivary expectorant of Ae. japonicus, but not Ae. triseriatus, which indicated the presence of salivary gland barriers. Although transmission for intrathoracically inoculated Ae. japonicus was significantly higher than orally infected mosquitoes, the rates were low. The low midgut infection and transmission rates lead us to believe that there are potential midgut and salivary gland barriers that limit Ae. japonicus and prevent Ae. triseriatus from ZIKV transmission. Although Ae. albopictus was capable of ZIKV transmission after oral infection, intrathoracic inoculation significantly increased its transmission rates, indicating the presence of a midgut barrier. Studies of virus and vector systems have shown that these barriers play an important role during the extrinsic incubation period and may limit the ability of the virus to infect the mosquito for successful transmission [53, 54]. In addition, gut microbiota and immune pathways may also be involved when the virus enters the midgut [55–57]. It has been hypothesized that midgut and salivary gland barriers are responsible for the geographical variation in vector competence seen in Ae. aegypti and Ae. albopictus for ZIKV [58–60].
Although Ae. japonicus was capable of ZIKV transmission, it is not an aggressive human biter and predominantly inhabits forested areas, which limits its role in ZIKV transmission. Surprisingly, Ae. triseriatus, Cx, pipiens and Cx. restuans were not competent for ZIKV even though they are competent vectors of other flaviviruses, such as WNV or SLEV [61–63]. Aedes albopictus, on the other hand, was highly competent for ZIKV and exhibits aggressive, anthropophilic behaviour. The likelihood for this species to contribute to ZIKV transmission in Virginia is much higher compared to other Aedes mosquitoes in this region.
This study also found that Ae. albopictus, Ae. triseriatus, Ae. japonicus and Ae. aegypti were susceptible to CVV infection and capable of virus transmission. The combination of high vector competence, previous isolations from the field, and anthropophilic behavior suggests that Ae. albopictus could play a major role in CVV transmission in endemic areas [64, 65]. Aedes triseriatus and Ae. japonicus were also competent for CVV and blood meal analysis has shown that all three species feed on deer, the primary amplifying vertebrate host for CVV [28, 66–69]. We used high and low CVV blood meal titers that bracketed the range of titers found in experimentally infected deer [22] and showed that Ae. albopictus, Ae. triseriatus and Ae. aegypti were susceptible to CVV infection and subsequently transmitted virus even when exposed to low titer blood meals. There is currently no evidence of field isolation of the virus from Ae. aegypti, but the distribution of CVV includes the southern USA where Ae. aegypti is commonly found [18]. Even though CVV has been isolated from wild Ae. japonicus [20], and this species has been shown to feed on deer [28], it is unclear if it serves as a major vector in enzootic or local transmission of the virus. We also tested vector competence of field-caught Cx. pipiens and Cx. restuans for CVV, and found no evidence of transmission by either species. The existence of a dose-dependent infection or escape barrier can determine how certain mosquito species and strains are refractory to infection. Studies looking at dose-dependent interactions between mosquito vectors and the virus typically find that high titers result in greater midgut infection and transmission potential while low titers result in low midgut infection and transmission rates [70–73]. The dose-dependent tissue barriers are often associated with midgut escape barriers or RNA interference (RNAi) pathways [56, 72], while incompatibility between the virus and cells of the midgut or salivary glands are dose-independent barriers [52, 53]. With laboratory evidence of low titer vector competency and abundant distribution throughout North America, Ae. aegypti, Ae. albopictus, Ae. japonicus and Ae. triseriatus could play major roles in CVV transmission.
Outbreaks of mosquito-borne diseases can have large economic and devastating impacts on human and animal health. Experimental vector competence studies allow us to understand the potential for a mosquito species to contribute to an outbreak and facilitate more targeted surveillance and control. Due to the wide variability of mosquito and virus infectivity, it is not possible to make the assumption that studies involving vectors from different geographical locations will have similar competencies. Therefore, it is not appropriate to extrapolate results from other studies for a single conclusion. Several studies clearly show considerable variability in the susceptibility of the same vector species for viral infection for DENV [74, 75], CHIKV [76] and ZIKV [59, 77]. In addition, when arboviruses are detected in field-caught mosquitoes, we cannot assume that it is competent and able to transmit the virus. The mosquito may have an undigested blood meal that was recently taken from an infected host, which can yield a false positive. Laboratory vector competence studies allow us to determine if a mosquito species is capable of transmitting the virus. When conducting laboratory vector competence studies, it is important to consider laboratory-reared versus field-caught mosquitoes. For example, some vector competence studies have shown that laboratory-reared Cx. quinquefasciatus is able to become infected and transmit ZIKV [78, 79], while studies using field populations were not able to transmit the virus [38].
