Abstract
Objectives
We sought to confirm in very early rheumatoid arthritis (ERA) a much greater superiority (30%) of first-line etanercept+methotrexate (ETN+MTX) over treat-to-target MTX (MTX-TT) than previously reported in ERA (14%); and explore whether ETN following initial MTX secures a comparable response to first-line ETN+MTX.
Methods
Pragmatic, open-label, randomised controlled trial of treatment-naïve ERA (≤12 months symptom), Disease Activity Score 28 joint (DAS28)-erythrocyte sedimentation rate (ESR) ≥3.2, rheumatoid factor (RF)+/−anticitrullinated peptide antibody (ACPA) positive or ultrasound power Doppler (PD) if RF and ACPA negative. Subjects were randomised 1:1 to ETN+MTX; or MTX-TT, escalated to ETN if week 24 DAS28-ESR ≥2.6 and intramuscular corticosteroid at protocolised time points. Primary endpoint of week 48 DAS28ESR remission with clinical and imaging secondary endpoints.
Results
We randomised 120 patients, 60 to each arm (71% female, 73% RF/84% ACPA positive, median (IQR) symptom duration 20.3 (13.1, 30.8) weeks; mean (SD) DAS28 5.1 (1.1)). Remission rates with ETN+MTX and MTX-TT, respectively, were 38% vs 33% at week 24; 52% vs 38% at week 48 (ORs 1.6, 95% CI 0.8 to 3.5, p=0.211). Greater, sustained DAS28-ESR remission observed with ETN+MTX versus MTX-TT (42% and 27%, respectively; p=0.035). PD was fully suppressed by week 48 in over 90% in each arm. Planned exploratory analysis revealed OR 2.84, 95% CI 0.8 to 9.6) of achieving remission after 24 weeks of ETN administered first line compared with administered post-MTX.
Conclusions
Compared with remission rates typically reported with first-line tumour necrosis factor inhabitor+MTX versus MTX-TT, we did not demonstrate a larger effect in very ERA. Neither strategy conferred remission in the majority of patients although ultrasound confirmed local inflammation suppression. Poorer ETN response following failure of MTX-TT is also suggested.
Trial registration number
Keywords: early rheumatoid arthritis, anti-TNF, etanercept, remission, ultrasound
Key messages.
What is already known about this subject?
In new onset, early rheumatoid arthritis (ERA), biological disease-modifying antirheumatic drug (bDMARD) (with mainly tumour necrosis factor inhibitor (TNFi) tested)+methotrexate (MTX) has not been shown to be superior to MTX+/−additional conventional synthetic DMARD in strategy trials to justify first-line use; although studies to date have not necessarily included all the elements of optimal treat-to-target (TT) strategies. Randomised controlled trial data of targeted synthetic DMARDs (janus kinase (JAK) inhibitors in MTX and bDMARD-inadequate response (IR)), suggest similar pragmatic evaluation is needed to inform on its place.
What does this study add?
This study did not confirm a large effect size (of 30%) suggested in previous exploratory analysis with first-line TNFi+MTX compared with MTX-TT. This highlights that despite incorporating all the recommended TT strategies in a real-life, treatment-naïve, early(≤12 months symptom) RA cohort, a ceiling effect with both first-line MTX-TT and etanercept-TNFi+MTX exists; that does not appear attributable to ongoing local inflammation (as evidenced by power Doppler ultrasound).
The data suggest that in a very ERA MTX-TT-IR cohort (compared with longer-duration cohort of previous pivotal MTX-IR trials), a proportion still may not respond to TNFi; implying preceding inflammation and drug exposure may lead to an acquired biology of less TNFi responsiveness.
How might this impact on clinical practice or future developments?
There is a continued need to understand the basis for this limited response rate and testing of alternative strategies to ensure more complete remission rates are achieved.
The exploratory observations support research to understand the biology of a very ERA MTX-TT-IR subgroup for future therapeutic opportunities acknowledgements.
Introduction
Biological disease-modifying antirheumatic drugs (bDMARDs) are established in the treatment of rheumatoid arthritis (RA) but failure of conventional synthetic DMARD (csDMARD), usually methotrexate (MTX), is a minimum hurdle requirement.1 Extensive evaluation of first-line csDMARD and bDMARD, mainly tumour necrosis factor inhibitor (TNFi),2 including pragmatic strategic studies in DMARD-naïve and MTX-naïve cohorts have been contradictory in demonstrating clear benefit of bDMARD.3–7 Therefore, bDMARDs are still restricted to MTX-inadequate response (IR), which avoids overtreatment.8 Nevertheless, with first-line bDMARD combination, remission is achieved earlier,9 10 with benefits for quality of life and jobs,11 and greater possibility of bDMARD tapering.12 Exploratory analysis in a previous study suggested a heightened difference in remission rate (of 30%) with first-line bDMARD compared with MTX in very early RA (ERA).13 None of the treatment strategies achieve remission in the majority and remission rates are virtually always higher when drug is used first-line.14
The Very early Etanercept and MTX versus MTX with Delayed Etanercept in RA (VEDERA) study aimed, in a real-life cohort with treat-to-target (TT) strategies, to determine whether initial etanercept (ETN) and MTX compared with MTX-TT, conferred a larger than standard effect (30%) in very ERA and to explore the performance of ETN when administered first-line or following MTX.
Patient and methods
VEDERA was a pragmatic investigator-initiated study conducted at Leeds Teaching Hospitals NHS Trust rheumatology outpatient department (full protocol details published15). All patients gave their written, informed consent to take part. Independent lay individual from our public and patient advocacy group provided input into study design.
Patients
Eligible patients were ≥18 years, had new-onset ERA fulfilling 2010 American College of Rheumatology/European League against Rheumatism (ACR/EULAR) RA classification criteria16; no prior DMARD therapy; ≤12 months symptom duration; disease activity score 28 joint (DAS28)-erythrocyte sedimentation rate (ESR) ≥3.2 with clinical evidence of synovitis; positive anticitrullinated peptide antibody (ACPA) and/or rheumatoid factor (RF), or if RF and/or ACPA negative, evidence on hand ultrasound (US) of power Doppler (PD) defined as grade ≥1 in at least one joint.
