Abstract
Aims
To assess visual function in young adults born preterm and compare with full-term individuals of the same age.
Methods
Young adults, born preterm (birth weight ≤1500 g) in 1988–1990, previously included in a population-based study on the incidence of retinopathy of prematurity (ROP) in Stockholm County, Sweden were included. A control group of participants born at term, in the same area during the same time period, was used for comparison. Best-corrected visual acuities were assessed at distance and near with logMAR charts. Distance visual acuity was also measured with single symbols to calculate crowding. Visual fields were measured with Humphrey 24-2 and the mean deviation was noted. Contrast sensitivity was assessed with Vistech contrast sensitivity test and the area under the curve was calculated.
Results
Fifty-nine preterm (females 37) and 44 full-term (females 18) individuals were included. All individuals were between 25 and 29 years of age. Preterm individuals had significantly lower distance visual acuity (mean −0.08 (SD 0.11) vs −0.14 (SD 0.07) logMAR, p=0.009), near visual acuity (mean −0.08 (SD 0.11) vs −0.13 (SD 0.06) logMAR, p=0.049), mean deviation (mean −1.09 (SD 1.13) vs −0.80 (SD 1.03) dB, p=0.05) and contrast sensitivity (mean 2.02 (SD 0.19) vs 2.16 (SD 0.14), p<0.001) in the better eye compared with full-term individuals. The differences in distance visual acuity and contrast sensitivity were also evident after excluding persons with previous ROP and neurological complications. In multivariable analyses, treated ROP was a risk factor for reduced near visual acuity and visual fields.
Conclusion
Visual function seems to be reduced in prematurely born individuals even in adulthood. The reason may be prematurity per se since individuals without previous ROP or neurological complications are also affected.
Synopsis
Visual function, assessed as visual acuity, visual fields and contrast sensitivity, was reduced in young adults born preterm and previously included in a population-based study on the incidence of retinopathy of prematurity, as compared with controls.
Keywords: long-term follow-up, prematurely born, retinopathy of prematurity (rop, visual function
Introduction
Ophthalmological and neurodevelopmental disorders are more common in prematurely born children than in children born at term.1 Previous studies have found a higher prevalence of refractive errors, strabismus, reduced visual acuity and cerebral visual impairment in preterm adolescents than in full-terms.2 3 However, few studies have reported the ophthalmological outcome in adults and most of those have described the complications of retinopathy of prematurity (ROP).4 5 Recently, Darlow et al 6 reported the visual outcome in former low birth weight (BW) adults previously screened for ROP, but not treated, since they were born before the introduction of treatment. The authors reported that former very low BW young adults with previous ROP had problems with vision affecting daily life. In Stockholm County, Sweden, a prospective population-based study of the incidence of ROP was performed in infants born during 1988 to 1990.7 The incidence of ROP was 40% and cryo-treatment was performed in 11% of the infants. The children were followed ophthalmologically until 10 years of age.8 The aim of the present study was to report the visual function (visual acuity, visual fields and contrast sensitivity) in the same cohort at 25–29 years of age and compare with individuals of the same age born at term. A secondary aim was to explore whether previous ROP or treated ROP had any impact on the ophthalmological outcome in adulthood.
Materials and methods
Materials
The original population-based cohort consisted of 260 infants born with a BW of ≤1500 g, between 1 November 1988 and 31 October 1990, in Stockholm County, Sweden. All infants were screened prospectively for ROP. Inclusion criterion for screening at that time was BW ≤1500 g. Forty per cent had ROP and 11% were treated with cryotherapy for ROP.7 At that time, the criterion for treatment in Sweden was ROP stage 3 in at least four clock hours in zone II, even in the absence of plus disease. Of the originally screened infants, 12 were excluded from the study. One child emigrated, seven died and four were excluded due to diseases unrelated to prematurity. Consequently, 248 were followed until 3.5 years of age.9 At 10 years of age, the children were called back for examination and 216 accepted the invitation.10–13 At 25 years of age, a new invitation was sent to the original cohort (248). As a control group, individuals born at term (39–41 weeks) between 1 November 1988 and 31 October 1990 with normal BW (3000–4000 g) in Stockholm County were randomly chosen from the Swedish National Board of Health and Social Welfare Register. All persons were located using personal identification numbers used in Sweden and were asked by letter if they wanted to participate in a 25-year ophthalmological follow-up study at the Department of Ophthalmology, Uppsala University Hospital. Of the prematurely born persons, 59 accepted the invitation (study group). Regarding the full-terms, 569 individuals received an invitation and 44 accepted (control group). The study was approved by the regional Ethical Review Board of Uppsala, Sweden and was performed in accordance with the Declaration of Helsinki. Those who agreed to participate signed a written consent before participation.
