INTRODUCTION
Cutaneous T-cell lymphomas (CTCLs) encompass a heterogeneous collection of non-Hodgkin lymphomas that arise from skin-tropic memory T lymphocytes. Among them, mycosis fungoides (MF) and Sézary syndrome (SS) are the most common malignancies. In its earliest stages, patients classically present with discrete skin lesions that resemble eczema or with widespread erythema. Patients with advanced disease may have fungating tumors or leukemic disease with eventual involvement of lymph nodes and viscera. Most patients with MF present with early stage disease and have an indolent disease course with a low risk of disease progression; however, cure is rarely achieved. The goal of treatment is to minimize symptomatic morbidity and limit disease progression. Increasingly, hematopoietic stem cell transplantation is being considered for patients with advanced stages, is the only therapy with curative intent.
EPIDEMIOLOGY
The overall incidence of CTCL is 10.2 per million persons.1 More than half of these cases are MF, with an incidence of 5.6 per million persons.1 The age-adjusted incidence rate for SS is 0.1 per million persons.2 Men are more commonly affected than women (incidence rate ratio [IRR] 5 1.6).2 The incidence increases with age, with the highest CTCL incidence at greater than 70 years of age. Blacks have a higher incidence rate than whites (IRR 5 1.57).2 Black patients are diagnosed at an earlier age (median age of diagnosis of 53 years, compared with 63 years for whites) and have worse survival than white patients regardless of age and stage of presentation.2
DIAGNOSIS
The diagnosis of MF/SS can be challenging and requires input from the clinical presentation, pathologic evaluation, and molecular studies. MF/SS can resemble benign inflammatory dermatoses, and characteristic histologic features of MF may be absent in early disease even after multiple biopsies. Moreover, traditional polymerase chain reaction (PCR) of the T-cell receptor (TCR) used to identify the presence of a T-cell clone in clinical samples has a significant false-negative rate in early stage disease.3 An algorithm to assist in the diagnosis of early MF has been proposed, although not formally validated.4 This algorithm emphasizes the importance of integrating the clinical presentation (persistent, progressive patches or plaques in non–sun-exposed location and morphology), histopathology (superficial lymphoid infiltrate, epidermotropism without spongiosis, lymphoid atypia), immunopathology (decreased expression of CD5, CD7 or epidermal-dermal discordance of CD2, CD3, CD5, CD7), and molecular evaluation of T-cell clonality.4 Pathologic criteria to assist in the diagnosis have been put forth but are not often used in clinical practice.5 To date there are no diagnostic molecular markers used clinically that can reliably identify malignant T-cell from benign T-cell. However, expression of TOX, a thymocyte selection-associated HMG box protein, may be a useful adjunct.6–8
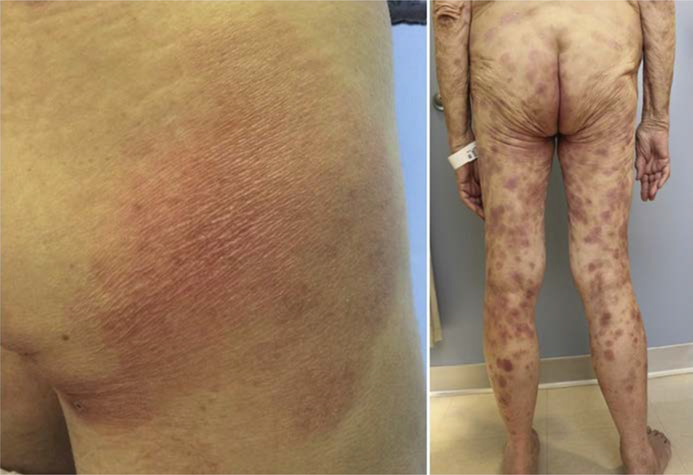
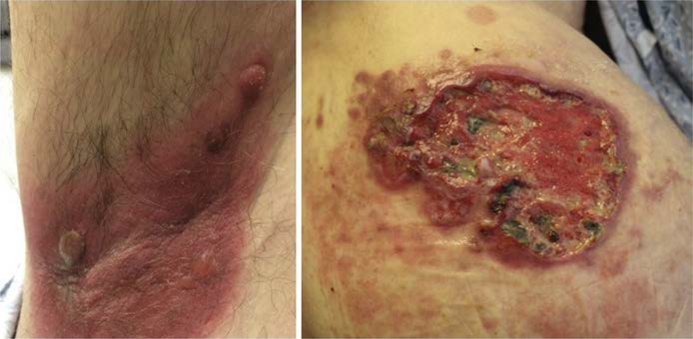
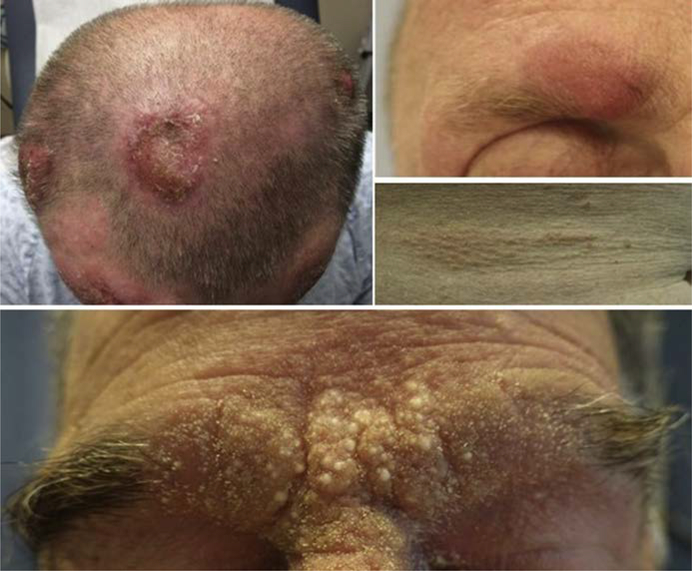
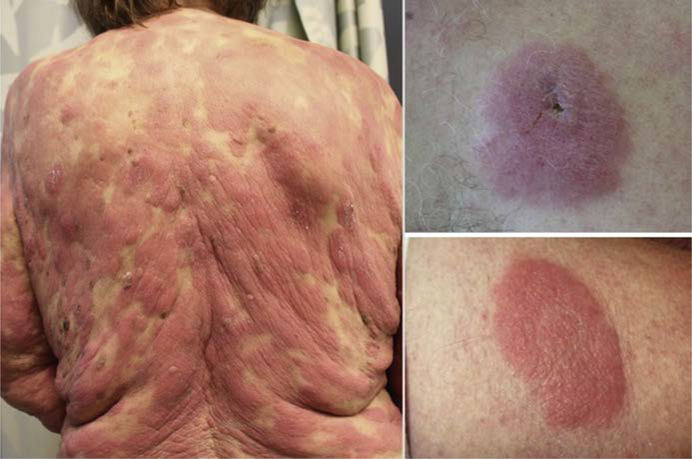
There are 3 clinical morphologies in MF: patch, plaque, and tumor (Fig. 1–3).9,10 Each is distinguished from the former by increasing thickness. There are 3 recognized subtypes of MF by the World Health Organization(WHO)/European Organization for Research and Treatment of Cancer (EORTC) with different clinical and histologic features (Table 1, Fig. 4).11 Clinicians must also be aware of the several distinct clinicopathologic variants of MF (Box 1, Fig. 5).12
Fig. 1.