Study design
VEDERA is a single-centre, phase IV, open-label, two-arm, randomised controlled trial in patients with ERA. Patients were block randomised 1:1 to first-line ETN+MTX or first-line MTX-TT for a total duration of 48 weeks.
ETN+MTX regimen: intramuscular (IM) depomedrone 120 mg, subcutaneous ETN 50 mg weekly and oral MTX 15 mg weekly, increased to 20 mg and 25 mg weekly at weeks 4 and 8, respectively. MTX-TT protocol: IM depomedrone and oral MTX monotherapy 15 mg weekly, increased to 25 mg weekly at 2 weeks. If not in low disease activity (LDA) (DAS28-ESR ≤3.2) weeks 8, 12, 16 or 20, oral sulfasalazine (SSZ) 1 g two times per day and hydroxychloroquine (HCQ) 200 mg daily were added to MTX. At week 24, if DAS28-ESR ≥2.6, ETN was added to MTX (MTX-TTb), and SSZ and HCQ were discontinued. IM depomedrone 120 mg was administered in both arms at week 12 if DAS28-ESR ≥3.2, weeks 24 and 36 if DAS28-ESR ≥2.6. Subcutaneous MTX was administered with intolerance to oral MTX. All patients received folic acid 5 mg each day (except day of MTX). Stable doses of oral glucocorticoids (≤10 mg/day of prednisone or equivalent) and/or a single non-steroidal anti-inflammatory drug were permitted.
All patients on ETN at week 48, stopped the ETN. Patients were treated as per standard practice with 48-week observational follow-up and established on bDMARD if they fulfilled National Institute for Health and Care Excellence (NICE) criteria (DAS28 >5.1)17 (with ETN prescribed unless already tried and failed during the trial).
Blinding
Trained research nurses blinded to allocation performed assessments throughout the study. US assessments were performed by an ultrasonographer blinded to allocation.
Outcomes and assessments
The primary endpoint was the proportion who at week 48 achieved DAS28-ESR remission (DAS28-ESR ≤0.6). Multiple secondary endpoints: proportion achieving at weeks 12, 24, 48 and 96 (only to be inferentially compared at week 96): DAS44 remission, DAS28 remission,18 Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI),19 ACR and EULAR response20 Boolean remission rates21; time to sustained remission (SR; defined as DAS28-ESR (or DAS44, SDAI, CDAI) remission observed at ≥2 consecutive visits within weeks 12, 24, 36 and 48); change from baseline in Health Assessment Questionnaire Disability Index (HAQ)22 and normalisation of HAQ (to <0.5); change from baseline in Visual Analogue Scales (VASs) for patient pain, and patient and physician global assessments of disease activity, EuroQoL-5 Dimensions-3 Level23 and Rheumatoid Arthritis Quality of Life24; cumulative steroid dose. High-resolution US of dominant hand MCPs 1–5/wrists (or hand with greater evidence of inflammation) at weeks 0, 12, 24 and 48 to assess for synovitis, using semiquantitative (0–3) scores of Grey Scale (GS) and PD, and for presence of erosions.25 One of two assessors scanned the participants, with a third scanning <10%. Plain radiology of hands and feet to determine change in total van der Heijde modified Sharp score26 at weeks 48 and 96. The mean of scores by two independent readers who knew the order of the films but were blind to allocation was used and any significant disagreement adjudicated by a third reader. Finally, change in MRI synovitis score at 12, 24, 48 and 96 weeks (to be reported separately).
Safety
Adverse events (AE) and serious AE (SAE) were recorded during the 48-week protocolised treatment strategies.
Statistical analysis
Sample size and power calculation
The Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET) studyubgroup remission rates of patients with ≤4 months since diagnosis treated with ETN+MTX or MTX monotherapy were 70% vs 35%, respectively.13 We expected remission rates in VEDERA patients, recruited at <12 months symptom onset rather than diagnosis, would be similar to the COMET very ERA subgroup (of less than 4 months since diagnosis). Therefore, remission rate in ETN+MTX was anticipated at 70% and 40% in MTX-TT (delayed or deferred ETN); at 1-beta=0.8, alpha=0.05 and accounting for 10% drop-out, 49 patients per arm were required, increased to 60 per arm to allow for an exploratory subgroup analysis of ETN+MTX compared with MTX-TTa (csDMARD throughout) and MTX-TTb (delayed ETN). We estimated that 50% of MTX-TT patients would require delayed ETN.
Analysis
Complete plan can be found in the online supplementary material.
annrheumdis-2019-216539supp001.pdf (652.5KB, pdf)
The full analysis set (intention to treat (ITT)), for efficacy and safety, included all patients randomised, as randomised, with per protocol (PP) set comprising all ITT patients with primary endpoint data available and no major protocol violations. Two-sided tests were conducted throughout at the 5% level of significance. The Holm correction (modified Bonferroni) to control for multiple comparisons of secondary outcomes set the critical p value for testing significance at the 5% level to p<0.00088.
Primary outcome
The primary analysis compared the proportions achieving DAS28ESR <2.6 between the ETN+MTX and MTX-TT arms using Pearson’s χ2 test. The ORs and 95% CI for the odds of achieving DAS28-ESR remission is reported. A number of planned sensitivity analyses of the primary outcome were conducted (detailed in results).
Secondary outcomes
Proportions achieving remission, ACR or EULAR response at 96 weeks were compared between groups using Pearson’s χ2 tests (with descriptive evaluation for the other time points). Changes in continuous variables were analysed using linear multilevel modelling. Baseline values were included as covariates. An exponential autoregressive within-subject covariance pattern was found to be optimal using Akaike information criterion values after inspection of correlations between repeated observations. Severely skewed US and radiographic variables were analysed using quantile regression. Time to SR (as defined in ‘outcomes and assessment’) was analysed using log-rank tests.