In the original study, ROP was divided into mild, severe untreated and severe treated ROP.12 Mild ROP was defined as ROP stage 1–2, and severe as ROP stage 3–5. At the 25-year follow-up, only one person with previous severe untreated ROP attended. Therefore, the group was divided into subgroups of ‘no ROP’, ‘untreated ROP’ (including mild ROP and one case of severe ROP) and ‘treated ROP’.
At 2.5 years, neurological complication was defined as an intraventricular haemorrhage in the neonatal period and/or obvious neurological sequelae (epilepsy, cerebral palsy or mental retardation). No further neurological examination was performed at 10 or 25 years of age.
Methods
Best-corrected distance visual acuity (VA) was assessed monocularly with the logarithmic ETDRS chart at 4 m. Chart R was used for refraction, Chart 1 for right eyes (RE) and Chart 2 for left eyes (LE). Best-corrected near VA was assessed binocularly with the logarithmic Near Visual Acuity ETDRS Chart at 40 cm. All correctly read letters were noted and the exact logMAR value was calculated.
Crowding was estimated monocularly using LEA optotypes. Line acuity was assessed with the LEA symbols 15-Line Distance Chart at a viewing distance of 3 m and single optotype acuity with the LEA symbols Single Symbol Book at the same distance. Crowding ratio was calculated as the measured single optotype acuity divided by line acuity.
Visual fields (VFs) were tested with the Humphrey Field Analyzer II 750i (Carl Zeiss Meditech, Germany) and the SITA standard 24-2 program. Tests with >15% false-positive responses were excluded.
Contrast sensitivity (CS) was determined for each eye with the Vistech Contrast Sensitivity Test System (VCTS 6500) (Vistech Consultants, USA), which measures the CS at five spatial frequencies, 1.5, 3, 6, 12 and 18 cycles per degree (c/deg), at a viewing distance of 3 m. The logarithmic values were used for the analysis.
Slit-lamp examination of the anterior segment and ophthalmoscopy of the posterior segment through dilated pupils were performed. Autorefractometry was performed under cycloplegia.
Right eyes were assessed before left eyes (LEs). The better eye was defined as the eye with the better best-corrected distance VA. If VA was equal in both eyes, the RE was chosen as the better eye. The better and worse eye according to this definition were used for analyses of VA, crowding, VF and CS.
Statistical methods
Power analysis was performed before the study, using power of 80% and a significance level of 0.05. Better and worse eyes were analysed separately. Comparison of VA (distance and near), crowding, VF, and CS between the premature and control groups were analysed using a series of linear regression models, all adjusted for gender and refraction. A similar regression model was used for the analysis of factors related to visual function within the study group, that is, gestational age (GA), BW, neurological complication at 2.5 years, gender, ROP (yes/no) and treated ROP. The analyses were conducted in two steps in which all factors were first analysed separately, and then multivariably. When comparing the distributions of VA and crowding ratio ≥1.5 between the premature and control groups, Fisher’s exact test was used. The within-subject restricted area under the curve (AUC) (from 1.5 to 18 c/deg) for log CS was estimated using a third-degree polynomial regression model. When comparing the preterm study and drop-out groups, an unpaired t-test was used with regard to GA, BW and VA at 10 years, and Pearson’s χ2 test for gender, neurological complication at 2.5 years, ROP (yes/no) and treated ROP. A p value <0.05 was regarded as statistically significant.