Patch-stage MF. Patches are flat to atrophic lesions, usually erythematous with variable amounts of scale, and may resemble eczema. Atrophic lesions have a cigarette-paper, wrinkled appearance. (Courtesy of J. O’Malley, MD, PhD, Boston, MA.)
Fig. 3.
Tumor-stage MF. Tumors exhibit a significant vertical growth phase and must measure at least 1 cm in diameter. They are often ulcerated.
Table 1.
Mycosis fungoides variants recognized by the World Health Organization/European Organization for Research and Treatment of Cancer
| MF Subtype | Clinical Presentation | Immunophenotype | Histology |
|---|---|---|---|
| Folliculotropic MF | Predilection for head/neck Alopecic skin lesions Follicular papules; Acneiform/comedonal-like papules or nodules (see Fig. 4) |
CD41CD31 T-cell Admixed CD30 1 blasts |
Perivascular and periadenexal lymphocytic infiltrates with infiltration of follicular epithelium (folliculotropism); variable infiltration of eccrine sweat glands by atypical lymphocytes with sparing of epidermis; follicular mucinosis |
| Pagetoid reticulosis | Predilection for extremities Localized psoriasiform patch or plaque Indolent clinical behavior |
CD41CD31 T-cell or CD81CD31 T-cell CD30 often positive |
Hyperplastic epithelium with marked pagetoid-like atypical lymphocytes; dermis with mixed reactive lymphocytes and histiocytes |
| Granulomatous slack skin | Predilection from axillae and groin Lax pendulous skin Indolent clinical behavior |
CD41CD31 T-cell | Dense granulomatous dermal infiltrates of atypical lymphocytes, macrophages, many multinucleated giants cells; destruction of elastic tissue |
Data from Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105(10):3768–85.
Fig. 4.
Folliculotropic MF. Lesions preferentially affect the head and neck area. When located within hair-bearing areas, it may cause alopecia. Patches or plaques may be composed of cyst-like or follicular-based papules.
Box 1.
Clinicopathologic variants of mycosis fungoides
| MF clinical variants |
| Bullous |
| Hypopigmented (see Fig. 5) |
| Ichthyosiform |
| MF palmaris et plantaris (keratoderma-like) |
| Pigmented purpuric dermatosis-like |
| Papular |
| Poikilodermatous |
| Psoriasiform |
| Pustular |
| Solitary/unilesional |
| Syringotropic |
| Verrucoid |
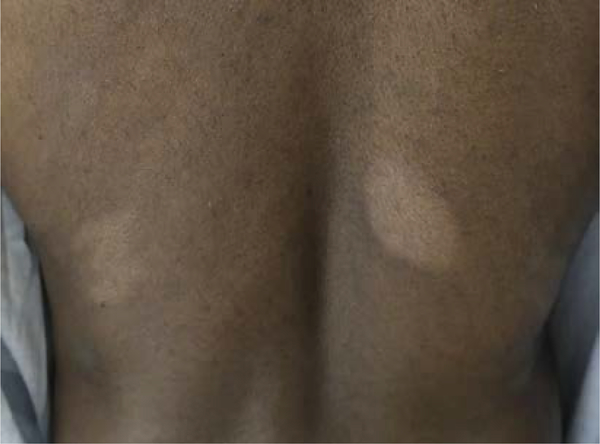
Fig. 5.
Hypopigmented MF. Predominately affects African Americans. It has an indolent disease course. Immunophenotype is classically of atypical CD81 T-cell.
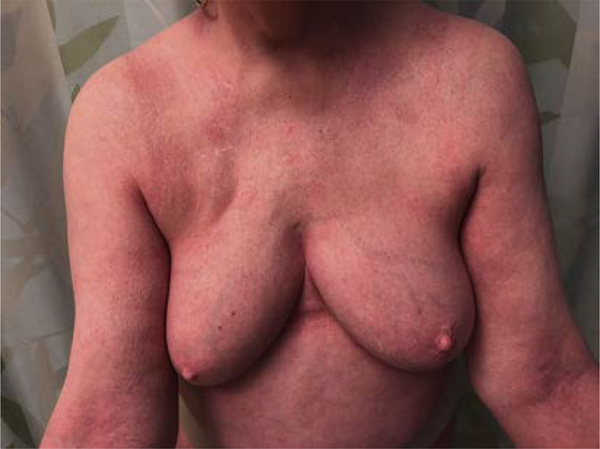
Patients with SS typically present with erythroderma, defined as diffuse erythema affecting at least 80% of the body surface area (Fig. 6).11 These patients must be distinguished from other benign causes of erythroderma (Box 2).