Additional planned analyses
The response in ETN+MTX over the first 24 weeks was compared with the response in MTX-TTb (delayed ETN) over weeks 24–48. Proportions requiring escalation to triple therapy and bDMARD have been presented, as has cumulative IM steroid dose up to week 48.
Additional unplanned remission analyses
The ACR/EULAR remission criteria27 were ‘provisional’ in 2011; hence not included as an outcome of the trial. Nevertheless, additional, unplanned, descriptive data comparing ACR/EULAR remission between groups at week 48 are presented.
The online supplementary file details handling of missing data.
Results
Patient disposition
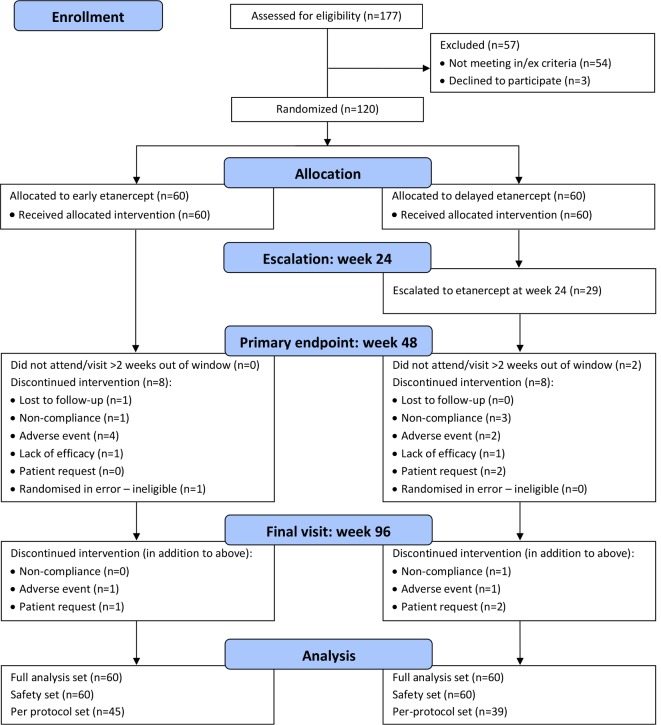
Of 177 patients screened between October 2011 and October 2015, 120 patients were recruited and randomly assigned to receive ETN+MTX (n=60) or MTX-TT strategy (n=60) (see online supplementary table S1). One hundred and four (87%) subjects reached week 48 and 98 (82%) week 96 (figure 1). Reasons for exclusion from PP efficacy set, withdrawals, inclusion and exclusion criteria for all screened patients are included in the online supplementary table S1.
Figure 1.
CONSORT flow diagram participant flow diagram up to week 96. CONSORT, Consolidated Standards of Reporting Trials.
Baseline demographics and characteristics
Patient demographics and baseline characteristics (table 1) were comparable between the two arms, and were representative of a new-onset, treatment-naïve, high disease activity ERA population. Seventy-two per cent (81/113) had evidence of erosive disease (any Sharp erosion score >0). Only four patients had any prior steroid exposure reflecting a treatment-naïve inception cohort.
Table 1.
Baseline demographics and disease profile for the entire group, ETN+MTX and MTX-TT
| Variable | All | ETN+MTX | MTX-TT |
| Demographics | |||
| Age, years Mean (SD) | 50.0 (12.8) | 49.6 (12.5) | 50.3 (13.2) |
| Female % (n/N) | 71 (85) | 65 (39) | 77 (46) |
| RA presenting history, % (n/N) (unless otherwise stated) | |||
| Symptom duration, weeks, median (Q1, Q3) | 20.3 (13.1 to 30.8) | 19.2 (12.5 to 28.1) | 20.8 (15.9 to 31.9) |
| Previous IM steroid | 1 (1/120) | 0 (0/60) | 2 (1/60) |
| Previous IA steroid | 0 (0/120) | 0 (0/60) | 0 (0/60) |
| Concomitant oral steroid | 3 (3/120) | 0 (0/60) | 5 (3/60) |
| Concomitant NSAID | 88 (105/120) | 92 (55/60) | 83 (50/60) |
| RA disease phenotype, % (n/N) | |||
| RF positive | 73 (87/120) | 70 (42/60) | 75 (45/60) |
| ACPA positive | 84 (101/120) | 82 (49/60) | 87 (52/60) |
| ANA positive | 15 (18/120) | 18 (11/60) | 12 (7/60) |
| RA disease activity components, Median (Q1, Q3) (unless otherwise stated) | |||
| TJC28 | 11.0 (7.0, 17.0) | 11.5 (6.0, 20.0) | 10.0 (7.0, 16.0) |
| SJC28 | 5.0 (2.0, 9.0) | 5.0 (3.0, 10.5) | 5.0 (2.0, 9.0) |
| ESR, mm/hour | 31.5 (18.5 to 51.0) | 30.5 (17.0 to 51.5) | 32.5 (20.5 to 51.0) |
| CRP, mg/L | 8.8 (2.3, 24.0) | 10.2 (1.8, 28.0) | 8.0 (2.7, 21.5) |
| Disease activity VAS, mm Mean (SD) | 57.1 (22.3) | 60.7 (21.6) | 53.6 (22.6) |
| RA disease activity scores, Mean (SD) | |||
| DAS28-ESR | 5.7 (1.1) | 5.8 (1.1) | 5.6 (1.0) |
| DAS44-ESR | 3.7 (0.8) | 3.7 (0.9) | 3.7 (0.7) |
| DAS28-CRP | 5.1 (1.2) | 5.2 (1.2) | 4.9 (1.1) |