Results
Demographics of the 59 prematurely born participants and 44 controls are shown in table 1.
Table 1.
Demographics of prematurely born individuals and those born at term
| N RE/LE | Gender M/F | GA at birth (weeks) | BW (g) | |
| Mean (SD) | Mean (SD) | |||
| Range | Range | |||
| Control subjects | 44/44 | 26/18 | 39–41 | 3000–4000 |
| Prematures | 59/59 | 22/37 | 29.3 (2.1) | 1167 (237) |
| 24–34 | 700–1490 | |||
| No ROP* | 34/36 | 16/18 | 29.8 (2.0) | 1264 (195) |
| 26–34 | 711–1490 | |||
| Untreated ROP* | 12/10 | 4/8 | 29.2 (1.6) | 1082 (258) |
| 27–32 | 750–1466 | |||
| Treated ROP* | 13/13 | 2/11 | 28.0 (2.4) | 993 (201) |
| 24–32 | 700–1380 |
*In the eye with the most severe stage of ROP.
BW, birth weight; F, female; GA, gestational age; LE, left eye; M, male; N, number; RE, right eye; ROP, retinopathy of prematurity.
All participants were between 25 and 29 years at the time of examination. There was a difference between the two groups regarding gender, the study group having a larger proportion of females (table 1). Eight prematurely born individuals had neurological complications according to the definition at 2.5 years.9 The spherical equivalent in the better and worse eyes had mean values of −0.52 (SD 2.07) and −0.66 (SD 2.47) in the study group and −0.19 (SD 1.53) and −0.16 (SD 1.47) in the control group.
The preterm drop-out group is presented in online supplementary eTable 1. There were no differences with regard to GA or BW between the individuals who agreed to and those who declined to participate at 25 years of age. Furthermore, there were no differences between the groups regarding previous ROP and previous neurological complications. However, a larger proportion of those previously treated for ROP attended the 25-year follow-up, and a larger proportion was female.
bjophthalmol-2019-314429supp001.pdf (37.4KB, pdf)
All subjects were able to complete the assessments for monocular distance VA in both eyes, and binocular near VA. In two of the prematurely born individuals, crowding and CS could not be measured in the worse eye. Regarding VF, the better eye in two preterms, and both eyes in one full-term, were excluded according to the criteria.
Mean values of best-corrected distance VA in the better and worse eyes are presented in table 2. There was a significant difference between the study and the control groups in the better (p=0.009) and worse eyes (p=0.01) (table 2). A difference remained if those with previous ROP (p=0.08 better eye, p=0.034 worse eye) and if those with neurological complications (p=0.016 better eye, p=0.015 worse eye) were excluded. The distributions of VA differed significantly between the study and control groups regarding the better (p<0.001) and worse eyes (p<0.001) (figure 1).
Table 2.
Mean values, SD and 95% CI of distance visual acuity, crowding and mean deviation (MD) of the visual fields and number and fraction of crowding ratio ≥1.5 in the better and worse eyes of prematurely born individuals and those born at term
| VA (logMAR) | CR | CR ≥1.5 | MD | ||||
| Mean (SD) | Mean (SD) | N | Mean (SD) | ||||
