Abstract
Objective
To study the antiviral activities of clemastanin B (CB), epigoitrin, phenylpropanoids portion (PEP) and the mixture of phenylpropanoids, alkaloids and organic acid fractions (PEP + ALK + OA) from Banlangen (Radix Isatidis).
Methods
The experiment consisted of four parts: therapeutic action, prophylaxsis action, inhibition of virus attachment, and direct virucidal action. Cytopathic effect (CPE) and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) were used to assess antiviral activity.
Results
CB, epigoitrin, PEP and PEP + ALK + OA fractions from Banlangen (Radix Isatidis) extract significantly increased the viability of MDCK cells pre-infected with the virus compared with the virus control group in all the dilutions (P < 0.01). Pretreated with either pure compounds or chemical fractions of Banlangen (Radix Isatidis) extract in all the dilutions significantly improved the viability of MDCK cells (P < 0.01). The inhibition of virus absorption to the host cells by CB, epigoitrin and PEP was in a dose dependent manner.
Conclusion
CB, epigoitrin, PEP and PEP + ALK + OA exert their anti-influenza activity by inhibiting the virus multiplication, prophylaxsis and blocking the virus attachment. The primary mode of action of PEP and PEP + ALK + OA is the inhibition of virus replication. The inhibitory effects on virus attachment and multiplication are the main modes for epigoitrin.
Key word: Radix Isatidis, Clemastanin B, Goitrin, Antiviral agents, Orthomyxoviridae
Introduction
Caused by influenza virus, influenza or flu is A seasonal disease affecing millions of people in the world every year. In addition, influenza virus has the potency to cause severe pandemic and economic loss.1 Currently, synthetic antiviral drugs or vaccines have limited use in developing countries due to the emergence of resistant strains, the high cost and the harmful side effects.2, 3 However, anti-influenza compounds derived from herbs have many advantages such as low cost, low toxicity and extensive source4, 5 Moreover, herbal drugs usually have multi-target effects, which not only act as antiviral agents but also stimulate immunity system.6 Therefore, medicinal plant extracts and phytochemicals have been attracting more and more attention as the potential sources for the development of new antiviral drugs in the recent years.
Banlangen (Radix Isatidis) is the dry root of plant Isatis indigotica Fort. Banlangen (Radix Isatidis) was firstly documented as the herbal medicine in The Divine Husbandman's Herbal Foundation Canon, a famous ancient medical book in the Han Dynasty of China (200 AD).7 It has been used in the treatment of cold, sore throat and headache for hundreds of years in China.8, 9 It was used for the prevention of severe acute respiratory syndrome (SARS) in 2003 and swine flu pandemic in 2009 in China and Japan.10, 11 Recently, the antiviral effect of the methanol, water and ethylacetate extract of Banlangen (Radix Isatidis) was confirmed in vitro test.11, 12 However, the modes of antiviral actions of these extract are still not clear. Additionally, few publications have been reported about the differences of antiviral action between clemastanin B and epigoitrin and the extract where the compounds were isolated from.
Phenylpropanoids (PEP), alkaloids and organic acids are three major chemical fractions in Banlangen (Radix Isatidis). Clemastanin B (CB) is the most abundant compound which belongs to phenylpropanoid and epigoitrin is the main alkaloid compound isolated from the Banlangen (Radix Isatidis) (Figure 1 ).13, 14 The latest studies showed that clemastanin B inhibited different subtypes of human and avian influenza viruses at different magnitudes of activity.15 Epigoitrin has been used as a marker of antiviral efficacy in Banlangen (Radix Isatidis) in the 2010 edition of the Chinese Pharmacopoeia.16 Our previous screening showed that CB and epigoitrin have strong inhibitory effects on influenza A1 virus FM1.17 The present study aimed to investigate the anti-influenza mechanisms of CB and epigoitrin and compare with the phenylpropanoids portion and the mixture of phenylpropanoids, alkaloids and organic acid fractions (PEP + ALK + OA).
Figure 1.
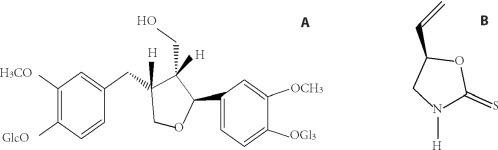
Chemical structure of clemastanin B and epigoitrin A: clemastanin B; B: epigoitrin.