| DAS44-CRP | 3.4 (0.8) | 3.5 (0.9) | 3.3 (0.8) |
| SDAI | 31.6 (13.7) | 34.2 (14.7) | 29.0 (12.3) |
| CDAI | 29.8 (12.7) | 32.2 (13.6) | 27.3 (11.2) |
| Patient-reported outcome measures, Mean (SD) (unless otherwise stated) | |||
| Global pain VAS, mm | 53.5 (24.5) | 59.0 (23.4) | 48.1 (24.6) |
| HAQ-DI | 1.2 (0.5) | 1.2 (0.5) | 1.1 (0.5) |
| RAQoL | 17.3 (7.3) | 16.8 (7.4) | 17.9 (7.2) |
| In paid work % (n/N) | 73 (88/120) | 82 (49/60) | 65 (39/60) |
| EQ-5D-3L index | 0.5 (0.3) | 0.4 (0.3) | 0.5 (0.3) |
| RAWIS | 18.2 (6.6) | 19.0 (6.7) | 17.3 (6.4) |
| Ultrasound scores Median (Q1, Q3) | |||
| Total GS score | 2.0 (0.0, 5.0) | 3.0 (0.5, 5.0) | 2.0 (0.0, 5.0) |
| Total PD score | 0.0 (0.0, 2.5) | 0.0 (0.0, 3.0) | 0.0 (0.0, 2.0) |
| Total erosion score | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Radiographic score median (Q1, Q3) | |||
| Total modified Sharp score | 2.5 (0.5, 6.0) | 2.0 (0.5, 5.0) | 2.5 (0.5, 6.3) |
ACPA, anticitrullinated protein antibody; ANA, antinuclear antibody; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS28, Disease Activity Score 28 joint; EQ-5D-3L, EuroQoL-5 Dimensions-3 Level; ESR, erythrocyte sedimentation rate; ETN, etanercept; GS, Grey Scale; HAQ-DI, Health Assessment Questionnaire Disability Index; IA, intra-articular; IM, intramuscular; MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug; PD, power Doppler; RA, rheumatoid arthritis; RAQoL, Rheumatoid Arthritis Quality of life Questionnaire; RAWIS, Rheumatoid Arthritis Work Instability Scale; RF, rheumatoid factor; SDAI, Simplified Disease Activity Index; SJC, swollen joint count; TJC, tender joint count; TT, treat-to-target; VAS, Visual Analogue Scale.
Primary endpoint
Of the full analysis set, 52% ETN+MTX vs 38% MTX-TT achieved DAS28-ESR remission at week 48 (OR 1.73, 95% CI 0.81 to 3.70) p=0.160) (figure 2A; online supplementary table S2). Sensitivity analyses supported this main analysis, except when assuming that those with missing data treated with ETN+MTX responded while those receiving MTX-TT did not (see online supplementary table S3). Only under this assumption did we observe the disproportionately large difference (30%; 63% ETN+MTX vs 33% MTX-TT) expected.
Figure 2.
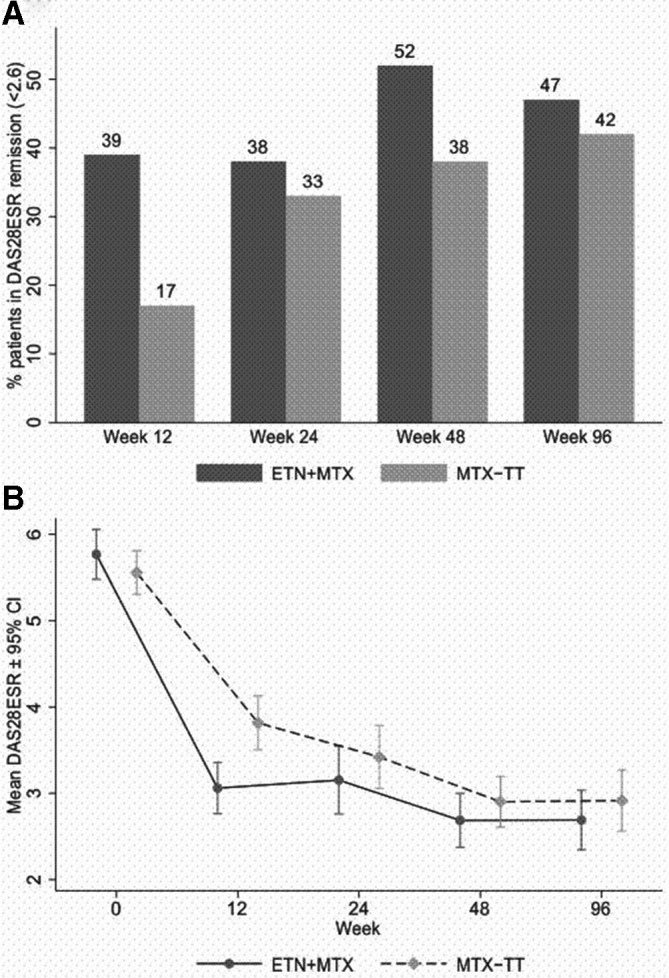
(A) DAS28-ESR remission rates in ETN+MTX (n=60) and MTX-TT (n=60). Percentage patient estimated via multiple imputation. (B) Individual DAS28-ESR scores over time in ETN+MTX (n=60) and MTX-TT (n=60). DAS28, Disease Activity Score 28 joint; ESR, erythrocyte sedimentation rate; ETN, etanercept; MTX, methotrexate; TT, treat-to-target.
Secondary endpoints
Clinical outcomes
DAS remission rates
Thirty-nine per cent receiving ETN+MTX achieved remission at week 12 vs 17% receiving MTX-TT (OR 3.18, 95% CI 1.35 to 7.50); figure 1B). By week 24, the groups were similar (see online supplementary table S2). Between-group differences in alternative DAS-based remission criteria were descriptively similar to DAS28-ESR remission (see online supplementary tables S4–S6). At week 96, there were no statistically significant differences between the groups in remission rates; continuous DAS scores (unplanned descriptive analysis) were similar across arms (see online supplementary table S7).