| 95% CI | 95% CI | Fraction | 95% CI | ||||
| Better eye | |||||||
| Control subjects | N=44 | −0.14 (0.07) | N=44 | 1.20 (0.26) | 4 | N=43 | −0.80 (1.03) |
| −0.16 to −0.12 | 1.13 to 1.28 | 0.09 | −1.12 to −0.48 | ||||
| Prematures | N=59 | −0.08 (0.11) | N=59 | 1.26 (0.30) | 16 | N=56 | −1.09 (1.13) |
| −0.11 to −0.05 | 1.18 to 1.34 | 0.27 | −1.39 to −0.79 | ||||
| No ROP | N=36 | −0.10 (0.09) | N=36 | 1.27 (0.25) | 9 | N=35 | −0.93 (0.92) |
| −0.13 to −0.07 | 1.18 to 1.35 | 0.25 | −1.25 to −0.62 | ||||
| Untreated ROP | N=10 | −0.08 (0.10) | N=10 | 1.38 (0.30) | 4 | N=9 | −1.10 (1.61) |
| −0.15 to −0.01 | 1.16 to 1.59 | 0.40 | −2.33 to 0.14 | ||||
| Treated ROP | N=13 | −0.02 (0.14) | N=13 | 1.16 (0.40) | 3 | N=12 | −1.54 (1.26) |
| −0.11 to 0.06 | 0.91 to 1.40 | 0.23 | −2.34 to −0.73 | ||||
| Worse eye | |||||||
| Control subjects | N=44 | −0.09 (0.08) | N=44 | 1.32 (0.27) | 14 | N=43 | −0.92 (1.16) |
| −0.12 to −0.07 | 1.23 to 1.40 | 0.32 | −1.28 to −0.57 | ||||
| Prematures | N=59 | 0.03 (0.27) | N=57 | 1.30 (0.30) | 16 | N=57 | −1.32 (1.19) |
| −0.04 to 0.10 | 1.22 to 1.38 | 0.28 | −1.63 to −1.00 | ||||
| No ROP | N=34 | 0.01 (0.30) | N=33 | 1.33 (0.31) | 8 | N=33 | −1.34 (1.15) |
| −0.10 to 0.11 | 1.22 to 1.44 | 0.24 | −1.75 to −0.94 | ||||
| Untreated ROP | N=12 | 0.01 (0.08) | N=12 | 1.28 (0.27) | 4 | N=12 | −1.07 (1.43) |
| −0.04 to 0.06 | 1.11 to 1.45 | 0.33 | −1.98 to −0.17 | ||||
| Treated ROP | N=13 | 0.09 (0.29) | N=12 | 1.25 (0.30) | 4 | N=12 | −1.49 (1.12) |
| −0.09 to 0.26 | 1.06 to 1.44 | 0.33 | −2.20 to −0.78 |
CR, crowding ratio; N, number; ROP, retinopathy of prematurity; VA, visual acuity.
Figure 1.
Distribution of distance visual acuity (VA) (logMAR) in prematurely born and full-term young adults.
Within the study group, there was a statistical difference between those with previously treated ROP and those without ROP in the better eye (p=0.022) (table 2). However, the difference disappeared in a multivariable analysis taking also GA, BW and neurological complications into account. Six preterm individuals had VA >0 logMAR, of which two had neurological complication at 2.5 years, three had previous ROP and one had neither. One of the controls had VA >0 logMAR.
At 10 years of age, VA could be assessed with optotypes in 213 children, and there was no statistical difference in VA between those who later attended the present follow-up and those who did not (online supplementary eTable 1). However, at 10 years, there were four children with visual impairment according to WHO’s definition, and none of them attended at 25 years.12
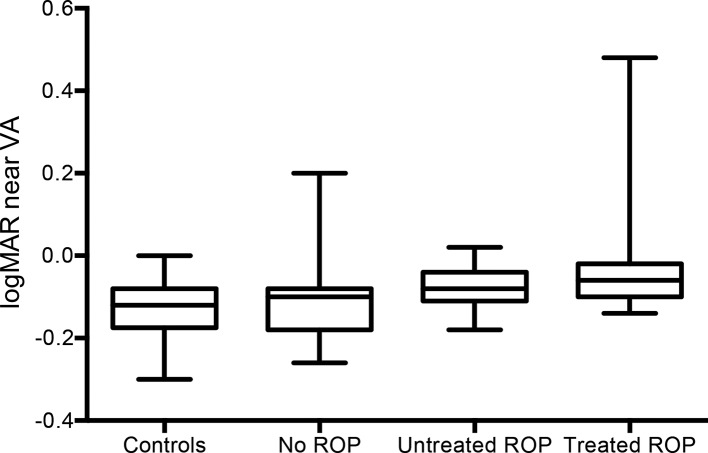
Binocular near VA is illustrated in figure 2. There was a statistical difference between the study (mean −0.08 (SD 0.11) logMAR) and control groups (mean −0.13 (SD 0.06) logMAR) (p=0.049). A difference remained if individuals with neurological complications (p=0.06) were excluded, but not when excluding those with previous ROP. Within the study group, there was a difference between previously treated persons and those without ROP in the multivariable analysis (p=0.022).