Materials and Methods
Viral strains, cell lines and reagents
Mouse lung-adapted variant of influenza virus (FM1) strain was obtained from the department of microbiology and immunology at Nanjing University of Chinese Medicine. The virus was propagated twice in allantoic cavity of 9 to 10-day-old embryonated hen's eggs at 35 °C for 48 h to enhance the virulence. The allantoic fluid was harvested for the measurement of its hemagglutinating activity. Once the hemagglutination titer reached 1 : 640, the virus was aliquoted and stored at −80 °C until use. The Madin-Darby canine kidney (MDCK) cells and human cervical cancer (HeLa) cells were obtained from the Institute of Cell Biology, Chinese Academy of Sciences. The positive control ribavirin (Batch No. 101018) was purchased from Baili Pharmaceutical Co., Ltd. (Sichuan, China).
Preparation of plant extracts
The Banlangen (Radix Isatidis) was collected from a medical market in Tongling city of Anhui province (China). Herb identification was confirmed through morphological and microscopic analysis according to the Chinese Pharmacopeia.18 clemastanin B and epigoitrin were separated and purified in our laboratory. The structures (Figure 1) were elucidated by their ultra violet (UV), infrared radiation (IR), mass spectrometry (MS), 1H-nuclear magnetic resonance (1H NMR), 13C-nuclear magnetic resonance (13C NMR) and 2 dimensional nuclear magnetic resonance (2D NMR) data.11, 12, 13 The purity of the two compounds was above 98% which was determined by HPLC-DAD-ELSD. The phenylpropanoids, alkaloids and organic acid fractions were prepared using our previous methods.19 According to yield of each fraction, the mixture of phenylpropanoids, alkaloids and organic acid fractions (PEP+ ALK+OA) was prepared by mixing each of the above fractions at a ratio of 1:2:2 (w/w). The lyophilized materials were directly resuspended in the cell culture medium and filter sterilized through the 0.22 µm membrane. Clemastanin B and epigoitrin were dissolved in fresh medium diluted to different concentrations.
Modes of anti-influenza action
The anti-influenza action of CB, epigoitrin and chemical fractions from Banlangen (Radix Isatidis) extract was investigated in four different modes: therapeutic action, prophylaxis, direct virus inactivation and inhibition of virus attachment.20, 21
Antiviral activity in preincubation
The cells were pre-infected with the virus before the pure compounds or chemical fractions of plant extract were added. The therapeutic action of the drugs was evaluated by both CPE reduction assay and cell MTT assay.
The CPE reduction assay was conducted as previously described with modifications.22 Briefly, quadruplicate MDCK monolayer cells in 96-well plates were infected with 0.1 mL suspension containing 100 TCID50 of virus for 2 h. Unabsorbed virus was then washed off using phosphate buffer solution (PBS). Quadruplicate cell monolayers were subsequently overlaid with 0.1mL medium containing different non-toxic two-fold serial dilutions of pure compounds or chemical fractions of Banlangen (Radix Isatidis) extract. Cells with virus infection without drug treatment and the cells without virus and drugs were used as controls. The plates were incubated at 37 °C under 5% CO2 for 72 h. The virus-induced CPE was observed under a light microscope in comparison with the parallel virus control and cell control.
The MTT reduction assay was performed according to the standard protocol.23 In short, the experimental setup was consistent with the procedures in CPE assay. After 3 days of incubation, 20 µL of MTT was added to each well and incubated at 37 °C for 4h. Subsequently, dimethyl sulfoxide (DMSO) was added and the absorbance was measured at 570 nm. The cells protection rate (%) was calculated by the following formula:22
where ODexp, ODvirus, and ODcell indicate the absorbencies of the test sample, the virus control and the cell control, respectively.