Fig. 6.
Erythroderma. Erythroderma is defined as diffuse erythema affecting 80% or greater body surface areas. It often appears eczematous with a variable amount of scale. Erythroderma is often a sign of leukemic disease.
Box 2.
Causes of erythroderma
| Differential diagnosis of erythroderma |
| Idiopathic |
| Atopic dermatitis |
| Psoriasis |
| Pityriasis rubra pilaris |
| SS |
| Systemic allergic contact dermatitis |
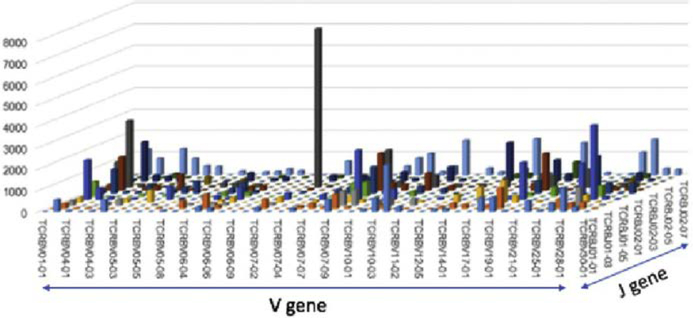
The single greatest advancement to aid in the diagnosis of MF/SS is the advent of high-throughput sequencing (HTS) of the TCRB gene, which permits identification of a T-cell clone through the sequence of its CDR3 region with superior sensitivity compared with traditional TCRG PCR (Fig. 7).3 It has also shown to be effective at discriminating between CTCL and benign inflammatory diseases when the frequency of the top T-cell clone is evaluated as the fraction of total nucleated cells.3 This analysis, however, is being used in a limited number of cancer centers at this time.
Fig. 7.
HTS of the TCR. TCR sequencing identifying expanded population of clonal malignant T-cell in a patient with patch-stage CTCL. The V versus J gene usages of T-cell from a patch MF lesion are shown. The gray peak includes the clonal malignant T-cell population and other benign T-cell that share the same V and J usage. (Image courtesy of Dr. John O’Malley.)
Given these diagnostic challenges, referral of patients to specialized multidisciplinary cutaneous lymphoma cancer centers is advised.
STAGING AND PROGNOSIS
Staging of MF/SS was initially set forth by the MF Cooperative Group of the American Joint Committee on Cancer.9 The International Society for Cutaneous Lymphomas (ISCL) and the EORTC in 2007 proposed a revision of the staging criteria, which was later validated in a single-center cohort of 1502 patients.9 The National Comprehensive Cancer Network (NCCN) has adapted the revised ISCL/EORTC recommendations for staging of MF/SS (Tables 2–4).
Table 2.
International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer classification of mycosis fungoides/Sézary syndrome
| TNMB Stages Definition | |
|---|---|
| T (Skin) | |
| T1 | Patches, papules, and/or plaques covering <10% BSA T1a patch only T1b plaque ± patch |
| T2 | Patches, papules, and/or plaques covering 2:10% BSA T2a patch only T2bplaque±patch |
| T3 | One or more tumors (at least one 1-cm-diameter solid or nodular lesion with evidence of depth and/or vertical growth |
| T4 | Confluence of erythema covering 2:80%BSA |
| N (Node) | |
| N0 | No clinically abnormal peripheral lymph nodes, biopsy not required |
| N1 | Clinically abnormal peripheral lymph nodes, histopathology Dutch grade 1 or NCI LN0–2 N1a clone negative N1b clone positive |
| N2 | Clinically abnormal peripheral lymph nodes, histopathology Dutch grade 2 or NCI LN3 N2a clone negative N2b clone positive |
| N3 | Clinically abnormal peripheral lymph nodes, histopathology Dutch grade 3–4 or NCI LN4, clone positive or negative |
| Nx | Clinically abnormal peripheral lymph nodes, no histopathologic confirmation |
| Visceral (M) | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathology, and organ is to be specified) |
| Blood |
|
| _____ | B0 |
| Absence of significant blood involvement: s5% of blood lymphocytes are Sézary cells B0a clone negative B0b clone positive |
|
| B1 | Low blood tumor burden: >5% of peripheral blood lymphocytes are Sézary cells but does not meet criteria for B2 disease B1a clone negative B1 b clone positive |
| B2* | High blood tumor burden: 2:100/uL Sézary cells with positive clone in blood (matching clone in skin) or positive clone and one of the following: 1. CD4/CD8 ratio of 10 or more 2. 2:40% CD41CD7- cells of total lymphocytes |
| 3. 2:30% CD41CD26- cells of total lymphocytes | |
Abbreviation: BSA, body surface area.
Republished with permission of American Society of Hematology, from Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110(6):1715; permission conveyed through Copyright Clearance Center, Inc.
Table 4.
World Health Organization/European Organization for Research and Treatment of Cancer staging of mycosis fungoides/Sézary syndrome
| Stage | T | N | M | B |
|---|---|---|---|---|
| IA | 1.0 | 0 | 0 | 0.1 |
| IB | 2.0 | 0 | 0 | 0.1 |
| II | 1.2 | 1.2 | 0 | 0.1 |
| IIB | 3.0 | 0–2 | 0 | 0.1 |
| IIIA | 4.0 | 0–2 | 0 | 0 |
| IIIB | 4.0 | 0–2 | 0 | 1.0 |
| IVA1 | 1–4 | 0–2 | 0 | 2.0 |
| IVA2 | 1–4 | 3.0 | 0 | 0–2 |
| IVB | 1–4 | 0–3 | 1 | 0–2 |
Republished with permission of American Society of Hematology, from Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110(6):1715; permission conveyed through Copyright Clearance Center, Inc.