Figure 2.
Near visual acuity (VA) (logMAR) in prematurely born and full-term young adults. The rectangles include 50% of the values; the horizontal line represents the median, and the whiskers minimum and maximum values. ROP, retinopathy of prematurity.
Mean values of crowding, and prevalence of crowding ratio ≥1.5, are given in table 2. Regarding mean values, there were no statistical differences between the study and control groups and no differences within the study group. However, in the better eye, there was a difference regarding the prevalence of crowding ratio ≥1.5 between prematures and controls, p=0.02. One of the 16 preterms with crowding ≥1.5 had a neurological complication.
Analyses of the mean deviation of the VFs were performed and are shown in table 2, and there was a statistically significant difference between the prematurely born individuals and those born at term in the better (p=0.05) and worse eyes (p=0.05) (table 2), but not when excluding those with neurological complications and previous ROP. Within the study group, there was a statistical difference between those with previously treated ROP and those without ROP in the better eye (p=0.007) in the multivariable analysis (table 2), but not in the worse eye.
The mean values of logarithmic contrast sensitivity in all spatial frequencies in the better and worse eyes together with the AUC are shown in online supplementary eTable 2 and figure 3 (better eye). The values of AUC differed statistically between the study and control groups in the better eye (p<0.001), and the difference remained when those with previous ROP (p<0.001) and neurological complication (p<0.001) were excluded. The results from the worse eyes were in line with the better eyes (p<0.001). Within the prematurely born group, no differences were found.
Figure 3.

Contrast sensitivity curve, mean and 95% CI, in better eye of prematurely born and full-term young adults. ROP, retinopathy of prematurity.
bjophthalmol-2019-314429supp002.pdf (45.1KB, pdf)
Three preterm individuals had VA >0.3 logMAR in their worse eyes, one due to glaucoma secondary to complications of severe ROP and two due to strabismic amblyopia. All previously treated individuals had retinal scars in the periphery; however, none had macular heterotopia. Two control subjects had macular pigmentations in their worse eyes, but the VA was <0 logMAR in both cases. One control subject had pigmentations on the corneal endothelium (VA RE −0.18 and LE 0.12 logMAR) and one had a lens opacity (VA 0.0 logMAR). Furthermore, one control subject had undergone LASIK because of refractive error.
Discussion
In the present study, visual function was affected in young adults who were born preterm. Distance and near VA, central VF and CS were reduced compared with individuals of the same age, born in the same area, but born at full-term. Except for near VA and VF, previous ROP or treated ROP had no impact on the outcome in the prematurely born individuals.
In this population-based study, infants had been prospectively screened for ROP. At the time of the screening, there were few prospective, population-based studies on the incidence of ROP. Darlow,14 Fledelius15 and Fielder et al 16 published the incidences from New Zealand, Denmark and UK, but their infants were born before the introduction of cryo-treatment. Consequently, the present cohort was the only one at that time in which infants had also been treated for their ROP. All four cohorts were followed up at school age: Darlow et al at 7–8 years,17 Fledelius at 7–10 years,18 19 O’Connor et al 20 at 12 years, and the present cohort first up to 3.5 years9 and then at 10 years of age.8 There were more ophthalmological problems in the prematurely born children of school age compared with children born at term in all the studies. A follow-up to 27–29 years has been performed by Darlow et al.6 To our knowledge, the present study and that by Darlow are the only population-based studies on the ophthalmological outcome in adults formerly born preterm in which the cohort had also been screened prospectively for ROP during the neonatal period.