Pre-treatment with drugs (prophylaxis)
To evaluate the effects of pure compounds and chemical fractions from woad extract on prophylaxis of cell infection, the MDCK monolayer cells in 96-well plates were overlaid with different non-toxic two-fold serial dilutions of the two bioactive compounds and chemical fractions of Banlangen (Radix Isatidis) extract. Four replicates were set up for each treatment and control. After 4 h, the test substances were removed from the wells and the monolayer cells were then infected with 100 TCID50 of influenza virus (FM1) at 37 °C for 2 h to allow virus absorption. Subsequently, the unabsorbed virus was washed off using PBS and equal amount of maintenance medium was added into each well. The plates were incubated at 37 °C under 5% CO2 for 72 h. The virus-induced CPE was observed under light microscope and graded following the same criteria described in the section “Antiviral activity in preincubation”.
Direct virucidal effect
The direct virucidal activity of the pure compounds and chemical fractions from woad extract was tested according to the methods described by Carlucci et al. 24 One hundred microliter of 100 TCID50 of virus was treated with equal volumes of two-fold diluted pure compounds or extract fractions for 2 h at 37 °C. The samples were then ten-fold serially diluted. When the confluent monolayer of MDCK cells was formed, the surviving virus in the mixtures was determined in CPE assay and titers (TCID50 values) were calculated according to the Reed-Muench method.
Inhibition of virus attachment assay
The MDCK cells were cultured in 96-well plates. One hundred microliter of different non-toxic two-fold serial dilutions of pure compounds, chemical fractions of Banlangen (Radix Isatidis) extract and equal volume of 100 TCID50 of virus were simultaneously added to MDCK cells.25 After 2 h incubation at 4 °C, the virus/extract mixture was removed from the wells, and then disrupted by freezing and thawing twice. The virus-induced CPE was observed under light microscope and graded following the same criteria described in above.
Statistical analysis
All the experiments were repeated for three times, each with quintuplicate determinations. The data were expressed as mean ± standard deviation ( ± s). Analysis of variance and Duncan's multiple range tests were performed to test significant differences between different groups. A value of P < 0.05 was considered as significant difference, and P < 0.01 was considered very significant.
± s). Analysis of variance and Duncan's multiple range tests were performed to test significant differences between different groups. A value of P < 0.05 was considered as significant difference, and P < 0.01 was considered very significant.
Results
Therapeutic action of the pure compounds and chemical fractions from Banlangen (Radix Isatidis) extract
Both the solvent blanks, CB, epigoitrin and chemical fractions of Banlangen (Radix Isatidis) extract had no obvious cytotoxicity (Data were not shown). The therapeutic action was evaluated by both CPE assay and MTT assay. Clear cytopathic effects were observed in MDCK cells infected with FM1 after 72 h such as increased gaps between cells, rupture of the cell nucleus and the partial or complete collapse of cells (Figure 2C virus control). In virus control group, 26%-50% CPE was observed (Table 1 ). However, MDCK cells grow well in the drug treatment groups (Figure 3B ribavirin and PEP + ALK + OA portion groups) and CPE formation was completely inhibited in all the dilutions (Table 1).
Figure 2.
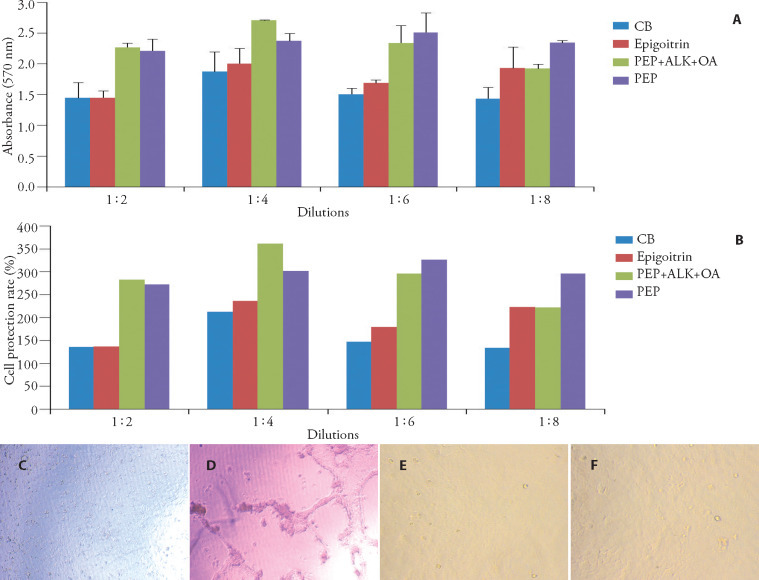
Effect of different dilutions of pure compounds and bioactive fractions from Banlangen (Radix Isatidis) on the viability of MDCK cells pre-infected with 100 TCID50 of influenza virus (FM1)
A: MTT assay; B: cell protection rate; C-F: × 100. C: cell control; D: virus control; E: Ribavirin (1: 4); F: PEP + ALK + OA portion (1: 4). CB: clemastanin B; PEP + ALK + OA: the mixtures of phenylpropanoids, alkaloids and organic acid fractions. Cell control: normal MDCK cells without virus infection and drugs treatment; virus control: cells infected with virus without drug treatments. Error bars represent standard deviation. The asterisks indicate a significant difference between the test samples and the virus control according to Duncan's multiple range tests, aP < 0.01.