There are many knowledge gaps in CVV dynamics, especially our understanding of its natural cycle of competent vectors and susceptible amplifying hosts. In addition, CVV infections are often misdiagnosed for other flu-like illness, which presents itself as a challenge for accurate reporting to local or state health departments. In contrast, the ZIKV outbreak in 2015 sparked high demand for all areas of research to understand and control the virus. Although cases have dropped significantly in the USA, ZIKV is still present in parts of Africa, Asia and South America, and may remain indefinitely [11, 80].
Conclusions
Our studies show that a species that has not been tested for ZIKV vector competency, Ae. japonicus, was able to transmit the virus, but at a low rate. Aedes japonicus, however, was competent for CVV transmission. Aedes albopictus, the most widespread anthropophilic mosquito in Virginia, was competent for both ZIKV and CVV. Aedes aegypti was competent for both viruses, but its inability to overwinter in colder climates reduces this species’ likelihood of ZIKV transmission in Virginia. With the abundance of highly competent mosquito species, there may be greater concern for increased CVV transmission in temperate regions of the USA.
Acknowledgements
We thank Andrea Bertke from the Virginia-Maryland College of Veterinary Medicine for providing ZIKV PRVABC59 and Fan Yang for his help on isolating and identifying CVV 4B.
Abbreviations
- ZIKV
Zika virus
- CVV
Cache Valley virus
- WNV
West Nile virus
- LACV
La Crosse virus
- DENV
dengue virus
- DPE
days post-exposure
- lt
low titer
- ht
high titer
Authors’ contributions
KC and SLP designed the initial project. KC conducted laboratory experiments. KC and SLP analyzed the results, interpreted data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant from Graduate Research Development Programme at Virginia Tech.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The datasets used for the present study are available from the corresponding author upon request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kevin K. Chan, Email: kchan90@vt.edu
Albert J. Auguste, Email: jauguste@vt.edu
Carlyle C. Brewster, Email: carlylb@clemson.edu
Sally L. Paulson, Email: spaulson@vt.edu
References
- 1.Roehrig JT. West Nile virus in the United States—a historical perspective. Viruses. 2013;5:3088–3108. doi: 10.3390/v5123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunze S, Koch LK, Kochmann J, Klimpel S. Aedes albopictus and Aedes japonicus—two invasive mosquito species with different temperature niches in Europe. Parasit Vectors. 2016;9:573. doi: 10.1186/s13071-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae) Annu Rev Entomol. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell CJ. Vector competence of North and South American strains of Aedes albopictus for certain arboviruses: a review. J Am Mosq Control Assoc. 1991;7:446–451. [PubMed] [Google Scholar]
- 6.Krauer F, Riesen M, Reveiz L, Odalpo OT, Martínez-Vega R, Progo TV, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: systematic review. PLoS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanluca C, de Melo VCA, Mosimann ALP, dos Santos GIV, dos Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayllón T, Campos RM, Brasil P, Morone FC, Câmara DCP, Meira GLS, et al. Early evidence for Zika virus circulation among Aedes aegypti mosquitoes, Rio de Janeiro, Brazil. Emerg Infect Dis. 2017;23:1411–1412. doi: 10.3201/eid2308.162007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayres CFJ. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016;16:278–279. doi: 10.1016/S1473-3099(16)00073-6. [DOI] [PubMed] [Google Scholar]
- 10.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). World map of areas with risk of Zika; 2017. https://wwwnc.cdc.gov/travel/page/world-map-areas-with-zika. Accessed 3 Mar 2018.