In the present study, distance VA was reduced in preterms compared with full-terms, in accordance with the study by Darlow et al.6 The distribution of VA between preterm and full-terms differed in the present study, the prevalence of better VA being higher in full-terms. However, overall, our individuals had better VA in the better eye, both in the prematurely born and the full-terms, than the individuals in the study by Darlow et al. When comparing the prevalence of logMAR >0 in the better eye in the two studies, Darlow et al found higher prevalence both in preterms (33% vs 10%) and in full-terms (13% vs 2.3%). In the study from New Zealand, no participant in the preterm group had been treated for ROP, which could possibly explain the difference between the two studies. Finally, it should be added that there were four persons in our cohort with visual impairment at 10 years of age who did not participate in the present study.12 However, the difference in VA between the studies regarding full-terms could not be fully explained.
The binocular near VA was reduced in the prematurely born individuals, as it was at 10 years of age.12 In contrast to the results at 10 years, individuals without previous ROP did not have worse near VA than the controls. The reason for this change over time cannot be explained. However, within the preterm group, the reduced VA was most obvious in individuals previously treated for ROP.
Crowding is the inability to separate objects presented closely together. In daily life, this can affect the ability to read or detect things in a crowded environment. We found no difference in the crowding ratio between preterm and full-term adults. However, a crowding ratio ≥1.5 was more common in the preterm individuals, as it was at 10 years of age.12 Crowding is more common in persons with brain lesions,21 but of those who had crowding, only one of the preterms had a neurological problem.
In the 10-year follow-up, VFs were constricted in children previously treated for ROP, and the neural capacity of the central VF was reduced in prematurely born children, regardless of previous ROP, as compared with full-terms.12 In the present study, at 25 years of age, the sensitivity in the central VF was also reduced in preterms in comparison with full-terms. In the multivariable analyses of risk factors within the preterm group, previously treated ROP was the main risk factor for reduced central VF, in contrast to the results at 10 years. It can only be hypothesised that the peripheral retinal treatment has also had a long-term effect on the central VF. This accords with the findings by Åkerblom et al 22 who found reduced retinal nerve fibre layer assessed with optical coherence tomography in preterm schoolchildren, but primarily in treated children.
The ability to perceive contrast is important for daily life activity. In the present study, the CS was reduced in adults born preterm, in contrast to the study by Darlow et al,6 although different CS tests were used in the two studies. Contrast sensitivity can be affected by neurological and retinal diseases.23 24 However, in the present cohort, the reduction was also evident when those with neurological complications and those with previous ROP were excluded, indicating the role of prematurity per se.
Despite the differences between preterm and full-term individuals, no risk factors for visual dysfunction such as GA, BW, ROP and neurological complication could be identified in the multivariable analyses within the preterm group, except for severe treated ROP regarding near VAs and VFs. We therefore speculate that prematurity per se might be a cause for reduced visual function throughout childhood up to adulthood in prematurely born individuals. If structural changes in the macular area as well as functional changes in rods and cones previously found in preterm children could be a reason for the reduction may only be speculated on.25–27 Also, microstructural changes in the optic nerve and/or visual pathway might affect visual function.28 Previous studies in adults with severe ROP in the neonatal period have shown long-term complications.4 Fledelius and Jensen speculated that eyes of preterms are more vulnerable.5 It cannot be concluded if the present finding of reduced visual function also in individuals without previous ROP may reflect a more vulnerable eye and be a cause of higher risk for other eye diseases later in life. Further follow-up is therefore warranted.
Strengths and limitations
The present study was strictly population-based both regarding prematurely born and full-term individuals. They were all born in the same area during the same time period. Further, the study was designed by paediatric ophthalmologists and was prospective with regard to ROP screening and follow-up. All prematurely born and full-term individuals were examined in exactly the same way, under the same conditions, and by the same paediatric ophthalmologist and ophthalmic research nurse. Another advantage was the analysis of several parameters included in the visual function, that is, VA, crowding, VF and CS. A limitation of the present study was the size of the preterm drop-out group. For ethical reasons we could not contact those who did not answer our letter of invitation. However, we compared those who attended the present study with those who did not attend (online supplementary eTable 1), and the groups differed only with regard to gender and the number of treated persons, which was considered in the analyses. Another limitation was the lack of MRI scans and neurological examinations in both of the groups.