Clinical stage is an important determinant of the risk of disease progression (RDP) and overall survival (OS).13 Patients with stage IA have a median survival of 35.5 years and a disease-specific survival (DSS) of 90% at 20 years, which is comparable with patients without MF. Although these patients have an indolent disease course, there is an 18% RDP at 20 years.13 Patients with stage IB have a median survival of 21.5 years, a DSS of 67%, and an RDP of 47% at 20 years.13 Patients with stage IIA have a median survival of 15.8 years, a DSS of 60%, and an RDP 41% at 20 years.13 Patients with stage IIB have a median survival of 4.7 years and a DSS of 56% at 5 years and 29% at 20 years.13 Their RDP is 48% by 5 years and 71% by 20 years.13 Patients with IIIA and IIIB have a median survival of 4.7 and 3.4 years, respectively, and a 10-year DSS of 45%.13 Their RDP is 53% and 82%, respectively.13 Patients with stage IVA1 have a median survival of 3.8 years, a DSS of 41% at 5 years and 20% at 10 years.13 Their RDP is 62% at 5 years.13 Patients with stage IVA2 have a median survival of 2.1 years and a DSS of 23% at 5 years and 20% at 10 years.13 Their RDP is 77% by 5 years.13 Patients with stage IVB have a median survival of 1.4 years with a DSS of 18% at 5 years.13
In this patient cohort, several prognostic factors were identified.13 Advanced age was associated with a higher RDP, poorer OS, and worse DSS. Skin (T) stage, B0b (compared with those with B0a), folliculotropic MF, large-cell transformation (LCT), and elevated lactate dehydrogenase (LDH) were independently associated with RDP, worse OS, and DSS. These prognostic factors gave rise to the prognostic index score, developed by the Cutaneous Lymphoma International Consortium study, for patients with advanced MF/SS.14 Stage IV, age greater than 60 years, large-cell transformation, and increased LDH were combined into a 3-tier prognostic index model. These risk groups had significantly different 5-year survival rates regardless of patient stage (IIB–IV): low risk (68%), intermediate risk (44%), and high risk (28%).
One of the greatest challenges in the management of MF is the identification of which early stage patients are at risk for disease progression. A significant advancement in identify these patients comes from the work of de Masson and colleagues.15 In this single-center retrospective study, the burden of malignant T-cell clone (tumor clone frequency [TCF]) in lesional skin predicted RDP and OS in early stage patients. A TCF of greater than 25% was significantly associated with progression-free survival (PFS) and OS. This measure was superior to predicting the PFS compared with stage (IB vs IA), presence of plaques, elevated LDH, age, and the presence of LCT. Furthermore, when patients at high risk of disease progression as determined by TCF were treated with radiation, a superior therapy capable of locally eliminating malignant disease, they had an improved OS (O’Malley and colleagues, submitted for publication).
Determination of malignant clonal burden by HTS has also been found important in determining outcomes following bone marrow transplantation.16
PATHOPHYSIOLOGY
Malignant T-Cell Origin
Although MF and SS have overlapping presentations and are not distinguished in the WHO/EORTC staging criteria, they are considered separate entities.11 The WHO/EORTC and the ISCL consider SS to be a clinical syndrome presenting with erythrodermic skin and leukemic disease.9 This consideration is in contrast to patients who initially present with classic skin lesions of MF and later meet the staging criteria for SS. The latter are referred to as leukemic MF, SS preceded by MF, or secondary SS. The NCCN considers patients with SS to be anyone who meets the criteria for a high blood burden of disease (B2 disease).
MF and SS classically arise from skin tropic memory CD41 T-cell (CD81 and CD4– CD8- subtypes may also be observed); but demonstration of different T-cell surface phenotypes and molecular profiles support the hypothesis that these malignancies originate from distinct memory T-cell subsets: the skin resident memory T-cell (TRM) in MF and the skin-tropic central memory T-cell (TCM) in SS.17
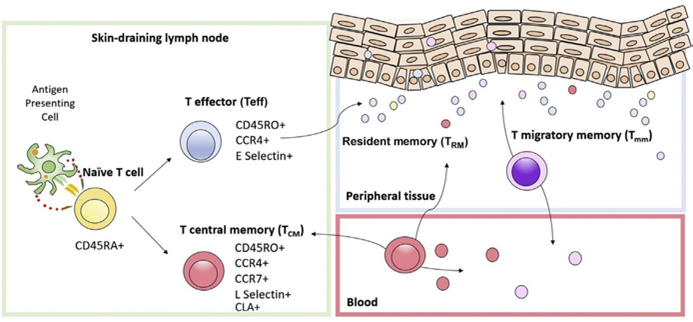
The average adult skin contains about 20 billion T-cell.18 These T-cell are normally present in noninflamed human skin.19 Most of these T-cell are memory T-cell; less than 5% are naïve.18 Naïve T-cell reside in the blood or lymph nodes.20 If naïve T-cell first encounter antigen in skin-draining lymph nodes, they proliferate clonally as effector T-cell and differentiate to express the skin homing addressin cutaneous lymphocyte antigen (CLA) and the C-C chemokine receptor 4 (CCR4) (Fig. 8).20 Once these effector T-cell eliminate their cognate antigen, they differentiate into memory T-cell (Fig. 8).20–22 Skin TCM cells are CCR41/CCR71/L-selectin1, which allows for circulation in skin, blood, and lymph nodes.22 Skin resident TRM cells are CCR41/CLA1 and lack CCR7 and L-selectin. They rarely circulate out of the skin.22 A subset of T-cell, termed the migratory memory T-cell (TMM), express CCR7 but not L-selectin and perhaps represent an intermediate phenotype recirculating more slowly out of the skin to blood compared with the TCM.22,23
Fig. 8.
Skin-tropic T-cell subtypes. Naïve T-cell differentiate into effector memory and central memory T-cell after binding to their cognate antigen on antigen presenting cells in skin-draining lymph nodes. Expression of surface ligand CCR4 determines their skin homing ability. Expression of CCR7/L-selectin determines their ability to re-circulate between blood and lymph node.