Table 1.
The formation of CPE in MDCK cells pre-infected with influenza virus (FM1)
| Group | Initial concentration |
Dilutions |
|||
|---|---|---|---|---|---|
| (μg/mL) | 1: 2 | 1:4 | 1:8 | 1: 16 | |
| CB | 50 | – | – | – | – |
| Epigoitrin | 50 | – | – | – | – |
| PEP portion | 1 | – | – | – | – |
| PEP+ALK+OA | 1 | – | – | – | – |
| Ribavirin | 100 | – | – | – | – |
| Cell control | 0 | – | – | – | – |
| Virus control | 0 | ++ | ++ | ++ | ++ |
Notes: the CPE was graded as follows: “-”= 0% CPE; “+”= 0%-25% CPE; “++”= 26%-50% CPE; “+++”= 51%-75% CPE; “++++” = 76%-100%. Cell control: normal cells without virus infection and drug treatments. Virus control: cells infected with influenza virus.
Figure 3.
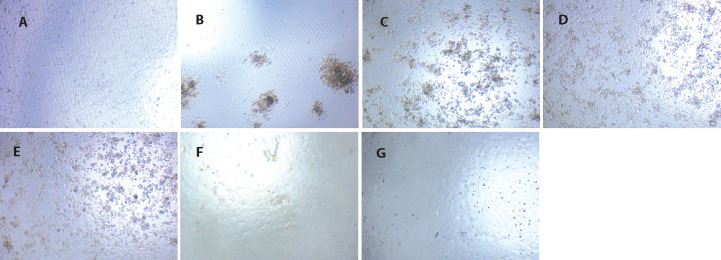
Direct virucidal effect of pure compounds and bioactive fractions from Banlangen (Radix Isatidis) extract on influenza virus (FM1, × 100)
A: cell control; B: virus control; C: CB (1: 2); D: Epigitrin (1: 16); E: PEP (1: 2); F: PEP + ALK + OA (1: 2); G: Ribavirin (1: 4). Cell control: normal MDCK cells without virus infection and drugs treatment; Virus control: cells infected with virus without drug treatments. CB: clemastanin B; PEP: phenylpropanoids portion; PEP + ALK + OA: the mixtures of phenylpropanoids, alkaloids and organic acid fractions.
MTT reduction assay showed that the addition of CB, epigoitrin, PEP and PEP + ALK + OA fractions from Banlangen (Radix Isatidis) extract significantly increased the viability of MDCK cells pre-infected with the virus compared with the virus control group in all the dilutions (P < 0.01) (Figure 2a). Interestingly, the protection rate in four treatment groups was significantly higher than that in the positive control ribavirin group under the same dilution (P < 0.05). This indicated that compounds and extract fractions from Banlangen (Radix Isatidis) have better therapeutic action against influenza A virus FM1 than the current commercial synthetic antiviral drug ribavirin. Additionally, the protective effect of CB, epigoitrin, PEP and PEP + ALK+OA fractions was not dose-dependent. The highest protection rate was observed in 1:4 dilution of CB, epigoitrin or the mixture of PEP + ALK + OA fractions, while, PEP diluted 1: 8 resulted in the highest cell viability (Figure 2b). In comparison of different treatment groups, the mixture of PEP + ALK + OA fractions (1: 4) has the highest cell protection rate.