Conclusion
Visual function was reduced in adults formerly born preterm and previously screened for ROP in comparison with full-term individuals. Except for near VA and VF, previous ROP or treated ROP had no impact on the outcome, indicating a role of prematurity per se.
Acknowledgments
We thank Eva Nuija, study nurse, for her skilful help with the study, and Marcus Thuresson, PhD, who assisted with the statistical analysis and created figure 3.
Footnotes
Contributors: The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. EL and GH contributed to the planning, conduct and reporting, and DP contributed to the conduct and reporting of the work described in the article.
Funding: The study was funded by the Crown Princess Margaretha Foundation for the Visually Impaired, The Carmen and Bertil Regnér Foundation, Synskadades Vänner i Uppsala län and Ögonfonden.
Disclaimer: The funders had not been involved in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: This study was performed in accordance with the ethical standards of the institutional and national research committee and with the Helsinki declaration. The study was approved by the Regional Ethical Review Board in Uppsala, Sweden (no. 2014/548).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on request.
References
- 1. Hellgren KM, Tornqvist K, Jakobsson PG, et al. Ophthalmologic outcome of extremely preterm infants at 6.5 years of age: Extremely preterm infants in Sweden study (EXPRESS). JAMA Ophthalmol 2016;134:555–62. 10.1001/jamaophthalmol.2016.0391 [DOI] [PubMed] [Google Scholar]
- 2. Lindqvist S, Vik T, Indredavik MS, et al. Visual acuity, contrast sensitivity, peripheral vision and refraction in low birthweight teenagers. Acta Ophthalmol Scand 2007;85:157–64. 10.1111/j.1600-0420.2006.00808.x [DOI] [PubMed] [Google Scholar]
- 3. Hellgren K, Aring E, Jacobson L, et al. Visuospatial skills, ocular alignment, and magnetic resonance imaging findings in very low birth weight adolescents. J Aapos 2009;13:273–9. 10.1016/j.jaapos.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 4. Smith BT, Tasman WS. Retinopathy of prematurity: late complications in the baby boomer generation (1946–1964). Trans Am Ophthalmol Soc 2005;103:225–34. [PMC free article] [PubMed] [Google Scholar]
- 5. Fledelius HC, Jensen H. Late subsequent ocular morbidity in retinopathy of prematurity patients, with emphasis on visual loss caused by insidious 'involutive' pathology: an observational series. Acta Ophthalmol 2011;89:316–23. 10.1111/j.1755-3768.2009.01707.x [DOI] [PubMed] [Google Scholar]
- 6. Darlow BA, Elder MJ, Kimber B, et al. Vision in former very low birthweight young adults with and without retinopathy of prematurity compared with term born controls: the NZ 1986 VLBW follow-up study. Br J Ophthalmol 2018;102:1041–6. 10.1136/bjophthalmol-2017-311345 [DOI] [PubMed] [Google Scholar]
- 7. Holmström G, el Azazi M, Jacobson L, et al. A population based, prospective study of the development of ROP in prematurely born children in the Stockholm area of Sweden. Br J Ophthalmol 1993;77:417–23. 10.1136/bjo.77.7.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holmström G, Larsson E. Long-term follow-up of visual functions in prematurely born children—a prospective population-based study up to 10 years of age. J Aapos 2008;12:157–62. 10.1016/j.jaapos.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 9. Holmström G, el Azazi M, Kugelberg U. Ophthalmological follow up of preterm infants: a population based, prospective study of visual acuity and strabismus. Br J Ophthalmol 1999;83:143–50. 10.1136/bjo.83.2.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson EK, Rydberg AC, Holmström GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Arch Ophthalmol 2003;121:1430–6. 10.1001/archopht.121.10.1430 [DOI] [PubMed] [Google Scholar]