Boolean remission and DAS28ESR LDA rates (unplanned analysis)
Differences in the proportions achieving ACR/EULAR Boolean remission and DAS28ESR LDA were consistent with those reported for DAS28ESR remission (see online supplementary tables S8–S9).
Sustained remission
Sustained (DAS28-ESR) remission was achieved earlier in the ETN+MTX group compared with MTX-TT (after 24 vs 36 weeks, in 42% vs 27%, respectively, p=0.035); but at the corrected significance threshold (p<0.0008) this was not statistically significant (see online supplementary table S10).
EULAR and ACR response rates
ETN+MTX arm achieved earlier EULAR and ACR responses compared with MTX-TT; but response rates were comparable by week 48, maintained at week 96 (see online supplementary tables S11–S15).
Planned exploratory analysis of early and delayed ETN + MTX
At week 24, 29 patients in MTX-TT arm had not achieved DAS28-ESR remission, and switched to ETN+MTX. One received only one dose of ETN and was excluded from subgroup analysis. Response to 24 weeks duration ETN+MTX exposure if received early (ETN+MTX) versus delayed (MTX-TTb), (with resetting of ‘baseline’ DAS28 in MTX-TTb to week 24) revealed an adjusted OR (95% CI) of achieving DAS28-ESR remission of 2.84 (0.84 to 9.60) (figure 3).
Figure 3.
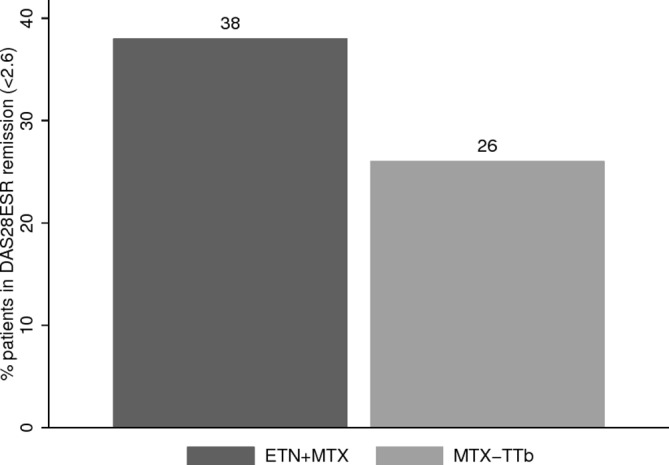
Proportion of patients achieving DAS28-ESR remission following 24 weeks ETN exposure, either received first-line (ETN+MTX) or following failure to achieve remission on MTX-TT (MTX-TTb). Percentage patient estimated via multiple imputation. DAS, Disease Activity Score 28 joint; ESR, erythrocyte sedimentation rate; ETN, etanercept; MTX, methotrexate; TT, treat-to-target.
MTX-TTb (delayed ETN) was on average, in a moderate disease activity state at week 48 (mean (SD) DAS28-ESR (3.21 (1.12)). MTX-TTa (csDMARD throughout) maintained remission state at week 48 (2.58 (0.97)) (see online supplementary figure S1).
Patient-reported outcome measures
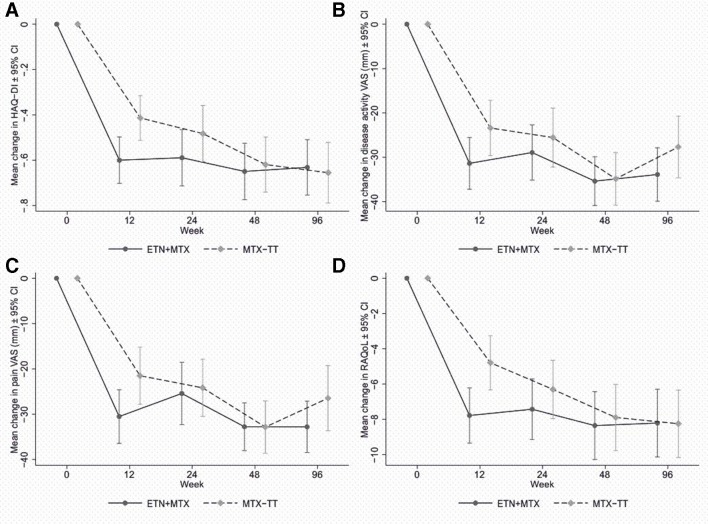
Tests of differences across the course of the trial revealed no statistically significant differences in functional improvement (figure 4A; online supplementary tables S16–S17), overall quality of life (see online supplementary table S18); patient VAS (figure 4B, C; online supplementary tables S19–S20), disease-specific quality of life (figure 4D; online supplementary table S21) or work instability (see online supplementary table S22).
Figure 4.
Patient-reported outcomes over time. ESR, erythrocyte sedimentation rate; ETN, etanercept; HAQ-DI, Health Assessment Questionnaire Disability Index; MTX, methotrexate; RAQoL, Rheumatoid Arthritis Quality of Life; TT, treat-to-target; VAS, Visual Analogue Scale.
Imaging outcomes
There were no statistically significant differences in total mTSS scores between the treatment arms at weeks 48 and 96, with minimal changes on average in both groups (see online supplementary table S23 and figure S2).
US GS and PD scores decreased at week 12 in both arms, with no notable differences (table 2; online supplementary figures S3–S4). Comparable proportions in each arm had GS >0 and PD >0 at each time point. Over 50% in each arm scored GS >0 from baseline to week 48. In contrast, the proportion with PD >0 diminished rapidly by week 12%–15% in each arm, maintained to week 48 (table 2). By week 48, the median number of erosions was 0 in both arms and the 90th percentile did not differ between ETN+MTX (0.38) and MTX-TT (0.78) (see online supplementary table S24 and figure S5).
Table 2.