- 12.Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:55–58. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 13.Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg Infect Dis. 2016;22:1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibernardo A, Turell MJ, Robbin Lindsay L, Loomer C, Iranpour M. Vector competence of some mosquito species from Canada for Zika virus. J Am Mosq Control Assoc. 2017;33:276–281. doi: 10.2987/17-6664.1. [DOI] [PubMed] [Google Scholar]
- 15.Campbell GL, Mataczynski JD, Reisdorf ES, Powell JW, Martin DA, Lambert AJ, et al. Second human case of Cache Valley virus disease. Emerg Infect Dis. 2006;12:854–856. doi: 10.3201/eid1205.051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JF, Livingston CW, Chung SI, Collisson EC. Ovine arthrogryposis and central nervous system malformations associated with in utero Cache Valley virus infection: spontaneous disease. Vet Pathol. 1989;26:33–39. doi: 10.1177/030098588902600106. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong PM, Andreadis TG, Anderson JF. Emergence of a new lineage of Cache Valley virus (Bunyaviridae: Orthobunyavirus) in the northeastern United States. Am J Trop Med Hyg. 2015;93:11–17. doi: 10.4269/ajtmh.15-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calisher CH, Francy DB, Smith GC, Muth DJ, Lazuick JS, Karabatsos N, et al. Distribution of Bunyamwera serogroup viruses in North America, 1956–1984. Am J Trop Med Hyg. 1986;35:429–443. doi: 10.4269/ajtmh.1986.35.429. [DOI] [PubMed] [Google Scholar]
- 19.Ngo KA, Maffei JG, Dupuis AP, Kauffman EB, Backenson PB, Kramer LD. Isolation of Bunyamwera serogroup viruses (Bunyaviridae, Orthobunyavirus) in New York State. J Med Entomol. 2006;43:1004–1009. doi: 10.1603/0022-2585(2006)43[1004:iobsvb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Chan K, Marek PE, Armstrong PM, Lui P, Bova JE, et al. Cache Valley virus in Aedes japonicus japonicus mosquitoes, Appalachian region, United States. Emerg Infect Dis. 2018;24:553–557. doi: 10.3201/eid2403.161275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreadis TG, Armstrong PM, Anderson JF, Main AJ. Spatial-temporal analysis of Cache Valley virus (Bunyaviridae: Orthobunyavirus) infection in anopheline and culicine mosquitoes (Diptera: Culicidae) in the northeastern United States, 1997–2012. Vector Borne Zoonotic Dis. 2014;14:763–773. doi: 10.1089/vbz.2014.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackmore CG, Blackmore MS, Grimstad PR. Role of Anopheles quadrimaculatus and Coquillettidia perturbans (Diptera: Culicidae) in the transmission cycle of Cache Valley virus (Bunyaviridae: Bunyavirus) in the midwest, USA. J Med Entomol. 1998;35:660–664. doi: 10.1093/jmedent/35.5.660. [DOI] [PubMed] [Google Scholar]
- 23.Ayers VB, Huang YS, Lyons AC, Park SL, Higgs S, Dunlop JI, et al. Culex tarsalis is a competent vector species for Cache Valley virus. Parasit Vectors. 2018;11:519. doi: 10.1186/s13071-018-3103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuill TM, Thompson PH. Cache Valley virus in the Del Mar Va Peninsula. IV. Biological transmission of the virus by Aedes sollicitans and Aedes taeniorhynchus. Am J Trop Med Hyg. 1970;19:513–519. doi: 10.4269/ajtmh.1970.19.513. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Zhou T, Lai Z, Zhang Z, Jia Z, Zhou G, et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes as Zika virus vectors. China. Emerg Infect Dis. 2017;23:1085–1091. doi: 10.3201/eid2307.161528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller BR, Mitchell CJ, Ballinger ME. Replication, tissue tropisms and transmission of yellow fever virus in Aedes albopictus. Trans R Soc Trop Med Hyg. 1989;83:252–255. doi: 10.1016/0035-9203(89)90667-6. [DOI] [PubMed] [Google Scholar]
- 27.Whitehorn J, Kien DT, Nguyen NM, Nguyen HL, Kyrylos PP, Carrington LB, et al. Comparative susceptibility of Aedes albopictus and Aedes aegypti to dengue virus infection after feeding on blood of viremic humans: implications for public health. J Infect Dis. 2015;212:1182–1190. doi: 10.1093/infdis/jiv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–214. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- 29.Sardelis MR, Turell MJ. Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Control Assoc. 2001;17:137–141. [PubMed] [Google Scholar]
- 30.Sardelis MR, Dohm DJ, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J Med Entomol. 2002;39:480–484. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- 31.Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J Med Entomol. 2002;39:635–639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- 32.Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J Am Mosq Control Assoc. 2003;19:159–162. [PubMed] [Google Scholar]
- 33.Sánchez-Trinidad A, Ordoñez-Sánchez F, Valdes-Perezgasga MT, Sánchez-Ramos FJ, Zavortink TJ, Cortés-Guzmán AJ, et al. Geographical distribution of the Aedes triseriatus Group (Diptera: Culicidae) in Mexico. J Vector Ecol. 2014;39:134–137. doi: 10.1111/j.1948-7134.2014.12079.x. [DOI] [PubMed] [Google Scholar]
- 34.European Centre for Disease Prevention and Control (ECDC). Aedes triseriatus—factsheet for experts; 2014. https://ecdc.europa.eu/en/disease-vectors/facts/mosquito-factsheets/aedes-triseriatus. Accessed 3 Mar 2019.