Prophylactic action of the pure compounds and chemical fractions from Banlangen (Radix Isatidis) extract
CPE assay showed that there was no obvious CPE formation in MDCK cells pretreated with CB, epigoitrin, PEP and PEP + ALK + OA fractions in all the dilutions (Table 2 ).
Table 2.
Formation of CPE in MDCK cells pre-treated with pure compounds and chemical portions of Banlangen (Radix Isatidis) extract
| Initial concentration (μg/mL) | Dilutions |
||||
|---|---|---|---|---|---|
| 1: 2 | 1:4 | 1:8 | 1:16 | ||
| CB | 50 | – | – | – | − |
| Epigoitrin | 50 | – | – | – | – |
| PEP portion | 1 | − | − | − | − |
| PEP+ALK+OA | 1 | − | − | − | − |
| Ribavirin | 100 | − | − | − | − |
| Cell control | 0 | − | − | − | − |
| Virus Control | 0 | +++ | +++ | +++ | +++ |
Notes: the CPE was graded as follows: “-” = 0% CPE; “+” = 0%-25% CPE; “++” = 26%-50% CPE; “+++” = 51%-75% CPE; “++++” = 76%-100%. Cell control: Normal cells without virus infection and drug treatments. Virus control: Cells infected with influenza virus (FM1).
As shown in Table 3 , pretreated with either pure compounds or chemical fractions of Banlangen (Radix Isatidis) extract in all the dilutions significantly improved the viability of MDCK cells (P < 0.01). Moreover, compared with ribavirin, natural compounds or extracts from Banlangen (Radix Isatidis) have higher prophylactic activity against influenza virus (FM1) (P < 0.01). The cell viability was dose-dependently increased by PEP and PEP + ALK + OA fractions (Table 3). In contrast, the protection rate and cell viability were not significantly changed by the dilution of CB and epigoitrin (from 1:2 to 1: 16). Among the four different samplesused in this study, PEP fraction showed the most significant protective effect on the cell protection rate of 263.467%.
Table 3.
Effect of prophylactic treatment on the viability and protection rate of MDCK cells ( ± s)
± s)
| Group |
OD570 |
Protection rate (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| 1:2 | 1: 4 | 1: 8 | 1: 16 | 1: 2 | 1: 4 | 1: 8 | 1: 16 | |
| CB (50 μg/mL) | 2.126±0.034a | 2.228±0.167a | 2.284±0.017a | 2.218±0.011a | 188.348 | 199.080 | 205.023 | 198.08 |
| Epigoitrin (50 μg/mL) | 2.197±0.152a | 2.239±0.050a | 2.202±0.102a | 2.041±0.036a | 195.871 | 200.290 | 196.344 | 214.435 |
| PEP portion (1 mg/mL) | 2.840±0.150a | 2.464±0.024a | 2.407±0.048a | 2.149±0.069a | 263.467 | 223.910 | 217.912 | 190.82 |
| PEP+ALK+OA (1 mg/mL) | 2.698±0.082a | 2.090±0.042a | 2.045±0.089a | 2.171±0.028a | 248.580 | 184.610 | 179.879 | 193.135 |
| Ribavirin (100 μg/mL) | 1.866±0.251a | 1.924±0.306a | 1.614±0.086a | 1.869±0.016a | 161.047 | 167.100 | 134.245 | 161.310 |
| Virus control | 0.335±0.073 | − | − | − | − | |||
| Cell control | 1.286±0.277 | − | − | − | − | |||
Notes: PEP: phenylpropanoids portion; PEP + ALK + OA: the mixtures of phenylpropanoids, alkaloids and organic acid portions. Cell control: Normal cells without virus infection and drug treatments. Virus control: Cells infected with influenza virus. Data of OD570 was mean ± SD. The asterisks indicate a significant difference between the test samples and the virus control according to Duncan's multiple range tests,
P < 0.01. CPE: cytopathic effect.
Direct virucidal action of the pure compounds and chemical fractions from Banlangen (Radix Isatidis) extract
The c = outcomesrevealed that CB, epigoitrin, PEP and PEP + ALK + OA could not directly inactivate influenza virus A FM1 even at the concentration of 1: 2 dilution.