Total Grey Scale (GS) and power Doppler (PD) ultrasound scores
| Total GS | ||||||
| Visit | Estimated % GS >0 | Estimated median (95% CI) | Difference (95% CI) | T value, p value | ||
| ETN+MTX (n=60) | MTX-TT (n=60) | ETN+MTX (n=60) | MTX-TT (n=60) | |||
| Baseline | 75 | 70 | 3.00 (1.96 to 4.04) | 2.00 (0.70 to 3.30) | ||
| Week 12 | 56 | 69 | 1.00 (0.48 to 1.52) | 2.00 (1.22 to 2.78) | 1.00 (0.14 to 1.86) | t=2.29, p=0.024 |
| Week 24 | 60 | 58 | 1.00 (0.22 to 1.78) | 1.18 (0.02 to 2.34) | 0.18 (−1.21 to 1.57) | t=0.26, p=0.797 |
| Week 48 | 53 | 53 | 0.96 (0.32 to 1.60) | 0.94 (0.21 to 1.67) | −0.02 (−0.98 to 0.94) | t=−0.04, p=0.967 |
| Total PD | ||||||
| Visit | Estimated % PD >0 | Estimated 90th percentile* (95% CI) | Difference (95% CI) | T value, P value | ||
| ETN+MTX (n=60) | MTX-TT (n=60) | ETN+MTX (n=60) | MTX-TT (n=60) | |||
| Baseline | 47 | 45 | 5.00 (1.93 to 8.07) | 4.00 (0.93 to 7.07) | ||
| Week 12 | 15 | 15 | 1.00 (−0.80 to 2.80) | 3.00 (−0.07 to 6.07) | 1.82 (−1.79 to 5.43) | t=1.00, p=0.320 |
| Week 24 | 13 | 18 | 1.02 (−1.19 to 3.23) | 1.68 (−0.66 to 4.02) | 0.42 (−2.77 to 3.61) | t=0.26, p=0.795 |
| Week 48 | 8 | 13 | 0.06 (−2.62 to 2.74) | 0.70 (−1.13 to 2.53) | 0.46 (−2.28 to 3.20) | t=0.33, p=0.739 |
*Median was 0 in both groups at all visits. Unplanned use of 90th percentile instead of median as point of comparison.
ETN, etanercept; MTX, methotrexate; TT, treat-to-target.
Intervention period DMARD changes
Comparable cumulative IM glucocorticoid doses were administered in each arm (see online supplementary table S25). In MTX-TT, 53/60 (88%) escalated to triple therapy by week 24 (three-quarters by week 12) in line with the 48-week randomised treatment protocol. In all patients receiving ETN at week 48, ETN was stopped (total 91; 60 and 31 in ETN+MTX and MTX-TT, respectively). Four patients (ETN+MTX) withdrawn prior to week 48 consented to continued observational follow-up; all four were escalated to double/triple csDMARD therapy by week 48 (see online supplementary table S26).
Observational period DMARD changes
On cessation of ETN (in ETN+MTX arm) at week 48 25/60 escalated to at least one additional csDMARD, with six on triple therapy by week 96. Three patients in ETN+MTX arm were commenced on a bDMARD (two adalimumab and one abatacept) as per NICE guidelines (DAS28 >5.1) and one patient in MTX-TT (ETN) (see online supplementary table S26).
On withdrawal of ETN in the ETN+MTX arm, DAS28-ESR remission rate from week 48 to week 96 dropped by only 4% (with addition of csDMARD as above; figure 2).
Safety
A number of AEs per 100 patient-years in the ETN+MTX and MTX-TT arms were 413.6 and 509.6, respectively, most frequently infections and gastrointestinal events (numerically higher in MTX-TT). A number of SAEs per 100 patient-years were 10.6 and 5.9 in ETN+MTX and MTX-TT, respectively. Withdrawals due to AE/SAE up to week 48 occurred in six subjects (three SAEs, pulmonary embolism (ETN+MTX), pneumonia and acute appendicitis (both MTX-TT); and three AEs, neutropaenia and palmoplantar pustulosis (both ETN+MTX) and general non-specific symptoms (MTX-TT)). Online supplementary table S27 details all AE and SAE.
Discussion
This study was not designed to demonstrate the standard level of superiority with first-line ETN-MTX such as was observed in COMET.10 We aimed to validate the post hoc analysis of COMET13 that suggested a much larger effect (30%) of ETN-MTX compared with MTX-TT in patients at the earliest stages of their RA, which we did not confirm in our study. A 14% difference was instead observed, which is consistent with the smaller, but still clinically relevant, effect reported for ERA.10 Escalation to ETN in those that failed to achieve remission with MTX-TT at 6 months may not secure a comparable response to first-line ETN, possibly suggesting reduced TNFi-responsiveness.
While remission is the goal in early, new-onset RA1 60% receiving MTX-TT did not achieve this by week 48 (comparable to other studies that report 30%–60%28–30; and only 50% in the ETN+MTX arm, lower than predicted.13 30 A more positive interpretation, namely, 40% and 50% achieving remission, respectively, still highlights what we would consider suboptimal rates for the contemporary era. Our study optimised design features that could contribute to reduced response including ERA defined by symptom as opposed to disease duration and all DMARD-naive not MTX-naive5 10 28 30; expedient MTX, csDMARD and bDMARD escalation and adjunctive corticosteroid use.31 MTX intolerance does not appear to explain the findings, with minimal drop-out in both arms (with n=2 AE and n=3 non-compliance in MTX-TT and n=4 and 1, respectively, ETN+MTX).
Our study eligibility aligned with clinical practice, representing a real-world population. Half the cohort had at least one comorbidity, and 20% at least two. This may have partly driven the generally poorer than expected performance32 33; the exact mechanisms for which are unclear.