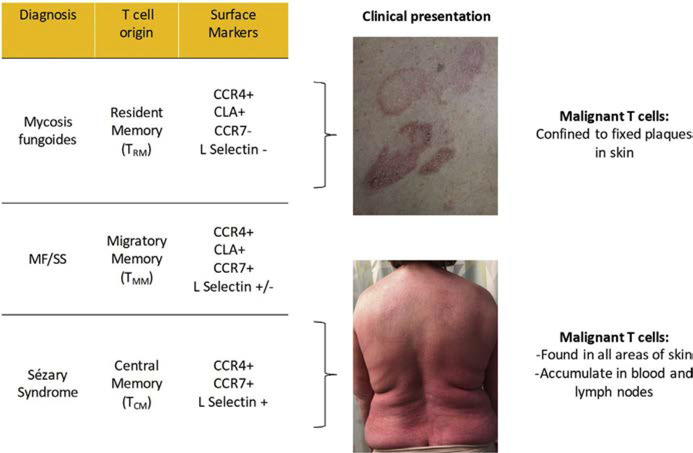
Campbell and colleagues17 showed that MF malignant T-cell are CCR41/CLA1/L-selectin-/CCR7-(TRM), whereas SS malignant T-cell are CCR41/L-selectin1/CCR71 (TCM). The molecular behavior of these T-cell types correlates with the clinical presentation of their malignant counterpart (Fig. 9). Skin TRM are nonmigratory populations, and clinically patients with MF have fixed skin lesions with discrete borders.20,24 In contrast, TCM recirculate between skin, blood, and lymph node; clinically patients with SS have diffuse erythema and leukemic disease.17,23 Patients with a TMM phenotype have ill-defined but discrete skin lesions.22,23 Interestingly, patients with a TMM phenotype do not respond to alemtuzumab as well as patients with a TCM phenotype. This therapy is effective only for leukemic disease; malignant TCM cells seem to recirculate into frequently.23
Fig. 9.
Distinct T-cell origins of MF and SS. Surface molecular phenotype correlates with clinical presentation and morphology of skin disease. TMM, migratory memory T-cell.
Genomic Alterations
MF/SS have diverse and complex genomic abnormalities, which have been best studied in SS. Striking findings include the discovery of many chromosomal abnormalities; somatic copy number variations (SCNVs) are favored over single nucleotide variants (SNVs) with 92% of all driver mutations arising from SCNVs.25 Chromosomal aberrations most often occur on chromosomes 8, 10, and 17.25–27 There is a high incidence of complex chromosomal structural rearrangements with more than 65% of patient samples exhibiting at least one chromothripsis-like rearrangement.25 Chromosomal instability may be favored because of abnormal DNA repair machinery, activation of RAG endonucleases, impaired cell cycle control, and widespread DNA hypomethylation.25,28,29 Most (74%) point mutations are C > T because of age-related and UVB-related mutagenesis.25
A meta-analysis of 220 genetically profiled patients with CTCL identified 55 driver mutations and implicated 14 biologically relevant pathways.28 Affected pathways broadly include those involved in T-cell activation, function, migration, and differentiation; chromatin modification; cell cycle, survival and proliferation; and DNA damage response (Table 5).25–28,30 Most genes are affected because of SCNV.25,30 Mutations within genes are comparably much less common across CTCL cohorts (Table 6).27,31 It is not surprising given the recurrent alterations of epigenetic modifiers that patients with SS exhibit marked hypomethylation and hypermethylation of CpG islands across the genome compared with patients with benign inflammatory dermatoses and solid tumor malignancies.29 Overall the SS methylome is most comparable with that of regulatory T-cell.29 Evaluation of open chromatin sites used to predict transcription factor binding sites in CTCL samples, using assay of transposase-accessible chromatin with sequencing, showed unique regulomes and chromatin dynamics in CTCL cells compared with benign host T-cell and healthy donor T-cell.32 Notable findings include decreased interferon gamma, interleukin (IL)-2, NFAT, and PIK3R1 (regulatory subunit of PI3K) expression in leukemic cells and gain of expression of HDAC9 and natural killer–kB in all samples with activation of 1 of 3 transcription factor motif patterns due to chromatin modification: Jun-AP1; CTCF; or EGR, SMAD, MYC, and KLF.32 Interestingly, differences in the chromatin accessibility landscape among leukemic cells predicted responses to HDAC inhibitors.32
Table 5.
Pathways affected in mycosis fungoldes/Sézary syndrome
| Biological Pathway | Affected Gene/Pathway |
|---|---|
| T-cell function, cytokine signaling | CD28, CARD11, PDCD1, PLCG1, RLTPR, PTPRN2, PRKCB, PRKCQ, CSNK1A1, CCR4, ZEB4, JAK1/2/3, STAT3/5B, TNFRSF1B, NFKB2, IRF4 |
| Chromatin modification | ARID1A, DNMT3A, KMT2C, KMT2D, SETDB2, TRRAP, TET1/2, KDM6A, NCOR1, BCOR, SMARCB1, CTCF |
| Cell cycle, survival, and proliferation | CDKN2A, CDKN1A, CDK4, MYC, RB1, RPSKA1, FAS, MAPK, and PI3K-Akt Dathwavs |
| DNA damage response | TP53, ATM |
Table 6.