Inhibitory activity of the pure compounds and chemical fractions from Banlangen (Radix Isatidis) extract on influenza virus A FM1 attachment
The results of CPE assay were listed in Table 4 . There was no CPE formation in all the drug treatment groups even at the lowest concentration (1: 16 dilution). The results suggested that CB, epigoitrin, PEP and PEP + ALK + OA fractions have strong inhibitory effect on binding of influenza A virus to MDCK cells. The viability of MDCK cells at 72 h after infection of virus and simultaneous treatment with natural compounds was determined by MTT reduction assay. As shown in Table 5 , the viability of MDCK cells was significantly increased as the result of drug treatments compared with virus control (P < 0.01). The inhibition of virus absorption to the host cells by CB, epigoitrin and PEP was in a dose dependent manner. The maximum inhibitory effect of CB, epigoitrin and PEP was observed with a 1:2 dilution, which resulted in 3.53, 3.99 and 4.43 times increases in cell viability, respectively. In contrast, the highest cell protection rate in PEP + ALK + OA and positive control ribavirin groups was found in a 1:4 dilution. Additionally, it was showed that CB, epigoitrin, PEP or PEP + ALK + OA are more effective than ribavirin on the inhibition of influenza A FM1 virus attachment (Table 5). When comparing four natural compounds and chemical fractions, it was observed that PEP portion diluted 1: 2 possessed the maximum inhibitory effect on virus absorption, which led to the highest protection of 273.218%.
Table 4.
The formation of CPE in MDCK cells induced by virus attachment ( ± s)
± s)
| Group | Initial concentration (μg/mL) | Dilutions |
|||
|---|---|---|---|---|---|
| 1: 2 | 1: 4 | 1: 8 | 1: 16 | ||
| CB | 50 | – | – | – | – |
| Epigoitrin | 50 | – | – | – | – |
| PEP portion | 1 | – | – | – | – |
| PEP+ALK+OA | 1 | – | – | – | – |
| Ribavirin | 100 | – | – | – | – |
| Cell control | 0 | – | – | – | – |
| Virus control | 0 | +++ | +++ | +++ | +++ |
Notes: the CPE was graded as follows: = 0% CPE; “+” = 0%-25% CPE; “++” = 26%-50% CPE; “+++” = 51%-75% CPE; “++++” =
76%-100%. Cell control: Normal cells without virus infection and drug treatments. Virus control: Cells infected with influenza virus (FM1).
Table 5.
Inhibitory effect of pure compounds and chemical portions from Banlangen (Radix Isatidis) extract on influenza virus (FM1) attachment ( ± s)
± s)
| Group | OD570 |
Protection rate (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| 1:4 | 1:8 | 1:16 | 1:2 | 1:4 | 1:8 | 1:16 | ||
| CB (50 μg/mL) | 1.940±0.262a | 1.665±0.058a | 1.599±0.161a | 1.368±0.210a | 201.267 | 161.455 | 151.973 | 118.458 |
| Epigoitrin (50 μg/mL) | 2.192±0.108a | 1.980±0.246a | 1.833±0.049a | 1.639±0.347a | 237.749 | 207.058 | 185.849 | 157.763 |
| PEP portion (1 mg/mL) | 2.437±0.190a | 2.340±0.123a | 2.020±0.319a | 1.695±0.041a | 273.218 | 259.175 | 212.921 | 165.798 |
| PEP+ALK+OA (1 mg/mL) | 2.043±0.072a | 2.055±0.009a | 1.743±0.282a | 1.599±0.069a | 216.178 | 217.988 | 172.819 | 151.900 |
| Ribavirin (100 μg/mL) | 1.614±0.093a | 1.736±0.307a | 1.441±0.001a | 1.279±0.018a | 154.144 | 171.734 | 129.099 | 105.646 |
| Virus control | 0.549±0.690 | − | − | − | − | |||
| Cell control | 1.240±0.675 | − | − | − | − | |||
Notes: Cell control: normal cells without virus infection and drug treatments. Virus control: cells infected with influenza virus. PEP: phenylpropanoids portion; PEP + ALK + OA: the mixtures of phenylpropanoids, alkaloids and organic acid portions. The asterisks indicate a significant difference between the test samples and the virus control according to Duncan's multiple range tests,
P < 0.01.