- 11. Larsson E, Martin L, Holmström G. Peripheral and central visual fields in 11-year-old children who had been born prematurely and at term. J Pediatr Ophthalmol Strabismus 2004;41:39–45. 10.3928/0191-3913-20040101-10 [DOI] [PubMed] [Google Scholar]
- 12. Larsson EK, Rydberg AC, Holmström GE. A population-based study on the visual outcome in 10-year-old preterm and full-term children. Arch Ophthalmol 2005;123:825–32. 10.1001/archopht.123.6.825 [DOI] [PubMed] [Google Scholar]
- 13. Larsson E, Rydberg A, Holmström G. Contrast sensitivity in 10 year old preterm and full term children: a population based study. Br J Ophthalmol 2006;90:87–90. 10.1136/bjo.2005.081653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darlow BA. Incidence of retinopathy of prematurity in New Zealand. Arch Dis Child 1988;63:1083–6. 10.1136/adc.63.9.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fledelius HC. Retinopathy of prematurity. Clinical findings in a Danish County 1982–87. Acta Ophthalmol 1990;68:209–13. [DOI] [PubMed] [Google Scholar]
- 16. Fielder AR, Shaw DE, Robinson J, et al. Natural history of retinopathy of prematurity: a prospective study. Eye 1992;6:233–42. 10.1038/eye.1992.46 [DOI] [PubMed] [Google Scholar]
- 17. Darlow BA, Horwood LJ, Mogridge N, et al. Prospective study of New Zealand very low birthweight infants: outcome at 7–8 years. J Paediatr Child Health 1997;33:47–51. 10.1111/j.1440-1754.1997.tb00990.x [DOI] [PubMed] [Google Scholar]
- 18. Fledelius HC. Pre-term delivery and subsequent ocular development. A 7–10 year follow-up of children screened 1982–84 for ROP. 1) Visual function, slit-lamp findings, and fundus appearance. Acta Ophthalmol Scand 1996;74:288–93. [DOI] [PubMed] [Google Scholar]
- 19. Fledelius HC. Pre-term delivery and subsequent ocular development. A 7–10 year follow-up of children screened 1982–84 for ROP. 2) Binocular function. Acta Ophthalmol Scand 1996;74:294–6. [DOI] [PubMed] [Google Scholar]
- 20. O'Connor AR, Stephenson T, Johnson A, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics 2002;109:12–18. 10.1542/peds.109.1.12 [DOI] [PubMed] [Google Scholar]
- 21. van Genderen M, Dekker M, Pilon F, et al. Diagnosing cerebral visual impairment in children with good visual acuity. Strabismus 2012;20:78–83. 10.3109/09273972.2012.680232 [DOI] [PubMed] [Google Scholar]
- 22. Åkerblom H, Holmström G, Eriksson U, et al. Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol 2012;96:956–60. 10.1136/bjophthalmol-2011-301010 [DOI] [PubMed] [Google Scholar]
- 23. Good WV, Hou C, Norcia AM. Spatial contrast sensitivity vision loss in children with cortical visual impairment. Invest Ophthalmol Vis Sci 2012;53:7730–4. 10.1167/iovs.12-9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolkstein M, Atkin A, Bodis-Wollner I. Contrast sensitivity in retinal disease. Ophthalmology 1980;87:1140–9. 10.1016/S0161-6420(80)35112-9 [DOI] [PubMed] [Google Scholar]
- 25. Molnar AEC, Rosén RM, Nilsson M, et al. Central macular thickness in 6.5-year-old children born extremely preterm is strongly associated with gestational age even when adjusted for risk factors. Retina 2017;37:2281–8. 10.1097/IAE.0000000000001469 [DOI] [PubMed] [Google Scholar]
- 26. Åkerblom H, Andreasson S, Larsson E, et al. Photoreceptor function in school-aged children is affected by preterm birth. Transl Vis Sci Technol 2014;3 10.1167/tvst.3.6.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molnar AEC, Andréasson SO, Larsson EKB, et al. Reduction of rod and cone function in 6.5-year-old children born extremely preterm. JAMA Ophthalmol 2017;135:854–61. 10.1001/jamaophthalmol.2017.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 2008;121:306–16. 10.1542/peds.2007-0414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2019-314429supp001.pdf (37.4KB, pdf)
bjophthalmol-2019-314429supp002.pdf (45.1KB, pdf)