Recurrently affected genes in mycosis fungoides/Sézary syndrome
| Affected by CNV (% of CTCL Samples) | Affected by SNV (% of CTCL Samples) |
|---|---|
| TP53 (92.5%) | MLL3 (4%–57%) |
| ZEB1 (65%) | TP53 (16%–43%) |
| STAT5B (63%) | ZEB1 (4%–27%) |
| ARID1A (58%) | STAT5B (2.77%–26.0%) |
| CDKN2A (40%) | ARID1A (8%–25%) |
| FAS (40%) | CARD11 (7%–22%) |
| DNMT3A (38%) | FAS (3%–19%) |
| ATM (30%) | PLCG1 (18%) |
| PRKCQ (30%) | CDKN2A (4%–17%) |
| TNFAIP3 (25%) |
Immunopathogenesis
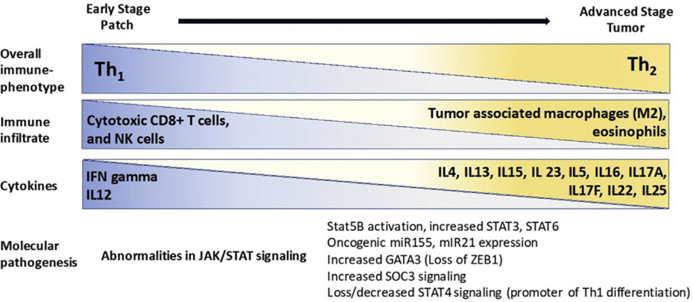
Patients with MF/SS are at increased risk of bacterial infection, especially in advanced stages, because of the disruption of the skin barrier by ulcerated tumors as well as depressed local and systemic immune response to pathogens.33 Immunosuppression is directly correlated with the malignant T-cell burden and is driven in part by abnormalities in the JAK/STAT signaling pathway (Fig. 10).27,34,35 The tumor microenvironment becomes skewed from a T-helper 1 to a T-helper 2 phenotype with advancing stages.34,36–38 These effects are reversible with depletion of malignant T-cell.39
Fig. 10.
Immunopathogenesis of MF/SS.
Cause
Although the cause of MF is unknown, the leading therapy is the chronic antigen stimulation theory first described in 1974 by Tan and colleagues.40 Chronic antigen or superantigen stimulation is thought to lead to clonal expansion of T-cell and malignant transformation. Several lines of observation support this theory. MF/SS is largely a malignancy of memory T-cell.17 Malignant T-cell depend on dendritic cells for survival and proliferation.41 The most frequent clonally expanded TCR vb gene is TRBV20 to 1, which is associated with recognition of Staphylococcus aureus.27 S aureus, in a series of patients with MF/SS, was able to act as a superantigen and stimulate proliferation of malignant T-cell.42 As yet, no heritable germ-line mutations have been identified. However, patients with psoriasis and atopic dermatitis, which has a known familial inheritance, are at a somewhat higher risk for MF/SS.43,44 Most patients with CTCL, however, have no antecedent T-cell–mediated skin disease. Many infectious agents have been investigated for putative roles in the cause of MF/SS. However, the data are limited and studies have yielded contradictory results to reliably implicate any single infectious agent, including HTLV-1 in CTCL.45,46 Recently, a more sophisticated genomic analysis of SS samples using VirCapSeq-VERT revealed no functional coding sequences for viral pathogens or unknown viruses or evidence for active infection.47 Interestingly, partially coding proviral sequences of human endogenous retroviruses (HERVs) were detected.47 Although these particles may play a pathogenic role in disease, given that HERVs are normally present in the human genome, demonstrating a causal role in CTCL will be challenging.48
TREATMENT
Treatment is often multidisciplinary, as it combines skin-directed (Table 7) and systemic therapies (Table 8). Although there are several therapies recognized by the NCCN for the treatment of MF/SS, there is a paucity of effective therapies providing durable responses. Targeted therapies have variable response rates ranging from 30% to 67%, with complete responses no higher than 41%.49–61
Table 7.
Skin-directed therapies for the treatment of mycosis fungoides/Sézary syndrome
| Skin-Directed Therapies | Overall Response Rate (%) |
|---|---|
| Topical superpotent corticosteroids | 75–95 |
| Bexarotene gel | 50–75 |
| Nitrogen mustard/mechlorethamine HCl gel | 50–90 |
| Imiquimod cream | 50 |
| Tazarotene cream | 58 |
| Narrow-band UVB | 54–90 |
| PUVA | 85–100 |
| Radiation therapy (local external electron beam, brachytherapy, total skin electron beam therapy) | — |
Abbreviations: HCl, hydrochloride; PUVA, psoralen UVA.
Table 8.
Systemic therapies used to treat mycosis fungoides/Sézary syndrome
| Systemic Therapies | Overall Response Rate |
|---|---|
| Bexarotene | ORR 45%, CR 13%14 |
| IFN a | ORR 64%; CR 27% in stage IA–IVA15 |
| Romidepsin | ORR 38%, CR 6%16 |
| Methotrexate | ORR 58%, CR 41% in erythrodermic MF17 ORR 33%, CR 12% in plaque-stage MF18 |
| Brentuximab vedotin | ORR 65%, CR 10%10 |
| Pralatrexate | ORR 41%, CR <1%19 |
| Doxorubicin | ORR 30% to 80%, CR 20% to 60%20,21 |
| Gemcitabine | ORR 51.0%–70.5%, CR 11.5%–23.0%22,23 |
| Pembrolizumab | ORR 38%, 1 CR24 |
| Bortezomib | ORR 67%, CR 17%25 |
Abbreviations: IFN, interferon; ORR, overall response rate.
Furthermore, although traditional chemotherapy may have a higher response rate, these gains are short-lived and associated with worse overall outcomes.62–64 Traditional nonmyeloablative allogeneic stem cell transplantation, the only potential cure for CTCL, has a 46% OS at 5 years.65 Recently, the Stanford transplantation regimen showed an overall response rate of 90% with a 2-year OS and PFS of 76% and 50%, respectively.66 There was a low incidence of graft-versus-host disease (GVHD) (23% grade II–IV acute GVHD and 23% with chronic GVHD at 2 years). Nonrelapse mortality due to GVHD or secondary malignancy at 1 year was 3%.66 An important predictor of successful transplantation is the degree of remission achieved before transplant. Because CR is more readily achieve in SS than in advanced MF, most successful transplants have been performed in patients with SS.