The suboptimal remission rates did not appear to be driven by joint-related inflammation; as evidenced by US PD suppressed in both arms to <13% with any PD by week 48. GS persisted in over half the cohort (in particularly, the wrist), likely indicating normal background GS in joints and fibrotic change. Radiographic and US erosion scores were comparable. Pain was also effectively suppressed by both strategies. Of note, only 50% of patients had PD at baseline despite a minimum of moderate DAS28 disease activity Discrepant observations between US findings and DAS are well recognised.34–36 Our clinical and US findings further highlight the complexities of achieving remission (see online supplementary figures S6–S7) plot DAS28 components and different DAS28 definitions for each treatment arm to illustrate some of these issues in this cohort).
Remission rate did not improve appreciably with escalation to bDMARD at week 24 in the MTX-TT cohort1 (with 60% still failing to achieve remission at week 48), and TNFi escalation unable to move this subgroup even to a low disease activity state. Planned exploratory analysis suggested 24 weeks exposure of ETN following MTX-TT-IR may be associated with a lower remission rate compared with first-line ETN+MTX. In comparison, a post hoc exploratory analysis of the Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab (OPTIMA) trial6 demonstrated little advantage to starting with adalimumab and MTX. To definitively confirm our findings would require comparison of strategies in (an as of yet undefined) patient population who were all likely to fail first-line MTX-TT; (where arguably unethical to include MTX-TT as a strategy). Finally, this study did not seek to address how to manage new onset RA and first-line TNFi-IR at week 24.
The open-label nature of this study is a legitimate source of bias (although would be expected to overestimate response). However, we sought to capture real-world practice. The use of blinded assessors ensured key components of the endpoints were free from such bias. Also, the drop-out rate was almost twice that anticipated. US of only the dominant hand may partly explain the discrepancy between suppression of PD in almost all subjects but failure to achieve remission in half the patients. Finally, ETN tapering protocol after week 48 would have been desirable; however, in England, NICE does not reimburse ETN until in high disease activity, forcing immediate cessation. In contrast to PRESERVE,37 we observed minimal (4%) drop in remission rates, likely attributable to the early, treatment-naïve cohort.
In summary, the VEDERA study did not demonstrate the larger than standard effect size (of 30%), which was proposed to exist in a previous exploratory subgroup analysis with first-line TNFi-MTX in very ERA. These data also highlight a ceiling effect in achieving remission in a real life, comorbid ERA cohort. The suggestion that expedient addition of ETN to MTX-TT-IR may not be as effective in a proportion as in treatment-naïve patients requires validation and further investigation.
Patient and public involvement
Independent lay individual from our public and patient advocacy group provided input into study design.
Acknowledgments
We would like to thank the patients for participating in this study. Also, the clinical rheumatology staff at Leeds Teaching Hospitals NHS Trust for identifying patients. Gratitude to Laura Horton and Kate Smith for performing consistent ultrasound and Katherine Russell, the principal study research nurse. Finally, the trials administration team led by James Goulding and the monitoring and source data verification team (led by Rebecca Leslie and supported by Nuria Navarro-Coy and Catherine Bruckner) for ensuring complete data integrity.
Footnotes
Handling editor: Josef S Smolen
Presented at: This article/paper/report presents independent research funded/supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC).
Correction notice: This article has been corrected since it published Online First. The corresponding address has been corrected.
Contributors: The VEDERA trial was conceived by MHB and PE. EMAH was principal statistician. EMAH and MHB provided overall responsibility for the research methodology and statistical analysis plan. SH was the principal clinical fellow who supported MHB to submit the trial ethics and setup. SH, RBD and KN were the clinical research fellows over the trial duration. DvdH oversaw the scoring and interpretation of X-ray data, and RJW oversaw ultrasound component of the study. MHB drafted the manuscript, with critical input from EMAH and PE. All authors had the opportunity to further revise the manuscript and approved the final version.
Funding: Pfizer supported the VEDERA study via an investigator sponsored research grant ref. WS1092499.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Pfizer did not have any role in the study design, study delivery, statistical analyses, interpretation of data or manuscript preparation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the report for publication.
Competing interests: PE has undertaken clinical trials and provided expert advice to Pfizer, MSD, Abbvie, BMS, UCB, Roche, Novartis, Samsung, Sandoz and Lilly. PE has received consultant fees from BMS, AbbVie, Pfizer, MSD, Novartis, Roche and UCB. PE has received research grants paid to his employer from AbbVie, BMS, Pfizer, MSD and Roche. MHB has provided expert advice and received consultant fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Pfizer, Roche, Sandoz, Sanofi and UCB and has received research grants paid to her employer from Pfizer Bristol-Myers Squibb Ltd, Roche, UCB. DvdH has provided expert advice and received consultant fees from Abbvie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingleheim, Celgene, Cyxone, Daichii, Eisai, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma.
Patient consent for publication: Not required.
Ethics approval: The National Research Ethics Service (Leeds (West) Research Ethics Committee) approved the protocol (reference 10/H1307/138); and its amendments. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. Additional data are available on reasonable request.