- 35.Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turell MJ, O’Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- 37.Amraoui F, Atyame-Nten C, Vega-Rúa A, Lourenço-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016;21:30333. doi: 10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RM, Barbosa da Silva KA, Castro MG, et al. Culex quinquefasciatus from Rio de Janeiro is not competent to transmit the local Zika virus. PLoS Negl Trop Dis. 2016;10:e0004993. doi: 10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC). Zika cases in the United States; 2019. https://www.cdc.gov/zika/reporting/case-counts.html. Accessed 1 Mar 2019.
- 40.Likos A, Griffin I, Bingham AM, et al. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward Counties, Florida, June-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1032–1038. doi: 10.15585/mmwr.mm6538e1. [DOI] [PubMed] [Google Scholar]
- 41.Texas Health and Human Services. Texas announces local Zika virus case in Rio Grande Valley; 2016. https://www.dshs.texas.gov/news/releases/2016/20161128.aspx. Accessed 6 Feb 2019.
- 42.Ventura CV, Albini TA, Berrocal AM. First locally transmitted Zika virus cases identified in the United States. JAMA Ophthalmol. 2016;134:1219–1220. doi: 10.1001/jamaophthalmol.2016.3623. [DOI] [PubMed] [Google Scholar]
- 43.Armistead JS, Nishimura N, Arias JR, Lounibos LP. Community ecology of container mosquitoes (Diptera: Culicidae) in Virginia following invasion by Aedes japonicus. J Med Entomol. 2012;49:1318–1327. doi: 10.1603/ME11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson BT, Brewster CC, Paulson SL. La Crosse virus infection alters blood feeding behavior in Aedes triseriatus and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2012;49:1424–1429. doi: 10.1603/ME12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 46.Aitken THG. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq News. 1977;37:130–133. [Google Scholar]
- 47.Barker CM, Paulson SL, Cantrell S, Davis BS. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J Med Entomol. 2003;40:403–410. doi: 10.1603/0022-2585-40.4.403. [DOI] [PubMed] [Google Scholar]
- 48.Shan G, Gerstenberger S. Fisher’s exact approach for post hoc analysis of a chi-squared test. PLoS ONE. 2017;12:e0188709. doi: 10.1371/journal.pone.0188709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenzie BA, Wilson AE, Zohdy S. Aedes albopictus is a competent vector of Zika virus: a meta-analysis. PLoS ONE. 2019;14:e0216794. doi: 10.1371/journal.pone.0216794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dodson BL, Pujhari S, Rasgon JL. Vector competence of selected North American Anopheles and Culex mosquitoes for Zika virus. PeerJ. 2018;6:e4324. doi: 10.7717/peerj.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Main BJ, Nicholson J, Winokur OC, Steiner C, Riemersma KK, Stuart J, et al. Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS Negl Trop Dis. 2018;12:e0006524. doi: 10.1371/journal.pntd.0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franz AW, Kantor AM, Passarelli AL, Clem RJ. Tissue barriers to arbovirus infection in mosquitoes. Viruses. 2015;7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 54.Paulson SL, Grimstad PR, Craig GB., Jr Midgut and salivary gland barriers to La Crosse virus dissemination in mosquitoes of the Aedes triseriatus group. Med Vet Entomol. 1989;3:113–123. doi: 10.1111/j.1365-2915.1989.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 55.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khoo CC, Piper J, Sanchez-Vargas I, Olson KE, Franz AW. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti. BMC Microbiol. 2010;10:130. doi: 10.1186/1471-2180-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muturi EJ, Ramirez JL, Rooney AP, Kim CH. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl Trop Dis. 2017;11:e0005377. doi: 10.1371/journal.pntd.0005377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calvez E, O’Connor O, Pol M, Rousset D, Faye O, Richard V, et al. Differential transmission of Asian and African Zika virus lineages by Aedes aegypti from New Caledonia. Emerg Microbes Infect. 