Discussion
The classically defined antiviral mechanisms for medicinal plants include inhibiting virus replication, blocking virus attachment, direct inactivating the virus and preventing from virus infection.28 In this study, it was clearly demonstrated that CB, epigoitrin, PEP or PEP+ ALK+OA showed the anti-influenza activities by therapeutic action (inhibition of virus multiplication), prophylaxsis and inhibition of virus attachment. However, the differences were observed on the major modes of antiviral action between different compounds and chemical fractions (Figure 4 ). For instance, the highest cell protection rate in PEP or PEP + ALK + OA was from its therapeutic action (Figure 4, PEP, A1; PEP + ALK + OA, A1). The main anti-influenza modes of epigoitrin are the inhibition of virus multiplication and virus attachment (Figure 4, epigoitrin A1, A3). In contrast, three modes of the antiviral action of CB contribute equally on the cell protection rate.
Figure 4.
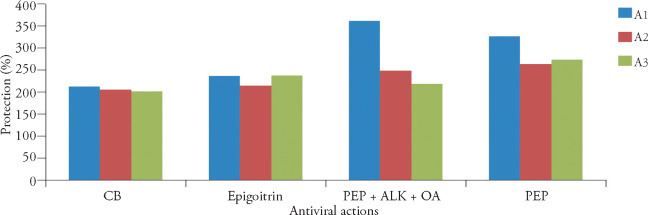
Differences on the major modes of antiviral action in the pure compounds and bioactive fractions from Banlangen (Radix Isatidis) extract
A1: therapeutic action; A2: prophylaxsis; A3: inhibition of virus attachment. CB: Clemastanin B; PEP + ALK + OA: the mixtures of phenylpropanoids, alkaloids and organic acid portions.
CB is the major phenylpropanoid compound in Banlangen (Radix Isatidis) and epigoitrin is an abundant alkaloid which is also an marker for the quality control of Banlangen (Radix Isatidis).18 Previously reported studies pointed that overall virus inhibitory effects of green tea were stronger in the plant total extract than any single pure compound from the extract due to the possible synergistic interactions between the ingredients.22, 29 However, in our study, the results showed that the antiviral activity changed which might result from the differences of antiviral mechanisms between the single compound and the mixture of the extract.
In the present study, the results suggested that CB, epigoitrin, PEP or PEP+ALK+OA demonstrated their anti-influenza activities by therapeutic action, prophylaxsis of cells and inhibition of virus attachment. All the compounds or chemical fractions did not have any direct virucidal activity. The main antiviral mode for PEP and PEP + ALK + OA is the therapeutic action while epigoitrin mainly inhibits the virus multiplication and attachment. This study may provide a theoretical basis to clarify the antiviral mechanism of Banlangen (Radix Isatidis).