References
- 1. Smolen JS, Landewé R, Bijlsma J, et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 2. Nam JL, Takase-Minegishi K, Ramiro S, et al. . Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review Informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017;76:1113–36. 10.1136/annrheumdis-2016-210713 [DOI] [PubMed] [Google Scholar]
- 3. Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Allaart CF, et al. . Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the best study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. 10.1002/art.21405 [DOI] [PubMed] [Google Scholar]
- 4. Nam JL, Villeneuve E, Hensor EMA, et al. . Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the idea study). Ann Rheum Dis 2014;73:75–85. 10.1136/annrheumdis-2013-203440 [DOI] [PubMed] [Google Scholar]
- 5. van Vollenhoven RF, Ernestam S, Geborek P, et al. . Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459–66. 10.1016/S0140-6736(09)60944-2 [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Emery P, Fleischmann R, et al. . Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled optima trial. Lancet 2014;383:321–32. 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 7. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. . Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343–55. 10.1016/S0140-6736(16)30363-4 [DOI] [PubMed] [Google Scholar]
- 8. Landewé RBM. Overdiagnosis and overtreatment in rheumatology: a little caution is in order. Ann Rheum Dis 2018;77:1394–6 10.1136/annrheumdis-2018-213700 [DOI] [PubMed] [Google Scholar]
- 9. Nam JL, Villeneuve E, Hensor EMA, et al. . A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the Empire trial. Ann Rheum Dis 2014;73:1027–36. 10.1136/annrheumdis-2013-204882 [DOI] [PubMed] [Google Scholar]
- 10. Emery P, Breedveld FC, Hall S, et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 11. Bejarano V, Quinn M, Conaghan PG, et al. . Effect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Rheum 2008;59:1467–74. 10.1002/art.24106 [DOI] [PubMed] [Google Scholar]
- 12. Schett G, Emery P, Tanaka Y, et al. . Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016;75:1428–37. 10.1136/annrheumdis-2016-209201 [DOI] [PubMed] [Google Scholar]
- 13. Emery P, Kvien TK, Combe B, et al. . Combination etanercept and methotrexate provides better disease control in very early (<=4 months) versus early rheumatoid arthritis (>4 months and <2 years): post hoc analyses from the COMET study. Ann Rheum Dis 2012;71:989–92. 10.1136/annrheumdis-2011-201066 [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 2015;11:276–89. 10.1038/nrrheum.2015.8 [DOI] [PubMed] [Google Scholar]
- 15. Dumitru RB, Horton S, Hodgson R, et al. . A prospective, single-centre, randomised study evaluating the clinical, imaging and immunological depth of remission achieved by very early versus delayed etanercept in patients with rheumatoid arthritis (VEDERA). BMC Musculoskelet Disord 2016;17:1–12. 10.1186/s12891-016-0915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aletaha D, Neogi T, Silman AJ, et al. . 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 17. NICE Technology appraisal guidance adalimumab, etanercept, inflfliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed, 2016. Available: nice.org.uk/guidance/ta375
- 18. Fransen J, van Riel PLCM. The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93–9. [PubMed] [Google Scholar]
- 19. Aletaha D, Smolen JS. The simplified disease activity index (SDAI) and clinical disease activity index (CDAI) to monitor patients in standard clinical care. Best Practice Res Research Rheumatol 2007;21:663–75. 10.1016/j.berh.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 20. Felson DT, Anderson JJ, Boers M, et al. . American College of rheumatology. preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 21. Felson DT, Smolen JS, Wells G, et al. . American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fries JF, Spitz P, Kraines RG, et al. . Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 23. EuroQol Group EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 24. Whalley D, McKenna SP, de Jong Z, et al. . Quality of life in rheumatoid arthritis. Br J Rheumatol 1997;36:884–8. 10.1093/rheumatology/36.8.884 [DOI] [PubMed] [Google Scholar]
- 25. Brown AK, Quinn MA, Karim Z, et al. . Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54:3761–73. 10.1002/art.22190 [DOI] [PubMed] [Google Scholar]
- 26. Pincus T, Callahan LF, Fuchs HA, et al. . Quantitative analysis of hand radiographs in rheumatoid arthritis: time course of radiographic changes, relation to joint examination measures, and comparison of different scoring methods. J Rheumatol 1995;22:1983–9. [PubMed] [Google Scholar]
- 27. Felson DT, Smolen JS, Wells G, et al. . American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. 10.1002/art.30129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emery P, Bingham CO, Burmester GR, et al. . Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis 2017;76:96–104. 10.1136/annrheumdis-2015-209057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atsumi T, Tanaka Y, Yamamoto K, et al. . Clinical benefit of 1-year certolizumab pegol (CZP) add-on therapy to methotrexate treatment in patients with early rheumatoid arthritis was observed following CZP discontinuation: 2-year results of the C-OPERA study, a phase III randomised trial. Ann Rheum Dis 2017;76:1348–56. 10.1136/annrheumdis-2016-210246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emery P, Hammoudeh M, FitzGerald O, et al. . Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 31. Detert J, Bastian H, Listing J, et al. . Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naïve patients with early rheumatoid arthritis: hit hard, an investigator-initiated study. Ann Rheum Dis 2013;72:844–50. 10.1136/annrheumdis-2012-201612 [DOI] [PubMed] [Google Scholar]
- 32. Radner H, Yoshida K, Frits M, et al. . The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease-modifying anti-rheumatic drugs. Rheumatology 2015;54:2076–84. 10.1093/rheumatology/kev239 [DOI] [PubMed] [Google Scholar]
- 33. Hitchon CA, Boire G, Haraoui B, et al. . Self-Reported comorbidity is common in early inflammatory arthritis and associated with poorer function and worse arthritis disease outcomes: results from the Canadian early arthritis cohort. Rheumatology 2016;55:1751–62. 10.1093/rheumatology/kew061 [DOI] [PubMed] [Google Scholar]
- 34. Brown AK, Conaghan PG, Karim Z, et al. . An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. 10.1002/art.23945 [DOI] [PubMed] [Google Scholar]
- 35. Horton SC, Tan AL, Freeston JE, et al. . Discordance between the predictors of clinical and imaging remission in patients with early rheumatoid arthritis in clinical practice: implications for the use of ultrasound within a treatment-to-target strategy. Rheumatology 2016;55:1177–87. 10.1093/rheumatology/kew037 [DOI] [PubMed] [Google Scholar]
- 36. Hensor EMA, McKeigue P, Ling SF, et al. . Validity of a two-component imaging-derived disease activity score for improved assessment of synovitis in early rheumatoid arthritis. Rheumatology 2019;58:1400–9. 10.1093/rheumatology/kez049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smolen JS, Nash P, Durez P, et al. . Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (preserve): a randomised controlled trial. Lancet 2013;381:918–29. 10.1016/S0140-6736(12)61811-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2019-216539supp001.pdf (652.5KB, pdf)