2018;7:159. doi: 10.1038/s41426-018-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Luna SM, Weger-Lucarelli J, Rückert C, Murrieta RA, Young MC, Byas AD, et al. Variation in competence for ZIKV transmission by Aedes aegypti and Aedes albopictus in Mexico. PLoS Negl Trop Dis. 2018;12:e0006599. doi: 10.1371/journal.pntd.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chamberlain RW, Sudia WD, Gillett JD. St. Louis encephalitis virus in mosquitoes. Am J Hyg. 1959;70:221–236. doi: 10.1093/oxfordjournals.aje.a120072. [DOI] [PubMed] [Google Scholar]
- 62.Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- 63.Erickson SM, Platt KB, Tucker BJ, Evans R, Tiawsirisup S, Rowley WA. The potential of Aedes triseriatus (Diptera: Culicidae) as an enzootic vector of West Nile virus. J Med Entomol. 2006;43:966–970. doi: 10.1093/jmedent/43.5.966. [DOI] [PubMed] [Google Scholar]
- 64.Armstrong PM, Anderson JF, Farajollahi A, Healy SP, Unlu I, Crepeau TN, et al. Isolations of Cache Valley virus from Aedes albopictus (Diptera: Culicidae) in New Jersey and evaluation of its role as a regional arbovirus vector. J Med Entomol. 2013;50:1310–1314. doi: 10.1603/ME13099. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell CJ, Haramis LD, Karabatsos N, Smith GC, Starwalt VJ. Isolation of La Crosse, Cache Valley, and Potosi viruses from Aedes mosquitoes (Diptera: Culicidae) collected at used-tire sites in Illinois during 1994–1995. J Med Entomol. 1998;35:573–577. doi: 10.1093/jmedent/35.4.573. [DOI] [PubMed] [Google Scholar]
- 66.Burkot TR. DeFoliart GR Bloodmeal sources of Aedes triseriatus and Aedes vexans in a southern Wisconsin forest endemic for La Crosse encephalitis virus. Am J Trop Med Hyg. 1982;31:376–381. doi: 10.4269/ajtmh.1982.31.376. [DOI] [PubMed] [Google Scholar]
- 67.Campbell GL, Eldridge BF, Hardy JL, Reeves WC, Jessup DA, Presser SB. Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg. 1989;40:428–437. doi: 10.4269/ajtmh.1989.40.428. [DOI] [PubMed] [Google Scholar]
- 68.Neitzel DF, Grimstad PR. Serological evidence of California group and Cache Valley virus infection in Minnesota white-tailed deer. J Wildl Dis. 1991;27:230–237. doi: 10.7589/0090-3558-27.2.230. [DOI] [PubMed] [Google Scholar]
- 69.Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1093/jmedent/43.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azar SR, Roundy CM, Rossi SL, Huang JH, Leal G, Yun R, et al. Differential vector competency of Aedes albopictus populations from the Americas for Zika Virus. Am J Trop Med Hyg. 2017;97:330–339. doi: 10.4269/ajtmh.16-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for Western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 73.Kuno G, Chang GJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett KE, Olson KE, Muñoz Mde L, Fernandez-Salas I, Farfan-Ale JA, Higgs S, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- 75.Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am J Trop Med Hyg. 1976;25:318–325. doi: 10.4269/ajtmh.1976.25.318. [DOI] [PubMed] [Google Scholar]
- 76.Tesh RB, Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- 77.Roundy CM, Azar SR, Rossi SL, et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis. 2017;23:625–632. doi: 10.3201/eid2304.161484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guedes DR, Paiva MH, Donato MM, Barbosa PP, Krokovsky L, Rocha SWDS, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6:e69. doi: 10.1038/emi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5:e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aliota MT, Bassit L, Bradrick SS, et al. Zika in the Americas, year 2: what have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Res. 2017;144:223–246. doi: 10.1016/j.antiviral.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included within the article. The datasets used for the present study are available from the corresponding author upon request.