Given the limited efficacy of existing therapies, patients with advanced disease are encouraged to participate in clinical trials. Several agents are in clinical development for the treatment of MF/SS (Table 9).
Table 9.
Therapies in clinical development for cutaneous T-cell lymphoma
| Investigational Agent | Clinical Development |
|---|---|
| Mogamulizumab (anti-CCR4 antibody) | Phase III ( NCT01728805) |
| E7777 (cytotoxic IL-2 fusion protein) | Phase II ( NCT01871727) |
| MRG-106 (miR-155 antagonist) | Phase II ( NCT02580552) |
| Duvelisib (PI3K inhibitor) | Phase II ( NCT02783625) |
| Ruxolitinib (JAK 1/2 inhibitor) | Phase II ( NCT02974647) |
| TTI-621 (SIRPaFc IgG4, anti-CD47) | Phase I ( NCT02663518) |
SUMMARY
This is an exciting time in cutaneous oncology. The advent of HTS for TCR has enhanced our ability to diagnose MF/SS earlier, to predict which patients are at risk for disease progression, and to predict the treatment response to bone marrow transplantation. Major insights into disease biology have been made through genomic and epigenomic studies of MF/SS. There are increasing numbers of clinical trials for novel therapies for MF/SS.
Fig. 2.
Plaque-stage MF. Plaques are raised or indurated lesions. Affected acral sites are considered plaques.
Table 3.
Histopathologic staging of lymph nodes
| Classification | Dutch System | NCI Classification |
|---|---|---|
| N1 | Grade 1 : Dermatopathic lymphadenopathy | LN0: No atypical lymphocytes LN1: Occasional, Isolated atypical lymphocytes LN2: many atypical lymphocytes In 3–6cellclusters |
| N2 | Grade2: Dermatopathic lymphadenopathy, early involvement of MF | LN3: Aggregates of atypical lymphocytes; nodal architecture preserved |
| N3 | Grade 3: Partial effacement of LN architecture; many atypical cells Grade 4: Complete effacement |
LN4: Partial to complete effacement of nodal architecture by atypical lymphocytes |
Republished with permission of American Society of Hematology, from Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110(6):1715; permission conveyed through Copyright Clearance Center, Inc.
KEY POINTS.
Mycosis fungoides and Sézary syndrome are the most common non-Hodgkin lymphomas to arise from skin-tropic clonal T lymphocytes.
Significant advances have been made in understanding the genetic and epigenetic aberrations in mycosis fungoides and Sézary syndrome.
Diagnosis requires a combination of clinical, pathologic, and molecular features.
Several prognostic factors have been recognized to identify patients with poor prognosis.
Treatment is intended to minimize morbidity and limit disease progression, as cure is rarely achieved.
REFERENCES
- 1.Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol 2013;149(11):1295–9. [DOI] [PubMed] [Google Scholar]
- 2.Imam MH, Shenoy PJ, Flowers CR, et al. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma 2013;54(4):752–9. [DOI] [PubMed] [Google Scholar]
- 3.Kirsch IR, Watanabe R, O’Malley JT, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med 2015; 7(308):308ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol 2005;53(6):1053–63. [DOI] [PubMed] [Google Scholar]
- 5.Guitart J, Kennedy J, Ronan S, et al. Histologic criteria for the diagnosis of mycosis fungoides: proposal for a grading system to standardize pathology reporting. J Cutan Pathol 2001;28(4):174–83. [DOI] [PubMed] [Google Scholar]
- 6.Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use of transcriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res 2015;21(12):2820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litvinov IV, Tetzlaff MT, Thibault P, et al. Gene expression analysis in Cutaneous T-Cell Lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunology 2017;6(5):e1306618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Wang Y, Yu R, et al. Molecular markers of early-stage mycosis fungoides. J Invest Dermatol 2012;132(6):1698–706. [DOI] [PubMed] [Google Scholar]
- 9.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110(6):1713–22. [DOI] [PubMed] [Google Scholar]
- 10.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol 2011;29(18):2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105(10):3768–85. [DOI] [PubMed] [Google Scholar]
- 12.Ahn CS, ALSayyah A, Sangueza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol 2014;36(12):933–48 [quiz: 949–51]. [DOI] [PubMed] [Google Scholar]
- 13.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010;28(31):4730–9. [DOI] [PubMed] [Google Scholar]
- 14.Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and sezary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol 2015;33(32):3766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Masson A, O’Malley JT, Elco CP, et al. High-throughput sequencing of the T cell receptor beta gene identifies aggressive early-stage mycosis fungoides. Sci Transl Med 2018;10(440) [pii:eaar5894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng WK, Armstrong R, Arai S, et al. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Sci Transl Med 2013;5(214):214ra171. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JJ, Clark RA, Watanabe R, et al. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 2010;116(5):767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA1 T cells are resident in normal skin. J Immunol 2006;176(7):4431–9. [DOI] [PubMed] [Google Scholar]
- 19.Wang XN, McGovern N, Gunawan M, et al. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J Invest Dermatol 2014; 134(4):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol 2010;130(2):362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med 2015;7(269):269rv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 2015;7(279):279ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe R, Teague JE, Fisher DC, et al. Alemtuzumab therapy for leukemic cutaneous T-cell lymphoma: diffuse erythema as a positive predictor of complete remission. JAMA Dermatol 2014;150(7):776–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not re-circulate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 2012;4(117):117ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet 2015;47(9):1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat Genet 2015;47(12): 1465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Ni X, Covington KR, et al. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet 2015;47(12):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Yang J, Wenzel AT, et al. Genomic analysis of 220 CTCLs identifies a novel recurrent gain-of-function alteration in RLTPR (p.Q575E). Blood 2017;130(12): 1430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of sezary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol 2016;136(9):1876–84. [DOI] [PubMed] [Google Scholar]