Footnotes
Supported by the National Natural Science Foundation of China Grant (No. 81073023) the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. ysxk-2010) and 2013 Program sponsored for scientific innovation research of college graduate in Jiangsu province (No. CXZZ13_0631)
References
- 1.Dawood FS, Jain S, Finelli L. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Bacon TH, Levin MJ, Leary JJ. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16(1):114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig S. Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J Antimicrob Chemother. 2009;64(1):1–4. doi: 10.1093/jac/dkp161. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Jia W, Zhao A, Wang X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Zhu Y. Anti-respiratory passage virus of Chinese herbal medicine. Health. 2010;2(12):1397–1400. [Google Scholar]
- 6.Wagner H. Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82(1):34–37. doi: 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Pan YL, Xue P, Li X, Chen JW, Li J. Determination of nucleosides and nucleobases in Isatidis Radix by HILIC-UPLC-MS/MS. Anal Methods. 2013;22(5):6395–6400. [Google Scholar]
- 8.Chung YC, Tang FY, Liao JW. Isatis indigotica induces hepatocellular cancer cell death via caspase-independent apoptosis-inducing factor translocation apoptotic pathway in vitro and in vivo. Integr Cancer Ther. 2011;10(2):201–214. doi: 10.1177/1534735410387420. [DOI] [PubMed] [Google Scholar]
- 9.Ho YL, Chang YS. Studies on the antinociceptive, anti-inflammatory and anti pyretic effects of Isatis indigotica root. Phytomedicine. 2002;9(5):419–424. doi: 10.1078/09447110260571661. [DOI] [PubMed] [Google Scholar]
- 10.Lin CW, Tsai FJ, Tsai CH. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Wang Y, Zhong S. In vitro inhibition of influenza virus infection by a crude extract from isatis indigotica root resulting in the prevention of viral attachment. Mol Med Rep. 2012;5(3):793–799. doi: 10.3892/mmr.2011.709. [DOI] [PubMed] [Google Scholar]
- 12.Hsuan SL, Chang SC, Wang SY. The cytotoxicity to leukemia cells and antiviral effects of isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol. 2009;123(1):61–67. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Yan J, Li HL. Studies on chemical constituents of compound indigowoad root granule by mass spectrometry. Gao Deng Xue Xiao Hua Xue Xue Bao. 2010;31(6):1137–1142. [Google Scholar]
- 14.Peng J, Fan G, Wu Y. Isolation and purification of clemastanin B and indigoticoside A from Banlangen (Radix Isatidis) by high-speed counter-current chromatography. J Chromatogr A. 2005;1091(1-2):89–93. doi: 10.1016/j.chroma.2005.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang ZF, Wang YT, Zheng ZG. Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int J Mol Med. 2013;31(4):867–873. doi: 10.3892/ijmm.2013.1274. [DOI] [PubMed] [Google Scholar]
- 16.Xu LH, Huang F, Chen T, Wu J. Anti-virus constituents in Banlangen (Radix Isatidis) Zhong Guo Tian Ran Yao Wu. 2005;3(6):359–360. [Google Scholar]
- 17.Ye WY, Li X, Cheng JW. Screening of eleven chemical constituents from Banlangen (Radix Isatidis) for antiviral activity. Afr J Pharm Pharmaco. 2011;5(16):1932–1936. [Google Scholar]
- 18.The State Pharmacopoeia Committee of People's Republic of China. Pharmacopoeia of People's Republic of China. Chemical Industry; Beijing: 2010. p. 191. [Google Scholar]
- 19.Chen H, Li J, L X, Chen JW, Xue P. Antioxidation of Different Extracts from Isatidis indigotica in vitro. Zhong Guo Shi Yan Fang Ji Xue Za Zhi. 2012;9(18):184–186. [Google Scholar]
- 20.Li Y, But PP, Ooi VE. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antiviral Res. 2005;68(1):1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Eo SK, Kim YS, Lee CK. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J Ethnopharmacol. 2000;72(3):475–481. doi: 10.1016/s0378-8741(00)00266-x. [DOI] [PubMed] [Google Scholar]
- 22.Serkedjieva J, Ivancheva S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J Ethnopharmacol. 1999;64(1):59–68. doi: 10.1016/s0378-8741(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, But PP, Ooi VE. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antiviral Res. 2005;68(1):1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Carlucci MJ, Ciancia M, Matulewicz MC. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Res. 1999;43(2):93–102. doi: 10.1016/s0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HJ, Kim HH, Yoon SY. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J. 2010;7:307. doi: 10.1186/1743-422X-7-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhtar M, Arshad M, Ahmad M, Roger J. Pomerantz, Brian Wigdahl, Zahida Parveen. Antiviral potentials of medicinal plants. Virus Res. 2008;131(2):111–120. doi: 10.1016/j.virusres.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68(2):66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
Uncited References
- 26.Larkin M. Flu vaccine: will scarcity improve compliance in USA. Lancet Infect Dis. 2004;4(12):715. doi: 10.1016/s1473-3099(04)01214-9. [DOI] [PubMed] [Google Scholar]
- 27.Chu VC, Whittaker GR. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc Natl Acad Sci USA. 2004;101(52):18153–18158. doi: 10.1073/pnas.0405172102. [DOI] [PMC free article] [PubMed] [Google Scholar]