- 30.Damsky WE, Choi J. Genetics of cutaneous t cell lymphoma: from bench to bedside. Curr Treat Options Oncol 2016;17(7):33. [DOI] [PubMed] [Google Scholar]
- 31.Chevret E, Merlio JP. Sezary syndrome: translating genetic diversity into personalized medicine. J Invest Dermatol 2016;136(7):1319–24. [DOI] [PubMed] [Google Scholar]
- 32.Qu K, Zaba LC, Satpathy AT, et al. Chromatin accessibility landscape of cutaneous T cell lymphoma and dynamic response to HDAC inhibitors. Cancer Cell 2017;32(1):27–41 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA 1992;267(10):1354–8. [PubMed] [Google Scholar]
- 34.Olsen EA, Rook AH, Zic J, et al. Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011;64(2):352–404. [DOI] [PubMed] [Google Scholar]
- 35.Netchiporouk E, Litvinov IV, Moreau L, et al. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014;13(21):3331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol 1994;103(5):669–73. [DOI] [PubMed] [Google Scholar]
- 37.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest 2005;115(4):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubio Gonzalez B, Zain J, Rosen ST, et al. Tumor microenvironment in mycosis fungoides and Sezary syndrome. Curr Opin Oncol 2016;28(1):88–96. [DOI] [PubMed] [Google Scholar]
- 39.Guenova E, Watanabe R, Teague JE, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res 2013;19(14):3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan RS, Butterworth CM, McLaughlin H, et al. Mycosis fungoides–a disease of antigen persistence. Br J Dermatol 1974;91(6):607–16. [DOI] [PubMed] [Google Scholar]
- 41.Berger CL, Hanlon D, Kanada D, et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood 2002;99(8):2929–39. [PubMed] [Google Scholar]
- 42.Krejsgaard T, Willerslev-Olsen A, Lindahl LM, et al. Staphylococcal enterotoxins stimulate lymphoma-associated immune dysregulation. Blood 2014;124(5): 761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelfand JM, Shin DB, Neimann AL, et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006;126(10):2194–201. [DOI] [PubMed] [Google Scholar]
- 44.Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol 2015;72(6):992–1002. [DOI] [PubMed] [Google Scholar]
- 45.Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. J Am Acad Dermatol 2011;64(2):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirvish JJ, Pomerantz RG, Falo LD Jr, et al. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clin Dermatol 2013;31(4):423–31. [DOI] [PubMed] [Google Scholar]
- 47.Anderson ME, Nagy-Szakal D, Jain K, et al. Highly sensitive virome capture sequencing technique VirCapSeq-VERT identifies partial noncoding sequences but no active viral infection in cutaneous T-cell lymphoma. J Invest Dermatol 2018;138(7):1671–3. [DOI] [PubMed] [Google Scholar]
- 48.Young GR, Stoye JP, Kassiotis G. Are human endogenous retroviruses pathogenic? An approach to testing the hypothesis. Bioessays 2013;35(9):794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2017. Eur J Cancer 2017;77:57–74. [DOI] [PubMed] [Google Scholar]
- 50.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001;19(9):2456–71. [DOI] [PubMed] [Google Scholar]
- 51.Olsen EA, Rosen ST, Vollmer RT, et al. Interferon alfa-2a in the treatment of cutaneous T cell lymphoma. J Am Acad Dermatol 1989;20(3):395–407. [DOI] [PubMed] [Google Scholar]
- 52.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010;28(29):4485–91. [DOI] [PubMed] [Google Scholar]
- 53.Zackheim HS, Kashani-Sabet M, Hwang ST. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. J Am Acad Dermatol 1996;34(4):626–31. [DOI] [PubMed] [Google Scholar]
- 54.Zackheim HS, Kashani-Sabet M, McMillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol 2003;49(5):873–8. [DOI] [PubMed] [Google Scholar]
- 55.Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390(10094):555–66. [DOI] [PubMed] [Google Scholar]
- 56.Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood 2012;119(18):4115–22. [DOI] [PubMed] [Google Scholar]
- 57.Quereux G, Marques S, Nguyen JM, et al. Prospective multicenter study of pegylated liposomal doxorubicin treatment in patients with advanced or refractory mycosis fungoides or Sezary syndrome. Arch Dermatol 2008;144(6):727–33. [DOI] [PubMed] [Google Scholar]
- 58.Zinzani PL, Baliva G, Magagnoli M, et al. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. J Clin Oncol 2000; 18(13):2603–6. [DOI] [PubMed] [Google Scholar]
- 59.Zinzani PL, Musuraca G, Tani M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol 2007;25(27):4293–7. [DOI] [PubMed] [Google Scholar]
- 60.Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol 2010; 21(4):860–3. [DOI] [PubMed] [Google Scholar]
- 61.Khodadoust M RA, Porcu P Pembrolizumab for treatment of relapsed/refrectory mycosis fungoides and Sezary Syndrome: clinical efficacy in a CITN multicenter phase 2 study [abstact]. Blood 2016;125(Abstract 181). [Google Scholar]
- 62.Hanel W, Briski R, Ross CW, et al. A retrospective comparative outcome analysis following systemic therapy in Mycosis fungoides and Sezary syndrome. Am J Hematol 2016;91(12):E491–5. [DOI] [PubMed] [Google Scholar]
- 63.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2017;92(10):1085–102. [DOI] [PubMed] [Google Scholar]
- 64.Hughes CF, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sezary syndrome: a comparative study of systemic therapy. Blood 2015;125(1):71–81. [DOI] [PubMed] [Google Scholar]
- 65.Duarte RF, Boumendil A, Onida F, et al. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol 2014;32(29):3347–8. [DOI] [PubMed] [Google Scholar]
- 66.Weng WKAR, Arai S, Johnston L, et al. Non-myeloablative allogeneic transplantation resulting in clinical and molecular remission with low Non-Relapse Mortality (NRM) in patients with advanced stage Mycosis Fungoides (MF) and Sézary Syndrome (SS). Blood 2014;124:2544.25171927 [Google Scholar]